Abstract
To assess the effect of the rapid removal of potentially infectious dogs on the prevalence and incidence of canine infections, a prospective study was undertaken in an area endemic for Leishmania infantum. We used serological testing based on the rapid DPP rK28 fusion protein chromatographic immunoassay for this dog screening-and-culling intervention trial. The outcome was evaluated by measuring seropositivity and sero-conversion/-reversion rates for canine infection. Our estimates indicated that concomitant detection and elimination of seropositive dogs with active disease may affect the numbers of canine infections and disease burden temporarily, although it is insufficient as a measure to interrupt the zoonotic L. infantum transmission. However, most of the asymptomatic, seropositive dogs continuously exhibit low levels of antibodies and/or reverted, remaining seronegative thereafter. In the process of waiting for an effective vaccine, one option for canine reservoir control may be to identify these possibly genetically resistant animals and promote their expansion in the population.
Introduction
Leishmaniasis caused by infection with the parasite genus Leishmania, is one of the major infectious diseases primarily affecting some of the poorest regions of the world. The disease is endemic in 98 countries or territories, and the World Health Organization (WHO)1 estimates that it is a threat to 350 million people with a worldwide prevalence of 12 million cases. Among the annual incidence of 2 million new cases of human infections, 0.5 million are life-threatening visceral leishmaniasis (VL). It should be noted that an estimated 2.4 million disability-adjusted life years, in addition to 59,000 lives were lost to leishmaniasis in 2001 alone.2 The three variations of the disease, visceral, mucocutaneous, and cutaneous leishmaniasis, consist of a wide range of symptoms, with VL causing the most severe clinical manifestations.
Zoonotic VL is found in areas of Leishmania infantum (syn. Leishmania chagasi) transmission (Latin America, southern Europe, North Africa, and West and Central Asia)1; the disease tends to be relatively chronic, and children are especially affected. In Brazil, VL is an increasingly important public health problem because of its frequency of occurrence (the reported human VL incidence in the 2000s averaged 3,362 cases/year) and its ability to spread rapidly.3 The parasite is transmitted from animals to arthropod vectors and then from vectors to humans. In Latin America, L. infantum is usually transmitted by Lutzomyia longipalpis phlebotomine sand flies,4 and the population density of these insects in the peridomestic setting can reach very high levels.5 The essential maintenance cycle of L. infantum involves foxes (Dusicyon vetulus and Cerdocyon thous),6,7 and domestic dog (Canis familiaris) populations serve as the peridomestic reservoirs of the parasite.4,8,9 Although opossums (Didelphis albiventris and Didelphis marsupialis) can be naturally infected,10,11 their epidemiologic role as sylvatic or peridomestic reservoir hosts remains unknown.
Measures used to control VL in Brazil have focused on disease surveillance through active case detection and treatment, the culling of seropositive dogs, and the use of residual insecticide spraying of houses and animal shelters.12 However, it is unclear which strategy provides the most cost-effective control of zoonotic VL, and a valid impact evaluation has not been conducted.13,14 Concomitant use of the three control methods was able to significantly reduce the numbers of human and canine cases in Minas Gerais, Brazil.15 The results of several intervention studies based on serological screening of dogs and killing of seropositive animals are equivocal.16–20 In theory, effective control would require a high proportion of infectious dogs to be removed immediately upon detection to achieve a marked reduction in disease transmission.8,9 Thus, it is postulated that if culling could target infectious (rather than simply infected) dogs, its effectiveness may be increased because the proportion of dogs that have never been infectious in the population may increase.9,21 On the basis of these observations, we conducted a prospective intervention study to determine whether concomitant detection and elimination of seropositive dogs developing clinical illness would decrease the prevalence and incidence of canine infection in a hyper-endemic area of rural southeast Brazil (Pancas, in the state of Espírito Santo). This report summarizes the effects of the control intervention targeting severe dog cases (which are likely to be highly infective to sand flies) on canine infection as determined by serological data in longitudinal studies.
Materials and Methods
Study area.
Field work was conducted in a rural area surrounding the municipality of Pancas in the state of Espírito Santo, Brazil. A complete census of domestic dogs was obtained in a small community made up of 45 well-dispersed houses situated along four streams, Córregos (Creeks) São Luiz I, Palmital, Roque, and Ubá. The study area has been previously described in detail.22 This L. infantum-endemic location was chosen because VL had been continuously transmitted there during the last 10 years.16 In 2003, nearly 57% of the indigenous dogs were seropositive (the seroprevalences through neighboring localities ranged from 42% to 76%),22 thus suggesting that L. infantum transmission in Pancas is higher than that reported for other regions in Brazil endemic for this parasite.15,18 Currently, there are many more cases of clinical disease in dogs than in people, but the regions are free of Chagas' disease and cutaneous leishmaniasis.
Dog surveys.
The house-to-house surveys ran from December 2009 to March 2011, during which time serological studies were performed on all available dogs. Sampling was conducted continuously over each 4-month sampling period. Data were analyzed at the end of each sampling period and again at the completion of all sampling periods. Consenting owners provided the age, sex, and breed of each animal in the study. The first cohort contained 123 dogs, and additional animals were enrolled into the study at sampling dates (Table 1). All dogs underwent gross physical examinations conducted by veterinary practitioners in the field. At the sampling times, animals (composed mainly of guard dogs, hunting dogs, pet dogs, and strays, with a mean age of 2.3 years) were scored clinically for 6 typical signs of canine VL (alopecia, dermatitis, chancres, conjunctivitis, lymphadenopathy, and onychogryphosis) on a semi-quantitative scale as previously described.22 Dogs with total scores of 0 to 2 were classified as asymptomatic, those with scores of 3 to 6 were classified as oligosymptomatic, and those with scores of 7 to 18 were classified as polysymptomatic.
Table 1.
Summary of canine surveys in the intervention area of Pancas, Espírito Santo, Brazil in 2009–2011*
| Outcome of the follow-up survey | |||||
|---|---|---|---|---|---|
| Sampling interval | No. (%) | Sero-positive | Sero-negative | Removed (euthanized) | Died or moved |
| Dec 2009–July 2010 | |||||
| Pos. | 38 (31) | 5 | 9 | 21 | 3 |
| Neg. | 85 (69) | 6 | 51 | 28 | |
| Total | 123 | 11 | 60‡ | ||
| July 2010–Oct. 2010 | |||||
| Pos. | 25 (23) | 2 | 8 | 13 | 2 |
| Neg. | 86 (77) | 6 | 58 | 22 | |
| Total | 111 | 8† | 66‡ | ||
| Oct. 2010–March 2011 | |||||
| Pos. | 15 (14) | 5 | 2 | 5 | 3 |
| Neg. | 94 (86) | 21 | 37 | 36 | |
| Total | 109 | 26† | 39‡ | ||
| March 2011 | |||||
| Pos. | 37 (40) | ||||
| Neg. | 56 (60) | ||||
| Total | (93) | ||||
Positive (Pos.) equals the total number of seropositive dogs in the follow-up group (†) plus new dogs that moved into the area that were seropositive. Negative (Neg.) equals the total number of seronegative dogs in the follow-up group (‡) plus new dogs that moved into the area that were seronegative.
Serology.
Serologic tests were performed using an innovative colloidal gold-based immunochromatography assay (namely, the rK28-based DPP CVL rapid test) designed to detect antibodies against the rK9/K26/K39 antigens of L. infantum (Biomanguinhos, Fiocruz, Rio de Janeiro, Brazil). The results of the ready-to-use disposable devices (which uses a 5-μL fresh blood sample) were read visually after 15 min first by two independent operators and then using a DPP optical reader device that measures reflectance in relative light units (RLU). Despite the DPP Evaluation Scales for evaluating test results provided by the manufacturer, any visible band in the test area (in addition to the control line) was considered a positive reaction if the specific antibody reactivity was above the cut-off value of 3 RLU; this value was established as the mean RLU plus five standard deviations obtained using the control sera from 59 health pets of various ages and breeds that had attended a veterinary clinic at the municipality of Vitória, ES (a VL-free area of Brazil). We chose this rapid test for our surveys because it is easily used in the field. It has also been reported23 to be as sensitive as rK26- and rK39-based ELISAs and superior to immunofluorescence assays24 in detecting clinically symptomatic and asymptomatic canine carriers of L. infantum.
Intervention and effect measures.
After informed consent was obtained from dog owners, the local public health service personnel removed all dogs with a DPP-determined K28-specific antibody reactivity of ≥ 15 RLU and/or with active disease to a veterinary public health post where they were eliminated within 8 days after being diagnosed. Before being euthanized using intravenous potassium oxalate, dogs were anaesthetized with 20-mg ketamine hydrochloride (Vetalar)/kg body weight, injected intramuscularly. Dogs with antibody levels lower than 15 RLU were not euthanized but were monitored serologically to follow the infection behavior in the local canine populations. The intervention began at the onset of the study and was maintained for the 15-month period. The elimination of a few seropositive stray dogs was justified for many reasons connected with health, the environment, and conservation.1 The existence of zoonotic VL in the studied sites provided additional justification. No human VL case treatment or vector control programs occurred during the study period. The outcome was evaluated by measuring seropositivity and seroconversion rates for canine L. infantum infection. In addition, on the basis of both the number of K28-specific antibody units and their changes over time, we were able to reliably identify dogs that were potentially noninfectious and infectious. A control group was not considered in this intervention trial because of the obvious ethical dilemma. Moreover, the heterogeneity of disease transmission within the study area could generate imbalances in the baseline comparisons among canine groups.
Statistical analysis.
Changes in prevalence and incidence of canine infection during the study period were compared using the χ2 test and the χ2 test for trend over time.
Ethical considerations.
This research has complied with all relevant Brazilian federal guidelines (Projeto de lei 3.964/97-www.planalto.gov.br). Informed consent (in either written or verbal form) was obtained from all owners to use their dogs. The Ethics Committee of Universidade Federal do Espírito Santo (UFES) sanctioned all clinical and experimental procedures.
Results
Detection of anti-Leishmania antibodies in dogs.
Dogs were considered to be infected if they tested positive by serology using the DPP-based chromatographic assay (i.e., showed any antibody reactivity above the threshold of 3 RLU). When seropositive dogs were subdivided based on disease severity, there was an increase in the ability of the serological test to detect cases with progressed disease. As expected, asymptomatic dogs (60%) displayed weak antibody responses (5.60 ± 0.76 RLU), and oligosymptomatic (15%) and polysymptomatic (25%) canines had moderate (30.43 ± 3.56 RLU) and high (55.67 ± 2.46 RLU) serum antibody reactivities, respectively, to the K28 fusion protein. DPP-tested dogs produced antibodies at various levels. Furthermore, dogs with latent infection (which are likely to be lowly infective to sand flies) could be easily distinguished from potentially infectious dogs (i.e., those developing clinical disease) based on both the levels of parasite-specific antibodies and their rates of change over time.
Prevalence and incidence of canine infection.
The data from the canine surveys are shown in Table 1. In December 2009, 123 dogs were surveyed and 38 (31%) of them were seropositive. Of the 102 dogs found to be asymptomatic, only 17 (17%) were seropositive. In contrast, all (21) symptomatic dogs were seropositive for rK9/K26/K39 antigens. Of these 38 infected dogs, 24 (63%) were euthanized (N = 21), died of other causes (N = 2), or could not be located (N = 1). By July 2010, 6 (7%) of the 85 seronegative dogs were seropositive, 51 were still seronegative, and 28 could not be located. At that time, 25 dogs were seropositive, including 14 newly recruited ones, and 86 were seronegative, including 26 newly recruited ones. Thus, at the 7-month time point, the incidence rate was 11%; this rate was estimated by dividing the number of converters to seropositive status (N = 6) by the number of dogs (both seronegative and seropositive) available for survey (N = 57).
In October 2010, 111 dogs were surveyed and 25 (23%) of them were seropositive (12 of 73 [16%] asymptomatic and 13 of 13 [100%] symptomatic). Of these 25 infected dogs, 15 (60%) were euthanized (N = 13), died of other cause (N = 1), or could not be located (N = 1). At that time, 6 (7%) of the 86 seronegative dogs identified in July had converted to a seropositive status, 58 were still seronegative, and 22 could not be located. Fifteen dogs were seropositive, including 7 newly recruited ones. Ninety-four were seronegative, including 28 newly recruited ones. At the 10-month period, the incidence rate was 9% (6 of 64).
Of the 109 dogs surveyed from October 2010 to March 2011, 15 (14%) were seropositive (10 of 89 [11%] asymptomatic and 5 of 5 [100%] symptomatic). Of these 15 infected dogs, 8 (53%) were euthanized (N = 5), died of other causes (N = 2) or relocated by their owners (N = 1). In March 2011, 21 (22%) of the 94 seronegative dogs had converted to a seropositive status, 37 dogs were still seronegative, and 36 could not be located. At that time, 37 dogs were seropositive, including 11 newly recruited ones. Fifty-six dogs were seronegative, including 17 newly recruited ones. At this 15-month period, 40% of the dogs surveyed (37 of 93) were seropositive and the incidence rate was 36% (21 of 58). Notably, 32 (31%) of 103 newly recruited (indigenous sentinel) seronegative dogs were infected during the 15-month period (mean seroconversion time was 8 months).
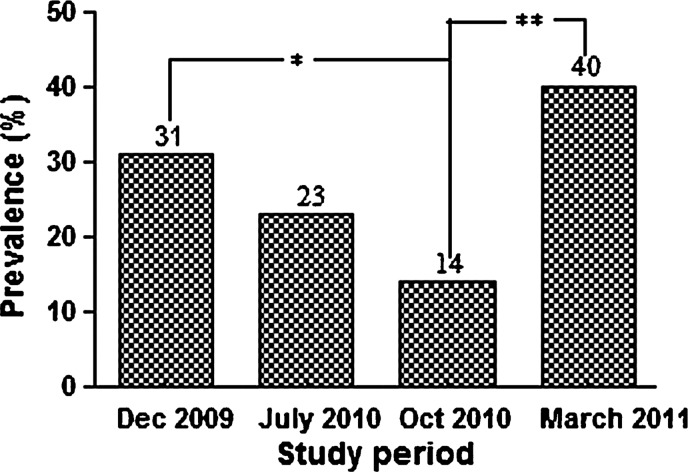
Figure 1 shows the change in prevalence of dog seropositivity over time. From an initial overall seroprevalence of 31%, the seropositivity rate decreased significantly to 14% (P = 0.002) at 10 months before again, rising significantly to 40% (P = 0.001) at the 15-month period after intervention. There were no statistically significant differences in either sex or age distribution between the infected (seroconverters) and non-infected (seronegative) dogs (data not shown). Differences in breed distribution could not be determined because breed miscibility prevailed in the local dog population. As expected, the seroprevalence of canine infection throughout contiguous localities was highly variable, ranging from 15% to 46% in the first survey (Table 2), suggesting a large heterogeneity in the transmission of L. infantum within the study area.
Figure 1.
Initial seroprevalence and seropositivity rates of canine Leishmania infantum infection in the intervention sites (Pancas, ES, Brazil, 2009–2011). Statistically significant differences between seropositive rates over time are indicated as *(P = 0.002) or **(P = 0.001).
Table 2.
Seropositivity rates of canine Leishmania infantum infection shown by the duration of exposure and by intervention site (Pancas, ES, Brazil, 2009–2011)
| rK26/rK39-specific antibodies detected by the DPP CVL rapid test | ||||
|---|---|---|---|---|
| Study sites* | No. initially seropositive no. tested (%) | No. seropositive at 7 mo/no. tested (%) | No. seropositve at 10 mo/no. tested (%) | No. seropositive at 15 mo/no. tested (%) |
| SL | 19/41 (46) | 7/35 (20) | 5/27 (19) | 10/22 (45) |
| P | 5/29 (17) | 3/22 (14) | 3/25 (12) | 12/22 (55) |
| R | 10/27 (37) | 9/29 (31) | 4/29 (14) | 4/22 (18) |
| U | 4/26 (15) | 6/25 (24) | 3/28 (11) | 11/27 (41) |
| Total | 38/123 (31) | 25/111 (23) | 15/109 (14) | 37/93 (40) |
Small, scattered settlements made up of well-dispersed houses located along four Creeks (SL = São Luiz I, P = Palmital, R = Roque, and U = Ubá) in the study area.
Note: At 7 months, seroprevalences were calculated for the follow-up group that was available for survey. At 10 and 15 months, seropositivity rates were calculated for the original follow-up group plus new canines that had moved into the area and remained there for at least 3 months.
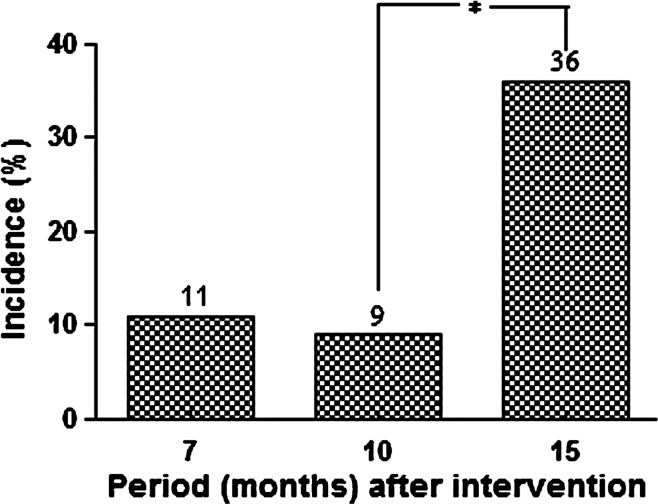
As presented in Figure 2, the cumulative seroconversion incidence for L. infantum infection among dogs also increased significantly from 9% to 36% (P < 0.001). Nonetheless, the elimination of symptomatic dogs that exhibited robust antibody responses led to a 62% reduction of the canine disease burden during the study period.
Figure 2.
Cumulative incidence of seroconversion for canine Leishmania infantum infection in the intervention sites (Pancas, ES, Brazil, 2009–2011). *Significant differences (P < 0.001) between incidence rates over time.
Serological reversions.
Serological reversion rates were calculated for the remaining dogs that were seropositive in the first survey and were available for repeat sampling. Overall, 50% (19 of 38) of the dogs reverted from a positive to negative serological status during the 15-month period. By July 2010, 14 of the original 38 seropositive dogs remained alive and 9 had reverted to displaying negative titers. Of these 9 dogs, 4 showed a single positive conversion following two negative readings. The 5 remaining dogs showed weak positive antibody reactivity, which subsequently fell below the threshold of 3 RLU. Of the 25 dogs that had converted to a seropositive status in October 2010, 10 dogs were still alive and 8 dogs had reverted to a seronegative status. Of the 15 seropositive dogs identified in March 2011, 7 survived and 2 of them had reverted to a seronegative status after three consecutive positive readings. The 5 remaining dogs had positive but low titers.
Discussion
In areas of Brazil in which VL is endemic, prophylactic control programs emphasize the serologic surveillance of canines and humans, and the elimination of seropositive animals.12,25 These culling campaigns are very expensive and labor intensive, and their relatively poor performance is aggravated by the absence of any good marker for infectious status in dogs.9 Hence, both the efficacy and acceptability of this control strategy are increasingly being challenged.13,14,26,27 Our data clearly show that attempting to remove seropositive dogs with active disease soon after detection may affect the cumulative incidence of seroconversion in dogs temporarily, although it is insufficient as a measure for eradicating canine VL. Indeed, the transmission of L. infantum was not interrupted, as evidenced by the detection of newly infected dogs every 4 months throughout the study. Comparable results were obtained previously during a controlled intervention study in dogs in northeast Brazil.18 These findings could be related to the efficiency and timing of the removal of dogs and the effect of these practices in relation to seasonal variations in transmission.8,28 Furthermore, the newly infected dogs could have moved into the area and brought the infection with them.
Although latent infections in dogs are typical,15,22 the epidemiologic relevance of these infections is poorly understood. The development of accurate assays, which are able to detect not only symptomatic parasitologically positive dogs but also seropositive asymptomatic dogs already infectious for sand flies, should be a research priority. Another explanation for the continued transmission observed during this study may be the incomplete elimination of infectious dogs, given that not all seropositive dogs were culled during the study period. Moreover, not all infected dogs were expected to be detected using serology,9 as the DPP CVL rapid test has a low sensitivity (47%) in identifying parasite-positive dogs that do not manifest clinical signs of VL.23 However, despite the infectious potential of naturally infected asymptomatic seropositive dogs for sand flies,29,30 these dogs are likely not epidemiologically significant. It is known that the probability of L. longipalpis becoming infected from an infected dog significantly increases with the strength of the dog's anti-Leishmania antibody response and its total clinical score.9,31–33 Theory also states that only a small proportion (17%) of highly infectious dogs are expected to be responsible for 88% of all transmission.9
It is generally acknowledged that humans are dead-end hosts for L. infantum in most cases9,26; this is because skin parasitism is more frequent in dogs and foxes than in humans.6 Nonetheless, the reservoir competence of the human host for this parasite species, as occurs in areas endemic for Leishmania donovani VL in Bangladesh, India, and Nepal,1 has also been suspected in Brazil.16,34 Accordingly, 11 of the 44 persons with active VL caused by L. infantum transmitted the infection to 0.7% of the total number of sand flies (26 of 3,747) that had fed on them, as characterized through xenodiagnosis; however, no sand flies acquired the infection from the 137 asymptomatic persons identified in the study.35 In a previous survey conducted in 2003 at the same intervention sites used for the current study, 42% of the human residents were leishmanin-positive reactors and 47% were seropositive.22 In this study, human-to-canine transmission of L. infantum infection by sand flies was unlikely, as no case of human VL was diagnosed during the study period.
Further studies are needed to determine the influence of reservoirs other than dogs on maintaining the long-term presence of the parasite in the study area. It is assumed that when infected foxes and opossums feed near human dwellings, they are bitten by L. longipalpis living in the peridomestic environment. These sand flies then become infected and subsequently transmit the parasite to dogs or humans living nearby.6,36 If opossums serve as an important peridomestic reservoir of L. infantum,10,11 VL may not be controlled in a community solely by eliminating infectious dogs. Canine habits could also be associated with susceptibility to L. infantum infection. In particular, guard or hunting dogs and strays experience a higher force of infection than pet dogs.21 In this study, most of the “owned” dogs were guard and hunting dogs. This could have increased a dog's chance of becoming infected, because working dogs usually sleep outdoors and are bitten more frequently than pet dogs by sand flies that have fed on roving sylvatic hosts near human dwellings.5 It should be noted that only a few seropositive stray dogs (either scored as symptomatic or asymptomatic) that were detected were all culled immediately upon detection.
The clinical expression of L. infantum infection in dogs is highly variable, depending on the immune status of the host30 and other genetic factors influencing canine susceptibility to VL.37,38 Severely affected dogs do not survive the disease, although subclinical infections may occur commonly in dogs22,26 as they do in humans.16,22,34 Hence, the removal of all seropositive dogs may be counterproductive in controlling VL; a proportion of infected dogs never become infectious, but these dogs may be replaced by susceptible puppies that do become infectious.9,39,40 In support of this hypothesis, the indigenous dog population in this study remained stable over the study period. This may have been because euthanized canines or those that died of natural causes were rapidly replaced by younger dogs (more often by puppies) that eventually underwent seroconversion. It has been postulated that the canine population in endemic areas is likely composed of four mutually exclusive groups of hosts: those susceptible, those resistant, those susceptible that become latent after a sand fly bite (asymptomatic), and those infectious to sand flies that emerge from latent canines at a constant rate.41,42 Dogs born resistant to L. infantum would be able to maintain an effective cellular immune response against the parasite and thus would not become infectious for sand flies.
The reasons some dogs maintain resistant infections while others show latent infections are unknown.9,15 However, our estimates of serological reversion rates indicated at a high recovery rate among the identified seropositive canine population, suggesting that efficacious immune mechanisms exist.43 Of importance, these possibly genetically resistant dogs could be identified in the field, as they continuously exhibit low levels of K9/K26/K39-specific antibodies and/or revert, remaining seronegative thereafter. Further research is required to confirm that canines displaying this serological profile actually constitute never-infectious reservoirs for sand flies. Such information is important as promoting population expansion of genetically determined resistant dogs would significantly improve VL control. However, the most feasible approach to controlling VL are likely the development of an effective vaccine44 to protect dogs from being infectious to the sand fly vector, and identifying new methods to prevent infection in canines and humans, such as the use of insecticidal dog collars,45,46 and insecticide-treated bednets,47,48 which are promising in reducing canine and human incidence. Future intervention trials should show whether these new tools could be used to develop a sustainable VL prevention and control strategy.
ACKNOWLEDGMENTS
We thank Clemilda A. de Paiva, Júlio M. Galdino, and Daniel Kiefer (Secretaria Municipal de Saúde de Pancas, Pancas, ES) for assistance in the field studies. The DPP CVL rapid test was kindly supplied by Bio-Manguinhos/Fiocruz (Rio de Janeiro, Brazil).
Footnotes
Financial support: This study was funded in part by Fiocruz and the National Council for Scientific and Technological Development of the Ministry of Science and Technology (the PRONEX 3/CNPq-66.1037/1998-3 and the INCT/CNPq-420067/2005-1), Brazil. Gabriel Grimaldi Jr. is a CNPq Fellow researcher.
Authors' addresses: Gabriel Grimaldi Jr., Laboratório de Patologia e Biointervenção, Centro de Pesquisas Gonçalo Moniz (CPqGM), Salvador, BA, Brazil, E-mail: grimaldi@bahia.fiocruz.br. Antonio Teva, Laboratório de Pesquisas em Leishmaniose, Instituto Oswaldo Cruz (IOC), Rio de Janeiro, RJ, Brazil, E-mail: teva@ioc.fiocruz.br. Claudiney B. Santos, Adelson L. Ferreira, and Aloísio Falqueto, Unidade de Medicina Tropical, Universidade Federal do Espírito Santo, Vitória, Brazil, E-mails: claudiney@ppgcf.ufes.br, adelsonlf@hotmail.com.br, and falqueto@npd.ufes.br.
References
- 1.World Health Organization Expert Committee . Control of the leishmaniases. Geneva: World Health Organization; 2010. Tech. Rep. Ser. 949. [PubMed] [Google Scholar]
- 2.Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasil, Ministério da Saúde . Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Brasília (DF): Editora do Ministério da Saúde; 2006. Manual de vigilância e controle da leishmaniose visceral. Available at. Accessed October 3, 2011. [Google Scholar]
- 4.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 5.Ward RD. Vector biology and control. In: Chang KP, Bray RS, editors. Leishmaniasis. New York: Elsevier; 1985. pp. 199–212. [Google Scholar]
- 6.Deane LM, Deane MP. Visceral leishmaniasis in Brazil: geographical distribution and transmission. Rev Inst Med Trop Sao Paulo. 1962;4:198–212. [PubMed] [Google Scholar]
- 7.Lainson R, Shaw JJ, Lins ZC. Leishmaniasis in Brazil. IV. The fox, Cerdocyon thous (L) as a reservoire of Leishmania donovani in Pará State, Brazil. Trans R Soc Trop Med Hyg. 1969;63:741–745. doi: 10.1016/0035-9203(69)90118-7. [DOI] [PubMed] [Google Scholar]
- 8.Quinnell RJ, Courtenay O, Garcez L, Dye C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology. 1997;115:143–156. doi: 10.1017/s0031182097001200. [DOI] [PubMed] [Google Scholar]
- 9.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis. 2002;186:1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- 10.Sherlock IA, Miranda JC, Sadigursky M, Grimaldi G., Jr Natural infection of the opossum Didelphis albiventris (Marsupialia, Didelphidae) with Leishmania donovani, in Brazil. Mem Inst Oswaldo Cruz. 1984;79:511. doi: 10.1590/s0074-02761984000400020. [DOI] [PubMed] [Google Scholar]
- 11.Corredor A, Gallego JF, Tesh RB, Morales A, Carrasquilla CF, Young DG, Kreutzer RD, Boshell J, Palau MT, Caceres E, Pelaez D. Epidemiology of visceral leishmaniasis in Colombia. Am J Trop Med Hyg. 1989;40:480–486. doi: 10.4269/ajtmh.1989.40.480. [DOI] [PubMed] [Google Scholar]
- 12.Lacerda MM. The Brazilian leishmaniasis control program. Mem Inst Oswaldo Cruz. 1994;89:489–495. doi: 10.1590/s0074-02761994000300036. [DOI] [PubMed] [Google Scholar]
- 13.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5:873–882. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 14.Romero GA, Boelaert M. Control of visceral leishmaniasis in Latin America: a systematic review. PLOS. 2010;4:1–17. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palatnik-de-Sousa CB, dos Santos WR, França-Silva JC, da Costa RT, Barbosa Reis A, Palatnok M, Mayrink W, Genaro O. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am J Trop Med Hyg. 2001;65:510–517. doi: 10.4269/ajtmh.2001.65.510. [DOI] [PubMed] [Google Scholar]
- 16.Dietze R, Barros GB, Teixeira L, Harris J, Michelson K, Falqueto A, Corey R. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis. 1997;25:1240–1242. doi: 10.1086/516096. [DOI] [PubMed] [Google Scholar]
- 17.Braga MD, Coelho IC, Pompeu MM, Evans TG, MacAullife IT, Teixeira MJ, Lima JW. Control of canine visceral leishmaniasis: comparison of results from a rapid elimination program of serum-reactive dogs using an immunoenzyme assay and slower elimination of serum-reactive dogs using filter paper elution indirect immunofluorescence. Rev Soc Bras Med Trop. 1998;31:419–424. doi: 10.1590/s0037-86821998000500001. [DOI] [PubMed] [Google Scholar]
- 18.Ashford DA, David JR, Freire M, David R, Sherlock I, Eulálio MC, Sampaio DP, Badaró R. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyg. 1998;59:53–57. doi: 10.4269/ajtmh.1998.59.53. [DOI] [PubMed] [Google Scholar]
- 19.Moreira ED, Jr, Mendes de Souza VM, Sreenivasan M, Nascimento EG, Pontes CL. Assessment of an optimized dog-culling program in the dynamics of canine Leishmania transmission. Vet Parasitol. 2004;122:245–252. doi: 10.1016/j.vetpar.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Costa CH, Tapety CM, Werneck GL. Control of visceral leishmaniasis in urban areas randomized factorial intervention trial. Rev Soc Bras Med Trop. 2007;40:415–419. doi: 10.1590/s0037-86822007000400009. [DOI] [PubMed] [Google Scholar]
- 21.Dye C, Killick-Kendrick R, Vitutia MM, Walton R, Killick-Kendrick M, Harith AE, Guy MW, Cañavate M-C, Hasibeder G. Epidemiology of canine visceral leishmaniasis: prevalence, incidence and basic reproduction number calculated from a cross-sectional serological survey on the island of Gozo, Malta. Parasitology. 1992;105:35–41. doi: 10.1017/s0031182000073662. [DOI] [PubMed] [Google Scholar]
- 22.Falqueto A, Ferreira AL, Santos CB, Porrozzi R, Santos da Costa MV, Teva A, Cupolillo E, Campos-Neto A, Grimaldi G., Jr Cross-sectional and longitudinal epidemiologic surveys of human and canine Leishmania infantum visceral infections in an endemic rural area of southeast Brazil (Pancas, Espírito Santo) Am J Trop Med Hyg. 2009;80:559–565. [PubMed] [Google Scholar]
- 23.Grimaldi G, Jr, Teva A, Ferreira AL, dos Santos CB, de-Souza Pinto I, de-Azevedo T, Falqueto A. Evaluation of a novel chromatographic immunoassay based on dual-path platform technology (DPP CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:54–59. doi: 10.1016/j.trstmh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Porrozzi R, Santos da Costa MV, Teva A, Falqueto A, Ferreira AL, Santos CD, Fernandes AP, Gazinelli RT, Campos-Neto A, Grimaldi G., Jr Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol. 2007;14:544–548. doi: 10.1128/CVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furtado Vieira JB, Coelho GE. Leishmaniose visceral ou calazar: aspectos epidemiológicos e de controle. Rev Soc Bras Med Trop. 1998;31((Suppl 2)):85–92. [PubMed] [Google Scholar]
- 26.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52:287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 27.Costa CH, Vieira JB. Changes in the control program of visceral leishmaniasis in Brazil. Rev Soc Bras Med Trop. 2001;34:223–228. doi: 10.1590/s0037-86822001000200013. [DOI] [PubMed] [Google Scholar]
- 28.Courtenay O, MacDonald DW, Lainson R, Shaw JJ, Dye C. Epidemiology of canine leishmaniasis: a comparative serological study of dogs and foxes in Amazon Brazil. Parasitology. 1994;109:273–279. doi: 10.1017/s0031182000078306. [DOI] [PubMed] [Google Scholar]
- 29.Vexenat JA, Fonseca de Castro JA, Cavalcante R, Tavares JP, da Silva MR, Batista WH, Furtado Campos JH, Howards MK, Frame I, McNerney R, Wilson S, Miles MA. Visceral leishmaniasis in Terezina, State of Piauí, Brazil: preliminary observations on the detection and transmissibility of canine and sandfly infections. Mem Inst Oswaldo Cruz. 1994;89:131–135. doi: 10.1590/s0074-02761994000200001. [DOI] [PubMed] [Google Scholar]
- 30.Alvar J, Molina R, San Andrés M, Tesouro M, Nieto J, Vitutia M, González E, San Andrés MD, Boggio J, Rodriguez F, Sainz A, Escacena C. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol. 1994;88:371–378. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- 31.Michalsky RM, Rocha MF, Rocha Lima AC, França-Silva JC, Pires MQ, Oliveira FS, Pacheco RS, dos Santos SL, Barata RA, Romanha AJ, Fortes-Dias CL, Santos Dias E. Infectivity of seropositive dogs, showing different clinical forms of leishmaniasis, to Lutzomyia longipalpis phlebotomine sand flies. Vet Parasitol. 2007;147:67–76. doi: 10.1016/j.vetpar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Araújo Soares MR, Mendonça IL, Bonfim JM, Rodrigues JA, Werneck GL, Costa CHN. Canine visceral leishmaniasis in Teresina, Brazil: relationship between clinical features and infectivity for sand flies. Acta Trop. 2010;117:6–9. doi: 10.1016/j.actatropica.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am J Trop Med Hyg. 2001;64:119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- 34.Badaró R, Jones TC, Lourenço R, Cerf BJ, Sampaio D, Carvalho EM, Rocha H, Teixeira R, Johnson WD., Jr A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986;154:636–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- 35.Costa CH, Gomes RB, Silva MR, Garcez LM, Ramos PK, Santos RS, Shaw JJ, David JD, Maguire JH. Competence of the human host as a reservoir for Leishmania chagasi. J Infect Dis. 2000;182:997–1000. doi: 10.1086/315795. [DOI] [PubMed] [Google Scholar]
- 36.Shaw JJ, Lainson R. Ecology and epidemiology: New World. In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in Biology and Medicine. Volume 1. London: Academic Press; 1987. p. 365. [Google Scholar]
- 37.Altet L, Francino O, Solano-Gallego L, Renier C, Sanches A. Mapping and sequencing of the canine NRAMP1 gene and identification of mutations in leishmaniasis-susceptible dogs. Infect Immun. 2002;70:2763–2771. doi: 10.1128/IAI.70.6.2763-2771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinnell RJ, Kennedy LJ, Barnes A, Courtenay O, Dye C, Garcez LM, Shaw MA, Carter SD, Thomson W, Ollier WE. Susceptibility to visceral leishmaniasis in the domestic dog is associated with MHC class II polymorphism. Immunogenetics. 2003;55:23–28. doi: 10.1007/s00251-003-0545-1. [DOI] [PubMed] [Google Scholar]
- 39.Paranhos-Silva M, Nascimento EG, Melro MC, Oliveira GG, dos Santos WL, Pontes-de-Carvalho LC, Oliveira-dos-Santos AJ. Cohort study on canine emigration and Leishmania infection in an endemic area for American visceral leishmaniasis. Implications for the disease control. Acta Trop. 1998;69:75–83. doi: 10.1016/s0001-706x(97)00116-2. [DOI] [PubMed] [Google Scholar]
- 40.Nunes CM, de Lima VM, de Paula HB, Perri SH, de Andrade AM, Dias FE, Burattini MN. Dog culling and replacement in an area endemic for visceral leishmaniasis in Brazil. Vet Parasitol. 2008;153:19–23. doi: 10.1016/j.vetpar.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Burattini MN, Coutinho FA, Lopes LF, Massad E. Modelling of the dynamics of leishmaniasis considering human, animal host and vector populations. Math Mod Anal Scient Comp. 1995;26:1–16. [Google Scholar]
- 42.Dye C. The logic of visceral leishmaniasis control. Am J Trop Med Hyg. 1996;55:125–130. doi: 10.4269/ajtmh.1996.55.125. [DOI] [PubMed] [Google Scholar]
- 43.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 44.Coler RN, Reed SG. Second-generation vaccines against leishmaniasis. Trends Parasitol. 21:244–248. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 45.David JR, Stamm LM, Bezerra HS, Souza RN, Killick-Kendrick R, Oliveira Lima JW. Deltamethrin-impregnated dog collars have a potent anti-feeding and insecticidal effect on Lutzomyia longipalpis and Lutzomyia migonei. Mem Inst Oswaldo Cruz. 2001;96:839–847. doi: 10.1590/s0074-02762001000600018. [DOI] [PubMed] [Google Scholar]
- 46.Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impregnated dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil? Int J Parasitol. 2004;34:55–62. doi: 10.1016/j.ijpara.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Bern C, Joshi AB, Jha SN, Das ML, Hightower A, Thakur GD, Bista MB. Factors associated with visceral leishmaniasis in Nepal: bed-net use is strongly protective. Am J Trop Med Hyg. 2000;63:184–188. doi: 10.4269/ajtmh.2000.63.184. [DOI] [PubMed] [Google Scholar]
- 48.Ritmeijer K, Davies C, van Zorge R, Wang S-J, Schorscher J, Dongu'du SI, Davidson RN. Evaluation of a mass distribution programme for fine-mesh impregnated bednets against visceral leishmaniasis in eastern Sudan. Trop Med Int Health. 2007;12:404–414. doi: 10.1111/j.1365-3156.2006.01807.x. [DOI] [PubMed] [Google Scholar]