Abstract
More than 25 years ago our laboratory reported sex-dependent relationships between social status and coronary artery atherosclerosis among cholesterol-fed cynomolgus monkeys (Macaca fascicularis) maintained in social groups of four to six animals each. Dominant males developed more atherosclerosis than subordinates, but only if housed in recurrently reorganized social groups. In contrast, dominant females developed significantly less atherosclerosis than subordinates, irrespective of social setting. Although we have continued to study these associations, no confirmatory investigations have been reported by other laboratories or using other atherosclerosis-susceptible monkey species. Accordingly, we conducted a meta-analysis of all relevant data sources developed in our laboratory since 1982 to determine whether the originally-reported relationships between social status and atherosclerosis reflected robust associations. The sentinel (first) studies were comprised of 16 females and 27 males. The current meta-analysis encompassed 419 animals (200 females, 219 males) derived from 11 separate investigations. The results confirmed that, among males, dominant individuals developed more extensive atherosclerosis than subordinates when housed in recurrently reorganized (unstable) social groups in which an estrogen-implanted female was also present. Dominant males in stable social groups tended to have less atherosclerosis than similarly housed subordinates, but this effect was not significant. In contrast, we found that dominant females developed reliably less atherosclerosis than subordinates.
Keywords: meta-analysis, dominance, monkey, atherosclerosis
INTRODUCTION
Old World monkeys have figured prominently in studies of atherosclerosis, the process by which fibrofatty plaques (atheromas) form within the inner lining of the arteries of susceptible individuals. In human beings, atherosclerosis typically begins to develop in the second and third decades of life and worsens progressively over time, contributing to risk of coronary heart disease and atherothrombotic stroke [Strong 1992; Strong et al. 1999]. The utility of nonhuman primates for modeling this disease process derives from the tendency of these animals following exposure to a diet moderately high in fat, cholesterol, and simple carbohydrates to develop atherosclerosis relatively rapidly and in a manner mirroring its natural history in people. Cynomolgus and rhesus macaques (Macaca fascicularis, M. mulatta), for example, develop atheromatous lesions that are similar in morphological characteristics and location to those seen in human beings, and these progress through the same stages of growth and complication [Kaplan et al. 1985]. As in people, males develop more extensive atherosclerosis than do similarly-aged, reproductively intact (i.e., premenopausal) female monkeys [Hamm et al. 1983], and when consuming a diet resembling that typically eaten in the US and other industrialized countries, male cynomolgus monkeys experience myocardial infarction at a rate similar to that seen in their human counterparts [Bond et al. 1980].
In addition to enhancing our understanding of biological factors that contribute to susceptibility to atherosclerosis (such as diet, age, and sex), studies of nonhuman primates – and specifically, cynomolgus monkeys – also document psychosocial influences on this disease process [Kaplan et al. 1985]. Much of this latter work has focused on individual differences in social status (i.e., whether animals are dominant or subordinate in their social groups) as a “risk factor” for atherosclerosis. Social status has long been thought to represent a fundamental component of sociality in nonhuman primates [Bernstein 1981; Silk 2002; Walters and Seyfarth 1987], and social dominance also comprises a primary axis of interpersonal behavior in the structure of human social relationships [Wiggins and Trapnell 1996].
We have conducted an extensive program in atherosclerosis research in our laboratories using cynomolgus monkeys, much of which has been directed at understanding the relationship between dominance status and lesion development [Kaplan et al. 1985; Kaplan et al. 1996; Kaplan and Manuck 1998; Kaplan and Manuck 2008]. Notably, our initial (sentinel) studies of social status and atherosclerosis revealed associations that differed markedly between males and females. Among males, dominant individuals were found to develop more extensive atherosclerosis than their subordinate counterparts, but only if housed in social groups of periodically organized (“unstable”) memberships. In groups of “stable” (unchanging) membership, dominant animals tended to develop less extensive lesions than subordinates, albeit not significantly (Figure 1). Moreover, the exacerbation of atherosclerosis among “unstable dominant” monkeys could be prevented by chronic administration of a beta-adrenoreceptor antagonist, suggesting that the behavioral demands of retaining pre-eminence in an unstable social environment may promote lesion development preferentially in dominant monkeys via recurrent sympathetic nervous system activation [Kaplan et al. 1987]. In contrast, dominant social status among reproductively intact female cynomolgus monkeys afforded a relative protection against atherosclerosis, compared to both males and subordinate females [Hamm et al. 1983] (Figure 2). The heightened lesion development seen among subordinate females appears to be mediated by the subclinical suppression of ovarian function frequently experienced by these animals [Kaplan et al. 1996]. We and others have suggested that such suppression may in turn reflect the stress occasioned by behavioral subordination in group-dwelling primates and other long-lived mammals (e.g., Kaplan and Manuck 2004; Wasser and Barash 1983).
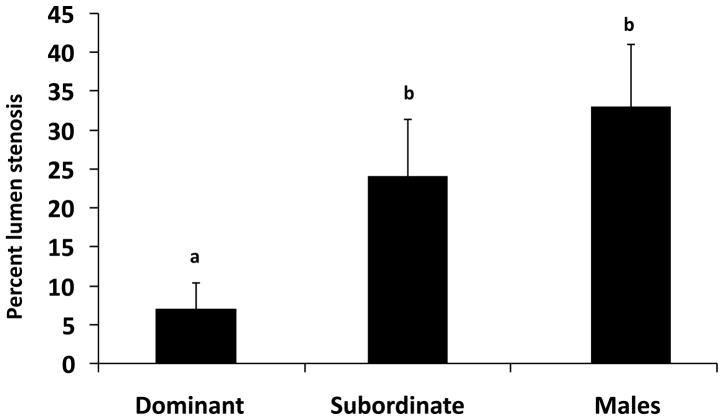
Figure 1.
Male sentinel study (MS1a and MS1b) showing that dominant monkeys housed in recurrently reorganized social groups develop significantly more coronary artery atherosclerosis than similarly housed subordinates or monkeys housed in stable social groups; all animals consumed an atherogenic diet [adapted from Kaplan et al. 1982].
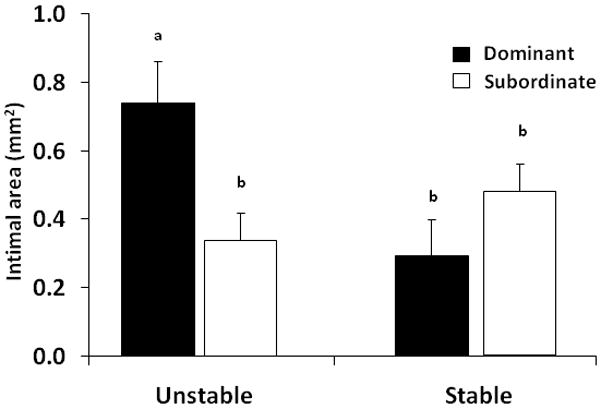
Figure 2.
Female sentinel study (FS4) showing that socially housed dominant animals develop significantly less coronary artery atherosclerosis than either similarly housed subordinates or males, with all animals consuming an atherogenic diet [adapted from Hamm et al., 1983].
Of course, confidence in the preceding findings and, by extrapolation, their potential implications for human disease, would be enhanced by corroboration from other laboratories and in other atherosclerosis-susceptible species of nonhuman primates. This would seem especially important in view of our unanticipated observation that social dominance was differentially associated with coronary atherogenesis in male and female monkeys. Unfortunately, despite the more than quarter century that has passed since our first studies were published, confirmatory evidence from other laboratories has not been published. Nor have other investigators pursued related studies of coronary artery disease and primate social status in this or other species.
During this same period, we have continued to study behavioral factors implicated in the pathophysiology of coronary disease, including effects of social stress on endothelial injury [Skantze et al. 1998], the influence of chronic social instability on abnormalities of coronary vasomotion [Williams et al. 1991; Williams et al. 1993], and the possible salutary effects of exercise and soy protein on atherosclerosis and vascular function [Adams et al. 2005; Walker et al. 2008; Williams et al. 2003]. Although social stratification of animals was not a primary variable of interest in many of these studies, the control conditions of some of our experiments entailed situations of housing and behavioral observations akin to those employed in our initial work addressing effects of social status on lesion development. Therefore in the absence of corroborating literature from other research centers, here we test the reliability of our associations of social dominance with atherosclerosis by subjecting to meta-analytic evaluation all compatible observations made since 1982 (the date of our first publication relating status to atherosclerosis). Subjects were socially housed male and female cynomolgus monkeys under comparable dietary and housing conditions regardless of whether social status was an identified parameter of study. The two primary objectives of this meta-analysis were to determine: 1) whether social status reliably influences atherosclerosis extent in monkeys; 2) whether the effect of social status on atherosclerosis is moderated by sex, and in males, by stability of social housing.
METHODS
Study Animals
All animals were imported as adults (as indicated by dentition) from commercial sources in Indonesia, the Philippines, Singapore, and Malaysia. Following importation and quarantine in single cages, monkeys were placed into social groups of between four and six animals each. The animals were distributed to insure that social groups within an experiment were comparable in body weight. Except where noted below, all groups were isosexual and stable in composition throughout a given investigation. We have no knowledge regarding the degree of relatedness or prior social familiarity among the animals comprising the individual studies. Finally, in all of the studies included in the meta-analysis, animals consumed a diet manufactured in our own diet laboratory and designed to model typical American consumption with respect to content of saturated fat and cholesterol [Kaplan 1985; Kaplan et al. 2002]. These diets are often termed “atherogenic”, as they adversely affect plasma lipids and lipoproteins and lead to exacerbation of atherosclerosis relative to diets with similar constituents, but low in saturated fat and cholesterol. Wake Forest University Health Sciences has been AAALAC accredited since 1966 and all studies were reviewed by the Wake Forest University Institutional Animal Care and Use Committee.
Criteria for Inclusion
Studies were drawn from among all published investigations of atherosclerosis conducted by the authors at Wake Forest University School of Medicine. The four major criteria for inclusion were that animals had been: 1) housed similarly in groups of four to six individuals that were social strangers at the start of the study; 2) fed diets that were comparably elevated in saturated fat and cholesterol and therefore provided a similar atherogenic stimulus; 3) evaluated for social status based on behavioral outcomes of competitive interactions (see below); and 4) subjected to no experimental manipulation affecting neuroendocrine or autonomic functioning (viz., premenopausal sex hormones, soy isoflavones, ovariectomy, chronic administration of beta-receptor antagonists, or aerobic conditioning). The 11 studies or portions thereof chosen for inclusion are enumerated in Table 1, which also contains a description of study design in relation to status and the stability of social groups. Animals totaling 200 females (91 dominant, 109 subordinate) and 219 males (114 dominant, 105 subordinate) provided data for the analyses presented here.
Table 1.
Studies Used in Meta-Analysis
| #Dominant | #Subordinate | Reference | |
|---|---|---|---|
| Studies with Males in Stable Groups | |||
| MS1a | 8 | 6 | Kaplan et al. 1982 |
| MS4a | 11 | 4 | Williams et al. 2003 |
| MS5a | 11 | 14 | Williams et al. 1993 |
| MS6 | 8 | 8 | Hamm et al. 1983 |
| MS7 | 13 | 17 | Adams et al. 2005 |
| Studies with Males in Reorganized Groups1 | |||
| MS1b2 | 6 | 9 | Kaplan et al. 1982 |
| MS2a2 | 6 | 6 | Kaplan et al. 1987 |
| MS2b | 16 | 13 | Kaplan et al. 1987 |
| MS4b | 10 | 8 | Williams et al. 2003 |
| MS5b | 23 | 22 | Williams et al. 1993 |
| Studies of Females | |||
| FS13 | 14 | 9 | Kaplan et al. 1984 |
| FS2a | 41 | 46 | Kaplan et al. 2002 |
| FS2b | 39 | 60 | Kaplan et al. 1995 |
| FS3 | 9 | 10 | Adams et al. 2000 |
| FS4 | 8 | 8 | Hamm et al. 1983 |
| FS5 | 21 | 22 | Walker et al. 2008 |
Each study consisted of groups of four to six animals that were rearranged in composition monthly, a manipulation that increased the amount and type of aggression over that observed in groups of unchanging composition
In addition to the monthly reorganization, an estrogen-implanted female was added to these groups.
Some of these animals were in groups that were reorganized every three months, a manipulation that did not affect social behavior and that was not considered in the original analysis; a vasectomized male was resident in all groups in this study.
Determination of Atherosclerosis
Coronary artery atherosclerosis was the dependent measure in most of the studies shown in Table 1 and was the primary outcome for the meta-analyses. At the end of each study, animals’ coronary arteries were perfused for one hour at a pressure of 100 mm Hg. We then took 15 blocks (each 3 mm in length) cut perpendicularly to the long axis of the arteries. Five of these were serial blocks from the left circumflex, five were from the left anterior descending and five were from the right coronary artery. Verhoeff van Gieson (VVG) – stained sections from each of the 15 blocks were projected onto an electronic plate and quantified using a hand-held stylus with a computer-assisted digitizer. The extent of atherosclerosis was measured as the mean cross-sectional area of plaque (the area between the internal elastic lamina and the arterial lumen) across the 15 sections of the artery segment as described in detail elsewhere [Clarkson et al. 2001]. Prior to widespread use of pressure fixation and digitizing equipment, atherosclerosis was evaluated in terms of the percent of luminal area occupied by plaque (“lumen stenosis”; MS6, FS4, reported in Hamm et al. 1983).
It is also possible to assess atherosclerosis in vivo using biopsy material taken from a surrogate peripheral artery, such as the iliac artery, which correlates well with atherosclerosis measured at the same time in the coronary arteries (r = 0.75, [Kaplan et al. 2002]). We used an iliac artery biopsy to estimate atherosclerosis in parts of two study cohorts (Table 1). Biopsy material was obtained by removal of a 3-cm section of the left common iliac artery after ligation distally and proximally. The section was perfusion fixed at a pressure of 100mm Hg for 10 minutes. To evaluate the extent of iliac atherosclerosis, we cut either one or three standard blocks (each approximately 3 mm in length) perpendicularly to the long axis of the artery. One VVG-stained section from each block was evaluated for atherosclerosis by measuring the area between the internal elastic lamina and the lumen. Either the single value (if one block were taken) or the mean of three sections were used to represent each animal [Kaplan et al. 2002; Walker et al. 2008].
Determination of Social Status
Both wild and captive macaques form hierarchies of social status in which some animals (dominants) reliably defeat others (subordinates) in competitive interactions [Kaplan and Manuck 1998]. Once such hierarchies are formed, fights among individuals are generally unambiguous in outcome, with the dominant monkey in any dyad exhibiting only attack gestures and subordinates only gestures associated with flight [Bernstein 1981]. In the studies enumerated in Table 1, the social status of each animal relative to the others in its social group was based on data collected during frequent (generally weekly) observations beginning after social group formation. These observation periods were usually 30 minutes in length, during which time all fights were recorded and the outcomes noted, irrespective of severity. Dominance and subordination were determined by the outcomes of these encounters, which are highly asymmetric in this species and yield clear winners and losers as judged by specific facial expressions, postures, and vocalizations [Kaplan et al. 1985]. The animal in each group that defeated all others was designated the first-ranking monkey. The animal that defeated all but the first-ranking monkey was designated second-ranking monkey, and so forth. For purposes of analysis, animals ranking first or second in groups of four or five were considered dominant, as were animals ranking one, two, or three in groups of six; the remainder of monkeys were labeled subordinate [e.g., Kaplan et al. 2002]. This procedure was employed in all of our studies and has also been applied elsewhere to small group studies of rhesus monkeys [Hoffman et al. 2007; Jarrell et al. 2008]. The rankings used were those reported in the original publications.
Notably, dominance relationships determined under these small-group conditions tend to be stable across long periods of time in both males and females [Kaplan et al. 1982; Kaplan et al. 1983; Kaplan and Manuck 1998], and even in groups that are periodically reorganized (see below). For example, in the largest female study conducted to date (Table 1, FS2a and FS2b), dominance status determined during a baseline period correlated significantly with that measured over the following 30 months (r = 0.96) [Kaplan et al., 1995], and with the ranks of the same animals measured six years later at the end of the study (r = 0.84) [Kaplan et al. 2002]. Changes in rank among these small social groups of fully adult male and female monkeys are rarely spontaneous, and they almost always follow the death or removal of another member of the social group.
The Social Instability Manipulation
In addition to their propensity to establish well-delineated dominance relationships, cynomolgus macaques respond antagonistically to new animals attempting to join their social groupings. This behavioral phenomenon is well suited to experimental manipulation, as it may be achieved by repeated reorganizations of social group memberships. The grouping of unfamiliar monkeys intensifies confrontations among individuals as monkeys attempt to reestablish hierarchic relationships and affiliative coalitions [Bernstein et al. 1974]. Such encounters with unfamiliar conspecifics are common in the natural history of macaque males, which typically disperse from their natal groups at adolescence and thereafter tend to be socially transient [Pusey and Packer 1987; Berard 1989]. The repeated exposure of males to unfamiliar conspecifics thus represents an ethologically salient stressor since it mimics the typical adult male experience of moving from one social group to another. In contrast, females typically remain with their group of birth throughout their lives and therefore encounter strangers (i.e., unfamiliar females) only on rare occasions. We manipulated social group membership experimentally in several of our studies (Table 1).
As noted above, social status tends to be relatively stable even when these studies included cohorts of 15 or 20 animals that were repeatedly reorganized into social groups of four or five. For example, in MS2a and MS2b (Table 1), animals were reorganized monthly over the course of a 36-month experiment, during which half of the animals also were treated with a beta-adrenoceptor antagonist. Differences in social status (dominant vs. subordinate) were relatively stable and equally so in both the treated and untreated groups. Hence, animals categorized as dominant were found to be high ranking in 81% of experimental periods among the treated monkeys, and in 82% of experimental periods among untreated animals (only the untreated animals are considered in the analyses reported here). Conversely, subordinate monkeys were found to be low ranking in 81% and 79% of experimental periods. We note that serial reorganization within a cohort of limited size requires that animals be repeatedly grouped with familiar individuals; nonetheless, it was generally possible in each reorganization to group animals together with three or four monkeys that were not together in the previous grouping. Importantly, in some but not all of the experiments applying this instability manipulation, an estrogen-implanted (sexually receptive), ovariectomized female was also housed in each social group for the last 2 weeks of each 4 week reorganization, which may have heightened intragroup competition. The presence of the female is noted in Table 1.
Statistical Techniques
The meta-analysis was conducted in stages. We first converted all effect measures to a common metric. Specifically, we calculated the effect size for each individual study as the standardized mean difference in atherosclerosis between the dominant and the subordinate animals. The initial analysis focused on all monkeys living in stable social groups, irrespective of sex. We then investigated the potential effect of covariates (moderator variables) such as sex, social stability, and the presence of an estrogen-implanted female on the relationship between social status and atherosclerosis.
Prior to the meta-analytic evaluation, a funnel plot (i.e., a scatterplot of treatment effect against a measure of study size) for all stable animal studies was generated to examine potential publication bias. Egger’s test was also used to examine the asymmetry of the funnel plot [Egger et al. 1997]. In this test, the normalized effect size was regressed against the inverse of its standard error, with an insignificant intercept providing support for the symmetry of the funnel plot. In addition, homogeneity analyses were conducted based on Q-statistics to examine whether the various effect sizes that were averaged into a mean value all estimate the same population effect size. Then for each subgroup meta-analysis (e.g., all animals in stable groups, all males in stable groups, all females in stable groups, all males in unstable social groups), individual study estimates were combined and weighted by estimates of their precision to obtain an overall effect size. Further, the 95% confidence intervals (CIs) and the corresponding p-values were obtained using a random effects model to account for random differences among studies and the subject-level sampling errors [Lipsey and Wilson 2001]. These results are presented in a forest plot, in which the effect sizes from each individual study and the overall meta-analysis, together with CIs, are displayed simultaneously. The sizes of squares in the forest plot are proportional to the sizes of various studies.
To investigate the potential effect of moderator variables, we fitted meta-regression models using a generalized least square method (GLS) [Sutton et al. 2000]. For all regression models, the goodness of fit tests was not rejected at the 0.10 level. Chi-Square tests were used for examining the significance of the covariates. Finally, we conducted a number of sensitivity analyses to evaluate whether the results were sensitive to changes in the pooling of studies of various characteristics. All statistical analyses were performed using SAS 9.2 (Cary, NC).
Each of the 11 published studies identified in Table 1 contributed to the primary or subsidiary analyses presented below. Individual studies varied in components that governed their inclusion in specific portions of the analysis. Hence, the males were in social groups that either were unmanipulated (MS1a, MS4a, MS5a, MS6, MS7) or recurrently reorganized (MS1b, MS2a, MS2b, MS4b, and MS5b), and if reorganized, either contained an estrogen-treated female (MS1b, MS2a) or did not (MS2b, MS4b, MS5b). Female studies varied in artery source (coronary in FS1, FS2a, FS3, and FS4, iliac in FS2b and FS5). Animals were housed in unisexual groups except for those in the reorganized portions of MS1a and MS2a (each group containing an estrogen-primed female), and in FS1 (each group contained a male as a part of a larger investigation involving separate cohorts in which animals were ovariectomized or allowed to become pregnant [Kaplan et al. 1996]). As stated above, all analyses and figures evaluated the atherosclerosis extent of dominant animals as a positive or negative deviation from the standardized mean (0) that was used to represent subordinate individuals.
RESULTS
Homogeneity
The funnel plot did not demonstrate significant asymmetry along the effect size axis. Further, Egger’s test showed that the intercept did not differ significantly from 0 (− 0.028 with 90% CI: − 2.43, 2.37). This result indicates that there is no evident relationship between the effect sizes and the study sizes. The tests for homogeneity all fell within acceptable bounds, justifying the pooling of studies for the meta-analyses described below.
Dominance Status and Atherosclerosis in Females and Socially Stable Males
Figure 3 depicts the confidence intervals associated with each study. A meta-analysis of all animals (100 males, 145 females) living in stable social groups revealed a significant protective effect of dominance on coronary artery atherosclerosis (estimated deviation of 123 dominants from 122 subordinates = − 0.50, CI = − 0.86 to − 0.17, p < 0.01; homogeneity p = 0.19). This was the largest analysis, as it encompassed portions of 9 studies (MS4a, MS7, MS5a, MS6, MS1a, MS6, FS2a, FS3, FS1, and FS4).
Figure 3.
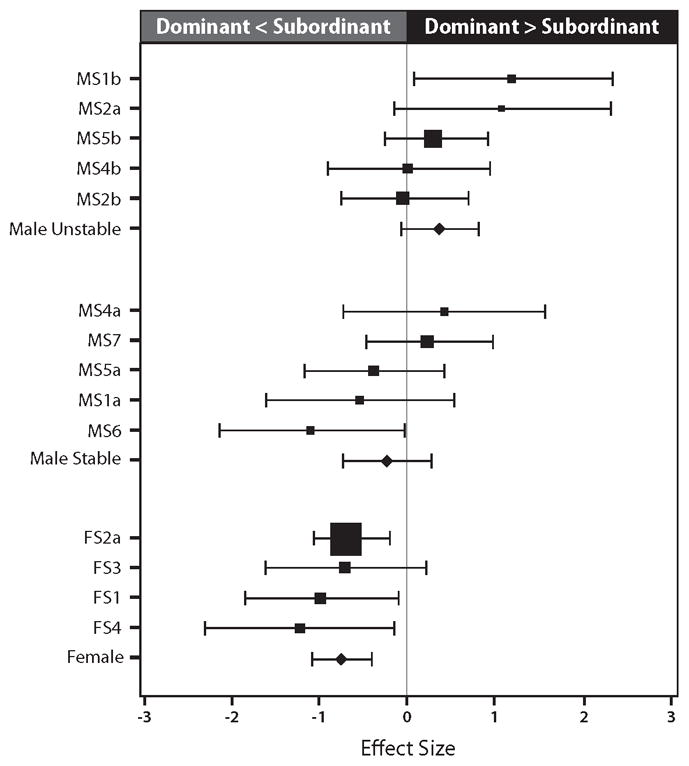
Forest plot showing the confidence intervals (CIs) for coronary artery atherosclerosis among dominant monkeys relative to subordinates for studies comprised of males living in stable social groups, males living in recurrently reorganized social groups, and females. Also shown are the CIs for these three subsets of individuals.
Previous publications indicated that the protective effect of dominance is moderated by sex. A formal test of this hypothesis by the GLS fixed models method demonstrated that there was a significant difference between the deviations of dominant males and females from the standardized, same sex, subordinate value (estimated mean deviation: males = − 0.18, females = − 0.73; goodness of fit = 0.41; difference between means p = 0.04). This outcome suggested that the sexes be evaluated separately. Indeed, a separate analysis of the five studies containing males housed in stable social groups (see Figure 3) indicates no significant deviation between dominants and subordinates, with a confidence interval that overlaps 0 (estimated deviation of 51 dominants from 49 subordinates = − 0.20, CI = − 0.72 to 0.29; p = 0.42, homogeneity p = 0.21).
In contrast, assessment of all available female data (Figure 3) shows that dominant females develop significantly less atherosclerosis than their subordinate counterparts (estimated deviation of 72 dominants from 73 subordinates = − 0.73, CI = − 1.07 to − 0.39; p < 0.0001; homogeneity p = 0.74). Among the females, we used the coronary arteries as the primary outcome because only this outcome measure was available for males. However, the addition of the animals with data from the iliac artery allowed inclusion of monkeys from F5 and the larger number of animals from FS2b, increasing the female N from 145 to 200. In this secondary analysis, results remained largely unchanged (estimated deviation of 91 dominants from 109 subordinates = − 0.60, CI = − 0.92 to − 0.27; p < 0.0001; homogeneity p = 0.33).
Male Dominance Status and Atherosclerosis under Conditions of Social Instability
The analyses thus far have considered females and stable males. As shown in Table 1, there were also five studies that subjected males to recurrent social group reorganization (i.e., social instability). Initial data with males had indicated that dominant monkeys in unstable social groups develop greater atherosclerosis than their subordinate counterparts in groups of similarly unstable membership, and in comparison to males in stable groups (irrespective of status). Figure 3 depicts the point estimate and confidence intervals of all of the male studies. A test of the hypothesis that the relationship between dominance and atherosclerosis is moderated by group stability was conducted by the fixed GLS model method. The results indicate a significant difference between the deviation of dominant stable and unstable males from the standardized value of comparably housed subordinates (estimated mean deviations: stable males = − 0.18, unstable males = + 0.37; goodness of fit p = 0.20; difference between the means p = 0.05).
The foregoing outcome suggests a separate evaluation in relation to stability, much as we conducted a separate evaluation in relation to sex. As already shown, a subset analysis including only males housed in stable groups revealed no significant difference in atherosclerosis between dominant and subordinate males (see CI in Figure 3). In contrast, a meta-analysis limited to animals in unstable groups suggested that dominant individuals developed more atherosclerosis than subordinates (estimated deviation between 61 dominants and 58 subordinates = 0.36, CI = − 0.04 to 0.82, p = 0.08). Although this subset meta-analysis did not achieve formal significance, the preceding GLS analysis demonstrated that the effect of status on atherosclerosis was moderated by social stability. Furthermore, the analysis showed that the mean atherosclerosis among dominants in unstable groups was greater than the standardized value (0) for similarly housed subordinates (p = 0.05). We therefore subjected the data from males living in unstable groups (N = 119) to a fixed GLS analysis to determine whether the addition of an estrogen-implanted, ovariectomized female to the groups (MS1b, MS2a) further moderated the effect of status. This analysis confirmed that, as suggested by Figure 3, dominant animals housed in unstable groups with a supplemental female (MS1b, MS2a) were substantially more affected than dominants housed in unstable groups not containing a female (MS2b, MS4, MS5) (estimated mean deviations: unstable males with female = 1.17, unstable males no female = 0.17; goodness of fit p = 0.88; difference between the means p = 0.04).
Taken together, the male data indicate a possible gradient between the degree of social stress (stable, unstable without female, unstable with female) and direction and extent of difference in atherosclerosis between dominants and subordinates. Hence, dominant males in stable groups tended (albeit not significantly) to have less atherosclerosis than comparably housed subordinates (− 0.21 difference from subordinates), those in unstable groups without females were more affected (+ 0.17 difference from subordinates), and those in unstable groups to which a female had been added were most affected (+ 1.17 difference from subordinates).
DISCUSSION
This meta-analysis sought to determine whether there exists a robust association between social status and atherosclerosis in a nonhuman primate model, and further, whether any such association is moderated by sex or qualities of the social environment. Our evaluation encompassed 419 animals (200 females, 219 males) derived from 11 separate investigations. The results confirmed that, among all males, dominant individuals developed more extensive atherosclerosis than subordinates, but only if they were housed in recurrently reorganized (unstable) social groups in which an estrogen-implanted female was also present. Although not significant, dominant males in stable groups tended to be less affected than subordinates. In contrast, dominant females developed substantially less atherosclerosis than subordinates, and this association was highly significant across studies.
We recognize that this is an unusual and somewhat limited meta-analysis because it focuses on the research of a single laboratory rather than on studies drawn from an entire literature. The results presented here are further limited by the fact that the original publications shown in Table 1 reported the results of analyses conducted in the context of complete experimental designs that are not duplicated in the current meta-analyses. For example, the sentinel studies suggesting an adverse effect of dominance status among males housed in unstable groups and a protective effect among females were MS1b and FS4. These studies were rather simple in design and analysis, with all statistical assessments limited to the stability × dominance interaction in the males and the dominance main effect in females. Figure 3 clearly shows the positive (MS1b) and negative (FS4) confidence intervals in these two studies.
In contrast to the sentinel investigations, subsequent studies had additional experimental components that contributed to published outcomes. Thus, although our publications described an adverse outcome for MS2a similar to that of MS1b, Figure 3 shows that the CI for MS2a crosses 0. A more pronounced effect was reported in the original study, in which these animals were contrasted with monkeys that had also been treated with a beta-adrenoceptor antagonist [Kaplan et al. 1987]. Moreover, while most studies using social group reorganization applied the manipulation throughout, one (MS5b) included monkeys that had been reorganized in one half of the study only. Despite these limitations, the meta-analysis furthers our understanding of the relationship between status and atherosclerosis, especially among males. It is now clear, for instance, that simple exposure to a novel social setting involving other males does not provoke a reliable exacerbation of atherosclerosis among dominants, as compared to subordinates. Rather, the adverse effect of dominant status is not expressed significantly unless the social setting is further perturbed, as by the introduction of a female to the manipulated groups. Importantly, because the estrogen-implanted female was only present in recurrently reorganized groups, it is not possible to determine whether the worsened lesions of the dominant males in this instance were induced by inclusion of females or by the presence of females in combination with social instability.
The situation among socially housed females is more robust, as three of the four coronary artery studies (Figure 3) reveal significant atheroprotection in dominant as compared to subordinate monkeys (i.e., the CIs do not cross 0). The major limitations to the female data relate to the relatively small number of separate investigations and some variability in design. For example, FS1 contained 13 animals that had been subjected initially to reorganization of group membership every three months. This manipulation, unlike the monthly manipulation of males, had no effect on behavior or physiology and therefore was not used to categorize the animals in the original analysis [Kaplan et al. 1984]. Similarly, one complete female study and a portion of another employed a surrogate artery (the iliac) to estimate atherosclerosis. However, results are unaffected by exclusion of the few females that had been housed in reorganized social groups or by addition of the animals whose atherosclerosis was characterized by iliac artery biopsy.
Finally, it is tempting to speculate on the reasons that low status is consistently pathogenic in female monkeys, while high status is pathogenic in males – albeit only under conditions of social instability. The answers, we believe, relate to differences in the social biology and natural history of male and female monkeys. For example, male macaques generally disperse from their natal groups at adolescence and tend to switch groups frequently during adulthood [see Berard 1989; Pusey and Packer 1987; Chapais and Berman 2004]. As a result, males must often decide when to challenge and break into an existing hierarchy and when to avoid confrontation. By temperament, some males are probably more aggressive (and successful) than others in challenge encounters (although perhaps at greater risk of injury). Our experimental instability manipulation forced males to confront social strangers repeatedly, either engaging and then winning these contests (i.e., the dominants), or engaging but losing or altogether avoiding competitive interactions (i.e., the subordinates). Such repeated contesting of rank was absent in the stable social groups, where status was decided on a single occasion at the beginning of each experiment.
In classic experimentation with mice, J.P. Henry and colleagues suggested that attempts to establish and maintain social control preferentially activate the sympathoadrenal medullary system, and may thereby increase risk of cardiovascular disease [Henry and Stephens 1977; Ely 1981]. Our data from the males support Henry’s suggestions. Hence, in one experiment, we not only showed that atherosclerosis was worsened among unstable dominants compared to subordinate animals, but also found that the lesion enlargement associated with social dominance could be inhibited by chronic administration of a beta-adrenoreceptor antagonist [Kaplan et al. 1987]. Several other investigations also implicated activation of the sympathetic nervous system in the mediation of arterial damage in male monkeys [Manuck et al. 1988 and 1995; Strawn et al. 1991; Skantze et al. 1998].
Low ranking males are also affected physiologically by their social environment, as adrenal hypertrophy is more prevalent in subordinate than dominant males, irrespective of the conditions of housing [Shively and Kaplan 1984]. Together, the exacerbation of atherosclerosis seen in dominant animals challenged by social instability, the atheroprotection afforded selectively to such animals by beta-adrenergic blockade, and the enlarged adrenal glands of subordinate animals are consistent with the hypothesis that social environments trigger different physiological responses in dominant and subordinate individuals and that those responses may vary in their pathobiological consequences.
Female macaques (and baboons) present a different picture than males, behaviorally as well as biologically. Female monkeys almost never leave the groups into which they are born, but structure all of their social interactions around their relatives and lifelong peers [e.g., Pusey and Packer 1987; Chapais and Berman 2004]. As a result, females tend to be more intolerant of same-sex social strangers than males. Moreover, even in established social groupings, dominant females intimidate subordinates and do so more persistently than males (Silk, 2002). It is also well-established that reproductive performance is often suppressed in subordinates, as indicated by reduced fecundity and infant survival, lengthened inter-birth intervals, and delayed maturation of female offspring; moreover, such suppression occurs in both captive and natural situations [e.g., Drickamer 1974; Sade et al. 1976; Dittus 1977 and 1980; Harcourt 1987; Altmann et al. 1988; van Noordwuijk and van Schaik 1999; Wasser 1999].
Notably, an extensive series of studies in our laboratory strongly implicates status-induced ovarian suppression as the mechanism underlying the exacerbation of atherosclerosis observed in subordinate, premenopausal monkeys. Hence, our earliest investigations indicated that the dominant – subordinate gradient in atherosclerosis was limited to reproductively intact, non-pregnant individuals, and that among such animals, subordinate individuals are reliably ovarian-impaired (reduced ovulatory estrogen, luteal phase progesterone deficits) compared to dominants [Adams et al. 1985; Kaplan et al. 2002]. Repeated pregnancy, a hyperestrogenic state, suppresses atherosclerosis equivalently in dominant and subordinate monkeys and to a level similar to that occurring among non-pregnant dominants. In contrast, ovariectomy (i.e., the elimination of estrogenic stimulation), exacerbates atherosclerosis in dominant animals, rendering them equivalent to subordinates in lesion extent. Interestingly, ovariectomy does not seem to worsen atherosclerosis in subordinate monkeys beyond that observed in similarly subordinate, reproductively-intact individuals [see review in Kaplan and Manuck 1998].
Further evidence supporting a role for ovarian hormones in atheroprotection is the observation that replacement with exogenous estrogen prevents the development of atherosclerosis in ovariectomized animals, irrespective of status. Finally, we have found that premenopausal estrogen supplementation (using oral contraceptives) prevents the subordination-associated increase in atherosclerosis without altering the protection afforded dominants [Kaplan et al. 1995; Kaplan et al. 2002]. We should note that hypercortisolemia and adrenal hypertrophy also distinguish subordinate from dominant females [Kaplan et al. 1986]. However, this effect is unchanged by ovariectomy and therefore seems unrelated to the association between subordinate social status and atherosclerosis.
We and others have suggested that stress-associated ovarian impairment is possibly adaptive, as it allows a long-lived female mammal to delay an energetically expensive pregnancy until safer, more propitious circumstances prevail. The same is not true of male mammals, whose reproduction is usually energetically inexpensive. A more extensive and complete discussion of evolutionary considerations and the health implications of ovarian suppression in primates and other mammals may be found in a series of reviews [e.g., Wasser and Barash 1983; Kaplan and Manuck 2004; Kaplan and Manuck 2008; Kaplan 2008].
Finally, variation in social status – like most behavioral adaptations – entails elements of benefit and cost to males as well as females. For dominant animals (irrespective of sex), the benefits are preferential access to resources, be they food, space or mates, and the costs are those associated with the behavioral demands of achieving and retaining preeminence against competitors in a social group. For subordinate animals, the costs are limited access to the same resources and ritualized or overt subjugation in encounters with more dominant animals. The benefits are less clear, but acquiescence to subordinate status probably reduces injury, ameliorates conflict until a time more advantageous for achieving higher status, and (in natural environments) may involve alternative, e.g., surreptitious, behavioral strategies for resource acquisition. As a result, we suggest that either dominant or subordinate status may have pathophysiologic consequences, depending on the balance of cost to benefit and the nature of accompanying neuroendocrine reactions.
Acknowledgments
The preparation of this manuscript was support in part by grants from the National Institutes of Health (PO1 HL 45666; PO1 HL 40962; RO1 HL 079421; and RO1 AG 027847). All procedures described in the foregoing research projects at Wake Forest University School of Medicine involving monkeys were conducted in accordance with state and federal laws, standards of the Department of Health and Human Services, and Institutional Animal Care and Use Committee guidelines.
References
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Adams MR, Golden DL, Williams JK, Franke AA, Register TC, Kaplan JR. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr. 2005;135:2852–2856. doi: 10.1093/jn/135.12.2852. [DOI] [PubMed] [Google Scholar]
- Altmann J, Hausfater G, Altmann SA. Determinants of reproductive success in savannah baboonsPapio cynocephalus. In: Clutton-Brock TH, editor. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Chicago: University of Chicago Press; 1988. pp. 403–418. [Google Scholar]
- Berard JD. Life histories of male Cayo Santiago macaques. Puerto Rico Health Sci J. 1989;8:61–64. [PubMed] [Google Scholar]
- Bernstein IS. Dominance: the baby and the bathwater. Behav Brain Sci. 1981;4:419–457. [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol. 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bond MG, Bullock BC, Bellinger DA, Hamm TE. Myocardial infarction in a large colony of nonhuman primates with coronary artery atherosclerosis. Am J Pathol. 1980;101:675–692. [PMC free article] [PubMed] [Google Scholar]
- Chapais B, Berman CM, editors. Kinship and Behavior in Primates. New York: Oxford University Press; 2004. [Google Scholar]
- Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- Dittus WPJ. The social regulation of population density and age-sex distribution in the toque monkey. Behaviour. 1977;63:281–322. [Google Scholar]
- Dittus WPJ. The social regulation of primate populations: A synthesis. In: Lindburg DG, editor. The Macaques: Studies in Ecology, Behavior and Evolution. New York: Van Nostrand Reinhold Co; 1980. pp. 263–286. [Google Scholar]
- Drickamer LC. A ten-year summary of reproductive data for free-ranging Macaca mulatta. Folia Primatol. 1974;21:61–80. doi: 10.1159/000155596. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely DL. Hypertension, social rank, and aortic atherosclerosis in CBA/j mice. Physiol Behav. 1981;26:655–661. doi: 10.1016/0031-9384(81)90140-2. [DOI] [PubMed] [Google Scholar]
- Hamm TE, Jr, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48:221–233. doi: 10.1016/0021-9150(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Harcourt AH. Dominance and fertility among female primates. Zool Soc Lond. 1987;213:471–487. [Google Scholar]
- Henry JP, Stephens PM. A Sociobiological Approach to Medicine. New York: Springer-Verlag; 1977. Stress, health and the social environment. [Google Scholar]
- Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31(2):202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93(4–5):807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Origins and health consequences of stress-induced ovarian dysfunction. In: Atsalis S, Marguilis SW, Hoff PR, editors. Primate Reproductive Aging Interdiscipl Top Gerontol. Vol. 36. Basel: Karger; 2008. pp. 162–185. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Monkeys, aggression, and the pathobiology of artherosclerosis. Aggr Behav. 1998;24:323–334. [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and heart disease: a monkey’s tale. In: Kessel F, Rosenfield P, Anderson N, editors. Interdisciplinary Research: Case Studies from Health and Social Science. Oxford, England: Oxford University Press; 2008. pp. 74–102. [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–735. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female “protection” among cynomolgus macaques. Atherosclerosis. 1984;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Prichard RW. Animal models of behavioral influences on atherogenesis. Adv Behav Med. 1985;1:115–163. [Google Scholar]
- Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fascicularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–1372. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Anthony MS, Morgan TM, Manuck SB, Clarkson TB. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15:2094–2100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: Lessons from animal models. Psychosom Med. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99(3):381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and heart disease: a monkey’s tale. In: Kessel F, Rosenfield P, Anderson N, editors. Interdisciplinary Research: Case Studies from Health and Social Science. Oxford, England: Oxford University Press; 2008. pp. 74–102. [Google Scholar]
- Lipsey MW, Wilson DB. Practical Meta-Analysis. Sage; Thousand Oaks, CA: 2001. [Google Scholar]
- Manuck SB, Kaplan JR, Adams MR, Clarkson TB. Effects of stress and the sympathetic nervous system on coronary artery atherosclerosis in the cynomolgus macaque. Am Heart J. 1988;116:328–333. doi: 10.1016/0002-8703(88)90110-x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: An example from coronary artery disease. Psychosom Med. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Packer C. Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 2150–2266. [Google Scholar]
- Sade DS, Cushing K, Cushing P, Dunaif J, Figueroa A, Kaplan JR, et al. Population dynamics in relation to social structure on Cayo Santiago. Yearbook Phys Anthropol. 1976;20:253–262. [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology in Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Silk JB. Practice random acts of aggression and senseless acts of intimidation: The logic of status contests in social groups. Evol Anthropol. 2002;11:221–225. [Google Scholar]
- Skantze HB, Kaplan J, Pettersson K, Manuck S, Blomqvist N, Kyes R, et al. Psychosocial stress causes endothelial injury in cynomolgus monkeys via α1-adrenoceptor activation. Atherosclerosis. 1998;136:153–161. doi: 10.1016/s0021-9150(97)00202-5. [DOI] [PubMed] [Google Scholar]
- Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, et al. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–1279. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- Strong JP. Atherosclerotic lesions: Natural history, risk factors, and topography. Arch Pathol Lab Med. 1992;116:1268–1275. [PubMed] [Google Scholar]
- Strong JP, Malcom GT, McMahan AC, Tracy RE, Newman WP, III, Herderick EE, et al. Prevalence and extent of atherosclerosis in adolescents andyoung adults. Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth. JAMA. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. Wiley; Chichester, England: 2000. [Google Scholar]
- van Noordwuijk MA, van Schaik CP. The effects of dominance rank and group size on female lifetime reproductive success in wild long-tailed macaques, Macaca fascicularis. Primates. 1999;40:105–130. doi: 10.1007/BF02557705. [DOI] [PubMed] [Google Scholar]
- Walker SE, Register TC, Appt SE, Adams MR, Clarkson TB, Chen H, et al. Plasma lipid-dependent and –independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15(5):950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Seyfarth RM. Conflicts and cooperation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 306–317. [Google Scholar]
- Wasser SK. Stress and reproductive failure: an evolutionary approach with applications to premature labor. Am J Obstet Gynecol. 1999;180:S272–S274. doi: 10.1016/s0002-9378(99)70716-7. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Barash DP. Reproductive suppression among female mammals: implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- Wiggins JS, Trapnell PD. A dyadic-interactional perspective on the five-factor model. In: Wiggins JS, editor. The Five-Factor Model of Personality. New York: Guilford Press; 1996. pp. 88–162. [Google Scholar]
- Williams JK, Vita JA, Selwyn AP, Manuck SB, Kaplan JR. Psychosocial factors impair vascular responses of coronary arteries. Circulation. 1991;84:2146–2153. doi: 10.1161/01.cir.84.5.2146. [DOI] [PubMed] [Google Scholar]
- Williams JK, Kaplan JR, Manuck SB. Effects of psychosocial stress on endothelium-mediated dilation of atherosclerotic arteries in cynomolgus monkeys. J Clin Invest. 1993;92:1819–1823. doi: 10.1172/JCI116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Kaplan JR, Suparto I, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:864–871. doi: 10.1161/01.ATV.0000067934.12783.6A. [DOI] [PubMed] [Google Scholar]