Abstract
The only effective intervention to slow onset and progression of glaucomatous blindness is to lower intraocular pressure (IOP). Among other modulators, adenosine receptors (ARs) exert complex regulation of IOP. Agonists of A3ARs in the ciliary epithelium activate Cl− channels, favoring increased formation of aqueous humor and elevated IOP. In contrast, stimulating A1ARs in the trabecular outflow pathway enhances release of matrix metalloproteinases (MMPs) from trabecular meshwork (TM) cells, reducing resistance to outflow of aqueous humor to lower IOP. These opposing actions are thought to be initiated by cellular release of ATP and its ectoenzymatic conversion to adenosine. This view is now supported by our identification of six ectoATPases in trabecular meshwork (TM) cells and by our observation that external ATP enhances TM-cell secretion of MMPs through ectoenzymatic formation of adenosine. ATP release is enhanced by cell swelling and stretch. Also, enhanced ATP release and downstream MMP secretion is one mediator of the action of actin depolymerization to reduce outflow resistance. Inflow and outflow cells share pannexin-1 and connexin hemichannel pathways for ATP release. However, vesicular release and P2X7 release pathways were functionally limited to inflow and outflow cells, respectively, suggesting that blocking exocytosis might selectively inhibit inflow, lowering IOP.
Key Words: Ciliary epithelium, Trabecular meshwork, Intraocular pressure, Pannexin-1 hemichannels, Connexin hemichannels, P2X7 ATP receptors, Vesicular release, A3 adenosine receptors, A1 adenosine receptors, Actin cytoskeleton
Introduction
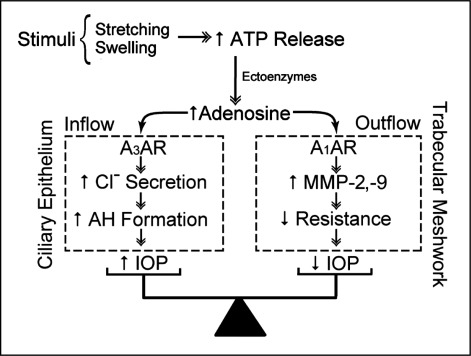
Glaucoma is a major cause of irreversible blindness world-wide [1] that is usually associated with elevated intraocular hydrostatic pressure (IOP). The symbols used in this paper are listed in Table 1. Reducing IOP is the only intervention documented to delay the onset and slow the progression of glaucomatous blindness, even if IOP is not abnormally high [2, 3, 4, 5, 6, 7]. In principle, IOP can be lowered by: slowing the rate of formation of aqueous humor, reducing the resistance to outflow through the pressure-sensitive (conventional) pathway, or shunting some of the trabecular outflow to exit through the relatively pressure-insensitive uveoscleral pathway. Among other regulators of intraocular pressure [8], adenosine receptors exert a complex modulation of aqueous humor dynamics (Fig. 1). Adenosine delivery to the aqueous humor face of the ciliary epithelium activates Cl− channels [9, 10], tending to enhance fluid inflow into the eye and increasing IOP [11]. Adenosine delivery to the trabecular meshwork (TM) cells of the trabecular outflow pathway can stimulate release of matrix metalloproteinases (MMPs) [12], reducing resistance to exit flow [13, 14], and thereby IOP [11, 13, 15, 16]. The integrative balance of these opposing effects on IOP through actions on inflow and outflow determines whether pressure will increase or decrease. As indicated in the cartoon of Fig. 1 and discussed below, the source of the adenosine delivered to both inflow [17] and outflow cells [18] is thought to be through autocrine/paracrine ATP release and subsequent metabolism by ectoATPases. If so, this raises the possibility that adenosine delivery to inflow and outflow tissues might be selectively modified pharmacologically, thereby permitting a novel strategy for lowering IOP. We have begun addressing this possibility in studies identifying the ATP release pathways and their regulation in inflow and outflow cells [18, 19, 20].
Table 1.
List of Abbreviations
| A1 and A3 ARs | A1 and A3 subtype adenosine receptors |
| AH | aqueous humor |
| AQP1 and AQP4 | aquaporin−1 and −4 water channels |
| BAF | bafilomycin A1 (inhibitor of vesicular ATP release) |
| bCE | bovine ciliary epithelial cell |
| CD73 | ecto-5′-nucleotidase |
| CE | ciliary epithelial cell |
| CFTR | cystic fibrosis transmembrane conductance regulator CI− channel |
| Cx | connexin hemichannel or gap junction |
| DTT | dithiothreitol (a reducing agent partially blocking PX1) |
| E-NPP1-3 | ecto-nucleotide pyrophosphatases/phosphodiesterases 1-3 |
| E-NTPD2, E-NTPD8 | ecto-nucleoside triphosphate diphosphohydrolases 2 and 8 |
| FGF | fibroblast growth factor |
| GM6001 | non-selective inhibitor of metalloproteinase activity |
| HCE | immortalized human nonpigmented ciliary epithelial cell |
| HEK293T | human embryonic kidney 293 cell line containing the SV40 large T-antigen |
| HEP | heptanol (relatively selective Cx inhibitor at 1 mM) |
| ICl,swell | swelling-activated CI− current |
| IOP | intraocular pressure |
| KN-62 | inhibitor of P2X7 |
| MMP | matrix metalloproteinase |
| NPE | nonpigmented ciliary epithelial cell or cell layer |
| P2X7 | P2X7 subtype ATP receptor |
| PX1 | pannexin-1 hemichannel |
| PE | pigmented ciliary epithelial cell or cell layer |
| PRO | probenecid (inhibitor of PX1 hemichannels) |
| Pxs | pannexins |
| RT-PCR | reverse-transcription polymerase chain reaction |
| TM | trabecular meshwork |
| TM5 | transformed human trabecular meshwork cell line |
Fig. 1.
Purinergic regulation of aqueous humor dynamics. Swelling or stretching of cells releases ATP, which can then be converted by ectoATPases to adenosine. Activation of A3 adenosine receptors of NPE cells of the ciliary epithelium will activate Cl− channels, favoring enhanced inflow and elevating IOP. In contrast, adenosine delivered to the trabecular meshwork cells of the outflow pathway activates A1 adenosine receptors, stimulating secretion of matrix metalloproteinases−2 and −9, lowering outflow resistance and IOP.
Formation of aqueous humor
The aqueous humor is formed by the bilayered ciliary epithelium comprising pigmented (PE) and nonpigmented (NPE) ciliary epithelial cell layers that cover the surface of the ciliary body. As with all secretory epithelia, fluid transfer depends on the net transfer of solute, here predominantly NaCl, with secondary passive transfer of water in response to the local osmotic gradient. Uptake of stromal Na+ and Cl− by the outer PE cell layer largely proceeds by electroneutral Na+−K+−2Cl− symports and paired Na+/H+ and Cl−/HCO3− antiports. Thereafter, solute and water permeate gap junctions to the NPE cells, which release Cl− through swelling-activated Cl− channels and extrude Na+ through Na+, K+− activated ATPase. AQP1 and AQP4 channels provide pathways for water release from NPE cells into the aqueous humor, but whether aquaporin channels play a role in uptake of water from stroma to PE cells is uncertain. The component plasma-membrane ion [21] and aquaporin [22] transporters subserving aqueous humor inflow are reviewed elsewhere.
Several considerations have suggested that NPE Cl−−channel activity limits the rate of aqueous humor secretion [23]. Selective agonists of A3 subtype adenosine receptors (ARs) activate Cl− currents [10] and cause cell shrinkage [9] of HCE immortalized human NPE cells [10]. The physiologic, non-selective A3AR agonist adenosine also shrank the HCE cells under conditions where K+ permeability was not rate-limiting. This shrinkage was abolished by preincubation with selective antagonists of A3ARs [9]. The Cl−−channel target of A3ARs shares macroscopic properties, and is likely identical, with swelling-activated Cl− channels (ICl,swell) of NPE cells [10] facing the aqueous humor [24]. Despite considerable experimental effort, the molecular identity of the conduit subserving ICl, swell is uncertain. Knockdown and blocking-antibody strategies have suggested that both ClC-3 [25, 26, 27] and pICln [28] play roles in functional expression of ICl,swell in NPE cells. As discussed elsewhere [21], whether these roles of ClC-3 [29, 30] and pICln [31, 32, 33] are direct or indirect in ciliary epithelial and other cells remains unresolved.
Based on the foregoing observations with isolated cells, we expect that agonists and antagonists of A3ARs will increase and decrease IOP, respectively. Measurements of IOP in the living mouse have confirmed these predictions [11], and A3-null mice display lowered IOP [34].
Outflow of aqueous humor
Outflow from the anterior chamber of the eye proceeds through two parallel pathways to eventually return to the venous system. The uveoscleral pathway begins with passage of aqueous humor between the ciliary muscle bundles, followed by permeation through several relatively pressure-insensitive routes [35]. Recent data suggest that uveoscleral exit may be more significant than previously believed. However, the parallel pressure-sensitive trabecular exit pathway is of particular interest since the elevated outflow resistance observed in glaucoma usually leads to an abnormally high IOP. The trabecular outflow pathway comprises, in series, the trabecular meshwork, juxtacanalicular tissue, Schlemm's canal and the collector channels. The site of, and mechanisms underlying, the resistance to trabecular outflow remain unclear [36]. Nonetheless, TM cells release metalloproteinases (MMPs) [12, 37], and increased MMP activity reduces outflow resistance of human [38] and bovine [14] preparations. One of adenosine's mechanisms of action in the outflow tract involves MMPs since agonists of A1 subtype ARs stimulate secretion of both MMP-2 [12, 20, 39] and MMP-9 [20]. The MMPs secreted after agonist activation of A1ARs are functional, reducing resistance within 20 min by 20-25%, an effect nearly completely abolished by the non-selective MMP inhibitor GM6001 [14]. The A1AR-mediated reduction in outflow resistance leads to decreased IOP, measured in multiple species, including non-human primates [11, 13, 15].
Balance of Purinergic Effects on IOP
The foregoing observations indicate that the physiologic agonist adenosine exerts opposing effects on IOP through its actions on inflow and outflow (Fig. 1). Adenosine activates A3ARs of NPE cells of the ciliary epithelium to increase Cl− release, tending to enhance inflow and thereby raising IOP. In contrast, adenosine stimulates A1ARs of the trabecular meshwork to enhance MMP-2 and MMP-9 secretion, reducing outflow resistance and thereby lowering IOP. In each case, the source of adenosine delivery to the ARs has been thought to be ATP release, with subsequent conversion of ATP to adenosine by ectoenzymes [17, 18].
This view has recently been supported by two new observations. First, gene expression of ectoATPases that metabolize ATP to adenosine [40] has been found [20] in both explant-derived human TM cells and the human TM5 cell line [41]. These ectoenzymes include: E-NPP1-3, members of the ecto-nucleotide pyrophosphatase/phosphodiesterase family converting ATP to AMP; E-NTPD2 and E-NTPD8 of the ecto-nucleoside triphosphate diphosphohydrolases converting both ATP to ADP and ADP to AMP; and ecto-5′-nucleotidase (CD73) converting AMP to adenosine. The identification of these six ectoATPases documents the plausibility that ATP release can modulate adenosine delivery to the ARs. Second, addition of ATP to the solution bathing TM5 cells stimulated MMP secretion, which was strongly blocked by inhibiting metabolism of ATP to adenosine (Li, A. et al., manuscript submitted). Given this experimental support, we have focused on identifying the mechanisms and regulation of ATP release by ciliary epithelial inflow cells and TM outflow cells.
Mechanisms of ATP Release
Multiple pathways have been documented to subserve ATP release [42], including channels, transporters such as P-glycoprotein, vesicular release and cell lysis. Under physiologic conditions, ATP is expected to be largely in the form of MgATP2-, with a minor fraction as ATP4-, so that channels must have large-diameter conduits of at least ∼12 Å [42]. Early evidence suggested that members of the ATP-binding cassette family such as the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) [43, 44] or P-glycoprotein [45, 46] might serve as conduits. Several additional candidates have been subsequently considered [42, 47], including the Volume-Sensitive Outwardly-Rectifying (VSOR) Cl− channel, the maxi-Cl−channel, and the connexin (Cx) and pannexin (Px) “hemichannels” and P2X7 ATP ionoreceptors described below. P-glycoprotein is an unlikely candidate since antibodies blocking whole-cell currents do not affect ATP release in HTC-R and NIH3T3/MDR1 cells over-expressing the protein [42]. The swelling-activated anion channel (aka VSOR, VRAC and VSAC) has been excluded since ATP release and activation of this channel can also be dissociated [42]. In contrast, the maxi-Cl− channel is a plausible pathway. Although the molecular structure of the maxi-Cl− channel is unknown [48], its inner bore radius is ∼13 Å, roughly twice that of ATP.
The term “hemichannels” is commonly applied to unpaired wide-bore channels that either form [connexins (Cxs), innexins] or were thought to form [pannexins (Pxs)], gap junctions in paired cells. Innexins form both gap junctions and hemichannels in protosomes, including nematodes, and connexins subserve the same dual function in deuterostomes, including vertebrates [48]. Connexin hemichannels have long been known [50]. More recently, the pannexins (Px1-3) have been identified [51]. The Pxs, Cxs and innexins share a common topology [52], but there is only a 20% homology between Pxs and innexins and no homology between Cxs and either Pxs or innexins. Although Px1, like Cxs, was initially thought to form gap junctions, the four (rather than six) cysteine sites in the extracellular loops and the rich glycosylation of pannexin proteins are currently thought to preclude Px-based gap-junctional formation [49, 52].
The role of Px1 as a conducting channel and conduit for ATP release has been documented by several laboratories [49, 53, 54, 55, 56]. The physiologic significance of Px2 as a homomeric hemichannel is less certain. Bruzzone et al. [57] found Px1 activity but no homomeric Px2 activity following heterologous expression of rat Px1 in Xenopus oocytes. Co-expression of Px1 and Px2 produced reduced currents that gated more slowly in comparison to the homomeric Px1 currents, suggesting that Px1/Px2 heteromeric channels had been formed. In contrast, Ambrosi et al. [52] reported that heterologous expression solely with a longer rat Px2 construct did produce currents without concurrent overexpression of Px1. However, the Px2-associated currents could not be stimulated at physiologically-relevant intracellular potentials, so that the potential in vivo importance of these currents is unknown. The final member of the Px family, Px3, has not been associated with channel activity [57]. Recent data suggest that Px3 plays a role in chondrocyte differentiation [58] and bone development [59].
ATP release is also associated with stimulation of the ionotropic ATP receptor P2X7. The cationic conduit of P2X7 is opened within milliseconds of its activation, but goes on to become permeable to large cations within subsequent seconds during continued stimulation [60]. P2X7 has been reported to trigger activation of both PX1-mediated and PX1-independent large-bore channels [61]. Whether the large-bore conduit associated with P2X7 is intrinsic to the receptor [62] or reflects the associated PX1 hemichannel [63, 64] has been controversial [60].
Many widely-applied blockers exert significant cross-channel inhibition [47], but several relatively selective agents have proved informative in pharmacologically distinguishing the ATP-releasing pathways. Probenecid inhibits PX1, but not Cx hemichannels [65]. The (-)-threo-(11R/2R) diastereomer of mefloquine also blocks PX1 with much higher potency than Cx hemichannels [66]. In contrast, both 1 mM heptanol [55] and extracellular Ca2+ [53] inhibit Cx, but not PX1, hemichannels. We have used bafilomycin A1 (BAF) to inhibit vesicular ATP release (Fig. 2). BAF acts by interfering with vesicular uptake of ATP, and is widely applied to block exocytosis of ATP [19]. We have complemented the pharmacologic approach with knockdown [18] and overexpression [18, 19] strategies in identifying the principle ATP release pathways in HEK293T and TM cells.
Fig. 2.
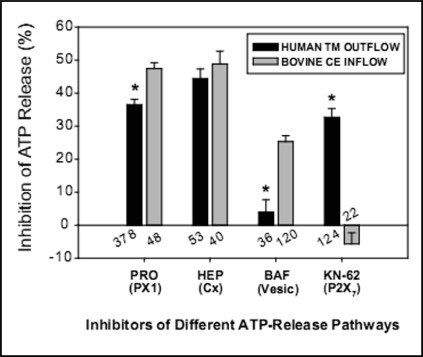
Comparison of swelling-activated pathways for ATP release in outflow and inflow cells. The inhibitors and, in parentheses, the release-pathway targets are presented along the abscissa. The numbers of wells studied are indicated for both outflow and inflow cells. P2X7 receptors mediated ATP release only in TM cells, whereas vesicular ATP release was displayed only by ciliary epithelial (CE) cells.
ATP Release Pathways from Trabecular Meshwork Outflow Cells
Both human explant-derived native TM cells and the human TM5 cell line displayed gene expression of multiple ATP-releasing conduits. PX1, Cx26, Cx31, Cx43, the P2X7 ionotropic ATP receptor, and CFTR were identified by the reverse-transcription polymerase chain reaction (RT-PCR). Responses observed to separate application of ∼20 inhibitors to TM5 cells indicated that PX1 hemichannels, Cx hemichannels and P2X7 receptors provided comparable contributions to ATP release, measured by the luciferin-luciferase reaction (Fig. 2). CFTR, swelling-activated anion channels and vesicular release played no significant role. Simultaneous block of PX1, Cx and P2X7 pathways inhibited ATP release by more than 96 ± 1%, so that cell lysis had a negligible effect. Identical responses were found in explant-derived TM cells after exposure to a selected number of inhibitors [18].
Estimation of the relative importance of the PX1, Cx and P2X7 pathways in releasing ATP was based on the pharmacologic responses (Fig. 2). The validity of this approach was tested by examining the pharmacologic profile after partial knockdown of PX1 hemichannels with a lentiviral shRNA strategy. As expected, PX1 knockdown reduced total ATP release and the efficacy of the PX1-blocker probenecid in further inhibiting release. In contrast, PX1 knockdown enhanced the percentage inhibition produced by blocking either Cx or P2X7 pathways of the residual ATP release [18].
Blocking PX1 and Cx pathways concurrently produced an additive inhibition of swelling-activated ATP release [18]. However, concurrent block of P2X7 together with either PX1 or Cx hemichannels produced a less-than-additive inhibition [20]. Thus, the contribution of P2X7 to ATP release appears greater when ATP is being concurrently released through parallel pathways. This view is consistent with the expectation that activation of the P2X7 ATP receptor will be greater in the presence of higher baseline ATP levels.
ATP Release Pathways from Ciliary Epithelial Inflow Cells
Primary cultures of bovine mixed NPE and PE (bCE) cells and transformed bovine NPE and PE cell lines were found by RT-PCR to express PX1-3, Cx43, Cx40 and P2X7. Expression of message for PX1 was confirmed by northern blots. Expression of bovine PX1 in HEK293T cells enhanced swelling-activated ATP release, and that increase was inhibited by the PX1 blocker probenecid [19].
Like TM cells, swelling-activated native and transformed bovine ciliary epithelial cells released ATP through three major pathways (Fig. 2). Similarly to TM cells, ciliary epithelial cells also utilized PX1 and Cx hemichannels. However, in contrast to TM cells, P2X7-mediated release was insignificant. Instead, approximately 20% of the release was vesicular (Fig. 2). Concurrent blockade of PX1, Cx and vesicular release pathways inhibited swelling-activated ATP release by >90% [19].
The foregoing observations suggest that applying blockers of vesicular release to the eye's anterior segment might lead to inhibition of inflow without affecting outflow resistance, thereby lowering IOP. We are pursuing this possibility, but two caveats must be kept in mind. First, because of the difficulty in obtaining very fresh post-mortem human ciliary epithelial cells, the inflow cells studied were bovine. Second, the studies described have been conducted with isolated cells. The regulatory volume responses, and perhaps other responses, of individual ciliary epithelial cells have proved to be highly region dependent, at least in rabbit ciliary epithelium [67].
Modulators of ATP Release
The regulation of ATP release is incompletely understood. At least six modulators of release have been implicated: cell volume, mechanical perturbation, redox state, intracellular Ca2+, growth factors, and cytoskeletal remodeling. Hypotonic cell swelling [68] and mechanical perturbation [54, 69, 70] are well-documented triggers of ATP release. In addition to measuring swelling-activated ATP release, stretching TM5 cells by 30% enhanced ATP release by twofold [20]. Separately blocking PX1, Cxs, P2X7 and vesicular release produced the same percentage inhibitions in stretch-activated ATP release as those observed in swelling-activated release. Combined inhibition of PX1, Cxs and P2X7 almost completely abolished stretch-activated ATP release. Evidently, cell swelling and mechanical perturbation trigger identical signaling pathways for ATP release in TM5 cells.
The redox state of the cell also modulates ATP release insofar as the reducing agent dithiothreitol (DTT) partially inhibits PX1 channels, an inhibitory effect that is precluded by interaction of PX1 with the K+ channel subunit Kvb3 [71]. In TM5 cells, DTT alone reduced swelling-activated ATP release by 26.4 ± 1.2%, but had no further effect when added to cells exposed to the PX1 blocker probenecid [18]
Whether or not intracellular Ca2+ activates PX1 has been controversial. Single-channel records of Xenopus oocytes heterologously expressing PX1 indicate that PX1 is activated within seconds of applying micromolar concentrations of cytosolic Ca2+ to inside-out patches [72]. In contrast, intracellular Ca2+ did not affect PX1 channels expressed in mammalian cells [55]. In TM5 cells, exposure to ionomycin did enhance ATP release but only after a prolonged lag time, 7-8 times longer than after hypotonic challenge. That release was abolished by blocking P2X7 with the large organic cation KN-62 [73] applied to either PX1-knockdown or mock-knockdown TM5 cells, but not by the PX1 inhibitor probenecid [18]. Evidently, change in cytosolic Ca2+ activity over the physiologic range does not activate PX1 hemichannels in TM cells. The P2X7-mediated increase triggered by ionomycin may reflect apoptotic magnification of ATP release initiated by ATP permeating membrane that was compromised by the high intracellular Ca2+ activity [18].
Growth factors have been reported to stimulate ATP release in other cells. Basic fibroblast growth factor (FGF-2) activates Cx hemichannels in Cx43-expressing C6 glioma cells [74]. Acidic fibroblast growth factor (FGF-1) initiates a series of events leading to stimulation of vesicular release and later of P2X7 receptors, and Cx and PX hemichannels in rat spinal astrocytes [75].
Remodeling of the actin cytoskeleton has been found to modulate swelling-evoked ATP release from TM5 cells [20]. Cytochalasin D-triggered depolymerization enhances, whereas prolonged exposure to dexamethasone reduces, swelling-activated ATP release. The effects of cytoskeletal modification were partly mediated by modulation of the duration of cell swelling after applying hypotonicity. The modulation of ATP release may regulate MMP secretion by adjusting ectoenzymatic generation of adenosine, activating A1ARs and thereby leading to MMP-mediated modification of outflow resistance.
In addition to these six modulators, we have very recently found that swelling-activated ATP release is also reduced by exposure to low concentrations of ouabain (≥10 nM) or to equivalent concentrations of the aglycone strophanthidin (Li et al., manuscript submitted). The effect on ATP release could be dissociated from the effects of the cardiotonic steroids on ion-exchange activity of the target, Na+, K+−activated ATPase, suggesting mediation through scaffolding and/or signaling functions of the enzyme [76, 77].
Conclusions
Adenosine receptors (ARs) of the inflow and outflow aqueous humor pathways exert opposing actions on IOP. Adenosine delivery to A3ARs in the ciliary epithelium activates Cl− channels, leading to increased IOP. In contrast, adenosine delivery to A1ARs in the TM cells stimulates MMP secretion, reducing resistance to aqueous humor outflow and decreasing IOP. In each case, adenosine is delivered by ectoenzymatic conversion of ATP released from the cells. In both inflow and outflow cells, PX1 and Cx hemichannels play major roles in ATP release. However, vesicular release is a major ATP-release mechanism only in the ciliary epithelial inflow cells, whereas P2X7 subserves ATP release only from human TM outflow cells (Fig. 2). This difference in ATP release mechanisms suggests that blocking vesicular release might selectively inhibit inflow without altering outflow resistance, thereby lowering IOP.
Although understanding of the regulation of ATP release is incomplete, release from TM cells is increased by cell swelling, cell stretch, and actin depolymerization. ATP release is inhibited by the reducing agent dithiothreitol. Fibroblast growth factors increase ATP release in other cells. Elevated intracellular Ca2+ has been reported to increase PX1 activity in some, but not other, cells. In TM5 cells, increasing cytosolic Ca2+ does not increase ATP release through PX1 hemichannels. Finally, cardiotonic steroids reduce swelling-activated ATP release, an effect likely mediated by scaffolding/signaling functions, rather than by the ion-exchange role, of Na+, K+−activated ATPase.
Acknowledgements
Supported by NIH Research Grant EY13624 (to M.M. Civan) and a Core Grant P30 EY001583 (to the University of Pennsylvania). We thank Dr. Iok-hou Pang and Alcon Laboratories Inc. for generously providing human TM5 cells and Dr. Miguel Coca-Prados for kindly providing the bovine ciliary epithelial cell lines.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 4.The AGIS investigators: The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon M. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 7.Higginbotham EJ, Gordon MO, Beiser JA, Drake MV, Bennett GR, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122:813–820. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]
- 8.Pang IH, Clark AF. Outflow Signaling Mechanisms and New Therapeutic Strategies for the Control of Intraocular Pressure. In: Civan MM, editor. The Eye's Aqueous Humor. Second Edition. San Diego: Elsevier; 2008. pp. 429–469. [Google Scholar]
- 9.Mitchell CH, Peterson-Yantorno K, Carré DA, McGlinn AM, Coca-Prados M, Stone RA, Civan MM. A3 adenosine receptors regulate Cl− channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276:C659–666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- 10.Carré DA, Mitchell CH, Peterson-Yantorno K, Coca-Prados M, Civan MM. Similarity of A(3)-adenosine and swelling-activated Cl(-) channels in nonpigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2000;279:C440–451. doi: 10.1152/ajpcell.2000.279.2.C440. [DOI] [PubMed] [Google Scholar]
- 11.Avila MY, Stone RA, Civan MM. A(1)-, A(2A)-and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001;134:241–245. doi: 10.1038/sj.bjp.0704267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3016–3020. [PubMed] [Google Scholar]
- 13.Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997;64:979–989. doi: 10.1006/exer.1997.0296. [DOI] [PubMed] [Google Scholar]
- 14.Crosson CE, Sloan CF, Yates PW. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005;46:3795–3799. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- 15.Crosson CE. Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther. 1995;273:320–326. [PubMed] [Google Scholar]
- 16.Crosson CE, Gray T. Characterization of ocular hypertension induced by adenosine agonists. Invest Ophthalmol Vis Sci. 1996;37:1833–1839. [PubMed] [Google Scholar]
- 17.Mitchell CH, Carré DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Mitchell CH, Civan MM. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2012;227:172–182. doi: 10.1002/jcp.22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol. 2010;299:C1308–1317. doi: 10.1152/ajpcell.00333.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7996–8005. doi: 10.1167/iovs.11-8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Civan MM. Formation of the Aqueous Humor: Transport Components and their Integration. In: Civan MM, editor. The Eye's Aqueous Humor. Second Edition. San Diego: Elsevier; 2008. pp. 2–45. [Google Scholar]
- 22.Stamer WD, Baetz NW, Yool AJ. Ocular Aquaporins and Aqueous Humor Dynamics. In: Civan MM, editor. The Eye's Aqueous Humor. Second Edition. San Diego: Elsevier; 2008. pp. 47–70. [Google Scholar]
- 23.Do C-W, Civan MM. Basis of chloride transport in ciliary epithelium. J. Membr Biol. 2004;200:1–13. doi: 10.1007/s00232-004-0688-5. [DOI] [PubMed] [Google Scholar]
- 24.Do CW, Peterson-Yantorno K, Civan MM. Swelling-activated Cl− channels support Cl− secretion by bovine ciliary epithelium. Invest Ophthalmol Vis Sci. 2006;47:2576–2582. doi: 10.1167/iovs.05-0851. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Chen L, Jacob TJ. The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. J Physiol. 2000;524:63–75. doi: 10.1111/j.1469-7793.2000.t01-1-00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vessey JP, Shi C, Jollimore CA, Stevens KT, Coca-Prados M, Barnes S, Kelly ME. Hyposmotic activation of ICl, swell in rabbit nonpigmented ciliary epithelial cells involves increased ClC-3 trafficking to the plasma membrane. Biochem Cell Biol. 2004;82:708–718. doi: 10.1139/o04-107. [DOI] [PubMed] [Google Scholar]
- 27.Do CW, Lu W, Mitchell CH, Civan MM. Inhibition of swelling-activated Cl− currents by functional anti-ClC-3 antibody in native bovine non-pigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:948–955. doi: 10.1167/iovs.04-1004. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Wang L, Jacob TJ. Association of intrinsic pICln with volume-activated Cl− current and volume regulation in a native epithelial cell. Am J Physiol. 1999;276:C182–192. doi: 10.1152/ajpcell.1999.276.1.C182. [DOI] [PubMed] [Google Scholar]
- 29.Hermoso M, Satterwhite CM, Andrade YN, Hidalgo J, Wilson SM, Horowitz B, Hume JR. ClC-3 is a fundamental molecular component of volumesensitive outwardly rectifying Cl− channels and volume regulation in HeLa cells and Xenopus laevis oocytes. J Biol Chem. 2002;277:40066–40074. doi: 10.1074/jbc.M205132200. [DOI] [PubMed] [Google Scholar]
- 30.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 31.Clapham DE. The list of potential volume-sensitive chloride currents continues to swell (and shrink) J Gen Physiol. 1998;111:623–624. doi: 10.1085/jgp.111.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strange K. Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln. J Gen Physiol. 1998;111:617–622. doi: 10.1085/jgp.111.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fürst J, Bottà G, Saino S, Dopinto S, Gandini R, Dossena S, Vezzoli V, Rodighiero S, Bazzini C, Garavaglia ML, Meyer G, Jakab M, Ritter M, Wappl-Kornherr E, Paulmichl M. The ICln interactome. Acta Physiol (Oxf) 2006;187:43–49. doi: 10.1111/j.1748-1716.2006.01549.x. [DOI] [PubMed] [Google Scholar]
- 34.Avila MY, Stone RA, Civan MM. Knockout of A(3) adenosine receptors reduces mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:3021–3026. [PubMed] [Google Scholar]
- 35.Toris CB, Camras CB. Aqueous Humor Dynamics II: Clinical Studies. In: Civan MM, editor. The Eye's Aqueous Humor. Second Edition. San Diego: Elsevier; 2008. pp. 231–272. [Google Scholar]
- 36.Freddo TF, Johnson M. Aqueous Humor Outflow Resistance. In: Civan MM, editor. The Eye's Aqueous Humor. Second Edition. San Diego: Elsevier; 2008. pp. 161–192. [Google Scholar]
- 37.Bradley JM, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 38.Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- 39.Husain S, Shearer TW, Crosson CE. Mechanisms linking adenosine A1 receptors and extracellular signalregulated kinase 1/2 activation in human trabecular meshwork cells. J Pharmacol Exp Ther. 2007;320:258–265. doi: 10.1124/jpet.106.110981. [DOI] [PubMed] [Google Scholar]
- 40.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994;13:51–63. doi: 10.3109/02713689409042398. [DOI] [PubMed] [Google Scholar]
- 42.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prat AG, Reisin IL, Ausiello DA, Cantiello HF. Cellular ATP release by the cystic fibrosis transmembrane conductance regulator. Am J Physiol. 1996;270:C538–545. doi: 10.1152/ajpcell.1996.270.2.C538. [DOI] [PubMed] [Google Scholar]
- 44.Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- 45.Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roman RM, Wang Y, Lidofsky SD, Feranchak AP, Lomri N, Scharschmidt BF, Fitz JG. Hepatocellular ATP-binding cassette protein expression enhances ATP release and autocrine regulation of cell volume. J Biol Chem. 1997;272:21970–21976. doi: 10.1074/jbc.272.35.21970. [DOI] [PubMed] [Google Scholar]
- 47.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 48.Sabirov RZ, Sheiko T, Liu H, Deng D, Okada Y, Craigen WJ. Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J Biol Chem. 2006;281:1897–1904. doi: 10.1074/jbc.M509482200. [DOI] [PubMed] [Google Scholar]
- 49.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–474. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- 52.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–24431. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 54.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–418. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, Yamada Y. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–18958. doi: 10.1074/jbc.M110.127027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J Bone Miner Res. 2011;26:2911–2922. doi: 10.1002/jbmr.509. [DOI] [PubMed] [Google Scholar]
- 60.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 61.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 62.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2×7 Receptor Channel Pore Dilates under Physiological Ion Conditions. J Gen Physiol. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. Embo J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iglesias R, Spray DC, Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes. 2009;16:131–137. doi: 10.3109/15419061003642618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaughlin CW, Zellhuber-McMillan S, Macknight AD, Civan MM. Electron Microprobe Analysis of Rabbit Ciliary Epithelium Indicates Enhanced Secretion Posteriorly and Enhanced Absorption Anteriorly. Am J Physiol Cell Physiol. 2007;293:C1455–1466. doi: 10.1152/ajpcell.00205.2007. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 70.Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004;287:C1389–1395. doi: 10.1152/ajpcell.00220.2004. [DOI] [PubMed] [Google Scholar]
- 71.Bunse S, Locovei S, Schmidt M, Qiu F, Zoidl G, Dahl G, Dermietzel R. The potassium channel subunit Kvß3 interacts with pannexin 1 and attenuates its sensitivity to changes in redox potentials. Febs J. 2009;276:6258–6270. doi: 10.1111/j.1742-4658.2009.07334.x. [DOI] [PubMed] [Google Scholar]
- 72.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 73.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 74.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garré JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Sáez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci USA. 2010;107:22659–22664. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am J Cardiovasc Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 77.Xie Z, Askari A. Na+/K+−ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]