Abstract
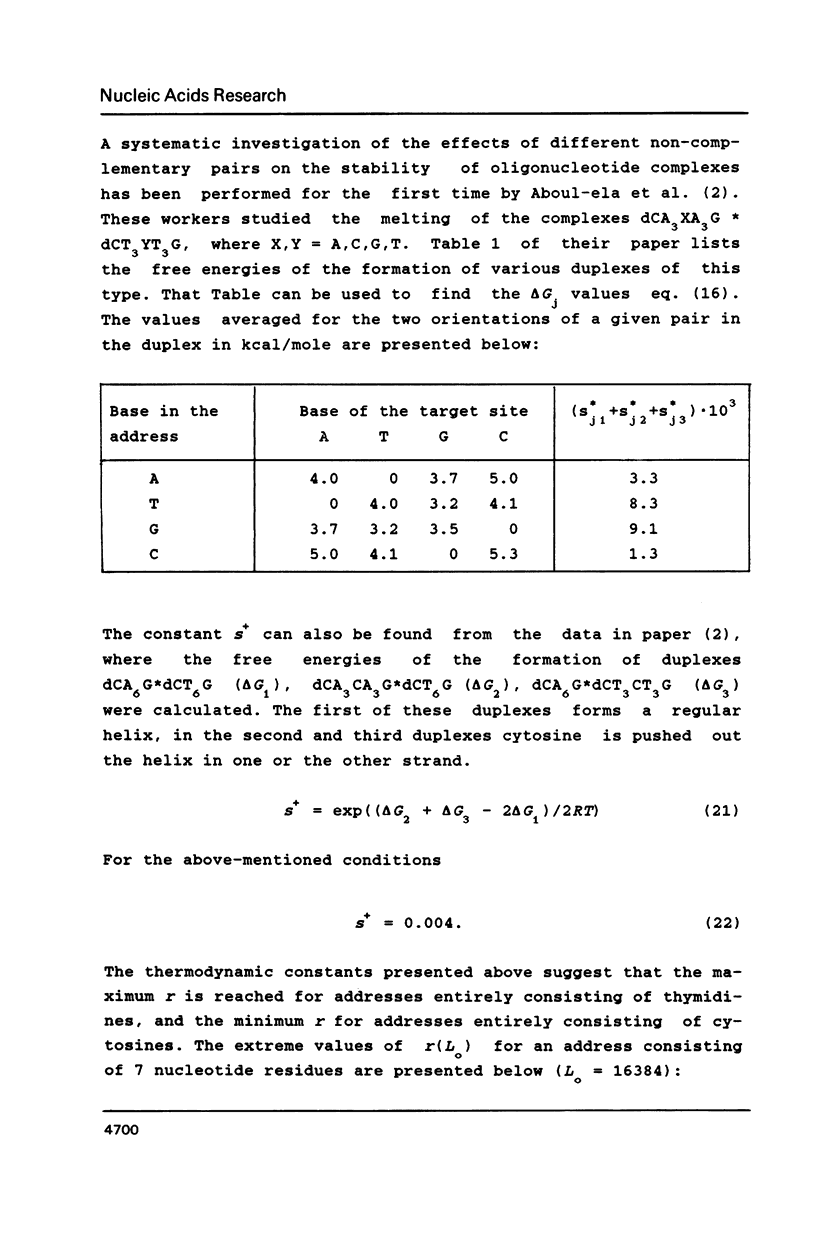
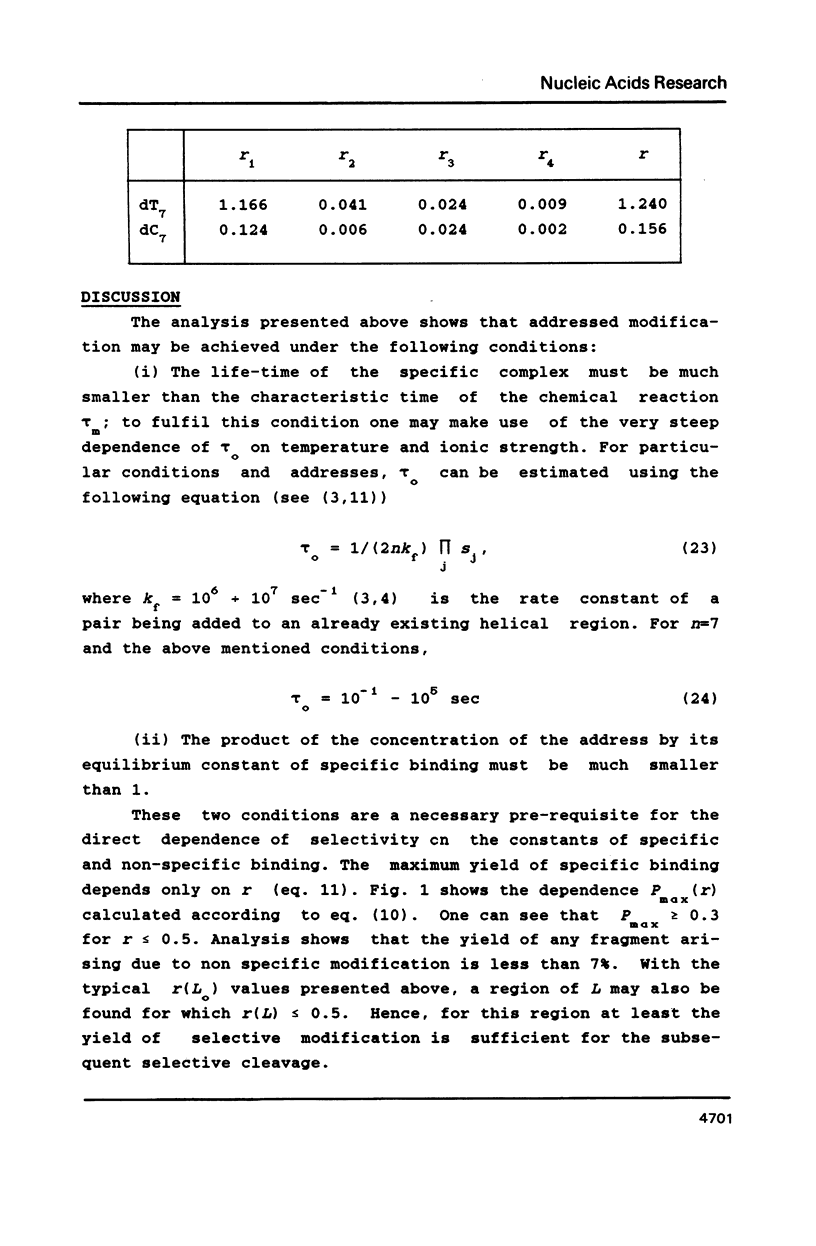
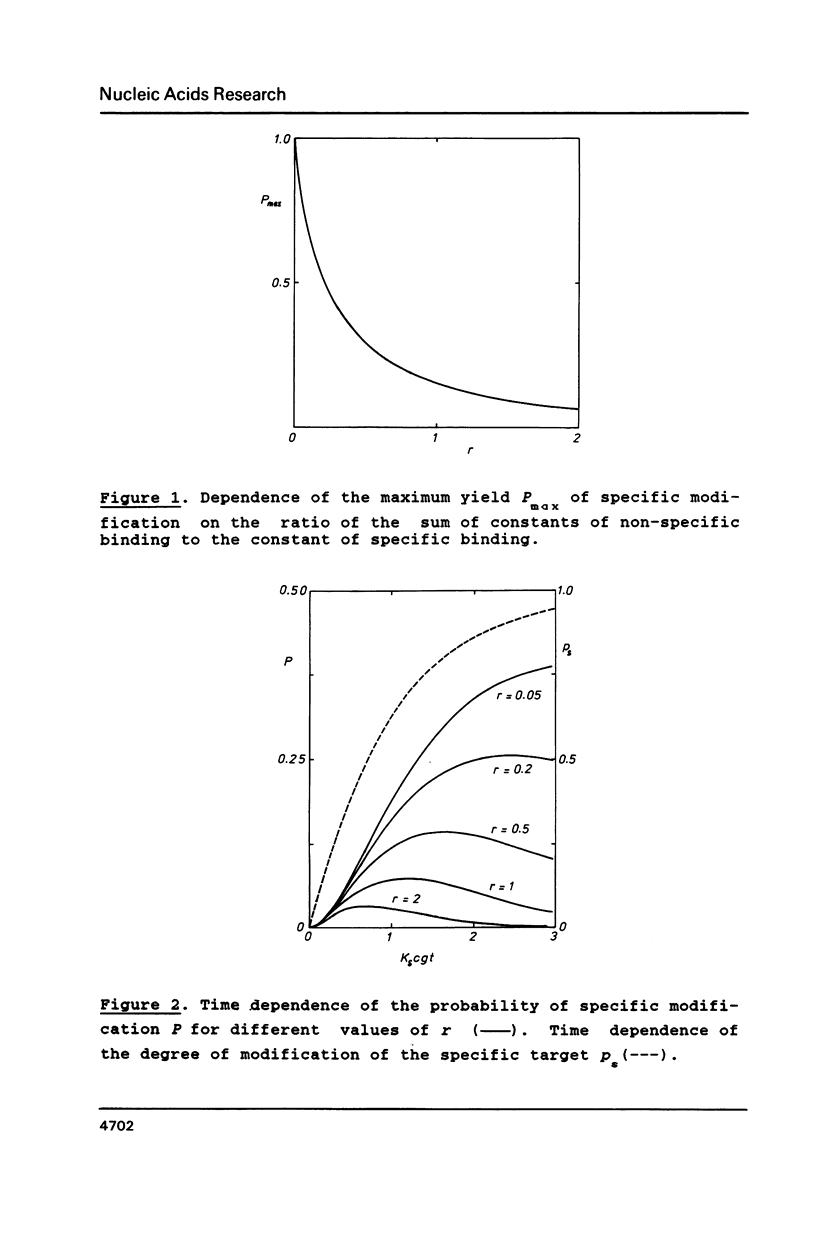
Chemical "addressed" modification of DNA involves treatment of single-stranded DNA with oligonucleotides complementary to certain target sequences in this DNA and bearing a groupings reactive towards DNA bases. The binding of oligonucleotides can occur both at completely (specific) and incompletely (nonspecific) complementary sites. We analyse the modification of a fragment that is flanked by two target sequences complementary to a given oligonucleotide address, contains no more such targets and has some randomly distributed sites for nonspecific binding. Conditions for the maximum ratio between specific and non-specific modification are determined. We find the probability of both target termini being specifically modified without any non-specific modification occurring within the fragment up to a given moment in time. Quantitative analysis is based on the use of known features of the specific and non-specific binding of an oligonucleotide to DNA sites. This analysis shows the possibility of specific cutting of DNA based on addressed modification.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anshelevich V. V., Vologodskii A. V., Lukashin A. V., Frank-Kamenetskii M. D. Slow relaxational processes in the melting of linear biopolymers: a theory and its application to nucleic acids. Biopolymers. 1984 Jan;23(1):39–58. doi: 10.1002/bip.360230105. [DOI] [PubMed] [Google Scholar]
- Craig M. E., Crothers D. M., Doty P. Relaxation kinetics of dimer formation by self complementary oligonucleotides. J Mol Biol. 1971 Dec 14;62(2):383–401. doi: 10.1016/0022-2836(71)90434-7. [DOI] [PubMed] [Google Scholar]
- Kozyavkin S. A., Mirkin S. M., Amirikyan B. R. The ionic strength dependence of the cooperativity factor for DNA melting. J Biomol Struct Dyn. 1987 Aug;5(1):119–126. doi: 10.1080/07391102.1987.10506380. [DOI] [PubMed] [Google Scholar]
- Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic--oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J Mol Biol. 1971 Dec 14;62(2):361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- Vologodskii A. V., Amirikyan B. R., Lyubchenko Y. L., Frank-Kamenetskii M. D. Allowance for heterogeneous stacking in the DNA helix-coil transition theory. J Biomol Struct Dyn. 1984 Aug;2(1):131–148. doi: 10.1080/07391102.1984.10507552. [DOI] [PubMed] [Google Scholar]
- Wada A., Yabuki S., Husimi Y. Fine structure in the thermal denaturation of DNA: high temperature-resolution spectrophotometric studies. CRC Crit Rev Biochem. 1980;9(2):87–144. doi: 10.3109/10409238009105432. [DOI] [PubMed] [Google Scholar]



