Abstract
Objective
The aim of this study is to determine if activation of β3-adrenoceptor (β3-AR) and downstream signaling of NOS isoforms protects the heart from failure and hypertrophy induced by pressure overload.
Background
β3-AR and its downstream signaling pathways are recognized as novel modulators of heart function. Unlike _1- and _2-ARs, _3-ARs are stimulated at high catecholamine concentrations and induce negative inotropic effects, serving as a “brake” to protect the heart from catecholamine overstimulation.
Methods
C57BL/6J and nNOS knock-out mice were assigned to receive transverse aortic constriction (TAC), BRL37344 (β3-agonist, BRL0.1 mg/kg/hour), or both.
Results
Three weeks of BRL treatment in wild type mice attenuated left ventricular dilation and systolic dysfunction, and partially reduced cardiac hypertrophy induced by TAC. This effect was associated with increased nitric oxide (NO) production and superoxide suppression. TAC decreased endothelial NO synthase (eNOS) dimerization, indicating eNOS uncoupling, which was not reversed by BRL treatment. However, nNOS protein expression was up-regulated 2-fold by BRL, and the suppressive effect of BRL on superoxide generation was abrogated by acute neuronal NO synthase (nNOS) inhibition. Furthermore, BRL cardioprotective effects were actually detrimental in nNOS−/− mice.
Conclusion
These results are the first to show in vivo cardioprotective effects of β3-AR specific agonism in pressure overload hypertrophy and heart failure, and support nNOS as the primary downstream NOS isoform in maintaining NO and reactive oxygen species (ROS) balance in the failing heart.
Keywords: β3-adrenergic receptor, heart failure, hypertrophy, oxidative stress, nitric oxide synthase
Introduction
There is accumulating evidence that β3-adrenergic receptors (β3-AR) play an important role in the modulation of cardiovascular function in heart failure (1–3). In contrast to the well-characterized β1/β2-AR, stimulation of β3-AR induces a negative inotropic effect that is associated with NO release via NOS activation (4). Chronic heart failure is associated with sustained over-activation of the sympathetic nervous system, which initially plays a compensatory role for depressed contractility, but worsens heart function over time. The positive inotropic response to β-AR stimulation is diminished during heart failure due to selective down-regulation and desensitization of β1/β2-AR (5). Conversely, β3-AR is up-regulated in failing hearts in both human and animal models (6–8). Whether this increase is a protective response to catecholamine over-expression or a contributor to heart failure has been controversial; however, increasing evidence suggests it functions as a “physiological brake” to reduce the effects of sympathetic overstimulation. Belge et al. showed that mice with cardiomyocyte specific over-expression of β3-AR had attenuated LV hypertrophy compared with WT mice in response to chronic isoprenaline administration (9). Our recent work revealed exacerbated pathologic remodeling and impaired cardiac functional compensation in mice lacking β3-AR (2,10). These studies support the idea that β3-AR serves a chiefly protective role in maladaptive remodeling and in the development of heart failure.
It is well established that the negative inotropic effect of β3-AR results from the downstream production of NO by NOS signaling (4,11). The effects of a β3-agonist, BRL 37344 (BRL), were inhibited by both NO and NOS inhibitors and could be reversed by an excess of the substrate for NO production, L-arginine. Several studies have suggested that eNOS is solely responsible for β3-induced negative inotropy (4,11–13). However, the relative involvement of nNOS, which is also constitutively expressed in cardiomyocytes, still remains unclear. Alterations of NOS-dependent regulation on cardiac function make this issue more complex. There is an apparent paradox between the observation that β3-AR stimulation induces a NO-dependent negative inotropic effect on failing ventricular myocardium despite the finding that eNOS-derived NO is decreased in this setting (14). These inconsistent findings may be reconciled by recent evidence indicating that nNOS-derived NO production was involved in altered contractile response by β3-AR stimulation in both diabetic and senescent heart (15,16).
In the present study, we hypothesized that the β3-AR preferential agonist BRL could reduce cardiac remodeling induced by pressure overload and preserve cardiac function through NO production, which may be largely attributable to activation of nNOS.
Methods
Experimental model
Thirty-eight C57BL/6J and 12 nnos−/− /B6129S male mice (9–10 weeks old, Jackson Laboratory, Bar Harbor, ME) were arbitrarily divided into 3 groups. Two-thirds of the mice underwent transverse aortic constriction (TAC) to induce cardiac hypertrophy and heart failure via pressure overload (17), while the remaining one-third had a sham procedure.
Cardiac function and geometry
In vivo cardiac geometry and function were serially assessed by transthoracic echocardiography (Acuson Sequoia C256, 13 MHz transducer, Siemens, Oceanside, CA) in conscious mice at baseline, 1 week and 3 weeks, respectively.
Histological evaluation and cellular morphometry
Myocardium was fixed in 10% formalin, processed by standard paraffin embedding, and serially sectioned at 5–8μm thickness, for hematoxylin and eosin (H&E) and trichrome staining.
Measurement of cardiac NO production
Cardiac NO production was determined as the measurement of nitrate plus nitrite using the Griess reaction assay (Cayman Chemical, Ann Arbor, MI) as previously described (18).
Measurement of cardiac superoxide generation
Lucigenin-enhanced chemiluminescence
Fresh-frozen myocardium was homogenized in 20 mM HEPES buffer and diluted in lucigenin buffer. Superoxide-generated signals were recorded as CPM by a liquid scintillation counter (LS6000IC, Beckman Instruments, Fullerton, CA).
Electron Paramagnetic Resonance
Fresh-frozen myocardium was homogenized in 1X PBS buffer containing 0.1mM DTPA (Sigma Aldrich). Samples were further diluted in 1x PBS with 1 mM 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl pyrrolidine (CMH, Enzo Life Sciences) and assayed for superoxide signals by Bruker E-Scan EPR spectrometer.
Western blot analysis
Snap-frozen LV tissue was homogenized in cell lysis buffer (Cell Signaling Technology, Danvers, MA) and subjected to western blot analysis with antibodies targeting the protein of interest. The densitometric volume of digitalized band was evaluated by Image J program.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Echocardiographic data were compared using repeated measures analysis of variance (RM-ANOVA). Group data were compared using one-way ANOVA with a Tukey’s post-hoc test for multiple comparisons. Student’s t-test with Welsh correction for multiple comparisons was used to compare the difference between BRL and vehicle treated nNOS−/− mice. P values less than 0.05 were considered to be statistically significant. GraphPad Prism 5.0 (La Jolla, CA) was used for statistical analysis.
Results
β3-AR stimulation prevents deterioration of cardiac function
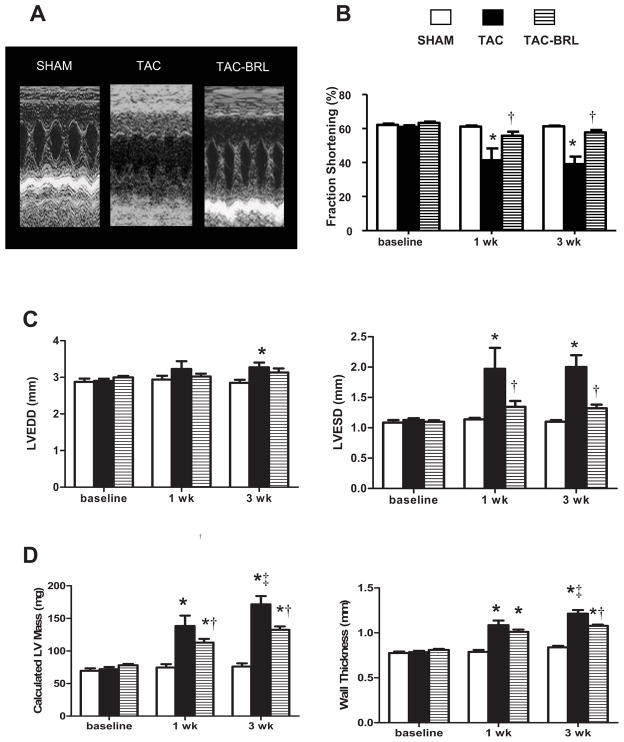
Mice developed increased LV chamber dilation and systolic dysfunction after 3 weeks of TAC (Fig. 1A), as evidenced by an 82% increase in LVESD and a 36% reduction in FS% compared to sham mice by echocardiography (Fig. 1B, C). Calculated LV mass and average wall thickness were increased vs. sham (Fig. 1D). Three weeks of BRL treatment totally prevented LV dilation , and cardiac systolic function remained normal. Calculated LV mass and average wall thickness were significantly lower in BRL treated mice compared to vehicle . We also treated sham mice with BRL, which had no significant effect (data not shown).
Figure 1. Effect of BRL on Left ventricular (LV) dilation and LV systolic function in transverse aortic constriction (TAC) mice.
(A) M-mode echocardiograms demonstrating increased LV dilation and wall thickness and decreased systolic function after 3 weeks of TAC, which were abrogated by BRL treatment. (B) BRL prevented cardiac systolic dysfunction induced by TAC. (C) BRL reduced LV chamber dilation. (D) TAC causes LV hypertrophy which was partially prevented by BRL treatment. *P<0.05 vs. sham; †P<0.05 vs. TAC; ‡P<0.05 vs. corresponding 1 week time point.
β3-AR stimulation reduces development of cardiac hypertrophy
Three weeks of TAC resulted in increased cardiac hypertrophy vs. sham, with a 44% higher heart weight to body weight ratio. BRL treated mice developed less hypertrophy (Appendix Fig. 1A). These findings were paralleled by similar changes in calculated LV mass by echocardiography (Fig. 1D). Both cardiomyocyte width by H&E staining and fibrosis scale (0–3 scale; 0= none, 3= severe) by Trichrome staining were significantly greater in TAC vs. sham. Interestingly, BRL reduced cardiomyocyte width but had no effect on fibrosis scale (Appendix Fig 1B, C).
β3-AR stimulation increases cardiac NO production and decreases ROS production in pressure-overloaded mice
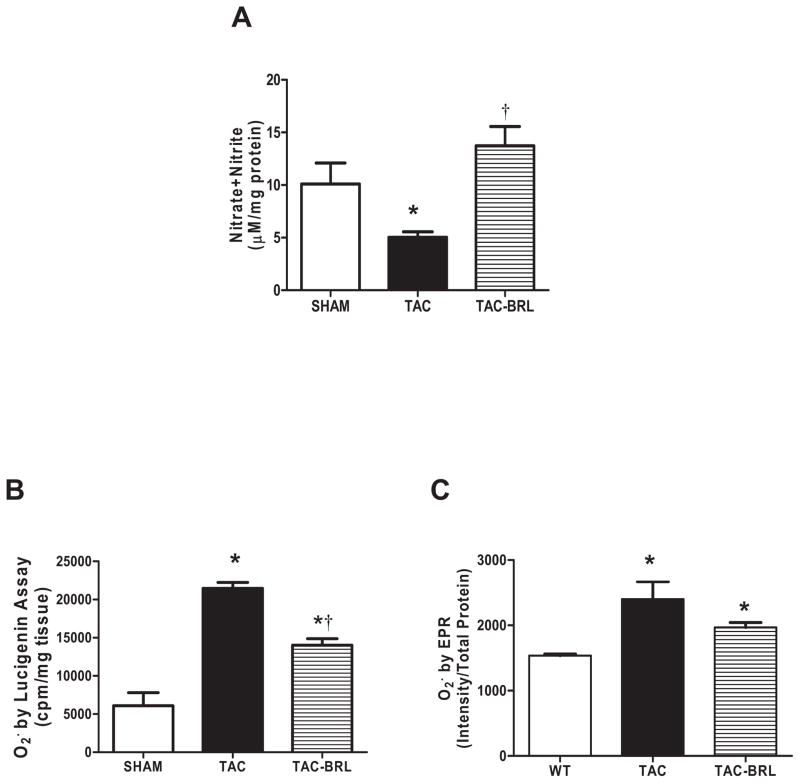
β3-AR-induced negative inotropic effects are thought to be associated with NO release via NOS (4). Our previous data showed that mice lacking β3-AR had lower NOS activity and generated more cardiac superoxide than WT mice after pressure-overload (2). We, therefore, tested NO production by measuring the sum concentrations of the NO metabolites (nitrate and nitrite) using the Griess assay, and superoxide generation by lucigenin-enhanced chemiluminescence and EPR to observe the effect of β3-AR agonism on NO and ROS production. Total nitrate + nitrite concentrations were decreased by 50% (Fig. 2A) and superoxide was increased ~3.5 fold (Fig. 2B) in TAC hearts over sham controls. Three weeks of BRL treatment completely prevented this decrease in NO metabolite concentration and partially inhibited superoxide generation. Superoxide studies were confirmed using EPR, which showed a 1.5 fold increase in ROS generation (Fig. 2C) and a downward trend in ROS production with BRL administration.
Figure 2. Changes in NO production and superoxide generation by BRL treatment.
(A) NO production, measured by Griess assay, was decreased by 50% after 3 weeks of TAC, but preserved at normal levels in the presence of BRL. (B) Lucigenin-enhanced chemiluminescence detected 3.5-fold increase in superoxide in 3-week TAC mice, which was significantly reduced BRL administration. (C) EPR detected a 1.5-fold increase in superoxide in TAC animals and downward trend in TAC+BRL. *P<0.05 vs. sham; †P<0.01 vs. TAC.
β3-AR stimulation regulates nNOS protein expression and superoxide generation
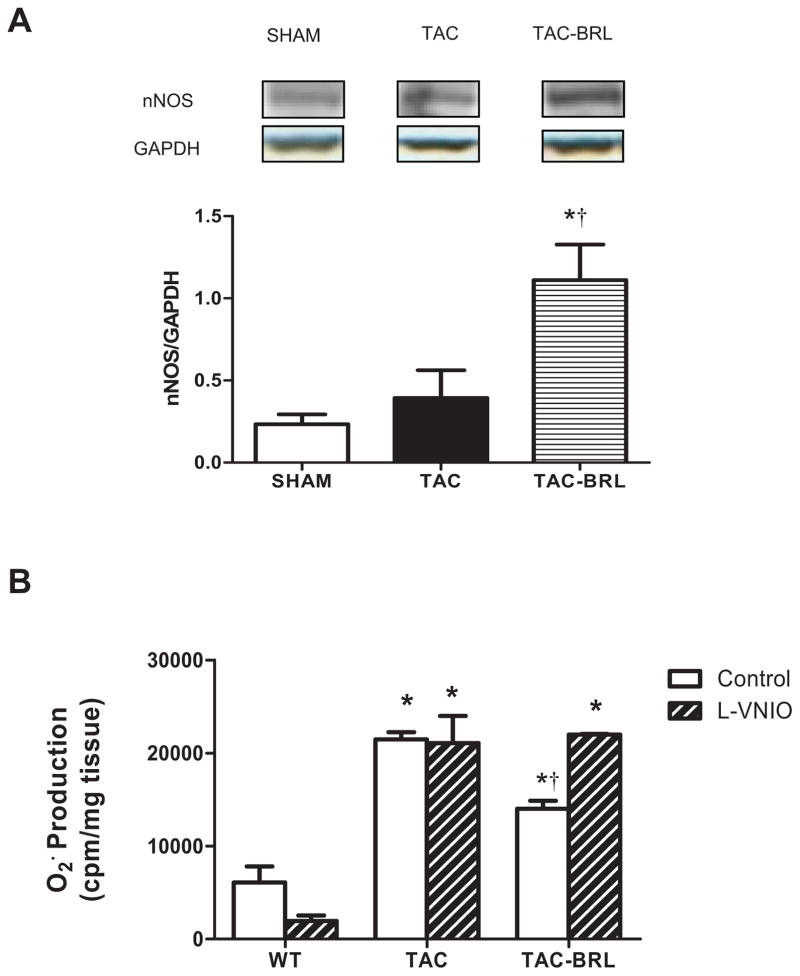
Recent studies demonstrated that nNOS-derived NO production was involved in altered contractile response by β3-AR stimulation in both diabetic and senescent hearts (15,16). We observed a 2-fold increase in nNOS protein expression in BRL-treated compared to vehicle-treated hearts (Fig 3A), although there was no difference between sham and untreated TAC. More interestingly, pretreating LV homogenates with 100 nM of the specific nNOS inhibitor L-VNIO completely abolished BRL suppression of superoxide generation (Fig 3B). This suggests that β3-AR reduces superoxide generation via a nNOS-dependent mechanism in the failing heart.
Figure 3. Effect of BRL on nNOS protein expression and the superoxide generation by acute nNOS inhibition.
(A) nNOS protein expression was significantly up-regulated by BRL treatment, though similar between sham and TAC. (B) Acute nNOS inhibition (striped bars) abrogated BRL-induced superoxide suppression in the presence of nNOS specific inhibitor Vinyl-L-NIO (L-VNIO, 100uM, 30 minutes in cold room), as compared with non-LVNIO treated samples (white bars). *P<0.01 vs. sham; †P<0.05 vs. TAC.
BRL modulation of eNOS and iNOS activation
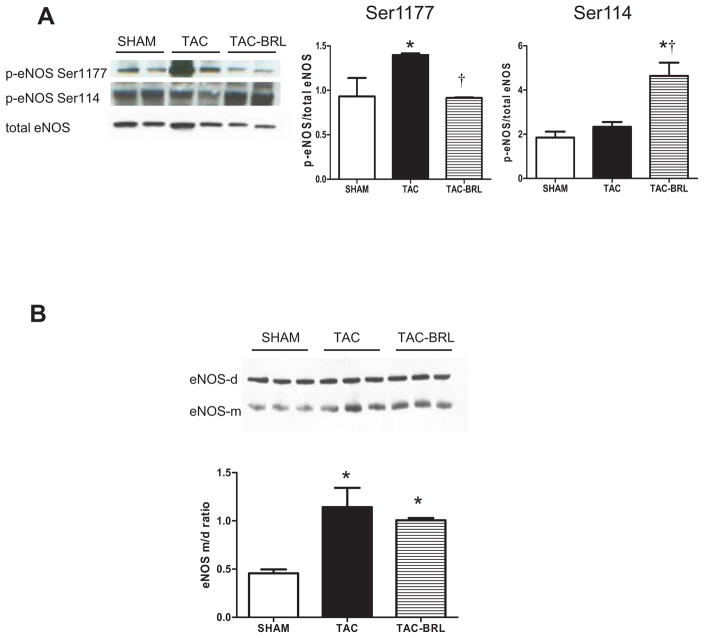
To investigate further the role of BRL on other NOS isoforms, we examined eNOS protein expression and phosphorylation. We focused on three enzyme phosphorylation sites that have been shown to modulate eNOS activity in the myocardium (19): eNOSSer1177, eNOSSer114 and eNOSThr495. After 3 weeks of TAC+BRL treatment, eNOSSer1177 phosphorylation, which indicates eNOS activation, was decreased by 50% as compared with TAC alone. In contrast, phosphorylation of eNOSSer114, which is an indication of eNOS deactivation, was increased 100% in BRL treated mice, while levels were similar between sham and untreated TAC (Fig. 4A). Total eNOS protein expression (Fig. 4A) and eNOSThr495 phosphorylation were unchanged between groups (Appendix Fig. 2A). In addition, no difference in iNOS expression was observed between groups (Appendix Fig. 2B)
Figure 4. Effects of BRL treatment on eNOS activation and dimerization.
(A) p-eNOS Ser1177/eNOS was decreased and p-eNOS Ser114/eNOS was increased after BRL treatment in TAC mice. (B) eNOS uncoupling indexed by increased eNOS monomer to dimer ratio was observed after 3 weeks of TAC and not reversed by BRL treatment. *P<0.05 vs. sham; †P<0.05 vs. TAC.
eNOS monomer-to-dimer ratio (m/d) is an indication of eNOS uncoupling, which results in NO generation switching to superoxide generation. Our data shows that 3 weeks of TAC resulted in increased eNOS uncoupling which is consistent with previous reports (10,17,20), athough 3 weeks of BRL treatment did not change the m/d ratio. (Fig 4B).
Lack of nNOS gene abolished the protective effect of β3-AR stimulation
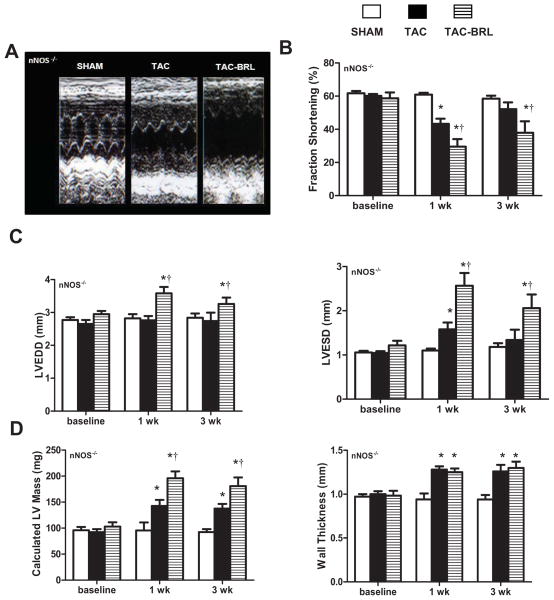
Because nNOS protein expression was up-regulated by BRL treatment, and BRL-induced superoxide suppression was attenuated by acute nNOS inhibition, we hypothesized that nNOS is pivotal in β3-AR-induced cardioprotection. To confirm this hypothesis, we used the 3- week TAC model in the absence or presence of BRL in mice genetically lacking nNOS. After 1 week, TAC mice developed LV systolic dysfunction (Fig 5B) and chamber dilation (Fig 5C) as compared with sham nNOS−/− mice. BRL treatment not only failed to restore LV function and dilation to baseline levels after 3 weeks of pressure overload, but exacerbated dysfunction (Fig 5B) and LV dilation (Fig 5C). LV mass (Fig 5D) was 33% greater in TAC nNOS−/− mice as compared with sham nNOS−/−, and worse in TAC+ BRL (Fig 5D) as compared with TAC alone mice. Wall thickness was also increased in TAC nNOS−/− mice as compared with sham (Fig 5D). There was no difference in wall thickness among BRL+TAC and TAC animals (Fig 5D).
Figure 5. Effect of BRL on LV hypertrophy and function in TAC mice lacking nNOS gene.
(A) M-mode echocardiograms demonstrated increased LV dilation and wall thickness and decreased systolic function after 3 weeks of TAC, which was worsened by BRL administration. (B) LV systolic function was decreased by TAC and worsened with BRL in nNOS−/− mice. (C) LV dilation was unaffected by TAC, but significantly increased by TAC with BRL treatment. (D) TAC resulted in LV hypertrophy which was exacerbated by BRL. *P<0.05 vs. sham; †P<0.05 vs. TAC.
Discussion
The novel findings in this study are that β3-AR activation prevents cardiac dysfunction through generating NO and reducing ROS via a nNOS-dependent mechanism in the failing heart.
Induction of cardioprotection by β3-AR
Despite a low level of myocardial expression and physiological insignificance under resting conditions (21), accumulating literature supports a significant role for β3-AR upregulation in the modulation of cardiac remodeling in heart failure. Until now direct evidence in vivo has been lacking. We have previously compared the cardiac response to pressure overload in both WT and β3−/− mice, displaying greater hypertrophy and cardiac systolic dysfunction in β3−/− mice as compared with WT controls (2). More recently, Aragon et. al. demonstrated that select β3-AR agonists, including the β1-blocker nebivolol, reduced cardiac infarct size in mice subjected to myocardial ischemia-reperfusion in WT, but were completely ineffective in β3-AR deficient mice (22). In the current study, we confirm that β3-AR agonism exerts a cardioprotective role in the failing heart (6,15,22,23). We observed that administering the β3-AR specific agonist BRL to C57BL/6 mice for 3 weeks totally prevented the deterioration of LV chamber dilation and cardiac dysfunction, and partially inhibited myocardial hypertrophy induced by chronic pressure-overload. This strongly suggests that specific β3-AR agonism may constitute an interesting and novel approach in treating cardiac hypertrophy and heart failure.
Primary role of nNOS in β3-AR cardioprotection
Studies have shown that the β3-AR-induced cardiac signaling is associated with NO release via eNOS (4,11). Chronic β3-AR stimulation in our model prevented the expected decrease in NO production during pressure overload, which confirming our previous findings that NOS activity is decreased in β3−/− mice after TAC (2). Although prior studies assumed that cardiac eNOS was the sole source of NO involved in the regulation of myocardial contraction (4,11), there remains great controversy over which NOS isoform is the chief player in β3-AR-signaling.
In the present study, eNOS and iNOS protein expressions were unchanged by BRL treatment. eNOS activity is generally modulated by either translocation or phosphorylation. Translocation has been observed by β3-AR stimulation only in right atrium, not in left ventricle (12,13). Ser1177 and Ser114 are two phosphorylation sites which can modulate eNOS activity. Phosphorylation at Ser1177 (or Ser1179 in human) activates eNOS, whereas phosphorylation at Ser114 deactivates eNOS (24–26). We observed a decrease in Ser1177 phosphorylation and an increase in Ser114 phosphorylation after BRL treatment, which suggested eNOS deactivation rather than activation by β3-AR stimulation. Similar results were recently observed in isolated human failing myocardium (14). The apparent paradox between the known β3-AR -mediated NO-dependent negative inotropic effect and clear eNOS deactivation in human failing myocardium could be explained by concomitant nNOS activation in cardiomyocytes. Paracrine negative inotropic effects via NO liberation from cardiac endothelial cells may be another explanation, but lacked in direct evidence until recently. The same group also reported that eNOS was activated through Ser1177 phosphorylation by BRL in human non-failing myocardium, which identified a different downstream signal of NOS isoform by β3-AR stimulation between failing and nonfailing hearts (12,13).
Emerging evidence indicates that nNOS-derived NO production plays a substantial part in the regulation of basal and β-AR myocardial contraction (27,28). nNOS was up-regulated in senescent rat hearts after myocardial infarction (MI) and in human failing hearts (22,27,29,30). Interestingly, it was shown that the positive inotropic response to non-specific β-AR stimulation was impaired in diabetic and aged rat hearts and restored by a β3-AR antagonist, a nonselective NOS inhibitor, and the selective nNOS inhibitor L-VNIO (15,16). nNOS gene deletion has been associated with more severe LV remodeling and functional deterioration in murine models of myocardial infarction, suggesting that nNOS-derived NO may also be involved in a protective myocardial response to injury (18,31). The present study revealed exclusive nNOS protein upregulation and activation by β3-AR agonism, suggesting nNOS-derived NO production is the primary source in the cardioprotective effect of β3-AR agonism. Importantly, cardiac hypertrophy, LV dysfunction, and reduced ejection fraction induced by TAC were actually worsened by BRL treatment in nNOS−/− mice, further confirming this mechanism. An ex vivo study from Idigo W. et al. also showed that the negative inotropic response to BRL in cardiomyocytes was absent in both nNOS−/− cardiomyocytes and WT cardiomyocytes with pharmacological inhibition of nNOS (32). These studies support nNOS-derived NO production as a primary factor in altered contractile response by β3-AR stimulation of the heart. We speculate that the pathway regulating cardiac contractility may be associated with nNOS post-translational modification such as translocation from sarcoplasmic reticulum (SR) to sarcolemma (27), or possible phosphorylation of select residues (33). This merits further investigation, and studies are ongoing in our laboratory.
Inhibition of oxidative stress
A significant number of animal studies and several clinical observations have demonstrated ROS activation in the cardiovascular system in response to various stressors and in the genesis of the hypertrophic and failing heart (34–36). Biomarkers for ROS have been detected in the pericardial fluid as well as in the peripheral blood of heart failure patients (37). Further experiments showed that ROS is increased in cardiac hypertrophy and adverse remodeling (38,39). However, the mechanism by which β3-AR stimulation affects ROS generation has not been clearly delineated. In our previous study using TAC in β3-AR−/− mice, we observed increased NOS-dependent generation of superoxide, implying that NOS-dependent ROS may be one of the downstream signaling pathways of β3-AR (2).
This was confirmed by the current study, which demonstrated a substantial reduction of TAC-induced superoxide generation by BRL treatment. β3-AR-induced suppression of ROS generation was abolished by acute inhibition of nNOS by preferential nNOS inhibitor, L-VNIO, at a concentration that only inhibits nNOS without affecting other NOS isoforms. These results revealed the antioxidant effect of β3-AR agonism is dependent on nNOS activation. Recently, Khan and Kinuga et al. have shown that deficiency of nNOS leads to profound increase in xanthine oxidoreductase (XOR)–mediated superoxide production without affecting XOR mRNA or protein abundance, which depresses myocardial excitation-contraction coupling in a manner reversible by XOR inhibitor (40,41). This suggests constrained XOR activity by nNOS as a possible connection between myocardial NOS and ROS systems.
We observed that eNOS was uncoupled after 3 weeks of TAC, which is in agreement with previous reports (17,20). However, eNOS was not recoupled by BRL treatment providing further evidence that eNOS is not the sole pathway for β3-AR, as previously thought. In addition, BRL-induced eNOS-Ser114 phosphorylation could indicate uncoupling and may explain the reason for exacerbated dysfunction seen in TAC-nNOS−/− animals treated with BRL. We speculate that β3-AR regulation occurs via balancing two opposing pathways when the heart starts to fail: a deleterious eNOS-dependent pathway and a protective nNOS-dependent pathway. Thus, the cardioprotective effect of β3-AR agonism on cardiac hypertrophy and heart failure could be attributed to nNOS activation, which maintains the equilibrium of myocardial NO and ROS production, despite uncoupled eNOS in this setting.
Clinical Implication
β1 blockers have become the standard treatment for chronic heart failure since 1990. We propose that β3-AR agonism can be considered similar to an endogenous β1 blocker, due to its protective and negative inotropic effect on human myocardium. This study strongly supports the notion that β3-AR plays a beneficial role in heart and highlights the potential therapeutic utility of β3-AR agonism. Although low basal expression levels of β3-AR in some tissues have resulted in disappointing outcomes from animal studies to clinical trials evaluating β3-AR agonists for obesity, type 2 diabetes and irritable bowel syndrome treatment (42–45), heart failure represents a more realistic promising therapeutic target for three main reasons. First, β3-AR has been demonstrated to be expressed at levels that can mediate physiological responses in healthy human myocardium (1). Second, β3-AR is up-regulated 2–3 fold in the progression of heart failure, while β1-AR is down-regulated (6). This increase in β3: β1-AR ratio likely plays a substantial role in activating β3-AR signaling. Lastly, co-treatment with conventional β-blockers can further increase the expression of β3-AR. A study in diabetic rats demonstrated that chronic treatment with metoprolol markedly increased the expression of cardiac β3-AR (46). Recent studies reported that the hemodynamic improvement obtained from third-generation β-blocker, nebivolol, in mice subjected to myocardial ischemia-reperfusion and heart failure patients is partially due to its NO-dependent negative inotropic effect by β3-AR stimulation (22,47).
In conclusion, we have shown that β3-specific agonism in vivo has substantial cardioprotective effects, and that these effects are largely attributable to nNOS activation. These findings have direct therapeutic implications for treating heart failure patients and for improving our understanding of the pathophysiology of heart failure.
Supplementary Material
Acknowledgments
Funding: This research was supported by the National Institutes of Health [K08-HL076220 to L.A.B.]; American Heart Association Beginning Grant-In-Aid [to L.A.B.]; American Diabetes Association [to L.A.B.]; National Institutes of Health [5T32HL007227 to V.L.W] and the China Scholarship Council (to X. N). We would like to thank Jonathan Jun for his expert assistance as well as Seva Polotsky for generously allowing us access to his laboratory facilities.
Footnotes
Disclosures
None of the authors has any conflicts of interest related to this manuscript.
There are no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gauthier C, Tavernier G, Charpentier F, et al. Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98:556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moens AL, Leyton-Mange JS, Niu X, et al. Adverse ventricular remodeling and exacerbated NOS uncoupling from pressure-overload in mice lacking the beta3-adrenoreceptor. J Mol Cell Cardiol. 2009;47:576–85. doi: 10.1016/j.yjmcc.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skeberdis VA, Gendviliene V, Zablockaite D, et al. beta3-adrenergic receptor activation increases human atrial tissue contractility and stimulates the L-type Ca2+ current. J Clin Invest. 2008;118:3219–3227. doi: 10.1172/JCI32519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier C, Leblais V, Kobzik L, et al. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–1384. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 6.Moniotte S, Kobzik L, Feron O, et al. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto A, Hasegawa H, Cheng HJ, et al. Endogenous beta3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol. 2004;286:H2425–33. doi: 10.1152/ajpheart.01045.2003. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Wu TG, Jiang ZF, et al. Effect of beta-blockers on beta3-adrenoceptor expression in chronic heart failure. Cardiovasc Drugs Ther. 2007;21:85–90. doi: 10.1007/s10557-007-6016-4. [DOI] [PubMed] [Google Scholar]
- 9.Belge C, Sekkali B, Tavernier G, et al. Cardiomyocyte-specific Overexpression of Beta3-adrenoceptors Attenuates The Hypertrophic Resposne to Catecholamines In Vivo. Circulation. 2007;116:II_148. [Google Scholar]
- 10.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 11.Varghese P, Harrison RW, Lofthouse RA, et al. beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. Journal of Clinical Investigation. 2000;106:697–703. doi: 10.1172/JCI9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brixius K, Bloch W, Pott C, et al. Mechanisms of beta 3-adrenoceptor-induced eNOS activation in right atrial and left ventricular human myocardium. Br J Pharmacol. 2004;143:1014–1022. doi: 10.1038/sj.bjp.0705983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brixius K, Bloch W, Ziskoven C, et al. Beta3-adrenergic eNOS stimulation in left ventricular murine myocardium. Can J Physiol Pharmacol. 2006;84:1051–1060. doi: 10.1139/y06-033. [DOI] [PubMed] [Google Scholar]
- 14.Napp A, Brixius K, Pott C, et al. Effects of the beta3-adrenergic agonist BRL 37344 on endothelial nitric oxide synthase phosphorylation and force of contraction in human failing myocardium. J Card Fail. 2009;15:57–67. doi: 10.1016/j.cardfail.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Amour J, Loyer X, Le Guen M, et al. Altered contractile response due to increased beta3-adrenoceptor stimulation in diabetic cardiomyopathy: the role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology. 2007;107:452–460. doi: 10.1097/01.anes.0000278909.40408.24. [DOI] [PubMed] [Google Scholar]
- 16.Birenbaum A, Tesse A, Loyer X, et al. Involvement of beta 3-adrenoceptor in altered beta-adrenergic response in senescent heart: role of nitric oxide synthase 1-derived nitric oxide. Anesthesiology. 2008;109:1045–1053. doi: 10.1097/ALN.0b013e31818d7e5a. [DOI] [PubMed] [Google Scholar]
- 17.Takimoto E, Champion HC, Li M, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest May. 2005;115:1221–31. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraiva RM, Minhas KM, Raju SV, et al. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 19.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. Journal of Molecular and Cellular Cardiology. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Moens AL, Takimoto E, Tocchetti CG, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:271–279. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germack R, Dickenson JM. Induction of beta3-adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. Journal of Pharmacology and Experimental Therapeutics. 2006;316:392–406. doi: 10.1124/jpet.105.090597. [DOI] [PubMed] [Google Scholar]
- 22.Aragón J, Condit M, Bhushan S, et al. β3-Adrenoreceptor Stimulation Ameliorates Myocardial Ischemia- Reperfusion Injury via eNOS and nNOS Activation. Journal of American College of Cardiology. doi: 10.1016/j.jacc.2011.09.033. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch LA, Cappola TP, Harrison RW, et al. Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mol Cell Cardiol. 2003;35:637–44. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 24.Bauer PM, Fulton D, Boo YC, et al. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–9. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 25.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 26.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie. doi: 10.1016/j.biochi.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Bendall JK, Damy T, Ratajczak P, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- 28.Barouch LA, Harrison RW, Skaf MW, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 29.Damy T, Ratajczak P, Robidel E, et al. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. Faseb J. 2003;17:1934–6. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- 30.Damy T, Ratajczak P, Shah AM, et al. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–7. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 31.Dawson D, Lygate CA, Zhang MH, Hulbert K, Neubauer S, Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112:3729–3737. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- 32.Idigo W, Zhang MH, Zhang YH, Casadei B. The negative inotropic effect of [beta]3-adrenergic receptor stimulation in nNOS−/− mice is restored by oxypurinol. Heart. 2006;92:e1 008. [Google Scholar]
- 33.Rameau GA, Tukey DS, Garcin-Hosfield ED, et al. Biphasic Coupling of Neuronal Nitric Oxide Synthase Phosphorylation to the NMDA Receptor Regulates AMPA Receptor Trafficking and Neuronal Cell Death. Journal of Neuroscience. 2007 doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol. 2002;34:379–88. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- 35.Sheeran FL, Rydstrom J, Shakhparonov MI, Pestov NB, Pepe S. Diminished NADPH transhydrogenase activity and mitochondrial redox regulation in human failing myocardium. Biochim Biophys Acta. doi: 10.1016/j.bbabio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Hamid T, Keith RJ, et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 121:1912–25. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–9. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 38.Kawai K, Qin F, Shite J, Mao W, Fukuoka S, Liang CS. Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol. 2004;287:H1003–12. doi: 10.1152/ajpheart.00797.2003. [DOI] [PubMed] [Google Scholar]
- 39.Bajcetic M, Kokic Nikolic A, Djukic M, et al. Effects of carvedilol on left ventricular function and oxidative stress in infants and children with idiopathic dilated cardiomyopathy: a 12-month, two-center, open-label study. Clin Ther. 2008;30:702–14. doi: 10.1016/j.clinthera.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Khan SA, Lee K, Minhas KM, et al. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–62. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 42.Arch JR. beta(3)-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 43.Arch JR. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:225–40. doi: 10.1007/s00210-008-0271-1. [DOI] [PubMed] [Google Scholar]
- 44.Clouse AK, Riedel E, Hieble JP, Westfall TD. The effects and selectivity of beta-adrenoceptor agonists in rat myometrium and urinary bladder. Eur J Pharmacol. 2007;573:184–9. doi: 10.1016/j.ejphar.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen HH, Figtree GA, Krum H, Bundgaard H. The use of beta3-adrenergic receptor agonists in the treatment of heart failure. Curr Opin Investig Drugs. 2009;10:955–62. [PubMed] [Google Scholar]
- 46.Sharma V, Parsons H, Allard MF, McNeill JH. Metoprolol increases the expression of beta(3)-adrenoceptors in the diabetic heart: effects on nitric oxide signaling and forkhead transcription factor-3. Eur J Pharmacol. 2008;595:44–51. doi: 10.1016/j.ejphar.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 47.Rozec B, Erfanian M, Laurent K, Trochu JN, Gauthier C. Nebivolol, a vasodilating selective beta(1)-blocker, is a beta(3)-adrenoceptor agonist in the nonfailing transplanted human heart. J Am Coll Cardiol. 2009;53:1532–8. doi: 10.1016/j.jacc.2008.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.