Abstract
In solutions of tetraalkylammonium salts the melting temperature of oligonucleotide duplexes is independent of nucleotide sequence and thus GC content. Data quantitating the destabilizing effects of various mismatches in these solvents are also presented. The results are in accord with theories on DNA melting and establish conditions under which oligonucleotides can be used as hybridization probes with predictable and controllable specificity.
Full text
PDF




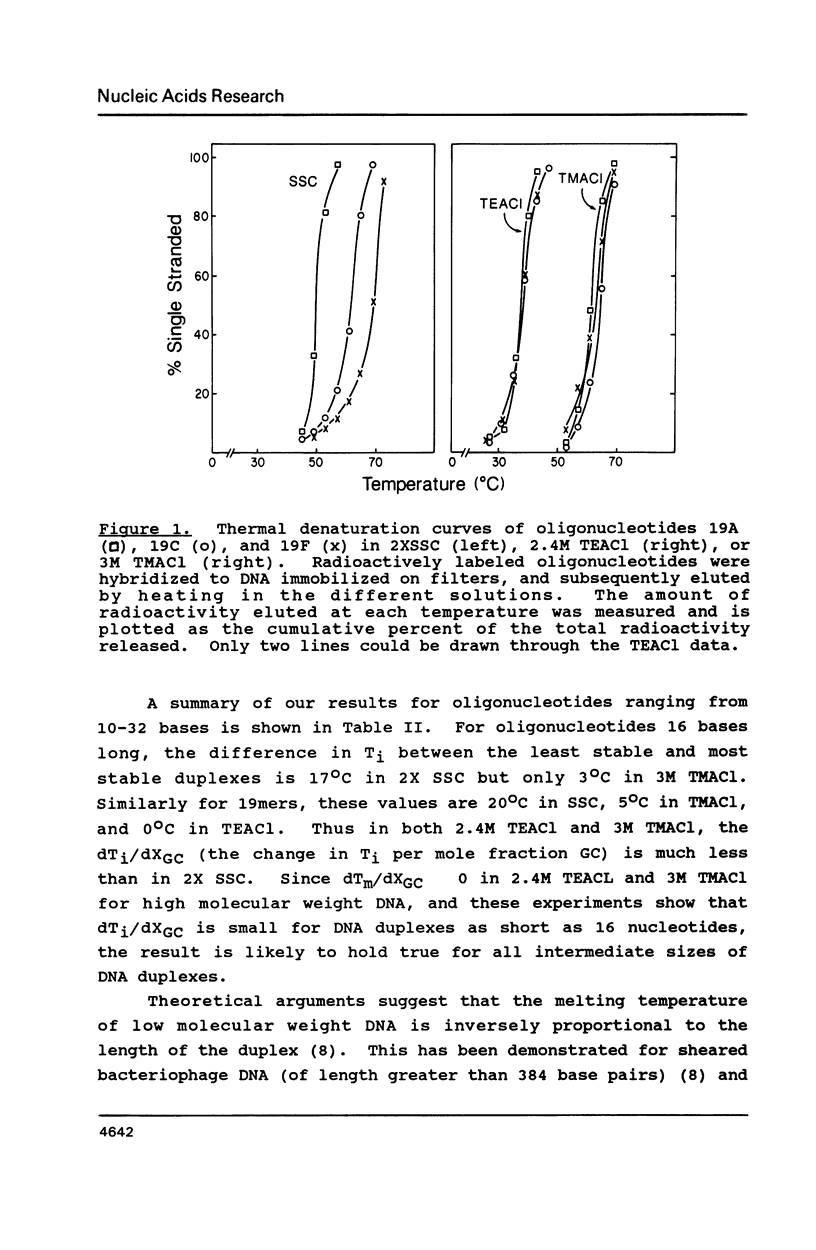
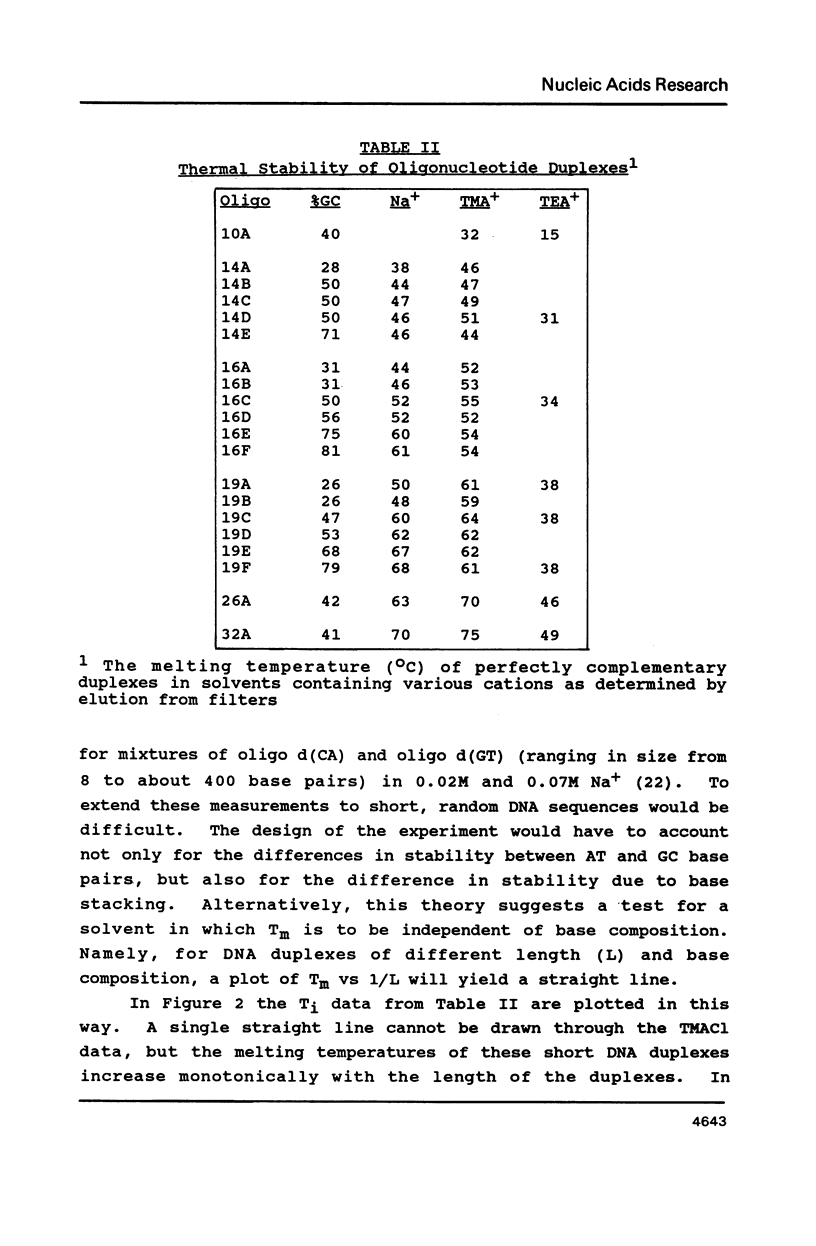
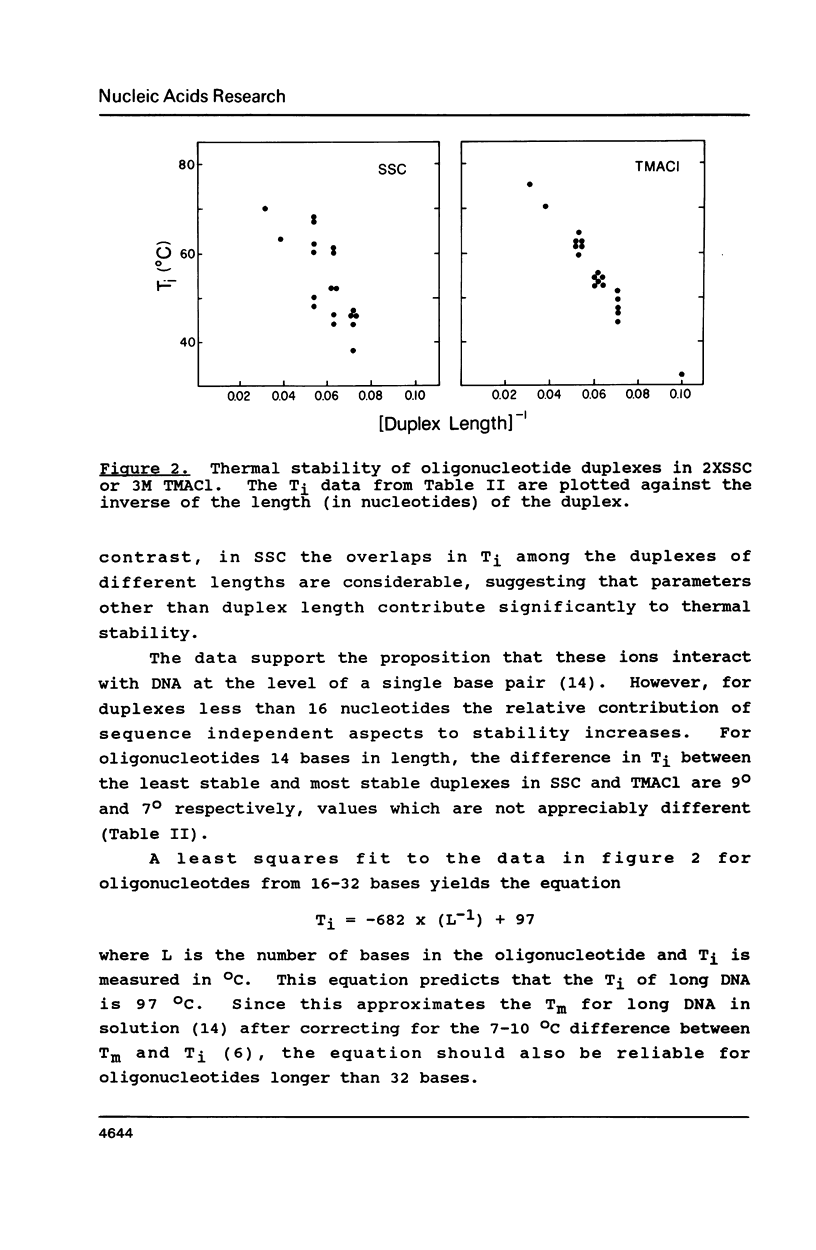
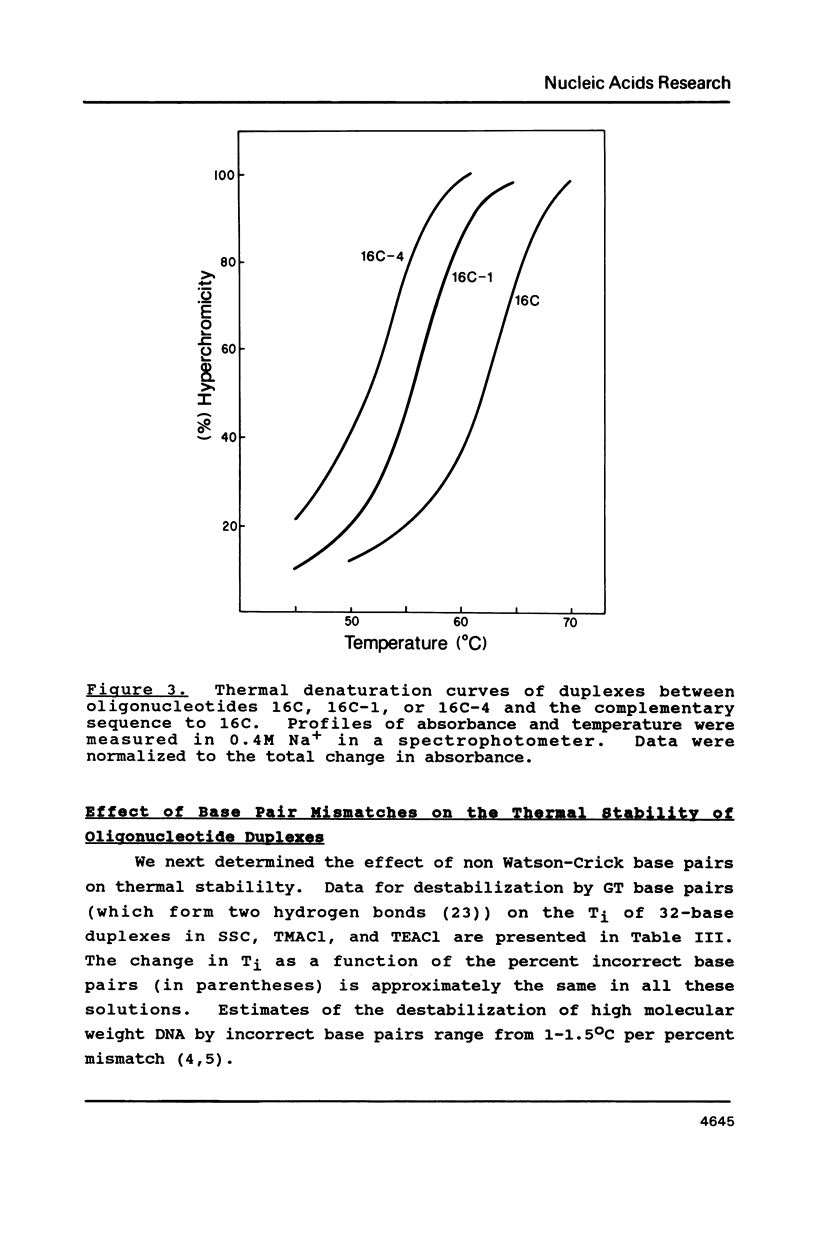





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Cetta A., Davidson E. H. The single-copy DNA sequence polymorphism of the sea urchin Strongylocentrotus purpuratus. Cell. 1978 Dec;15(4):1175–1186. doi: 10.1016/0092-8674(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- CROTHERS D. M., KALLENBACH N. R., ZIMM B. H. THE MELTING TRANSITION OF LOW-MOLECULAR-WEIGHT DNA: THEORY AND EXPERIMENT. J Mol Biol. 1965 Apr;11:802–820. doi: 10.1016/s0022-2836(65)80037-7. [DOI] [PubMed] [Google Scholar]
- CROTHERS D. M., ZIMM B. H. THEORY OF THE MELTING TRANSITION OF SYNTHETIC POLYNUCLEOTIDES: EVALUATION OF THE STACKING FREE ENERGY. J Mol Biol. 1964 Jul;9:1–9. doi: 10.1016/s0022-2836(64)80086-3. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Hain T. C., Hutton J. R., Wetmur J. G. Effects of microscopic and macroscopic viscosity on the rate of renaturation of DNA. Biopolymers. 1974;13(9):1847–1858. doi: 10.1002/bip.1974.360130915. [DOI] [PubMed] [Google Scholar]
- Hayes F. N., Lilly E. H., Ratliff R. L., Smith D. A., Williams D. L. Thermal transitions in mixtures of polydeoxyribodinucleotides. Biopolymers. 1970;9(9):1105–1117. doi: 10.1002/bip.1970.360090911. [DOI] [PubMed] [Google Scholar]
- Hunter W. N., Kneale G., Brown T., Rabinovich D., Kennard O. Refined crystal structure of an octanucleotide duplex with G . T mismatched base-pairs. J Mol Biol. 1986 Aug 20;190(4):605–618. doi: 10.1016/0022-2836(86)90246-9. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. K., Suggs S., Lin C. H., Browne J. K., Smalling R., Egrie J. C., Chen K. K., Fox G. M., Martin F., Stabinsky Z. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Von Hippel P. H. Alteration of the relative stability of dA-dT and dG-dC base pairs in DNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):298–302. doi: 10.1073/pnas.70.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein R. L., Fresco J. R. Correlation of Tm and sequence of DNA duplexes with delta H computed by an improved empirical potential method. Biopolymers. 1983 Aug;22(8):1979–2000. doi: 10.1002/bip.360220811. [DOI] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. DNA melting temperatures and renaturation rates in concentrated alkylammonium salt solutions. Biopolymers. 1977 Jun;16(6):1183–1199. doi: 10.1002/bip.1977.360160603. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Simmer R. L., Keith D. H., Shively L., Teplitz M., Itakura K., Gartler S. M., Riggs A. D. Isolation of a cDNA clone for human X-linked 3-phosphoglycerate kinase by use of a mixture of synthetic oligodeoxyribonucleotides as a detection probe. Proc Natl Acad Sci U S A. 1983 Feb;80(3):802–806. doi: 10.1073/pnas.80.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G. Hybridization and renaturation kinetics of nucleic acids. Annu Rev Biophys Bioeng. 1976;5:337–361. doi: 10.1146/annurev.bb.05.060176.002005. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


