Abstract
The endomembrane system of eukaryotic cells uses membrane-enclosed carriers to move diverse macromolecules among different membrane-bound compartments, a requirement for cells to secrete and take up molecules from their environment. Two recycling pathways—biosynthetic and endocytic, each with specific lipid components—make up this system, with the Golgi apparatus mediating transport between the two. Here, we integrate lipid-based mechanisms into the description of this system. A partitioning model of the Golgi apparatus is discussed as a working hypothesis to explain how membrane lipids and proteins that are segregated based on lateral lipid partitioning support the unique composition of the biosynthetic and endocytic recycling pathways in the face of constant trafficking of molecular constituents. We further discuss how computational modeling can allow for interpretation of experimental findings and provide mechanistic insight into these important cellular pathways.
Keywords: sphingolipids, microdomains, Golgi, secretion, endocytosis partitioning, modeling
INTRODUCTION
Eukaryotic cells have evolved a complex endomembrane system that uses membrane-enclosed transport carriers to move diverse molecules among membrane-bound compartments involved in the cell’s secretory, endocytic, and degradative processes. The various organelles comprising this system, both exocytic [endoplasmic reticulum (ER), Golgi apparatus and secretory vesicles] and endocytic (endosomes and lysosomes), have distinct identities and functions (12, 13, 41, 45). Moreover, the organelles are dynamic, being generated and maintained in the face of continual exchange of protein and lipid components. This results in their disappearance and reformation in response to specific conditions and during particular phases of the cell cycle (4, 43, 90). To understand how cells regulate this complex, intercommunicating array of membrane structures is a major goal of cell biology. Current thinking derives primarily from work that has examined, at a mechanistic level, how endomembranes operate and are organized overall. These studies have demonstrated an essential role for protein-based machinery, including coat proteins and their adaptors, small GTPases, tethering factors, and fusion proteins (12, 13, 22, 23, 45, 78, 92). This machinery helps change the membranes’ overall shape, sorts proteins into differentiated subdomains within membranes, and permits membrane carriers to translocate through the cytoplasm and fuse with specific acceptor membranes, a process critical for the emergence of distinct compartments. More recent studies have revealed that endomembranes are differentially populated by specific classes of lipids with distinct physical properties, which make the bilayer stiff or flexible, and which prefer association with tubular or vacuolar membrane elements (37, 79, 86, 87). It now appears that through intimate collaboration with embedded proteins in the bilayer and cytosolic proteins that insert into membranes, these lipids help drive the underlying complex pattern of membrane organization and the associated circulation of molecular species. These processes are essential for the generation and maintenance of the endomembrane system as a whole.
Here we focus primarily on how lipid sorting and trafficking can help account for transport of proteins along exocytotic and endocytic pathways of the endomembrane system. We begin by providing a general overview of the recycling pathways of the endomembrane system. These build and maintain the Golgi apparatus, the central sorting station within the system. We then discuss the lessons from a perturbant of membrane trafficking, which by disrupting membrane flow pathways causes the Golgi and other organelles to disassemble. An important role for lipid mixing regulated by coat proteins in maintaining organelle structure is suggested by these studies. Next, we examine the basic lipid species found in endomembranes, the interplay among them, and how their interaction may lead to protein sorting. Finally, we consider at the mechanistic level how the properties of lipids can be integrated with live cell imaging and biochemical findings to describe protein sorting and transport, particularly within the Golgi apparatus. Our overall goal is to summarize how three disciplines—protein trafficking, computational cell biology, and the biophysics of membrane lipids—hold great promise for collaborative work to explain transport within and between the exocytic and endocytic pathways.
OVERVIEW OF THE ENDOMEMBRANE SYSTEM
The endomembrane system serves two fundamental roles: export of secretory cargo and import of endocytic material. Export involves vectoral delivery of newly synthesized secretory proteins from the ER to the Golgi apparatus and then to the plasma membrane (PM) (41); import involves uptake of macromolecules from the PM into endosomes and then to lysosomes (21, 54). For the cell to continually export and import molecules, the endomembrane system must engage in extensive membrane recycling to retrieve functional components of organelles that are redistributed during the export and import processes. This permits organelles to maintain their identity amid constant flow of material. At the same time, the recycling permits bidirectional exchange of components between organelles. The endomembrane system thereby develops synergistic, functional connections among its various elements, enabling the cell to respond to different physiological conditions through changes in secretory and/or endocytic activities.
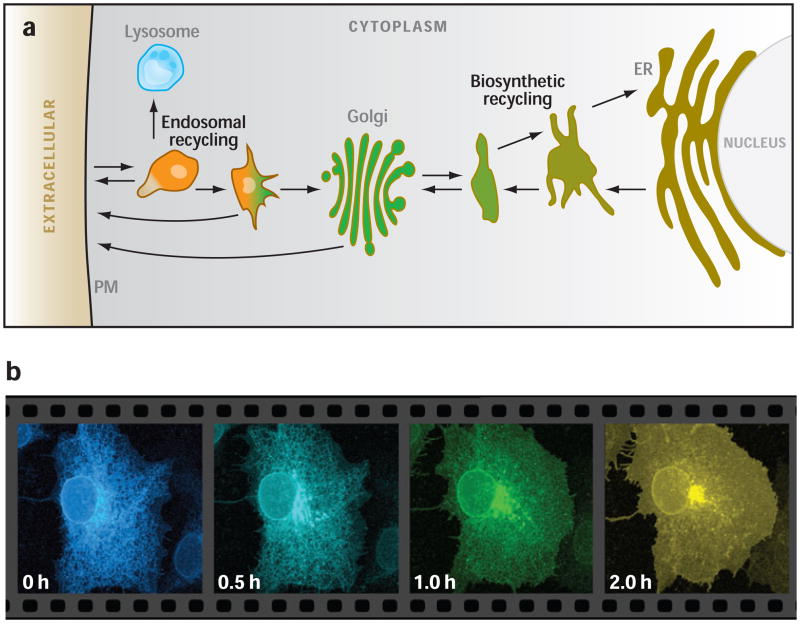
Notably, there are two major membrane recycling pathways within the endomembrane system (reviewed in Reference 73): (a) the biosynthetic membrane recycling pathway, whose trafficking routes interconnect ER, the ER/Golgi intermediate compartment (ERGIC) and early Golgi (12, 73); and (b) the endocytic recycling pathway, whose trafficking circuit interconnects the late Golgi, PM, and an ever-growing collection of named endosomes, lysosomes, and the endocytic recycling compartment (ERC) (21, 54) (Figure 1a). The biosynthetic and endocytic recycling pathways are morphologically and functionally symmetric. Each is composed of pleiomorphic, highly dynamic membrane-bounded structures that transport molecules from compartment to compartment in the pathway.
Figure 1.
(a) Diagram of endomembrane system showing biosynthetic and endocytic recycling subsystems. (b) Time-lapse images showing movement of ts045 vesicular stomatitis viral G protein tagged with green fluorescent protein as it moves vectorially through the secretory pathway after release from the endoplasmic reticulum (ER) by temperature shift (courtesy of George Patterson, NIH). PM, plasma membrane.
Situated at the midpoint of these two recycling pathways, and ideally placed to mediate communication and control between them, is the Golgi apparatus. With an elaborate morphology of flattened saccuoles with surrounding tubules and vesicles (44, 66), the Golgi operates as a carbohydrate factory for the processing and modification of proteins and lipids, and serves as the central sorting station of the endomembrane system (40, 41, 69). Movement through the Golgi is the only way that integral membrane proteins generated or modified in one recycling pathway can pass into the other. This is illustrated in Figure 1b, which shows the trafficking of newly synthesized vesicular stomatis viral G protein (VSVG) after release from the ER by temperature shift.
In both biosynthetic and endocytic recycling pathways, work involving membrane recruitment of cytosolic coat proteins and their adaptors is involved in the initial membrane outgrowth from the membrane source comprising each pathway (i.e., ER for secretory recycling pathway and PM for endocytic recycling pathway). The coat complexes bind in an energy-dependent, reversible manner to the cytosolic surfaces of the ER or PM, producing transport intermediates shaped as vesicles or tubules and enriched in specific types of proteins and lipids. The transport intermediates fuse together in a homotypic fashion, creating larger structures that, in turn, merge with more downstream structures to convey cargo along either exocytic or endocytic routes.
To preserve the identity and function of the ER and PM during this process, resident proteins and lipids of the ER or PM inadvertently sorted into transport intermediates are returned back to their target organelle by membrane retrieval routes. This process plays an important role in refining the contents of transport intermediates after they have budded off from the ER or PM. Thus, within transport intermediates moving from ER toward the Golgi, ERGIC proteins recycle back to the ER while PM-directed proteins are retained (20, 73). Likewise, within endocytic structures derived from the PM, endocytosed ligands are retained for degradation in lysosomes while their receptors are retrieved back to the PM (21, 54, 55).
CURVATURE-BASED SORTING WITHIN THE ENDOMEMBRANE SYSTEM
As mentioned above, the transport intermediates comprising the endomembrane system are highly pleiomorphic, exhibiting tubular and vacuolar morphologies. The opposite surface-to-volume ratio of these elements (with tubules having a high surface-to-volume ratio and vacuoles having a low surface-to-volume ratio) makes them adapted for carrying either more membrane material or more lumenal material. Consequently, tubules convey mostly integral membrane proteins and lipids, whereas vacuolar portions of an organelle tend to carry soluble content (73, 85). This adaptation greatly facilitates the sorting of membrane and lumenal content carried within transport intermediates. Both biosynthetic and endocytic recycling pathways utilize this geometry-based sorting. In the biosynthetic recycling pathway, transmembrane cargo receptors recycle back to the ER in tubules while soluble secretory cargos move within vacuolar elements toward the Golgi (13). Similarly in the endocytic recycling pathway, endocytosed membrane-bound receptors sort into narrow tubules that recycle back to the PM while their dissociated ligands remain in vacuolar structures that ultimately fuse with lysosomes (54).
In addition to facilitating the sorting of soluble and integral membrane proteins as described above, the tubule/vacuolar geometry of endomembranes leads to lipid sorting. This is because highly curved membranes, such as tubules, attract different lipid components relative to less curved bilayer surfaces, such as vacuoles, to accommodate the changes in bilayer compression and bending arising from high curvature (5, 9, 10, 70, 79). For example, when narrow membrane tubules are pulled from giant unilamellar vesicles, lipid molecules having high bending rigidity [e.g., sphingomyelin and sphingolipids (SL)] are largely excluded from the tubules, whereas lipids with low bending rigidity [e.g., glycerophospholipids (GPLs)] readily enter the tubules (79, 53). Lipid sorting in this system is amplified in the presence of lipid clustering proteins such as cholera toxin (79, 53). In this way, the tubule/vacuole geometries of transport intermediates play an important role in driving both lipid and protein sorting within the endomembrane system.
MEMBRANE DEFORMATION BY CYTOSOLIC COAT PROTEINS
Given that different geometric membrane shapes (e.g., tubular versus vacuolar) can cause segregation of soluble proteins, lipids, and integral membrane proteins, it is important to ascertain what controls the formation of these shapes. The major contributors appear to be cytosolic proteins that bind to membranes. These consist of coat proteins (e.g., COPI, COPII, and clathrin) and their adaptors, small GTPases (e.g., Arf1 and Sar1), lipid-modifying enzymes such as phosphatidylinositol kinases and phospholipases, “tethering factors,” and fusion proteins (12, 13, 17, 23, 32, 49, 59, 77, 78). Together, these molecules collaborate to produce coated buds on membranes that give rise to vesicle/tubule carriers and/or partitioned domains on membrane surfaces (4, 6).
Coat proteins, in particular, have the capacity to deform membranes (46, 56). This occurs through their ability, in conjunction with other proteins, to induce membrane curvature (6, 56). In the case of COPII coats, the N-terminal amphipathic helix of Sar1p is able to bend membranes (6). For clathrin-coated vesicles, some curvature inducers/stabilizers contain BAR superfamily domains that form dimeric coiled-coil modules, while others contain Epsin N-terminal homology (ENTH) or C2 domains (56). The latter two domain types, in addition to Sar1p, insert an amphiphatic helix or bulky hydrophobic residue into the bilayer causing nearby lipid headgroups to splay apart, and thereby inducing membrane curvature (56). BAR domains have a banana shape that acts as a scaffold to stabilize a curved membrane. The BAR domain can also sense membrane curvature. The ALPS motif, found in a number of proteins involved in trafficking in the early secretory pathway, is another type of membrane curvature sensor (6). It is unstructured in the absence of a lipid bilayer, but forms an amphipathic helix upon contact with a highly curved membrane (25). Proteins containing the ALPS motif are adapted to bind to the surface of lipid membranes as a result of defects in lipid packing, arising in response to changes in membrane curvature or lipid composition (6). Proteins that have the capacity to sense membrane curvature are very important, in that the cell can use membrane shape as a signal to trigger downstream events. For example, the ALPS motifs within ArfGAP1, which inactivates Arf1 (59), ensures that release of the COPI coat and vesicle tether, GMAP210, occurs only on highly curved membranes (11, 25). To extend curvature over a wide distance across a membrane surface, proteins containing these modules often oligomerize or cluster with other proteins by avidity interactions.
The cytosolic coat proteins work in conjunction with other factors to stabilize and dynamically modulate membrane shape changes. Binding of cytoskeletal motor proteins, for example, can help stabilize domains and cause extension of long tubules (3). Lipid-based partitioning of transmembrane proteins into tubule or vacuolar portions of a membrane, in turn, can induce further cytosolic protein recruitment (through binding motifs on their cytosolic tails) that through subsequent lipid clustering could drive further lipid sorting and cytosolic protein recruitment (4, 13). Thus, a complex interplay of protein-protein interactions can synergistically drive protein sorting: Cytosolic proteins initiate the membrane deformation process by binding to membrane and driving geometric shape by inducing membrane curvature. This leads to lipid sorting; partitioning of transmembrane proteins into sorted lipid domains can then induce further lipid sorting and cytosolic protein recruitment.
Although cytosolic coat complexes and their effectors play an essential role in membrane deformation and sorting within the endomembrane system, as described above, they do not explain how the endomembrane system emerges as a steady-state system nor why it comprises two distinct recycling pathways intersecting at the Golgi apparatus. To help clarify these questions, it is useful to begin by considering the effects of a widely studied cause of perturbation of membrane traffic, brefeldin A (BFA).
LESSONS FROM BREFELDIN A
A hallmark of the morphological effects of BFA is the rapid and dramatic induction of tubules derived from the Golgi, endosomes, lysosomes, and trans-Golgi network (43, 75). The morphological changes within the organelles are significant. Within seconds of BFA treatment, the stack of cisternae that form the Golgi converts into a tubule network and then fuses with the ER leaving no recognizable Golgi structure behind (75). Both biosynthetic and endocytic recycling systems transform as well, becoming highly tubular with little or no vacuolar elements (43). Remarkably, membrane transport, although altered, still continues in the presence of BFA. However, only specific membrane transport pathways are allowed. Newly synthesized secretory proteins that normally would move to the plasma membrane remain confined to the ER and its recycling system. Endocytosed transferrin receptor can still recycle to and from the plasma membrane, but now other receptors follow the same route, including the mannose-6-phosphate receptor, which normally recycles between late endosomes and Golgi (43). The main effect, therefore, is that under BFA treatment trafficking involves only mixing of tubular membranes within a particular recycling subsystem (i.e., biosynthetic or endocytic), in which the targets of fusion of tubules depends on the site of origin of the tubule. For example, Golgi tubules uniquely find ER and ERGIC, whereas endosomal tubules mix only with the PM and ERC. Neither tubule system, however, can “transfer’ components from one system to another.
What can explain these effects? The extraordinary progress made in elucidating the biochemical characteristics of cytosolic coat proteins, and their regulation and role in membrane traffic provides some clues. BFA acts as an uncompetitive inhibitor to GBF1 (61), the exchange factor at the Golgi that activates Arf1, a small GTPase (68). By preventing Arf1 binding to membrane, BFA leads to dissociation of downstream cytosolic effectors of Arf1, which includes the coat complexes of COPI (localized on Golgi and biosynthetic recycling membranes) and GGA (localized on late Golgi and endosomal recycling membranes). The resulting absence of coat activity leads to the loss of vacuolar elements of transport intermediates and the Golgi. Thus, it may be by displacing coats that drive curvature changes in the endomembrane system that BFA disrupts membrane flow patterns and membrane geometry, which in turn leads to organelle disassembly. The morphological and functional states of endomembrane organelles, therefore, are at a fine balance maintained by the assembly of Arf1, coats, and their numerous downstream effectors.
At the same time, because the membrane trafficking occurring in the absence of Arf1-dependent coats is limited to tubular networks that communicate with the ER or plasma membrane, but not with each other (43), the Arf1-based coat system may also provide the mechanistic basis for “jumping” between biosynthetic and endocytic recycling subsystems. But what could the Arf1-based coat system be doing to serve such a structural and regulatory role in trafficking between biosynthetic and endocytic recycling systems? As discussed below, we suggest the answer relates to the lipid composition of the two recycling systems, which bestows distinct physical properties that coat activity modulates to serve a variety of structural and regulatory roles, including the generation and maintenance of Golgi apparatus function.
LIPID TYPES, DISTRIBUTION, AND CIRCULATION WITHIN THE ENDOMEMBRANE SYSTEM
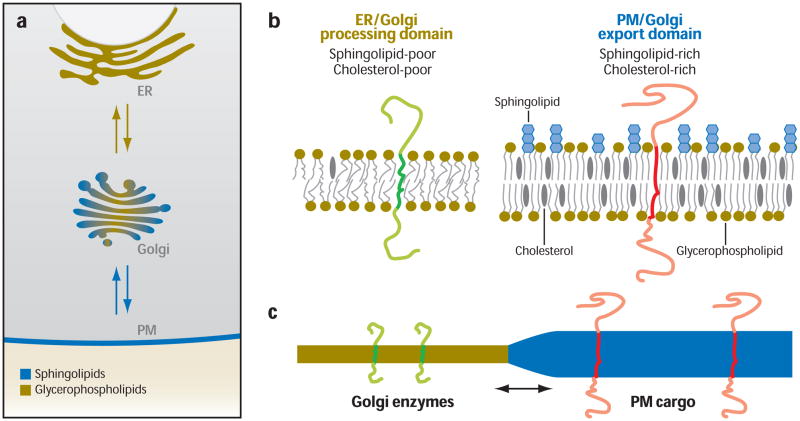
All eukaryotic cells use three major types of lipid in their endomembranes: GPLs, SLs and sterols (e.g., cholesterol) (37, 76, 86). When embedded within a membrane bilayer, these lipids have self-associative properties that facilitate selective overall lateral segregation of lipids and proteins in the membrane. GPLs are composed of fatty acyl chains attached to a glycerol backbone. Different GPLs include neutral (e.g., phosphatidylcholine and phosphatidylethanolamine) and anionic (e.g., phosphatidylinositol, phosphatidylglycerol and phosphatidylserine) forms that vary in their hydrophilic head moiety, degree of fatty acid unsaturation, and fatty acid length. Because the fatty acyl chains of GPLs are mostly unsaturated, the bilayers in which they are enriched are highly flexible, or liquid disordered (Ld) (Figure 2a). SLs are composed of sphingosine with a nonpolar tail of fatty acid chains and a headgroup of phosphocholine (found in sphingomyelin) or different sugar structures (found in glycosphingolipids). The fatty acid chains of SLs are usually saturated and can associate with themselves in the plane of the membrane through extensive inter- and intramolecular hydrogen bonding, packing more tightly and extending deeper into the bilayer than GPLs. As a result, bilayers enriched in SLs are more ordered and are stiffer and thicker than those enriched in GPLs (Figure 2a). Cholesterol contains a four-membered ring structure, a hydroxyl group, and a short hydrophobic tail. Its rigid, flat steroid backbone is readily stabilized between SL’s saturated hydrocarbon chains owing to van der Waals attraction. When present in the same bilayer as SLs, cholesterol is attracted to SLs and forms lateral lipid assemblies or microdomains enriched in these lipids (1, 2, 29, 30). Bilayers enriched in SLs and cholesterol are often called liquid-ordered (Lo) because they are more ordered. These properties of GPL, SL, and cholesterol lead to an overall nonrandom lipid distribution within the bi-layer of an organelle. This helps organize integral membrane proteins, since each seeks a membrane domain with a bilayer thickness that matches the length of its transmembrane segments (42) (Figure 2b).
Figure 2.
Lipid gradient across the secretory pathway, and its role in membrane partitioning and protein sorting. (a) Diagram illustrating the differential distribution of lipids across the endomembrane system. The endoplasmic reticulum (ER) is enriched in glycerophospholipids (GPL) (yellow), which are carried into the Golgi through secretory membrane trafficking. In the Golgi, sphingolipids (SL, blue) are synthesized. Due to interactions with cholesterol, SLs form phase-partitioned domains, referred to as export domains (blue), from which transport intermediates bud out to deliver cargo to the plasma membrane. Golgi domains enriched in GPL are referred to as processing domains (yellow) because enzymes involved in modifying the carbohydrate side chains of proteins reside here. (b) The lipid composition of ER/Golgi processing domains is similar in being poor in SL and cholesterol. This makes their bilayer thicknesses thinner (left) than that of plasma membrane/Golgi export domains (right), which are enriched in SL and cholesterol. (c) Because of the differential bilayer thicknesses in Golgi processing versus export domains, transmembrane proteins sort in the Golgi. Proteins with long transmembrane domains (i.e., plasma membrane cargo) sort into thick bilayers enriched in SL and cholesterol (blue), whereas proteins with short transmembrane domains (i.e., Golgi enzymes) prefer residing in thinner bilayers enriched in GPLs (yellow). Abbreviations: ER, endoplasmic reticulum; GPL, glycerophospholipids, PM, plasma membrane; SL, sphingolipids.
GPL, SL, and cholesterol are not distributed at equivalent levels among the organelles of the endomembrane system. Instead, there is a gradient in concentration, with the ER having a low concentration of SL and cholesterol (relative to GPL), the Golgi having an intermediate concentration, and the PM having the highest concentration (37, 86) (Figure 2c).
One reason why eukaryotic cells maintain this variability in lipid content within the biosynthetic and endocytic recycling pathways and Golgi is to generate lipid environments within organelles that are compatible with their distinct cellular functions (14, 37). Owing to low levels of SLs and cholesterol, the membranes in the ER and the biosynthetic recycling pathway are composed mainly of GPLs whose flexible acyl chains are loosely packed and deformable. This provides a lipid environment suitable for insertion and folding of proteins in the ER bilayer. In the PM and endocytic recycling system, by contrast, the high concentrations of SLs and cholesterol make the membranes thicker and less permeable to small molecules. This allows the PM to act as an impermeable barrier between the cytoplasm and cell exterior. On the other hand, the intermediate concentration of sterols and SLs in the Golgi make it ideal to serve as a transition station between the biosynthetic and endocytic cycling pathways.
Because integral membrane proteins always seek a lipid bilayer with a thickness that matches the length of its transmembrane segments (42), the presence of a gradient of lipids having different bilayer thicknesses within the endomembrane system leads to integral membrane proteins differentially distributing across it. For example, membrane proteins with short transmembrane segments (~15 amino acids) typically reside in the biosynthetic recycling pathway, whereas those with longer segments (~20–25 amino acids) reside in the endocytic recycling pathway (14, 20).
Given the significance of having a lipid gradient across the endomembrane system, how do cells generate and maintain it? Three characteristics of GPL, SL, and cholesterol are likely involved: (a) the self-organizing properties of these lipids (i.e., they tend to partition into distinct SL/cholesterol- and GPL-enriched domains when residing in the same bilayer), (b) the differential sites of synthesis of these lipids (GPLs and cholesterol are synthesized in the ER, whereas SLs are synthesized in the Golgi), and (c) their different preferences for curved or less curved bilayers. These characteristics lead GPLs, SLs, and cholesterol to follow different overall circulation routes within the endomembrane system. Specifically, GPL and cholesterol are synthesized in the ER, and forward secretory transport conveys these lipids into the Golgi system. Once cholesterol reaches the Golgi, it binds to SLs and partitions into domains depleted of GPLs. Whereas a significant fraction of GPLs traffic back to the ER (via narrow tubules), the SL and cholesterol do not (18). Instead, they traffic out of the biosynthetic recycling pathway into the endocytic recycling pathway, moving toward the PM in large pleiomorphic structures. After arriving at the PM, SL and cholesterol circulate within the endocytic recycling pathway, including back to the Golgi (64), resulting in high levels of these lipids within the pathway (55, 89). Anionic GPLs (PI and PS) are enriched in membrane structures of the endocytic recycling pathway and Golgi (22) owing to their affinity for SL and cholesterol (47). This helps high curvature intermediates (i.e., tubules) form within the endocytic recycling pathway despite the presence of elevated levels of SL and cholesterol.
A central step in producing the lipid gradient across the endomembrane system described above is the sorting of GPL, cholesterol, and SL within the Golgi apparatus. The next section describes a model for how such sorting may occur within the elaborately stacked cisternal system of the Golgi and its consequences for Golgi trafficking and function.
SORTING IN THE GOLGI APPARATUS AND THE MEMBRANE PARTITIONING MODEL
For decades, the focus of Golgi membrane trafficking studies has been on membrane proteins and cytosolic regulatory machinery. This was the natural initial approach because post-translational processing and sorting of proteins to their correct destinations are major functions of the secretory pathway in general, and the Golgi apparatus in particular. Moreover, it was thought that control of sorting would be mediated entirely by protein-protein interactions. Gradually, however, it became clear that lipids were potent regulators of the secretory pathway and lipid composition gradients and specialized membrane microdomains were functionally significant. This shift is reflected in a number of recent reviews (4, 27, 35, 36, 85).
A new model of the Golgi, called the rapid partitioning model, incorporates lipid trafficking pathways and the self-organizing properties of lipids as an integral part of the organelle (60). A key assumption of the model is that the self-associative properties of GPL, SL, and cholesterol in Golgi membranes lead to phase partitioning of these lipids into two types of domains: one having low SL/cholesterol levels and thin bilayer thickness, and one having high SL/cholesterol levels and thick bilayer thickness. This, in turn, facilitates the selective lateral segregation of integral membrane proteins residing in or passing through the Golgi because the integral membrane proteins sort by their transmembrane domain thickness (14). Various new technologies support these assumptions regarding self-organization of lipids and proteins (50). Indeed, dual-color imaging of long transmembrane-spanning secretory cargo and short transmembrane-spanning resident Golgi enzymes in the Golgi reveals the two proteins never fully overlap (60, 91), consistent with their phase partitioning at all levels of the Golgi.
In addition to having two classes of membrane domains within every cisterna formed by partitioning, the partitioning model assumes bidirectional trafficking of protein and lipid between Golgi cisternae and that cargo can exit the Golgi from all cisternae. These assumptions are supported by prior observations. In particular, morphological studies have shown rapid movement of proteins and lipids throughout the Golgi (19, 38, 60, 91) and cargo exiting the Golgi from both cis and trans sides (88). The model also assumes that cargo and enzymes have an optimal lipid environment with which they preferentially associate within the Golgi. This is consistent with previous data on the sorting of resident enzymes by their transmembrane thickness (14, 42, 57).
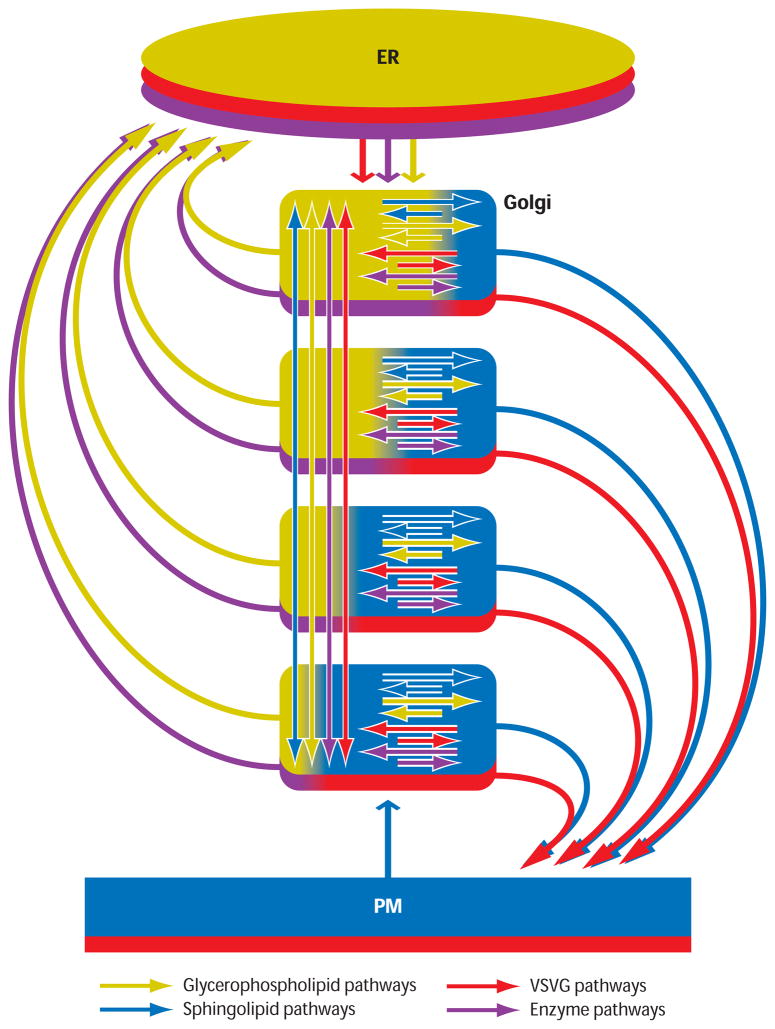
A spatially resolved version of the membrane partitioning model, shown in Figure 3, was generated to test whether the model could account for key characteristics of Golgi transport and organization (including cis-to-trans gradients in GPLs and SLs, differential cis/trans distributions of resident enzymes, and exponential release of cargo) (60). In the model, two classes of Golgi membrane domains are present in all Golgi cisternae. One class of domains, called Golgi-Processing Domains (GPDs), are enriched in Golgi-resident proteins and enzymes and contain less cholesterol and SL than do the second class of domains, the Golgi Exit Domains (GEDs). GEDs are enriched in cargo proteins destined for post-Golgi target membranes (e.g., plasma membrane and lysosomes) as well as cholesterol and SLs. Both domains contain GPLs. Molecules in each domain follow different trafficking routes: Those in GPDs (which are GPL rich) either return to the ER or circulate to other GPDs, whereas those in the GEDs (which are SL-rich) either are exported to the plasma membrane or continue to circulate in the Golgi.
Figure 3.
The rapid partitioning model of the Golgi. Each cisternae of the Golgi stack is modeled as a partitioning unit having one component consisting of glycerophospholipids (GPL, yellow) and another component consisting of cholesterol and sphingolipids(SL, blue). These components correspond to processing domains (yellow) and export domains (blue) as described in Figure 2. Transmembrane cargo proteins (red) move between both lipid environments but concentrate in the export domain, whereas transmembrane Golgi enzymes (green) are excluded from export domains and diffuse within the processing domain. The steady-state ratio of SL and GPL levels upon simulation gives rise to a lipid gradient across the stack with SL concentrations highest in the trans-most cisternae (i.e., bottom cisternae of stack) and lowest in the cis-most cisternae (i.e., top cisternae of stack). Colored arrows indicate the circulation pathways of lipids and proteins comprising this system. Abbreviations: ER, endoplasmic reticulum; GPL, glycerophospholipids, PM, plasma membrane; SL, sphingolipids; VSVG, vesicular stomatitis virus G protein.
Simulation and experimental testing of the rapid partitioning model revealed it could explain many of the major features of the Golgi apparatus (60). The model generated a gradient in SL/GPL compositions across the stack at steady state, with the ratio lowest in the cis cisternae and highest in the trans cisternae. Resident proteins with different SL/GPL preferences were enriched in different Golgi cisternae despite their rapid movement between cisternae. Cargo exited the Golgi with exponential kinetics, a finding consistent with experimental measurements of cargo export in living cells. Finally, a cargo wave pattern across the Golgi stack was observed in response to simulation of a short, low-temperature block and release of membrane traffic, consistent with prior electron microscopy experiments (82). Thus, the rapid partitioning model generates, through a self-organizing mechanism, all of the well-known asymmetries of cellular membrane lipid and protein composition in the Golgi. These are a consequence of lipid partitioning in concert with bidirectional vesicular/tubule trafficking of molecules between cisternae achieved by the simultaneous activity of many intracellular membrane trafficking pathways.
A deeper understanding of the mechanisms and consequences of membrane partitioning is necessary for the rapid partitioning model to expand its explanatory potential by accounting for new information about Golgi structure and function. Coats and membrane trafficking machinery are envisioned to play an essential role in partitioning by inducing geometric shape changes (i.e., membrane curvature) that enable protein and lipid sorting, and thus must be specifically integrated into the model. In addition, the role of particular lipid species passing through the Golgi needs further consideration. Below we discuss the possibility that cholesterol plays a special role in this system by serving as the lipid driver of membrane partitioning and regulator of protein traffic.
SPECIAL ROLE OF CHOLESTEROL/STEROLS IN MEMBRANE SORTING
In parallel with the increasing interest in membrane lipids, and beginning with the classic membrane thickness hypothesis (14), there has been unwavering interest in the role of cholesterol in membrane trafficking. Recently, cholesterol-SL-rich rafts have been intensively studied (31, 37, 50, 76). Cholesterol is uniquely capable of prompting the formation of segregated lipid domains in artificial bilayers. Equimolar mixtures of phospholipid, sphingomyelin, and cholesterol form domains that do not form in the absence of cholesterol (24). Biochemical evidence for SL-rich domains in the Golgi has been published from several groups (33). Cellular cholesterol content is exquisitely regulated (15, 16, 50, 76). Precise regulation is necessary, in part, because cholesterol is necessary for export from the Golgi (63, 89), but overloading cellular cholesterol pools with cyclodextrin-delivered cholesterol also results in a significant inhibition of Golgi export to the plasma membrane and other organelles (93). Cholesterol also regulates caveolin trafficking through the Golgi [though, in contrast to other studies, not vesicular stomatitis viral G (VSVG)] (62), to promote dynamin-dependent Golgi vesiculation, and to control intra-Golgi protein transport (80). Recently, cholesterol regulation of VSVG export from the ER has been examined in detail (71). A model in which cholesterol modulates entry of cargo into ER exit sites was found consistent with the data and is, in turn, consistent with a requirement for cholesterol in protein export to transport intermediates (67).
Despite this progress, it is not yet clear how cholesterol is trafficked or whether lipid transfer proteins mediate net cholesterol transport or even how steady-state lipid gradients are established and maintained. This may be an area in which computational cell biology or systems biology can accelerate progress. By integrating cholesterol traffic with models of protein, GPL, and SL traffic, one could not only provide quantitative tests of many mechanistic hypotheses, but also link membrane traffic with the ER/Golgi-based sterol regulatory element-binding protein (SREBP) control system. SREBP-based transcription regulation of genes has been elegantly described (15, 16), but we do not yet understand why evolution has chosen a cholesterol-based regulator, SREBP1-c, to control the genes of glycerolipid synthesis. Understanding the mechanistic rationale for this evolution-selected feedback system is likely to have profound implications for lipid metabolism, especially in cells that secrete plasma lipoproteins or accumulate tri-acylglycerols and cholesteryl esters in cytosolic lipid droplets. The Golgi is the final decision and control center for all of these processes. SREBPs will never reach the nucleus, even if they have been released from the ER by low cholesterol, unless the Golgi-resident proteases are active (28, 74). Consequently, an understanding of cholesterol’s role in membrane traffic is broadly important.
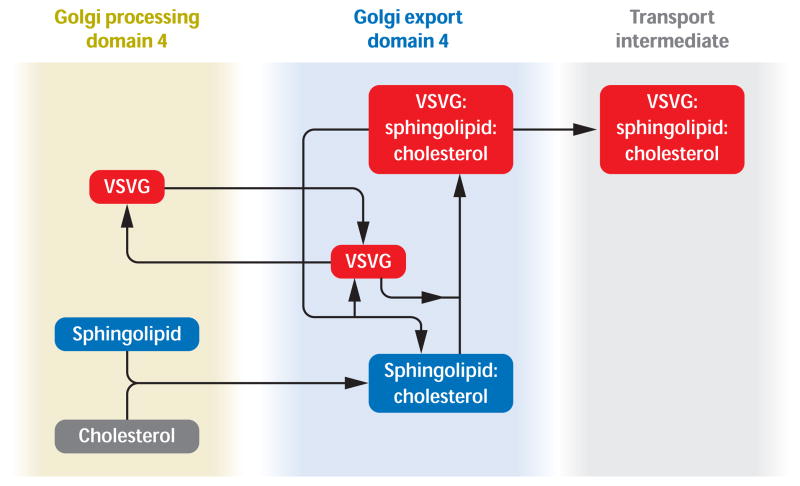
One working hypothesis, emerging from this literature, is that cholesterol is the membrane component that drives partitioning and thus controls protein sorting and export from the Golgi. This is illustrated in Figure 4. This idea is based on the dramatic demonstration that cholesterol initiates domain formation (9, 10) and the dynamic demonstration that cholesterol oxidase destroys such domains (72). The next step is initiated by membrane proteins stochastically exploring the Golgi membrane and the idea (6) that the minimum free energy state of a membrane protein is achieved when the length of its hydrophobic transmembrane domain matches the bilayer thickness. This idea has been experimentally tested (26) and the results are consistent with that shown in Figure 4..
Figure 4.
Working hypothesis concerning the role of cholesterol in Golgi membrane trafficking. For clarity, only one Golgi cisterna (the fourth) is represented. Golgi processing domain 4 and Golgi export domain 4 represent Golgi Processing and Export domains in the 4th Golgi cisterna. Processing and export domains are hypothesized in all cisternae. Cholesterol binds to sphingolipid and becomes part of a cholesterol and sphingolipid-enriched export domain. Cargo proteins [represented here by the widely studied prototype, vesicular stomatitis virus G (VSVG) protein] partition into exit domains because of the free-energy gradient inherent in moving to a sphingolipid:cholesterol-rich bilayer environment whose thickness matches the length of their transmembrane domains. Cargo proteins are thus concentrated in exit domains.
In the above hypothesis, cholesterol is an efficient solution to two important unanswered questions about Golgi function. First, it provides a driving force for partitioning. Second, it offers a physical chemical explanation for the observed ability of the Golgi to export cargo proteins at a rate proportional to their Golgi abundance (38, 60) (assuming transmembrane cargo proteins, by virtue of their bilayer thickness preference, help stabilize partitioned domains). Testing this hypothesis in the context of the full endomembrane system requires a more detailed examination of what is known about intracellular cholesterol traffic. This is because the dominant current view of intracellular cholesterol transport is that the vast majority of cholesterol is transported from the ER to the PM independent of the Golgi apparatus (76). Moreover, the involvement of the Golgi in retrograde traffic of endocytosed or lysosomal cholesterol is based on studies of Niemann-Pick cells (83), and an alternative pathway, independent of the Golgi, has been identified (51).
There is an enormous literature on intra-cellular cholesterol processing. One important review (76) summarizes 87 publications covering 26 distinct cholesterol trafficking pathways. This is the sort of complexity for which modeling is a natural approach. The most prominent fact, however, about cellular cholesterol delivery from its site of synthesis in the ER to the PM is that the literature is essentially unanimous in concluding that the majority of cholesterol traffic from ER to PM is independent of the secretory pathway (51, 76). Based on biosynthetic radiotracer studies involving various means of halting secretory traffic, the dogma has been established that most cholesterol reaches the PM by a Golgi-bypass route mediated by cytosolic lipid transfer proteins (LTPs) (8). Unlike GPLs and SLs, cholesterol can be transported between intracellular membranes by well-documented nonvesicular pathways, so a Golgi-bypass route cannot be excluded. On biophysical grounds, however, it appears entirely feasible to explain the tracer data by invoking a model in which LTPs mediate high-flux bidirectional transport between ER and PM, but with the net cholesterol flux returning to, not exiting from, the ER.
Alternatively, it might be hypothesized that this Golgi-bypass pathway evolved to allow the Golgi to regulate its cholesterol content independent of the need to transport large amounts of ER-synthesized cholesterol to the PM. If the partitioning model is correct and Golgi cholesterol content is a controller of Golgi export, this hypothesis makes teleological sense. If, however, LTPs and other cytosolic cholesterol-binding proteins such as the Golgi-targeted oxysterol-binding protein (OSBP) are only able to equilibrate cholesterol among Ld domains of organelles (as is currently thought), the ER-Golgi-cholesterol control system, discovered by Brown and Goldstein, can have little influence on the cholesterol content of Lo domains. Mass action will still increase or decrease cholesterol in Lo domains, but changes relative to Ld will necessarily be controlled by local processes. In this sense, Ld domains act as cholesterol buffers. Thus, the ER portion of the SREBP control system can only control Ld cholesterol within an unknown factor. It is intriguing that, depending on the informatics tool chosen, the transmembrane domains of SREBP cleavage-activating protein (SCAP) are between 21 and 23 amino acids long, likely targeting this cholesterol sensor to Golgi Lo regions. Whether this has functional consequences for the Golgi is currently unknown.
Clearly, there are many potential cholesterol transport pathways and regulatory mechanisms acting simultaneously (34). This is exactly the kind of situation in which the tools of computational cell biology are most useful. It should be possible, for example, to simulate a simple cholesterol trafficking model for a wide range of LTP abundances with binding constants in the reported range of 50–300 nM and provide a quantitative test of the hypothesis that net transfer of cholesterol on LTP is from the PM to the ER while remaining consistent with the classical data (84) that established the LTP-anterograde paradigm. Such a model must also predict the somewhat greater PM–specific activity obtained in the presence of BFA (84). This seems possible because LTPs could carry very large forward and reverse fluxes of cholesterol no matter which direction the net flux is traveling, and the biosynthetic tracer (3H-acetate) may have traced primarily the anterograde pathway.
Given that the mechanism of LTP interaction with membranes is only beginning to be understood and given the likelihood of accessory proteins and differing lipid compositions of the target membranes, it would be important to explore the influence of the LTP-cholesterol-binding constants at both membranes. Simulation should address this question easily. If it turns out that LTPs must carry a PM–directed flux of cholesterol, total cholesterol delivery to the PM could not exceed the rate of cholesterol synthesis. Because membrane traffic carries perhaps tenfold more lipid than is synthesized, such an outcome would require another retrieval pathway, such as caveolin (52), to accommodate the substantial changes in secretory pathway cholesterol traffic that must necessarily accompany changes in cell function.
Any theory of cholesterol trafficking must also account for cellular cholesterol efflux (7, 81) and also for the well-documented sterol gradients across the endomembrane system. Because mammalian cells express no enzymes capable of catalyzing cholesterol breakdown, a mechanism for cholesterol efflux is essential. Otherwise a steady state in cellular cholesterol content would be impossible. A classic result in this area of research is that cellular cholesterol efflux to an extracellular acceptor is at least biexponential (58). Again, it should be possible to test various models of cholesterol traffic against these data by simulating the presence of an extracellular cholesterol acceptor such as apolipoprotein A-I or methyl-beta-cyclodextrin or by examining the steady-state sterol gradients established by each model.
A computational cell biology approach could raise the intriguing possibility that LTPs are the long-sought cholesterol retrieval pathway that supports constitutive and acute traffic through the secretory pathway while maintaining a steady state of organellar and PM cholesterol composition. Moreover, because the ER and Golgi together constitute the control center for cellular cholesterol metabolism, this retrieval mechanism is essential to maintaining a “closed loop” for the SCAP:SREBP controller. The Brown & Goldstein (15) SREBP controller can only sense ER cholesterol, but it needs to regulate cellular cholesterol. From a modeling perspective, LTPs seem a likely mechanism to feed back the PM-cholesterol signal (where the vast majority of cellular cholesterol resides) to the ER and Golgi-resident SREBP control system.
The above examples focus on steady-state tracer kinetics and on acute responses to perturbations of membrane traffic, such as BFA. Brief mention must be made of longer-term experiments, if only because so much of the scientific interest in cholesterol is driven by disease-related research in which experimental durations are often days or weeks. To relate the simple cholesterol models discussed above to these more protracted experiments, a reasonable hypothesis is that ER-based cholesterol sensors monitor only cholesterol in the liquid disordered (Ld) domains of membranes. Several reports suggest the possibility that cytosolic lipid transfer proteins equilibrate these high-chemical-potential pools of cholesterol. This would allow the ER to regulate total cell cholesterol while still allowing the lipid and protein composition of each membrane to determine the cholesterol content of its liquid ordered (Lo) domains. This hypothesis derives from the Maxfield-Menon (55) and Lange-Steck (72) models and explicitly interfaces with the Brown-Goldstein SREBP control system (34). Although it should be possible to test simple models of cellular cholesterol trafficking against radiotracer data or data collected using fluorescent analogs of cholesterol such as BODIPY-cholesterol (39, 48), longer-term experiments will require models that include the SREBP transcription control system in order to account for changes in gene expression that commonly mediate longer-term responses. This is still well within the ambit of computational cell biology and represents a fertile area for biophysical approaches to membrane trafficking.
Computational cell biology is a natural tool for the analysis of complex systems and membrane trafficking is demonstrably complex. Let us close by returning full circle. An initial model of the endomembrane system described here or by Saraste & Goud (73) could focus on any one, or even several, of the major membrane constituents. The approach would be to write ordinary differential equations for each molecular species in each cellular location. The cellular locations would be ER, ER exit sites, the several (typically seven) Golgi cisternae [perhaps divided into lipid domains as in Patterson et al. (60)], the biosynthetic recycling compartment described by Saraste & Goud (73), the trans-Golgi network, vesicular and tubular transport intermediates, the PM (perhaps subdivided into liquid-ordered and liquid-disordered phases based on local lipid composition), specialized membrane domains such as coated pits and caveolae, early endosomes, recycling endosomes, late endosomes, and the endocytic recycling compartment. It is naturally easier to list the places than it is to list the processes by which molecules and information are transferred from one place to another. Places can be seen; processes must be inferred. We close by recalling a point alluded to earlier—that more scientific attention must be paid to retrograde trafficking, that is, to recycling. Correct kinetic models of cellular systems must be capable of steady states. In the case of cholesterol traffic, abundant experimental results demonstrate that cholesterol entering the cell by endocytosis, whether initially a component of the PM or entering as low-density lipoprotein cholesteryl esters, by some means, is transported to the ER. It is, of course, possible that this process is in part mediated by lipid transfer proteins (65), but there is also evidence for involvement of endosomes, lysosomes, ERC, PM, and possibly Golgi (51, 58). Membrane trafficking moves cholesterol at a rate far in excess of the rate at which cholesterol is synthesized. This means recycling pathways carry just as much cholesterol as anterograde pathways. A thoughtful, biophysically inclined cell biologist, interested in this field, might be well advised to pursue identification, characterization, and quantification of these essential retrograde pathways and the mechanisms that control them.
Acknowledgments
We would like to thank Drs. Cathy Jackson and Bruno Antonny for helpful comments and discussions regarding the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Jennifer Lippincott-Schwartz, Email: jlippin@helix.nih.gov.
Robert D. Phair, Email: rphair@integrativebioinformatics.com.
LITERATURE CITED
- 1.Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–53. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 2.Aittoniemi J, Niemela PS, Hyvonen MT, Karttunen M, Varrulainen I. Insight into the putative specific interactions between cholesterol, sphingomyelin, and palmitoyl-oleoyl phosphatidylcholine. Biophys J. 2007;92:1125–37. doi: 10.1529/biophysj.106.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan VJ, Thompson HM, McNiven MA. Motoring around the Golgi. Nat Cell Biol. 2002;4:E236–42. doi: 10.1038/ncb1002-e236. [DOI] [PubMed] [Google Scholar]
- 4.Altan-Bonnet N, Sougrat R, Lippincott-Schwartz J. Molecular basis for Golgi maintenance and biogenesis. Curr Opin Cell Biol. 2004;16:364–72. doi: 10.1016/j.ceb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–30. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 6.Antonny B. Membrane deformation by protein coats. Curr Opin Cell Biol. 2006;18:386–94. doi: 10.1016/j.ceb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32:172–93. doi: 10.1016/j.tibs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Pottekat A, et al. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 2005;44:5816–26. doi: 10.1021/bi048296z. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci USA. 2007;104:3165–70. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–24. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 11.Bigay J, Gounon P, Robineau S, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. Nature. 2003;426:563–66. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–66. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 13.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–14. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 14.Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–81. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- 15.Brown MS, Goldstein JL. Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J Lipid Res. 2009;50(Suppl):S15–27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–98. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 17.Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–21. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 18.Brugger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, et al. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–18. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- 20.Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins. J Cell Biol. 1998;140:1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 22.De Matteis MA, Godi A, Corda D. Phosphoinositides and the Golgi complex. Curr Opin Cell Biol. 2002;14:434–47. doi: 10.1016/s0955-0674(02)00357-5. [DOI] [PubMed] [Google Scholar]
- 23.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–84. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, et al. Lipid rafts reconstituted in model membranes. Biophys J. 2001;80:1417–28. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drin G, Casella JF, Gautier R, Boehmer T, Schartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Science. 2008;320:670–73. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 26.Dukhovny A, Yaffe Y, Shepshelovitch J, Hirschberg K. The length of cargo-protein transmembrane segments drives secretory transport by facilitating cargo concentration in export domains. J Cell Sci. 2009;122:1759–67. doi: 10.1242/jcs.039339. [DOI] [PubMed] [Google Scholar]
- 27.Echard A. Membrane traffic and polarization of lipid domains during cytokinesis. Biochem Soc Trans. 2008;36:395–99. doi: 10.1042/BST0360395. [DOI] [PubMed] [Google Scholar]
- 28.Espenshade PJ, Cheng D, Goldstein JL, Brown MS. Autocatalytic processing of site-1 protease removes propeptide and permits cleavage of sterol regulatory element-binding proteins. J Biol Chem. 1999;274:22795–804. doi: 10.1074/jbc.274.32.22795. [DOI] [PubMed] [Google Scholar]
- 29.Feigenson GW. Phase behavior of lipid mixtures. Nat Chem Biol. 2006;2:560–63. doi: 10.1038/nchembio1106-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feigenson GW. Phase boundaries and biological membranes. Annu Rev Biophys Biomol Struct. 2007;36:63–77. doi: 10.1146/annurev.biophys.36.040306.132721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–69. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- 32.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 33.Gkantiragas I, Brugger B, Stuven E, Kaloyanova D, Li XY, et al. Sphingomyelin-enriched microdomains at the Golgi complex. Mol Biol Cell. 2001;12:1819–33. doi: 10.1091/mbc.12.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Haucke V, Di PG. Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol. 2007;19:426–35. doi: 10.1016/j.ceb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holthuis JC, Pomorski T, Raggers RJ, Sprong H, van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol Rev. 2001;81:1689–723. doi: 10.1152/physrev.2001.81.4.1689. [DOI] [PubMed] [Google Scholar]
- 38.Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, et al. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtta-Vuori M, Uronen RL, Repakova J, Salonen E, Vattulainen I, et al. BODIPY-cholesterol: a new tool to visualize sterol trafficking in living cells and organisms. Traffic. 2008;9:1839–49. doi: 10.1111/j.1600-0854.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- 40.Jackson CL. Mechanisms of transport through the Golgi apparatus. J Cell Sci. 2009;122:443–52. doi: 10.1242/jcs.032581. [DOI] [PubMed] [Google Scholar]
- 41.Kepes F, Rambourg A, Satiat-Jeunemaitre B. Morphodynamics of the secretory pathway. Int Rev Cytol. 2005;242:55–120. doi: 10.1016/S0074-7696(04)42002-6. [DOI] [PubMed] [Google Scholar]
- 42.Killian JA. Hydrophobic mismatch between proteins and lipids in membranes. Biochim Biophys Acta. 1998;1376:401–15. doi: 10.1016/s0304-4157(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 43.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–80. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–49. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 46.Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–17. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 47.Leventis PA, Grinstein S. The distribution of phosphatidylserine phospholipids between the plasma and internal membranes in a cell. Annu Rev Biophys. 2010:39. doi: 10.1146/annurev.biophys.093008.131234. In press. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Mintzer E, Bittman R. First synthesis of free cholesterol-BODIPY conjugates. J Org Chem. 2006;71:1718–21. doi: 10.1021/jo052029x. [DOI] [PubMed] [Google Scholar]
- 49.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006;119:605–14. doi: 10.1242/jcs.02855. [DOI] [PubMed] [Google Scholar]
- 50.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 51.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 52.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–89. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 53.Manneville JB, Casella JF, Ambroggio E, Gounon P, Bertherat J, et al. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc Natl Acad Sci USA. 2008;105:16946–51. doi: 10.1073/pnas.0807102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 55.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–85. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 56.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–96. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 57.Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, et al. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–13. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- 59.Nie Z, Hirsch DS, Randazzo PA. ArfGAPs and membrane traffic. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 60.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–67. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peyroche A, Antonny B, Tobineau S, Acker J, Cherfils J, Jackson CL. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–85. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- 62.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, et al. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prydz K, Simons K. Cholesterol depletion reduces apical transport capacity in epithelial Madin-Darby canine kidney cells. Biochem J. 2001;357:11–15. doi: 10.1042/0264-6021:3570011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puri V, Watanabe R, Dominguez M, Sun X, Wheatley CL, et al. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. Nat Cell Biol. 1999;1:386–88. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173:107–19. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rambourg A, Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol. 1990;51:189–200. [PubMed] [Google Scholar]
- 67.Ridsdale A, Denis M, Gougeon PY, Ngsee JK, Presley JF, Zha X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol Biol Cell. 2006;17:1593–605. doi: 10.1091/mbc.E05-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robineau S, Chabre M, Antonny B. Binding site of brefeldin A at the interface between the small G protein ADP-ribosylation factor 1 (ARF1) and the nucleotide-exchange factor Sec7 domain. Proc Natl Acad Sci USA. 2000;97:9913–18. doi: 10.1073/pnas.170290597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rothman JE. The Golgi apparatus: two organelles in tandem. Science. 1981;213:1212–20. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- 70.Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–45. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Runz H, Miura K, Weiss M, Pepperkok R. Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G. EMBO J. 2006;25:2953–65. doi: 10.1038/sj.emboj.7601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samsonov AV, Mihalyov I, Cohen FS. Characterization of cholesterol-sphingomyelin domains and their dynamics in bilayer membranes. Biophys J. 2001;81:1486–500. doi: 10.1016/S0006-3495(01)75803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saraste J, Goud B. Functional symmetry of endomembranes. Mol Biol Cell. 2007;18:1430–36. doi: 10.1091/mbc.E06-10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheek S, Brown MS, Goldstein JL. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proc Natl Acad Sci USA. 1997;94:11179–83. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sciaky N, Presley J, Smith C, Zaal KJ, Cole N, et al. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–55. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–26. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 77.Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–95. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Sonnichsen B, Lowe M, Levine T, Jamsa E, Dirac-Svejstub B, Warren G. A role for giantin in docking COPI vesicles to Golgi membranes. J Cell Biol. 1998;140:1013–12. doi: 10.1083/jcb.140.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorre B, Callan-Jones A, Manneville J-B, Nassoy P, Joanny J-F, et al. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA. 2009;14:5622–26. doi: 10.1073/pnas.0811243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuven E, Porat A, Shimron F, Fass E, Kaloyanova D, et al. Intra-Golgi protein transport depends on a cholesterol balance in the lipid membrane. J Biol Chem. 2003;278:53112–22. doi: 10.1074/jbc.M300402200. [DOI] [PubMed] [Google Scholar]
- 81.Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta. 2009;1791:563–72. doi: 10.1016/j.bbalip.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, et al. Secretory traffic triggers the formation of tubular continuities across Golgi subcompartments. Nat Cell Biol. 2004;6:1071–81. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 83.Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, et al. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc Natl Acad Sci USA. 2008;105:16513–18. doi: 10.1073/pnas.0807450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urbani L, Simoni RD. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990;265:1919–23. [PubMed] [Google Scholar]
- 85.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–21. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 86.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16:373–78. doi: 10.1016/j.ceb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Volchuk A, Amherdt M, Ravazzola M, Brugger B, Rivera VM, et al. Megavesicles implicated in the rapid transport of intracisternal aggregates across the Golgi stack. Cell. 2000;102:325–48. doi: 10.1016/s0092-8674(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y, Thiele C, Huttner WB. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic. 2000;1:952–62. doi: 10.1034/j.1600-0854.2000.011205.x. [DOI] [PubMed] [Google Scholar]
- 90.Ward T, Polishchuk R, Hirschberg K, Barr F, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol. 2001;155:557–70. doi: 10.1083/jcb.200107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White J, Keller P, Stelzer EH. Spatial partitioning of secretory cargo from Golgi resident proteins in live cells. BMC Cell Biol. 2001;2:19. doi: 10.1186/1471-2121-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whyte JR, Munro S. Vesicle tethering complexes in membrane traffic. J Cell Sci. 2002;115:2627–37. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 93.Ying M, Grimmer S, Iversen TG, van DB, Sandvig K. Cholesterol loading induces a block in the exit of VSVG from the TGN. Traffic. 2003;4:772–84. doi: 10.1034/j.1600-0854.2003.00134.x. [DOI] [PubMed] [Google Scholar]