Abstract
Pregnancy in sickle cell disease (SCD) patients is associated with increased risk of maternal and fetal mortality. This study determines pregnancy outcomes among women with SCD delivering at Korle-Bu Teaching Hospital, Accra, Ghana. Nine hundred sixty (960) medical records of pregnant women (131 HbSS, 112 HbSC, and 717 comparison group) from 2007 to 2008 were reviewed. The HbSS women were at increased risk of eclampsia (adjusted odds ratio [AOR] = 10.56, 95% confidence interval [CI] = 3.60–30.96, P < 0.001), intrauterine growth restriction (AOR = 4.00, 95% CI = 1.38–11.64, P = 0.011), and placenta previa (AOR = 22.03, 95% CI = 9.87–49.14, P < 0.001) compared with the comparison group. The HbSC women had increased risk for intrauterine fetal death (AOR = 3.38, 95% CI = 1.15–9.96, P = 0.027) and decreased risk of delivering low birth weight babies (AOR = 0.21, 95% CI = 0.06–0.73, P = 0.014). Women with SCD in Ghana are at a greater risk of morbidity and mortality in pregnancy compared with women without hemoglobinopathies. Improved maternal and fetal outcomes in Ghanaian women with SCD can be achieved through effective intervention by health care providers with thorough knowledge about predisposing factors toward adverse outcomes.
Introduction
Sickle cell disease (SCD) is the most common monogenetic disorder worldwide, affecting an estimated 30 million individuals and representing a major public health concern because of its associated significant morbidity and mortality.1 Sickle cell disease occurs in individuals homozygous for the βS globin gene (SS) or heterozygous for the βS allele and different abnormal β globin gene alleles, such as βC (SC), Sβ0 thalassemia, or Sβ+ thalassemia.2 Pregnant women with SCD are known to be at high risk of obstetrical complications and perinatal mortality as well as sickle-related complications.3–5 The maternal and fetal complications include prepartum and postpartum painful crises, pulmonary complications, anemia, preeclampsia, eclampsia, premature delivery with associated risks, and intrauterine growth restriction (IUGR).5–9 In developed countries, SCD pregnancies have better outcomes because of improvements in active SCD management throughout the pregnancy.2,4,10,11 However, even though sub-Saharan countries such as Ghana and Nigeria have a high prevalence of SCD and maternal mortality rates exceeding 9% such improvements are yet to be observed.7 Furthermore, lack of adequate management during pregnancy is thought to be the major factor responsible for the poor maternal and fetal outcomes among women with SCD in sub-Saharan countries compared with developed countries such as Great Britain and the United States.9,12–15 Despite improved management in SCD pregnancies, higher rates of cesarean delivery and mortality have been observed among women with SCD in the United States population.5
Pregnant SCD women are at an increased risk of sickle cell crisis, urinary tract infections, gestational diabetes, pneumonia, and anemia.12 Several studies on SCD pregnancy have focused on the risks to the fetus including preterm labor and IUGR; however, there are limited data on the maternal outcomes.5,11,15 Surprisingly, studies in Saudi Arabia and Jamaica found a significantly higher rate of preeclampsia and eclampsia in pregnancies complicated by SCD, even though better pregnancy outcome was observed compared with the studies conducted in Africa.16,17 The higher mortality rate among women with SCD in countries like Ghana may be caused by inadequate healthcare support particularly for pregnant women.18
In Ghana about 2% of neonates are affected by SCD leading to 14,000 new cases annually.18 Currently, there is no active management for SCD during pregnancy in Ghana and this may be due to a lack of data on the pregnancy outcomes among women with SCD.18 Examining the possible complications in pregnancy associated with SCD may provide insight into the management of SCD pregnancies in this country. The purpose of this study was to identify association between SCD and the occurrence of adverse maternal and fetal outcomes that are associated with pregnancy at Korle-Bu Teaching Hospital (KBTH), Accra, Ghana. Determining the association of SCD to maternal and perinatal outcomes among pregnant women will provide understanding of the unique reproductive health burden of SCD on maternal and infant health in Ghana, which may contribute to the basis for reducing the maternal and fetal mortality in the country. This study is the first report of obstetric and pregnancy outcomes of women with SCD in Ghana.
Methodology
This retrospective cohort study identified pregnancies among women with SCD (HbSS or HbSC genotypes) who received prenatal care and gave birth at the department of obstetrics and gynecology at KBTH, Accra, Ghana compared with pregnancies among women without any hemoglobinopathies (comparison). After ethical approval from Morehouse School of Medicine Institutional Review Board for human subject research and Ghana Health Service ethical review committee, a review was made of all available medical records of women delivering at the department of obstetrics and gynecology between January 2007 and December 2008 for prenatal and postnatal information. The exclusion criteria were women < 18 years of age, non-singleton pregnancies, incomplete or unavailable medical records, and presence of co-morbidities such as malaria or human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS). Hemoglobin genotypes were characterized by acid-agar electrophoresis. The genotypes were characterized as HbSS and HbSC, and women with no hemoglobinopathies were characterized as a comparison group. The following information was recorded for each woman: age, gravidity (number of pregnancies), and parity (number of birth with a gestational age of 24 weeks or more). Obstetric outcomes measured were gestational age at delivery, mode of delivery, preterm delivery (defined as a delivery < 37 weeks), premature rupture of membrane (PROM), antepartum hemorrhage (APH), gestational diabetes, intrauterine growth restriction (IUGR), cephalopelvic disproportion (CPD), pregnancy-induced hypertension (PIH), placenta previa, intrauterine fetal death (IUFD), intrapartum stillbirth, preeclampsia, and eclampsia. Perinatal outcomes included: birth weight (low birth weight [LBW] defined as birth weight < 2.5 kg), fetal heart rate, complications, and mortality. The SCD-related complications were also recorded. The following are the definitions of the measured outcomes; PROM – breaking of the membranous sac that surrounds the fetus (amniotic sac) before labor; APH – bleeding from the vagina during pregnancy from the 24th week gestational age to term; gestational diabetes –condition in which women without previously diagnosed diabetes exhibit high blood glucose levels during pregnancy; IUGR – poor growth of a baby while in the mother's womb during pregnancy; CPD – when a baby's head or body is too large to fit through the mother's pelvis; PIH – high blood pressure during pregnancy; preeclampsia – condition in which high blood pressure and protein in the urine develop after the 20th week of pregnancy; eclampsia – seizures (convulsions) in a pregnant woman that are not related to a preexisting brain condition; placenta previa – complication of pregnancy in which the placenta grows in the lowest part of the womb (uterus) and covers all or part of the opening to the cervix; IUFD – fetal deaths that occur after 28 weeks gestation and the fetus weighs more than 500 g; intrapartum stillbirth – a baby born dead without signs of skin disintegration or maceration and the death is assumed to have occurred < 12 hours before delivery.
Statistical Analysis
Data entry and management was conducted using SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL). Data were analyzed with Stata version 9.2 (Stata Corp., College Station, TX). Chart extraction characteristics and effect of SCD on pregnancy were compared using Pearson Chi-square (χ2) or one-way analysis of variance where appropriate to determine statistical differences between the groups of women (HbS and comparison). For instances in which there were too few subjects per cell for the χ2 test to be used, Fisher's exact test was used to compare discrete outcomes. Multivariate logistic regression was used to determine the odds ratio with 95% confidence of obstetrics outcomes. A P value of ≤ 0.05 was considered significant for all analysis.
Results
Study population.
There were 18,488 deliveries including multiple births such as twins and triplets made by 17,781 women at the Obstetrics and Gynecology department of KBTH from 2007 to 2008 of which 253 (1.42%) were women with SCD. There were 21 deaths among the women with SCD, which was ∼7.2% of all maternal deaths. The mortality rate for women with SCD was 83 deaths per 1,000 deliveries compared with a mortality rate of 6.9 deaths per 1,000 deliveries for women without any hemoglobinopathies. Following the selection criteria, 960 antenatal records were selected of which 243 were women with SCD (131 with HbSS and 112 with HbSC genotypes) and 717 randomly selected women without any hemoglobinopathy (comparison group) with a complete record to match the SCD group in terms of age, gravidity, and parity. The age distribution of the women ranged from 18 to 44 years of age with a mean of 28.8 years. The majority of the women were between 25 and 34 years of age. Women with SCD (mean age, 27.7 years for HbSS and 27.8 years for HbSC) were slightly younger than the comparison women with a mean age of 29.1 years. This difference, although small, was statistically significant (P < 0.001). About 56% of the women were multigravidae (woman who has been pregnant more than one time) and 44% were nulliparous (women who have not given birth to a baby) (Table 1). A majority of the HbSS women (58%) were primigravida (first time or have been pregnant one time) and nulliparous (58%) compared with comparison group (40.3%), P < 0.001. However, there were no significant differences in gravidity and parity among HbSC and comparison women (Table 1).
Table 1.
Characteristics of pregnant women at Korle-Bu Teaching Hospital 2007–2008
| Variable | HbSS (N = 131) | HbSC (N = 112) | Comparison group (N = 717) |
|---|---|---|---|
| Age | |||
| Mean ± SD | 27.7 ± 5.1* | 27.8 ± 4.9† | 29.1 ± 5.7 |
| 18–24 | 38 (29.0%)* | 32 (28.6%)† | 161 (22.5%) |
| 25–34 | 80 (61.1%)* | 69 (61.6%)† | 407 (56.8%) |
| ≥ 35 | 13 (9.9%)* | 11 (9.8%)† | 149 (20.8%) |
| Gravidity | |||
| Primigravida | 76 (58.0%)* | 53 (47.3%) | 284 (40.3%) |
| Multigravidae | 55 (42.0%)* | 59 (52.7%) | 420 (59.7%) |
| Parity | |||
| Nulliparous | 76 (58.0%)* | 53 (47.3%) | 284 (40.3%) |
| Primiparous | 29 (22.1%)* | 29 (25.9%) | 190 (27.0%) |
| Multiparous | 26 (19.8%)* | 30 (26.8%) | 230 (32.7%) |
Pearson χ2 test adjusting for Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few subjects per cell for the Pearson χ2 test to be used, Fisher's exact test was used to compare discrete outcomes. Statistical significance was set at P ≤ 0.05. SCD = sickle cell disease; SD = standard deviation.
P < 0.001 HbSS vs. Comparison.
P < 0.001 HbSC vs. Comparison.
Antenatal and pregnancy-related complications.
The major complications that were present among the pregnant women are shown in Table 2. Regarding hematologic complications, anemia defined as hemoglobin < 11 g/dL, was present in 8.4% of the HbSS pregnancies compared with 2.8% comparison pregnancies (P < 0.001). However, anemia was not observed among HbSC women. HbSS and HbSC women did not differ significantly in their SCD-related complications (Table 2). There were 19 women in sickle cell crisis (12 HbSS and 7 HbSC). About 3% of the HbSS women had urinary tract infection (UTI), pyelonephritis, or pneumonia and this is comparable with the comparison group. However, UTI is the only infection that was observed among the HbSC women (1.8%). The IUGR, eclampsia, preeclampsia, PIH, PROM, and placenta previa rates were significantly higher among HbSS women compared with the comparison group (P < 0.001) (Table 2). However, complications such as APH, gestational diabetes, CPD, and meconium staining were comparable between the two groups (HbSS and comparison group). The rate of eclampsia was higher in HbSC women (5.4%) compared with comparison group (1.0%), P < 0.001. The prevalence of PIH, PROM, and placenta previa were significantly higher among HbSS women compared with HbSC women (P < 0.0001). Although, the prevalence of other pregnancy-related complications was lower among HbSC women compared with HbSS and the comparison group, the differences were not significant (Table 2).
Table 2.
Antenatal and pregnancy-related complications reported among women delivering at Korle-Bu Teaching Hospital 2007–2008
| Complications | HbSS (N = 131) | HbSC (N = 112) | Comparison group (N = 717) |
|---|---|---|---|
| Hematologic | |||
| Anemia | 11 (8.4%)* | – | 20 (2.8%) |
| Sickle cell crisis | 12 (9.2%) | 7 (6.3%) | – |
| Infections | |||
| UTI | 2 (1.5%) | 2 (1.8%) | 6 (0.8%) |
| Cellulitis | – | – | 1 (0.1%) |
| Pyelonephritis | 1 (0.8%) | – | 1 (0.1%) |
| Pneumonia | 1 (0.8%) | – | 2 (0.3%) |
| Pregnancy-related complications | |||
| APH | 3 (2.3%) | 1 (0.9%) | 15 (2.1%) |
| Gestational diabetes | 1 (0.8%) | – | 7 (1.0%) |
| IUGR | 9 (6.9%)* | 2 (1.8%) | 14 (2.0%) |
| Eclampsia | 13 (9.9%)* | 6 (5.4%)‡ | 7 (1.0%) |
| Preeclampsia | 10 (7.6%)* | 7 (6.3%) | 20 (2.8%) |
| CPD | 6 (4.6%) | 1 (0.9%) | 22 (3.1%) |
| PIH | 23 (17.6%)*† | 4 (3.6%) | 19 (2.6%) |
| PROM | 18 (13.7%)*† | 5 (4.5%) | 15 (2.1%) |
| Placenta previa | 26 (19.8%)*† | 2 (1.8%) | 10 (1.4%) |
| Meconium staining | 1 (0.8%) | – | 8 (1.1%) |
Pearson Chi-square (χ2) test adjusting for Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few subjects per cell for the Pearson χ2 test to be used, Fisher's exact test was used to compare discrete outcomes. Statistical significance was set at P ≤ 0.05. APH = antepartum hemorrhage; CPD = cephalopelvic disproportion; UTI = urinary tract infection; IUGR = intrauterine growth restriction; PIH = pregnancy-induced hypertension; PROM = premature rapture of membrane.
P < 0.0001 HbSS vs. Comparison.
P < 0.0001 HbSS vs. HbSC.
P < 0.001 HbSC vs. Comparison.
From the logistic regression analysis using the comparison group as the reference group, pregnancy-related complications such as anemia (adjusted odds ratio [AOR] = 3.20, 95% confidence interval [CI] = 1.23–8.31, P < 0.017); preeclampsia (AOR = 5.58, 95% CI = 2.14–14.53, P < 0.001); eclampsia (AOR = 10.56, 95% CI = 3.60–30.96, P < 0.001); IUGR (AOR = 4.00, 95% CI = 1.38–11.64, P < 0.011); PIH (AOR = 10.54, 95% CI = 4.94–22.47, P < 0.001); PROM (AOR = 9.11, 95% CI = 4.06–20.45, P < 0.001); and placenta previa (AOR = 22.03, 95% CI = 9.87–49.14, P < 0.001) were associated with increased likelihood of occurrence in pregnant HbSS women (Table 3). However, there were no significant associations of APH, CPD, gestational diabetes, or meconium staining with HbSS phenotype (Table 3). Interestingly, eclampsia was the only pregnancy-related complication that was associated with HbSC phenotype (AOR = 5.39, 95% CI = 1.74–16.65, P < 0.003); however, the risk is lower than for the HbSS phenotype (Table 3).
Table 3.
Logistic regression analysis of antenatal and pregnancy-related complications occurring at time of delivery among women with SCD
| Diagnosis | HbSS | HbSC | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | P value | AOR | 95% CI | P value | |
| APH | 1.79 | 0.43–7.40 | 0.424 | 0.53 | 0.07–4.10 | 0.543 |
| Anemia | 3.20 | 1.23–8.31 | 0.017 | – | – | – |
| Preeclampsia | 5.58 | 2.14–14.53 | < 0.001 | 2.33 | 0.93–5.83 | 0.070 |
| Eclampsia | 10.56 | 3.60–30.96 | < 0.001 | 5.39 | 1.74–16.65 | 0.003 |
| CPD | 1.14 | 0.33–3.97 | 0.839 | 0.28 | 0.04–2.11 | 0.216 |
| IUGR | 4.00 | 1.38–11.64 | 0.011 | 0.89 | 0.20–3.99 | 0.876 |
| Gestational diabetes | 0.39 | 0.02–6.71 | 0.513 | – | – | – |
| PIH | 10.54 | 4.94–22.47 | < 0.001 | 1.51 | 0.49–4.59 | 0.472 |
| PROM | 9.11 | 4.06–20.45 | < 0.001 | 2.08 | 0.72–6.01 | 0.175 |
| Placenta previa | 22.03 | 9.87–49.14 | < 0.001 | 1.30 | 0.28–6.06 | 0.739 |
| Meconium staining | 0.12 | 0.00–33.72 | 0.461 | – | – | – |
The adjusted odds ratio (AOR) and 95% confidence interval (CI) were obtained with logistic regression in multivariate analyses adjusted for age, parity, and other variables in the study. Statistical significance was set at P ≤ 0.05. Model I was used for the logistic regression analysis. The reference for hypertension was pregnant women without hypertension, anemia was pregnant women without anemia, eclampsia was pregnant women without eclampsia, and that of intrauterine fetal death was women without intrauterine fetal death. APH = antepartum hemorrhage; CPD = cephalopelvic disproportion; IUGR = intrauterine growth restriction; PIH = pregnancy-induced hypertension; PROM = premature rapture of membrane.
Maternal and perinatal outcomes.
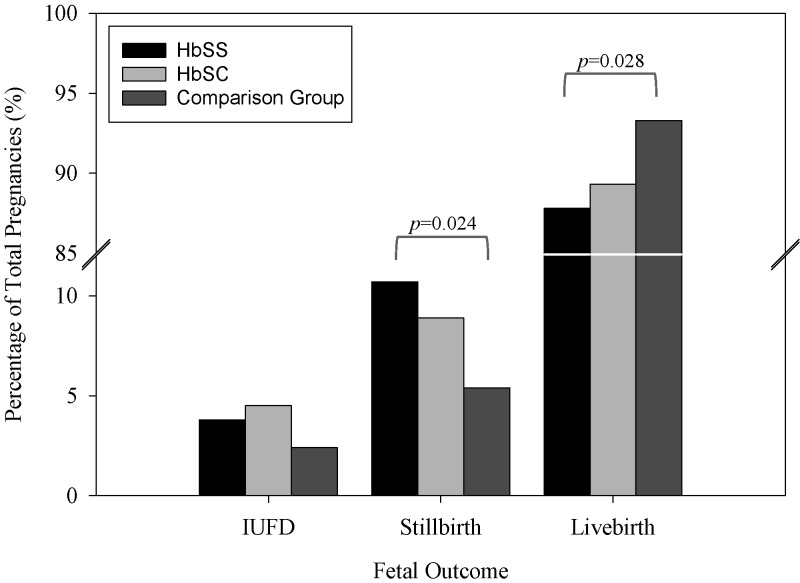
Table 4 shows the maternal and perinatal outcomes among the women at KBTH. There were a total of 884 live births (115 HbSS, 100 HbSC, and 669 comparison group) in the study population (Table 4 and Figure 1). However, the rates were lower among HbSS women (87.8%) compared with the comparison group (93.3%), P = 0.028 (Figure 1). The occurrence of preterm delivery (birth at < 37 weeks gestation) and the mean birth weight of infants delivered to the women were comparable across the groups (Table 4). However, the prevalence of LBW babies was lower among HbSC women (3.0%) compared with HbSS (12.2%) and comparison women (13.8%), P < 0.05 (Table 4). There was no difference in the rate of LBW deliveries between the HbSS and comparison groups. In addition, there were higher rates of transfers of infants to the neonatal intensive care unit (NICU) caused by fetal complications among the HbSS (25.2%) compared with comparison group (14.9%), P < 0.001 (Table 4). Furthermore, the incidence of fetal distress was higher among infants delivered to HbSS women (9.6%) compared with comparison group (2.5%), P < 0.001 (Table 4). There were no significant differences across the groups with regard to fetal complications such as grunting respiration and asphyxia. Nearly 50% of both HbSS and SC women had cesarean section (CS) compared with 33% of the comparison group (P < 0.001), Table 4. There were no significant differences between the groups with regard to IUFD (Figure 1). However, the rates of intrapartum stillbirth were higher among HbSS women (10.7%) compared with comparison group (5.4%), P = 0.024 (Figure 1). Logistic regression analysis confirmed that HbSS (AOR = 1.76, 95% CI = 1.20–2.68, P = 0.008) and HbSC women (AOR = 2.60, 95% CI = 1.60–4.22, P < 0.001) were more likely to undergo CS compared with comparison group (Table 5). Infants delivered by HbSS women are more likely to experience fetal distress (AOR = 3.26, 95% CI = 1.43–7.44, P = 0.005). Additionally, HbSS (AOR = 2.43, 95% CI = 1.23–4.82, P = 0.011) and HbSC women (AOR = 2.25, 95% CI = 1.04–4.90, P = 0.040) were more likely to experience intrapartum stillbirth and HbSC women (AOR = 3.38, 95% CI = 1.15–9.96, P = 0.027) were at an increased risk of experiencing IUFD (Table 5). Interestingly, HbSC (AOR = 0.21, 95% CI = 0.06–0.73, P = 0.014) women had a reduced risk of delivering LBW babies compared with the comparison group (Table 5).
Table 4.
Maternal and perinatal outcomes at Korle-Bu Teaching Hospital 2007–2008
| Outcomes | HbSS | HbSC | Comparison group |
|---|---|---|---|
| No. of live births (n) | 115 | 100 | 669 |
| Gestational age* | |||
| Full-term birth | 85 (73.9%) | 78 (78.0%) | 485 (74.4%) |
| Preterm birth | 30 (26.1%) | 20 (20.0%) | 167 (25.6%) |
| Birth weight (kg)* | |||
| Mean ± SD | 3.0 ± 0.6 | 3.0 ± 0.7 | 3.0 ± 0.7 |
| < 2.5 | 14 (12.2%)† | 3 (3.0%)‡ | 92 (13.8%) |
| ≥ 2.5 | 89 (77.4%)† | 79 (79.0%)‡ | 525 (78.5%) |
| Fetal heart rate* | |||
| Mean ± SD | 130.1 ± 34.3 | 133.0 ± 31.7 | 135.0 ± 25.7 |
| Complication* | |||
| NICU | 29 (25.2%)§ | 16 (16.0%) | 100 (14.9%) |
| Grunting respiration | 3 (2.6%) | – | 8 (1.2%) |
| Asphyxia | 3 (2.6%) | 3 (3.0%) | 10 (1.5%) |
| Fetal distress | 11 (9.6%)§ | 3 (3.0%) | 17 (2.5%) |
| Mode of delivery* | |||
| Vaginal | 59 (51.3%)§ | 51 (51.0%)‡ | 448 (67.0%) |
| Cesarean | 56 (48.7%)§ | 49 (49.0%)‡ | 221 (33.0%) |
Pearson Chi-square (χ2) test adjusting for Yates correction for continuity where appropriate were used to compare for statistical differences among the pregnant women. For instances in which there were too few subjects per cell for the Pearson χ2 test to be used, Fisher's exact test was used to compare discrete outcomes. Statistical significance was set at P ≤ 0.05. NICU = neonatal intensive care unit; IUFD = intrauterine fetal death; SD = standard deviation.
Live births only.
P < 0.02 HbSS vs. HbSC.
P < 0.001 HbSC vs. Comparison.
P < 0.001 HbSS vs. Comparison.
Figure 1.
Birth outcomes at Korle-Bu Teaching Hospital 2007–2008. Pearson Chi-square (χ2) test adjusting for Yates correction for continuity where used to compare for statistical differences among different groups of birth outcomes. For instances in which there were too few subjects per cell for the Pearson χ2 test to be used, Fisher's exact test was used. Statistical significance was set at P ≤ 0.05. IUFD = intrauterine fetal death.
Table 5.
Logistic regression analysis of maternal and perinatal outcomes among patients with SCD
| Diagnosis | HbSS | HbSC | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | P value | AOR | 95% CI | P value | |
| Cesarean | 1.76 | 1.20–2.68 | 0.008 | 2.60 | 1.60–4.22 | < 0.001 |
| Preterm birth | 1.14 | 0.66–1.97 | 0.648 | 1.06 | 0.54–2.08 | 0.872 |
| LBW | 0.85 | 0.44–1.61 | 0.612 | 0.21 | 0.06–0.73 | 0.014 |
| NICU | 1.63 | 0.95–2.80 | 0.079 | 1.12 | 0.58–2.18 | 0.732 |
| Grunting respiration | 1.11 | 0.25–5.01 | 0.889 | – | – | – |
| Asphyxia | 1.09 | 0.72–4.37 | 0.903 | 1.62 | 0.41–6.46 | 0.492 |
| Fetal Distress | 3.26 | 1.43–7.44 | 0.005 | 0.78 | 0.20–3.00 | 0.716 |
| IUFD | 1.20 | 0.34–4.21 | 0.779 | 3.38 | 1.15–9.96 | 0.027 |
| Stillbirth | 2.43 | 1.23–4.82 | 0.011 | 2.25 | 1.04–4.90 | 0.040 |
The adjusted odds ratio (AOR) and 95% confidence interval (CI) were obtained with logistic regression in multivariate analyses adjusted for age, parity, and other variables in the study. Statistical significance was set at P ≤ 0.05. Model I was used for the logistic regression analysis. The reference for cesarean section was vaginal delivery. LBW = low birth weight; NICU = neonatal intensive care unit.
Discussion
It is well established that women with SCD have an increased risk of maternal and fetal complications during pregnancy compared with healthy women.11,19 In Africa, a mortality rate of 7–12% persists7,13,20,21 as a result of SCD in pregnancy, reflecting limited services and inadequate antenatal care.19 We report for the first time the outcome of pregnant women with SCD delivering at KBTH and compare these outcomes with a comparison group of women with no hemoglobinopathies. Unlike other studies in developed countries such as in the United States,11,15,22 this study indicates that there is still a significantly higher maternal mortality in women with SCD compared with women having no documented hemoglobinopathies. The SCD mortality rate was ∼7.2% of all maternal mortality, which is similar to what has been observed in other studies in Africa,7,13 suggesting that in sub-Saharan Africa, the health of women with SCD is severely compromised during pregnancy.
Pregnancy has been shown to exacerbate sickle cell crises5 and increase the rate of hospitalization. Recent study indicates that sickle cell crisis occurred in over 50% of the pregnant women with SCD.23 However, about 8% of sickle cell crises were observed in this study, which is consistent with a cooperative study conducted in the United States.11
Although the mechanisms for preterm delivery among women with SCD remain unknown, several studies have shown an increased risk of preterm delivery among women with SCD.5,22,24,25 However, in the current study there were no associations between SCD and preterm delivery. The CS deliveries were more likely to be performed for pregnant women with SCD than for the comparison group. The CS deliveries are likely to be prearranged more often in SCD because of fetal compromise and previous history. Closer fetal monitoring and a lower threshold for tolerating abnormal fetal heart rate patterns, may also contribute to this trend.5 The CS deliveries may also be performed as a result of fetal distress, failure of labor to progress, or discretionary repeated need for surgery. However, because of the retrospective nature of this study, differences between elective and emergency CS deliveries could not be established. It is known that women with SCD have increased risk of IUGR (abnormally slow growth).3,6,16,19,20,22,26–28 In this study the occurrence of IUGR was higher among HbSS women than HbSC and comparison women. In addition, HbSS women were at increased risk of IUGR compared with comparison women. This increased risk could be caused by chronic maternal anemia and increased blood viscosity or degree of sickling and vaso-occlusion in the placental circulation.27,29 Reduced oxygen content of blood in maternal anemia can affect placental perfusion and thereby increase the risk of IUGR.30 We did not observe associated risk of IUFD among HbSS women. One explanation could be improved fetal monitoring and the increased likelihood of interventions.5 However, there was an increased risk of IUFD among HbSC women. The SCD women are more at risk of intrapartum stillbirth compared with the comparison group. The pathological basis for an increased intrapartum stillbirth rate and IUGR in women with SCD is unclear.16 Blockage of blood flow caused by vaso-occlusion in the placenta may lead to placental infarction and insufficient placental function affecting the supply of nutrients and metabolic exchange to the growing fetus.16,31 In addition, the effect of chronic maternal anemia and high incidence of PIH in SCD women may contribute to increased risk of intrapartum stillbirth.16
Urinary tract infection and placenta previa have been reported as more common among women with SCD in some studies but was not confirmed in this study.19,28 However, incidence of PIH was high among HbSS women and they were more at risk compared with the comparison group.
The HbSS women were at a greater risk of being anemic. Anemia is one of the major complications of sickle cell disease and may be caused by hemolysis or trapping of the red blood cells in the spleen.30 Anemia in pregnancy has been found to be associated with increased risk for PROM, spontaneous preterm labor, preterm delivery, poor intrauterine growth, and LBW infants,32–38 which in turn results in higher perinatal morbidity and mortality, and a higher infant mortality rate.
Preeclampsia was higher for the HbSS group than among the comparison group, which is consistent with previous studies.39,40 The HbSS women were also more at risk of preeclampsia, which was not observed among the HbSC group. Eclampsia is an acute and life-threatening complication of pregnancy, characterized by the appearance of tonic-clonic seizures, usually occurs in patients who have developed preeclampsia41 and was observed 10 times more among HbSS and 5 times more among HbSC women than the comparison group. This finding is consistent with other reports from the literature.5,6,42
SCD in pregnancy has been associated with LBW infants and has been observed in 28–42% of cases in Jamaica19,43 and 38% of cases in the United States.11 Factors associated with LBW among SCD mothers are varied and inconsistent but include lower gestational age, lower maternal age, lower maternal weight, history of preeclampsia, history of acute anemic episodes, and a number of SCD-related complications during pregnancy.11,19 In this study, there was a lower incidence of LBW deliveries among HbSC women compared with HbSS women and the comparison group. Surprisingly, HbSC women had a reduced risk of delivering LBW babies. The higher rate of LBW babies among HbSS women may be caused by lower maternal weight, history of preeclampsia, and history of acute anemic episodes, which could be influenced by a high frequency of early surgical delivery or premature induction, which are common interventions in management of pregnancy among women with SCD.31 Women with HbSS genotype are known to have a higher incidence of abnormal velocimetry in the uteroplacental circulation,44 which may be related to the sickling process in the uteroplacental vessels and abnormally high blood viscosity.2 This is consistent with the persistence of higher rates of preeclampsia and LBW babies in HbSS women.2
It is known that HbSC women have lower mortality and morbidity rates than HbSS women.45,46 Pregnancy outcome in HbSC disease is reported to be generally benign compared with HbSS disease.2 In a Jamaican cohort study followed from birth, pregnancy outcome in HbSC women did not differ from that of the comparison group for the prevalence of preeclampsia.47 The study also found more live births and greater birth weight in the HbSC women compared with HbSS women. The current study shows better obstetrical outcomes for HbSC women than HbSS women, which is consistent with reported studies.47 There were no significant differences in antenatal and pregnancy-related complications and pregnancy and fetal outcomes between HbSC women and the comparison group, except for eclampsia, LBW babies (lower in HbSC), and mode of delivery (number of cesareans). However, sickle-related complications were fewer for HbSC than HbSS women.
We acknowledge that there are practical concerns limiting our ability to fully interpret these results, in particular, the retrospective chart review limited the nature and amount of data collected and thus, the conclusions that can be drawn. Complete data were unavailable for most of the pregnancies, especially among comparison women, which could potentially introduce some level of bias to the conclusions. Certainly a retrospective cohort study of this nature, would often exclude such details as close monitoring of SCD impact during pregnancy and/or fetal outcome after birth. However, the study provides insights into the risk of complications that can burden pregnant women with SCD in Ghana. In addition, this study illustrates how longitudinally linked data are useful for assessing factors associated with adverse conditions such as SCD. There is certainly need for a further detailed prospective study in this population.
Conclusion
This is the first study examining the effects of SCD on pregnancy in Ghana. The study has identified association between SCD and the occurrence of adverse maternal and fetal outcomes that are associated with pregnancy at KBTH, Accra, Ghana. These results raise concern about the quality of medical care received by patients with SCD in Ghana. Although further research is needed to understand the specific biologic mechanisms that contribute to SCD and adverse pregnancy outcomes, there are current opportunities to improve care to women with SCD through improved preconception observation and counseling, education and prevention of high-risk behaviors, early and adequate prenatal care, and appropriate identification and treatment of pregnancy complications. Comprehensive care may promote awareness of SCD among affected women to present early for assessment and management of symptoms to medical and midwifery staff, who can provide continuity of care. This approach should result in early identification and management of acute problems by the appropriate caregivers in the appropriate place.
ACKNOWLEDGMENTS
We thank the pregnant women delivering at Korle-Bu Teaching Hospital whose medical records were reviewed for this study, the nurses of Obstetrics and Gynecology Department of Korle-Bu Teaching Hospital, the staff of Ghana Ministry of Health, Reproductive and Child Health Department, and the staff of Ghana National Malaria Control Program for their assistance. The support of Center for Disease Diagnosis and Research in Ghana is acknowledged.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention “Reproductive Epidemiological” grant to the Master of Public Health Program, Morehouse School of Medicine; the Minority International Health Disparities Research Training Program at Howard University; National Institutes of Health grants NIH-RCMI (RR03034), NIHNIGM-MBRS (SO6GM08248), and NIH-FIC (R21TW006804-01).
Authors' addresses: Nana O. Wilson, Jacqueline M. Hibbert, Adel Driss, and Jonathan K. Stiles, Morehouse School of Medicine, Department of Microbiology, Biochemistry and Immunology, Atlanta, GA, E-mails: nwilson@msm.edu, jhibbert@msm.edu, adriss@msm.edu, and jstiles@msm.edu. Fatou K. Ceesay, Morehouse School of Medicine, Department of Community Health and Preventive Medicine, Atlanta, GA, E-mail: fceesay@msm.edu. Samuel A. Obed, University of Ghana Medical School, Department of Obstetrics and Gynecology, Korle-Bu, Accra, Ghana, E-mail: obedamenyi@yahoo.com. Andrew Adjei and Richard K. Gyasi, University of Ghana Medical School, Department of Pathology, Korle-Bu, Accra, Ghana, E-mails: andrewanthonyadjei@yahoo.com and rkg539us@yahoo.com. Winston A. Anderson, Howard University, Department of Biology, Washington, DC, E-mail: wanderson@howard.edu.
References
- 1.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 2.Ngo C, Kayem G, Habibi A, Benachi A, Goffinet F, Galacteros F, Haddad B. Pregnancy in sickle cell disease: maternal and fetal outcomes in a population receiving prophylactic partial exchange transfusions. Eur J Obstet Gynecol Reprod Biol. 2010;152:138–142. doi: 10.1016/j.ejogrb.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Hassell K. Pregnancy and sickle cell disease. Hematol Oncol Clin North Am. 2005;19:903–916. doi: 10.1016/j.hoc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Koshy M. Sickle cell disease and pregnancy. Blood Rev. 1995;9:157–164. doi: 10.1016/0268-960x(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 5.Villers MS, Jamison MG, De Castro LM, James AH. Morbidity associated with sickle cell disease in pregnancy. Am J Obstet Gynecol. 2008;199:125. doi: 10.1016/j.ajog.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Afolabi BB, Iwuala NC, Iwuala IC, Ogedengbe OK. Morbidity and mortality in sickle cell pregnancies in Lagos, Nigeria: a case control study. J Obstet Gynaecol. 2009;29:104–106. doi: 10.1080/01443610802667112. [DOI] [PubMed] [Google Scholar]
- 7.Dare FO, Makinde OO, Faasuba OB. The obstetric performance of sickle cell disease patients and homozygous hemoglobin C disease patients in Ile-Ife, Nigeria. Int J Gynaecol Obstet. 1992;37:163–168. doi: 10.1016/0020-7292(92)90376-t. [DOI] [PubMed] [Google Scholar]
- 8.El-Shafei MA, Dhaliwal JK, Sandhu AK. Pregnancy in sickle cell disease in Bahrain. Br J Obstet Gynaecol. 1992;99:101–104. doi: 10.1111/j.1471-0528.1992.tb14463.x. [DOI] [PubMed] [Google Scholar]
- 9.Poddar D, Maude GH, Plant MJ, Scorer H, Serjeant GR. Pregnancy in Jamaican women with homozygous sickle cell disease. Fetal and maternal outcome. Br J Obstet Gynaecol. 1986;93:727–732. [PubMed] [Google Scholar]
- 10.Koshy M, Chisum D, Burd L, Orlina A, How H. Management of sickle cell anemia and pregnancy. J Clin Apher. 1991;6:230–233. doi: 10.1002/jca.2920060412. [DOI] [PubMed] [Google Scholar]
- 11.Smith JA, Espeland M, Bellevue R, Bonds D, Brown AK, Koshy M. Pregnancy in sickle cell disease: experience of the Cooperative Study of Sickle Cell Disease. Obstet Gynecol. 1996;87:199–204. doi: 10.1016/0029-7844(95)00367-3. [DOI] [PubMed] [Google Scholar]
- 12.Howard RJ, Tuck SM, Pearson TC. Pregnancy in sickle cell disease in the UK: results of a multicentre survey of the effect of prophylactic blood transfusion on maternal and fetal outcome. Br J Obstet Gynaecol. 1995;102:947–951. doi: 10.1111/j.1471-0528.1995.tb10900.x. [DOI] [PubMed] [Google Scholar]
- 13.Odum CU, Anorlu RI, Dim SI, Oyekan TO. Pregnancy outcome in HbSS-sickle cell disease in Lagos, Nigeria. West Afr J Med. 2002;21:19–23. [PubMed] [Google Scholar]
- 14.Omo-Aghoja IO, Okonofua FE. Pregnancy outcome in women with sickle cell: a five year review. Niger Postgrad Med J. 2007;14:151–154. [PubMed] [Google Scholar]
- 15.Sun PM, Wilburn W, Raynor BD, Jamieson D. Sickle cell disease in pregnancy: twenty years of experience at Grady Memorial Hospital, Atlanta, Georgia. Am J Obstet Gynecol. 2001;184:1127–1130. doi: 10.1067/mob.2001.115477. [DOI] [PubMed] [Google Scholar]
- 16.Al Jama FE, Gasem T, Burshaid S, Rahman J, Al Suleiman SA, Rahman MS. Pregnancy outcome in patients with homozygous sickle cell disease in a university hospital, eastern Saudi Arabia. Arch Gynecol Obstet. 2009;280:793–797. doi: 10.1007/s00404-009-1002-7. [DOI] [PubMed] [Google Scholar]
- 17.Serjeant GR, Serjeant BE, Mason KP, Hambleton IR, Fisher C, Higgs DR. The changing face of homozygous sickle cell disease: 102 patients over 60 years. Int J Lab Hematol. 2009;31:585–596. doi: 10.1111/j.1751-553X.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization Management of Haemoglobin Disorders; Report of a Joint WHO-TIF Meeting; Nicosia, Cyprus. 16–18 November 2007.2008. [Google Scholar]
- 19.Serjeant GR, Loy LL, Crowther M, Hambleton IR, Thame M. Outcome of pregnancy in homozygous sickle cell disease. Obstet Gynecol. 2004;103:1278–1285. doi: 10.1097/01.AOG.0000127433.23611.54. [DOI] [PubMed] [Google Scholar]
- 20.Hendrickse JP, Watson-Williams EJ, Luzzatto L, Ajabor LN. Pregnancy in homozygous sickle-cell anaemia. J Obstet Gynaecol Br Commonw. 1972;79:396–409. doi: 10.1111/j.1471-0528.1972.tb14177.x. [DOI] [PubMed] [Google Scholar]
- 21.Ndugwa CM. Pregnancy in sickle cell anaemia in Uganda (1971–1980) East Afr Med J. 1982;59:320–326. [PubMed] [Google Scholar]
- 22.Barfield WD, Barradas DT, Manning SE, Kotelchuck M, Shapiro-Mendoza CK. Sickle cell disease and pregnancy outcomes: women of African descent. Am J Prev Med. 2010;38:S542–S549. doi: 10.1016/j.amepre.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Ocheni S, Onah HE, Ibegbulam OG, Eze MI. Pregnancy outcomes in patients with sickle cell disease in Enugu, Nigeria. Niger J Med. 2007;16:252–255. [PubMed] [Google Scholar]
- 24.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 25.Nabukera SK, Wingate MS, Owen J, Salihu HM, Swaminathan S, Alexander GR, Kirby RS. Racial disparities in perinatal outcomes and pregnancy spacing among women delaying initiation of childbearing. Matern Child Health J. 2009;13:81–89. doi: 10.1007/s10995-008-0330-8. [DOI] [PubMed] [Google Scholar]
- 26.Adam S, Jonassaint J, Kruger H, Kail M, Orringer EP, Eckman JR, Ashley-Koch A, Telen MJ, De Castro LM. Surgical and obstetric outcomes in adults with sickle cell disease. Am J Med. 2008;121:916–921. doi: 10.1016/j.amjmed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charache S, Niebyl JR. Pregnancy in sickle cell disease. Clin Haematol. 1985;14:729–746. [PubMed] [Google Scholar]
- 28.Seoud MA, Cantwell C, Nobles G, Levy DL. Outcome of pregnancies complicated by sickle cell and sickle-C hemoglobinopathies. Am J Perinatol. 1994;11:187–191. doi: 10.1055/s-2008-1040742. [DOI] [PubMed] [Google Scholar]
- 29.Serjeant GR. Treatment of sickle cell disease in early childhood in Jamaica. Am J Pediatr Hematol Oncol. 1985;7:235–239. doi: 10.1097/00043426-198507030-00005. [DOI] [PubMed] [Google Scholar]
- 30.Yu CK, Stasiowska E, Stephens A, Awogbade M, Davies A. Outcome of pregnancy in sickle cell disease patients attending a combined obstetric and haematology clinic. J Obstet Gynaecol. 2009;29:512–516. doi: 10.1080/01443610903003175. [DOI] [PubMed] [Google Scholar]
- 31.Thame M, Lewis J, Trotman H, Hambleton I, Serjeant G. The mechanisms of low birth weight in infants of mothers with homozygous sickle cell disease. Pediatrics. 2007;120:e686–e693. doi: 10.1542/peds.2006-2768. [DOI] [PubMed] [Google Scholar]
- 32.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 33.Baker WF., Jr Iron deficiency in pregnancy, obstetrics, and gynecology. Hematol Oncol Clin North Am. 2000;14:1061–1077. doi: 10.1016/s0889-8588(05)70171-4. [DOI] [PubMed] [Google Scholar]
- 34.Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. Am J Clin Nutr. 2000;71:1288S–1294S. doi: 10.1093/ajcn/71.5.1288s. [DOI] [PubMed] [Google Scholar]
- 35.Blot I, Diallo D, Tchernia G. Iron deficiency in pregnancy: effects on the newborn. Curr Opin Hematol. 1999;6:65–70. doi: 10.1097/00062752-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Rao R, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatr Suppl. 2002;91:124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- 37.Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. J Nutr. 2000;130:443S–447S. doi: 10.1093/jn/130.2.443S. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Ananth CV, Li Z, Smulian JC. Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol. 2009;38:1380–1389. doi: 10.1093/ije/dyp243. [DOI] [PubMed] [Google Scholar]
- 39.Koshy M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red-cell transfusions in pregnant patients with sickle cell disease. A randomized cooperative study. N Engl J Med. 1988;319:1447–1452. doi: 10.1056/NEJM198812013192204. [DOI] [PubMed] [Google Scholar]
- 40.Oteng-Ntim E, Chase AR, Howard J, Khazaezadeh N, Anionwu E. Sickle cell disease in pregnancy. Obstetrics, Gynaecol Reprod Med. 2008;18:272–278. [Google Scholar]
- 41.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 42.Tuck SM. Sickle-cell disease and pregnancy. Br J Hosp Med. 1982;28:125–127. [PubMed] [Google Scholar]
- 43.Morris JS, Dunn DT, Poddar D, Serjeant GR. Haematological risk factors for pregnancy outcome in Jamaican women with homozygous sickle cell disease. Br J Obstet Gynaecol. 1994;101:770–773. doi: 10.1111/j.1471-0528.1994.tb11944.x. [DOI] [PubMed] [Google Scholar]
- 44.Anyaegbunam A, Langer O, Brustman L, Damus K, Halpert R, Merkatz IR. The application of uterine and umbilical artery velocimetry to the antenatal supervision of pregnancies complicated by maternal sickle hemoglobinopathies. Am J Obstet Gynecol. 1988;159:544–547. doi: 10.1016/s0002-9378(88)80003-6. [DOI] [PubMed] [Google Scholar]
- 45.Nagel RL, Fabry ME, Steinberg MH. The paradox of hemoglobin SC disease. Blood Rev. 2003;17:167–178. doi: 10.1016/s0268-960x(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 46.Powars DR, Hiti A, Ramicone E, Johnson C, Chan L. Outcome in hemoglobin SC disease: a four-decade observational study of clinical, hematologic, and genetic factors. Am J Hematol. 2002;70:206–215. doi: 10.1002/ajh.10140. [DOI] [PubMed] [Google Scholar]
- 47.Serjeant GR, Hambleton I, Thame M. Fecundity and pregnancy outcome in a cohort with sickle cell-haemoglobin C disease followed from birth. BJOG. 2005;112:1308–1314. doi: 10.1111/j.1471-0528.2005.00678.x. [DOI] [PubMed] [Google Scholar]