Abstract
Background
Adherence to hepatitis C virus (HCV) therapy with pegylated interferon and ribavirin has been incompletely examined.
Objectives
To evaluate the relationship between adherence to HCV therapy and early and sustained virologic response, assess changes in adherence over time, and examine risk factors for non-adherence.
Design
Retrospective cohort study.
Setting
National Veterans Affairs Hepatitis C Clinical Case Registry
Patients
5,706 HCV-infected patients (genotypes 1, 2, 3, or 4) with at least one prescription for pegylated interferon and ribavirin between 2003 and 2006 and HCV RNA results prior to and after treatment initiation.
Measurements
Adherence was calculated over 12-week intervals using pharmacy refill data. Endpoints included early virologic response (decrease of ≥2 log10 HCV RNA at 12 weeks) and sustained virologic response (undetectable HCV RNA 24 weeks after end of treatment).
Results
Early virologic response increased with higher levels of ribavirin adherence over the initial 12 weeks of therapy (genotype 1, 4: 25/68 [37%] with the lowest category [≤40% adherence] versus 1,367/2,187 [63%] with the highest category [91–100% adherence], p<0.001; genotype 2, 3: 12/18 [67%] with ≤40% adherence versus 651/713 [91%] with 91–100% adherence, p<0.001). Among genotype 1 and 4 patients, sustained response increased with higher ribavirin adherence over the second, third, and fourth 12-week intervals. Results were similar for interferon adherence. Mean adherence to interferon and ribavirin decreased 3.4% and 6.6% per 12-week interval, respectively (test for trend, p<0.001 for each drug). Patients prescribed growth factors or thyroid medications during treatment had higher mean antiviral adherence.
Limitations
Observational study without standardized timing for outcomes measurements.
Conclusions
Early and sustained virologic responses increased with higher levels of adherence to interferon and ribavirin. Adherence to both antivirals declined over time, but more so for ribavirin.
Keywords: Adherence, hepatitis C virus, HCV, antiviral therapy
INTRODUCTION
Pegylated interferon alfa-2a or -2b plus ribavirin is the current standard of care for the treatment of chronic hepatitis C virus (HCV) infection (1–5). This therapy can eradicate HCV infection, halt or regress hepatic fibrosis, and reduce the risks of cirrhosis, hepatic decompensation, and hepatocellular carcinoma (6–9). However, it requires a moderately complex regimen of weekly subcutaneous injections of interferon, twice-daily oral ribavirin, and frequent monitoring of laboratory results and adverse effects. As seen with other chronic viral illnesses, particularly human immunodeficiency virus (HIV), suboptimal adherence reduces the likelihood of treatment response (10, 11).
Despite its acknowledged importance, there are few data examining adherence to combination HCV therapy, particularly in real-world settings (12). Such information might help clinicians improve virologic response to antiviral therapy and facilitate the design of interventions to improve adherence. Prior studies have reported that decreased drug exposure from physician-directed dosage reductions of interferon and ribavirin can reduce virologic response rates (13–16), but data are limited on reduced drug exposure arising out of missed doses (17, 18). A prior study demonstrated the validity of using pharmacy refill records to measure adherence to HCV therapy (18). In that study, 85% or more adherence to pegylated interferon and ribavirin was associated with substantially greater declines in HCV viral load over the initial 12 weeks of therapy. We undertook this study to determine the relationship between adherence to combination HCV therapy and early and sustained virologic response, assess changes in adherence over the course of HCV treatment, and evaluate risk factors for non-adherence.
METHODS
Study Design and Data Source
We performed a retrospective cohort study using data from the National Veterans Affairs (VA) Hepatitis C Clinical Case Registry, an extract of electronic medical records for HCV-infected patients receiving care at VA medical facilities (19). This registry contains demographic information, laboratory results, pharmacy data, and diagnoses (recorded using International Classification of Diseases, Ninth Revision diagnostic codes) from U.S. veterans with confirmed HCV infection. The pharmacy database includes information on medication fill dates, dosages, numbers dispensed, and dosing frequency. Dispensing of the two drugs in the VA healthcare system is not linked (i.e., ribavirin is not automatically filled when requesting a refill for interferon or vice versa), and veterans must contact the pharmacy to obtain refills (i.e., refills are not automatically sent).
Study Patients
Patients were included if they: 1) were infected with HCV genotype 1, 2, 3, or 4; 2) received at least one prescription each for pegylated interferon and ribavirin between January 2003 and December 2006; 3) had a quantitative viral load prior to HCV therapy; and 4) had a viral load after treatment initiation. Patients were excluded if they received treatment within a clinical trial, switched from standard to pegylated interferon (since dosing schedules differ), or were HIV-infected (since response rates differ). If patients had multiple treatment courses (defined as a >20-week gap between interferon fills), only the first course was included.
Main Outcome Measures
The frequency and timing of HCV RNA measurements were performed at the discretion of the treating physician using the assay available at their site. Early virologic response (EVR) was defined as a ≥2 log10 decrease from pre-treatment baseline in HCV RNA IU/mL at 12 weeks, since guidelines recommend testing at this time to determine response to therapy and treatment continuation (2, 5). HCV RNA results obtained between weeks 9 and 15 were used to determine EVR. If multiple viral loads were available, the result closest to week 12 was selected.
Sustained virologic response (SVR) was defined as an undetectable viral load in all follow-up measurements 24 weeks after treatment end date (2, 5), defined as the final day of the supply of interferon from the last recorded prescription. Patients with no viral load measured 24 weeks after treatment end date were classified as not having SVR.
Data Collection
Data collected from visits prior to or at HCV treatment initiation included: age at start date; sex; race; site; height; weight; HCV genotype; quantitative HCV RNA; bipolar disorder/depression; schizophrenia; interferon and ribavirin dosages, numbers dispensed, and dosing frequency; and methadone use (dispensed within 90 days of treatment initiation). Diagnoses of bipolar disorder/depression and schizophrenia were defined by either a diagnosis code or antidepressant or anti-psychotic prescription (respectively).
Follow-up to ascertain virologic response ended on June 30, 2008. A viral load result was considered undetectable if it was below the lower limit of detection of the least sensitive quantitative assay (Versant HCV RNA 3.0 Quantitative Assay, Bayer; lower limit, 615 IU/mL) or negative on qualitative assay. Since treatment with pegylated interferon plus ribavirin can result in anemia, leukopenia, thyroid dysfunction, and depression, hemoglobin values during therapy and new prescriptions for growth factors (epoetin, darbepoetin, granulocyte macrophage / granulocyte colony-stimulation factor), thyroid hormone replacement (levothyroxine), anti-thyroid medication (methimazole, propylthiouracil), and antidepressants were collected to identify on-treatment toxicities.
Interferon and ribavirin dosages, numbers dispensed, and frequency of administration were collected at each fill date. Pegylated interferon alfa-2a dosages below 180 mcg/week and alfa-2b dosages below 1.4 mcg/kg/wk were classified as below recommended (2, 5). Ribavirin dosage was classified as below recommended if: <1,000 mg/day for genotype 1 and 4 patients weighing ≤75 kg; <1,200 mg/day for genotype 1 and 4 patients weighing >75 kg; or <800 mg/day for genotype 2 and 3 patients (2, 5). Since some clinicians might have reduced ribavirin dosages due to anemia but not updated prescriptions to reflect the change, we classified patients as “at risk” for ribavirin dosage reduction if: 1) hemoglobin declined below 10 gm/dL during therapy, or 2) they received epoetin or darbepoetin during therapy (5).
Data Analysis
Measurement of adherence
Adherence was calculated over 12-week intervals (0–12, 13–24, 25–36, and 37–48 weeks), since shorter periods may not reflect a stable measure of medication-taking behavior (20). Details appear in Appendices A, B, and C. Briefly, percent adherence was calculated as the sum of the days’ supply of antiviral dispensed divided by the number of days between the first and final fills of that interval (Appendix A) (21). Fills obtained closest to weeks 13, 25, and 37 represented initial fills in the second, third, and fourth adherence intervals. Patients were included in adherence analyses for an interval if they filled a prescription for that antiviral during the interval. For genotype 1 and 4 patients, the last recorded antiviral fills constituted the end date for the 37–48 week adherence interval. Since 24 weeks of therapy is recommended for HCV genotype 2 and 3 patients (rather than 48 weeks as for genotypes 1 and 4) (2, 5), the last antiviral fills constituted the end date for the 13–24 week interval for genotype 2 and 3 patients. For those who did not obtain a full 12 weeks’ supply for an interval, only observed fills were used to calculate adherence (Appendix B). We assessed the robustness of the adherence:outcome relation by using modified adherence definitions (see Appendices B and C).
Adherence and virologic response
The proportions with EVR and SVR were estimated for interferon and ribavirin within seven pre-defined adherence strata for each 12-week interval: 1) ≤40% adherence, 2) 41% to 50%, 3) 51% to 60%, 4) 61% to 70%, 5) 71% to 80%, 6) 81% to 90%, and 7) 91% to 100%. Analyses were stratified by HCV genotype (1 and 4; 2 and 3).
We used logistic regression to estimate the association between adherence over the initial 12-weeks and EVR, controlling for age, race, and treatment duration. For analyses examining the relationship between adherence and SVR, we included only patients who achieved EVR as well as those who had had no viral load measurement between weeks 9 and 15 but who had an undetectable result between weeks 15 and 24. For SVR analyses, we implemented repeated measures logistic regression models using generalized estimating equations to estimate the association of adherence over intervals 2, 3, and 4 and SVR, adjusting for age, race, and time on therapy (22). We assessed effects of potential confounders by examining changes in odds ratios as confounders were included in models (23). Analyses were stratified by genotype.
Since suboptimal antiviral dosages and dosage reductions can compromise virologic response, subanalyses evaluated virologic outcomes among patients prescribed recommended dosages of antivirals and who were not identified as “at risk” for ribavirin dosage reduction. P-values for testing associations of adherence and response were a priori one-sided, since it was biologically implausible that higher adherence could lead to lower response. A sample size of 2,000 persons provided 90% power to detect 10% point differences in EVR and SVR between good and poor (<80% of doses) adherers.
Interferon and ribavirin adherence over time
Percentage adherence to interferon and ribavirin within person over each interval was compared using paired t-tests. A subanalysis evaluated the mean change in ribavirin adherence among patients initiated and maintained on the recommended dosage of ribavirin and who were not “at risk” for ribavirin dosage reduction. We also compared mean rates of adherence of pegylated interferon alfa-2a to alfa-2b across patients over each interval using unpaired t-tests. Mixed effects models were used to estimate changes in adherence to each medication over time (22).
Factors associated with adherence
A longitudinal mixed effects linear regression model with patient as a random intercept evaluated associations between hypothesized risk factors and adherence to each antiviral over all intervals, adjusting for potential confounding by site (24). Hypothesized risk factors for non-adherence included: bipolar disorder/depression; schizophrenia; methadone use; and new initiation of a growth factor, antidepressant, or thyroid medication during therapy. A sample size of 2,000 patients provided 90% power to detect a 5% difference in mean adherence between patients with and without risk factors of interest, assuming a 12% standard deviation of adherence (18). Data were analyzed using SAS 9.0 (SAS Institute Inc., Cary, NC). Additional details of statistical models appear in Appendix D.
Regulatory Approvals
This study was approved by the Institutional Review Boards of the University of Pennsylvania and Philadelphia VA Medical Center.
Role of the Funding Sources
The study was funded by a VA Competitive Pilot Project Fund, the National Institute of Allergy and Infectious Diseases, and Penn Center for Education and Research on Therapeutics. The funding sources had no role in data collection, analysis, or interpretation or in the decision to submit the manuscript for publication.
RESULTS
Patient and regimen characteristics
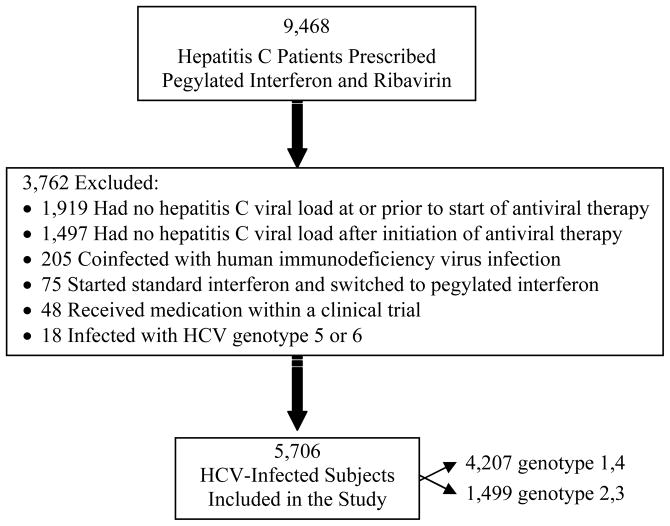
A total of 9,468 patients were prescribed pegylated interferon plus ribavirin during the observation period. Of these, 3,762 were excluded because they did not meet eligibility criteria (Figure 1). Excluded persons less commonly had a diagnosis of bipolar disorder/depression (1,136 [30%] versus 2,832 [50%]). All other characteristics (listed in Table 1) were similar between excluded and included patients. The final sample included 5,706 patients. This cohort was predominantly male and Caucasian, and bipolar disorder/depression and schizophrenia were relatively common (Table 1). The majority had HCV genotype 1 and a high (>400,000 IU/mL) baseline viral load.
Figure 1.
Selection of chronic hepatitis C virus-infected patients for inclusion in the study.
Table 1.
Characteristics of the study population at initiation of combination hepatitis C virus therapy and during follow-up.
| Characteristic | (n=5,706) |
|---|---|
| Mean age (years, standard deviation) | 51.6 (5.7) |
| Male sex (no., %) | 5,482 (96%) |
| Race (no., %) | |
| African-American | 1,080 (19%) |
| Caucasian | 3,397 (60%) |
| Other | 119 (2%) |
| Missing | 1,110 (19%) |
| Mean body weight (kg, standard deviation) | 83.6 (14.6) |
| Missing | 263 (4%) |
| Body mass index (no., %) | |
| <25 kg/m2 | 1,177 (23%) |
| 25–29 kg/m2 | 2,075 (41%) |
| >30 kg/m2 | 1,824 (36%) |
| HCV genotype (no., %) | |
| 1 or 4 | 4,207 (74%) |
| 2 or 3 | 1,499 (26%) |
| HCV viral load > 400,000 IU/mL (no., %) | 3,158 (55%) |
| Mean HCV viral load (log10 IU/mL, standard deviation) | 5.38 (1.4) |
| History of psychiatric diagnosis (no., %) | |
| Bipolar disorder/depression | 2,431 (43%) |
| Schizophrenia | 1,435 (25%) |
| Pegylated interferon formulation initiated (no., %) | |
| PEG-interferon alfa-2a | 3,824 (67%) |
| PEG-interferon alfa-2b | 1,882 (33%) |
| Initial pegylated interferon alfa-2a dosage (no., %) | |
| 180 mcg/week | 3,820 (99%) |
| <180 mcg/week | 4 (0.07%) |
| Initial pegylated interferon alfa-2b dosage (no., %) | |
| ≥1.4 mcg/kg/week | 1,376 (76%) |
| 1.2–1.3 mcg/kg/week | 370 (21%) |
| ≤1.1 mcg/kg/week | 61 (3%) |
| Pegylated interferon dosage below recommended (no., %) | |
| Starting dosage below recommended | 439 (8%) |
| Dosage reduced below recommended | 71 (1%) |
| Dosage below recommended at start or during follow-up | 458 (8%) |
| Initial ribavirin dosage (no., %) | |
| <800 mg/day | 450 (8%) |
| 800 mg/day | 1,198 (21%) |
| 1,000 mg/day | 1,334 (23%) |
| 1,200 mg/day | 2,706 (47%) |
| Ribavirin dosage below recommended (no., %) | |
| Starting dosage below recommended | 1,601 (28%) |
| Dosage reduced below recommended | 41 (1%) |
| Dosage below recommended at start or during follow-up | 1,642 (29%) |
| “At risk” for ribavirin dosage reduction (no., %)* | 1,722 (30%) |
| Active methadone use (no., %) | 420 (7%) |
| Newly initiated growth factors during treatment course (no., %) | |
| Erythrocyte growth factor | 1,381 (24%) |
| Leukocyte growth factor | 304 (5%) |
| Newly initiated thyroid supplement or anti-thyroid medication (no., %) | 465 (8%) |
| Newly initiated antidepressant during treatment course (no., %) | 1,174 (21%) |
Patients were classified as “at risk” for ribavirin dosage reduction if: 1) their hemoglobin declined below 10 gm/dL during treatment, or 2) they received epoetin or darbepoetin during therapy. HCV=hepatitis C virus
Receipt of pegylated interferon alfa-2a, alfa-2b, and ribavirin; initial dosages of these medications; and dosages below recommended at initiation and during therapy are presented in Table 1. A total of 5,706 patients obtained prescription fills during the first 12-week adherence interval, 2,834 during the second interval, 1,605 during the third interval, and 1,411 during the fourth interval. Ninety-five percent of pegylated interferon prescriptions and 93% of ribavirin prescriptions were for 30 days’ supply. New initiation of growth factors, thyroid hormone replacement, anti-thyroid medication, and antidepressants are also shown in Table 1.
Adherence and EVR
Seventy percent (3,992) of patients had a viral load measured between weeks 9 and 15. These patients were slightly older (52 versus 51 years; p=0.01), more commonly African-American (799 [20%] versus 281 [16.4%]; p=0.001), and more frequently infected with HCV genotype 1 (3,014 [76%] versus 1,193 [70%]; p<0.001) than those with no viral load measured between weeks 9 and 15.
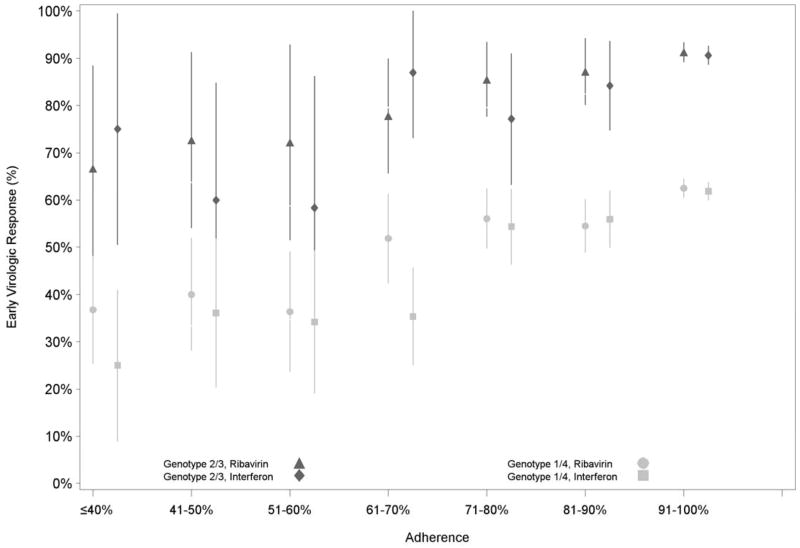
Among patients with a week 9–15 viral load, 1,787/3,014 (59%) of genotype 1 and 4 and 867/978 (89%) of genotype 2 and 3 patients achieved EVR. The proportion of patients with EVR was correspondingly higher with higher levels of adherence to interferon and ribavirin (Figure 2; Appendix E [Table E.1.]). This association remained after adjustment for age, race, and VA site (p<0.001 for both strata of genotypes). Similar results were obtained with sensitivity analyses using alternative adherence definitions. Subanalyses among patients prescribed recommended antiviral dosages and not identified as “at risk” for ribavirin dosage reduction demonstrated a stronger association between adherence and EVR compared to results from primary analyses (data not shown).
Figure 2.
Proportion of patients with early virologic response at each level of adherence to pegylated interferon and ribavirin over the initial 12 weeks of therapy, by hepatitis C genotype.
Adherence and SVR
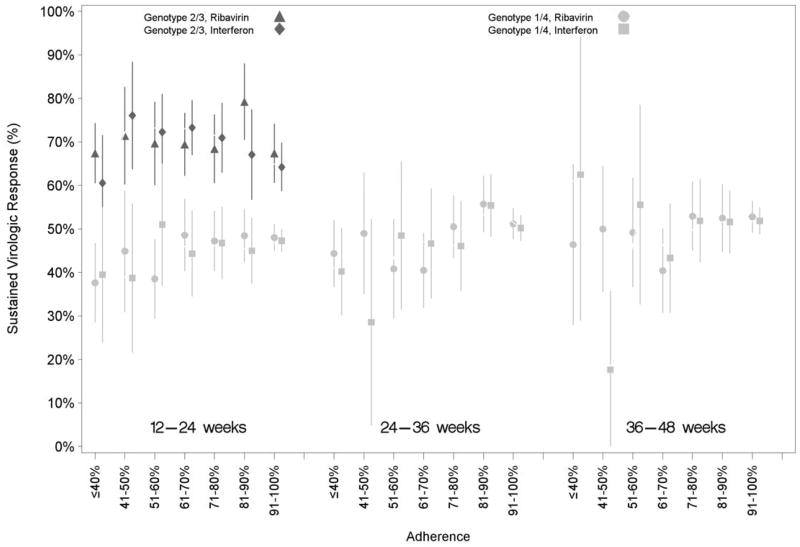
In addition to the 2,654 patients identified with EVR, 180 (genotype 1 or 4: 113; genotype 2 or 3: 67) had no available week 9–15 viral load but did have an undetectable viral load between weeks 15 and 24 and were included in SVR analyses. Among these 2,834 patients, 901/1,929 (47%) genotype 1 or 4 and 620/905 (69%) genotype 2 or 3 patients achieved SVR. The proportion of patients with SVR increased with higher levels of interferon and ribavirin adherence over the second, third, and fourth adherence intervals for genotype 1 and 4 patients (Figure 3; Appendix E [Table E.2.]).). However, among genotype 2 and 3 patients, SVR did not increase with higher antiviral adherence over the 12–24 week interval. Results were unchanged after adjustment for age, race, time on therapy, and VA site. Sensitivity analyses using alternative adherence definitions did not change results. Subanalyses among patients prescribed recommended antiviral dosages and who were not “at risk” for ribavirin dosage reduction showed stronger associations between adherence and SVR compared to primary analyses (data not shown).
Figure 3.
Proportion of patients with sustained virologic response at each level of adherence to pegylated interferon and ribavirin over latter 12-week adherence intervals for hepatitis C genotypes 1 / 4 and genotypes 2 / 3.
Interferon and ribavirin adherence over time
Mean adherence to both antiviral medications was high during the initial 12 weeks but declined over the course of treatment, and interferon adherence remained higher than for ribavirin over each interval (0 – 12 weeks: 100 % interferon adherence versus 97.1% ribavirin adherence, p<0.001; 13 – 24 weeks: 95.4% interferon adherence versus 86.1% ribavirin adherence, p<0.001; 25 – 36 weeks: 95.4% interferon adherence versus 84.4% ribavirin adherence, p<0.001; 37 – 48 weeks: 89.3% adherence versus 76.1% ribavirin adherence, p<0.001; Appendix E [Table E.3.]). There was a mean decline in ribavirin adherence of 6.6% points and in interferon adherence of 3.4% points per 12-week interval (test for trend, p<0.001 for each drug). Subanalyses among patients who were not “at risk” for ribavirin dosage reduction demonstrated a similar mean decline in ribavirin adherence (7.3% points per 12-week interval; test for trend, p<0.001). No clinically or statistically significant differences were observed in mean adherence to pegylated interferon alfa-2a versus alfa-2b during each interval (0–12 weeks: 100.7% adherence versus 102%, p=0.057; 13 – 24 weeks: 95.2% adherence versus 95.4%, p=0.89; 25 – 36 weeks: 95.9% adherence versus 94.1%, p=0.072; 37 – 48 weeks: 89.2% adherence versus 89.4%, p=0.94).
Factors associated with adherence
Few risk factors were associated with adherence, after we adjusted for possible differences in rates of risk factors across sites. Patients newly prescribed growth factors or thyroid medication had slightly higher mean adherence to both antivirals than those not prescribed them (Appendix E [Table E.4.]). Although some differences were statistically significant, all were small.
DISCUSSION
Our study demonstrates that in a real-world setting, higher levels of adherence to pegylated interferon and ribavirin are associated with higher rates of early and sustained virologic response. Interferon adherence was higher than that of ribavirin for all adherence intervals. Moreover, adherence to both antiviral medications declined during HCV therapy, but more so for ribavirin. Current methadone use was associated with reduced adherence.
These results support the concept that adherence should be a focus of clinical care teams prior to and throughout HCV treatment to help achieve virologic response. Identifying suboptimal adherence using pharmacy refill records might allow clinicians to counsel patients to improve their adherence during therapy. Although interventions to increase adherence to HCV therapy have not been tested, providers could ask patients about barriers to adherence and help them identify potential solutions. Linking pegylated interferon and ribavirin prescriptions might facilitate increased antiviral adherence, but this strategy has not been tested. Further, a policy of linked prescriptions would result in added costs for unnecessary additional pegylated interferon refills if clinicians rewrite the ribavirin prescription when changing dosage due to treatment-induced anemia.
Our results extend findings from retrospective analyses of clinical trials data (13–16). In two studies, receipt of less than 80% of total interferon or ribavirin doses for at least 80% of the duration of therapy resulted in reduced SVR rates among genotype 1, but not genotype 2 or 3, patients (13, 14). Two other analyses showed that receipt of less than 60% cumulative ribavirin dose (15) and premature ribavirin discontinuation (16) adversely affect SVR. However, these studies focused on decreased drug exposure due to interferon and ribavirin dosage reductions and discontinuations rather than due to missed antiviral doses.
Within individuals, adherence to ribavirin was lower than to interferon over each 12-week interval. These adherence differences might be due to ribavirin’s twice daily dosing frequency compared to the once-weekly administration of pegylated interferon. Additionally, prior to the start of therapy, patients may select a day of the week on which they administer their interferon injection, and this weekly routine might facilitate higher levels of adherence for interferon than ribavirin.
Adherence to both medications declined during treatment, but more so for ribavirin. The higher frequency of ribavirin administration may make it more burdensome to remember and more vulnerable to drop-offs in adherence over time. Declines in medication adherence over time have been reported for other chronic diseases, such as antiretroviral therapy for HIV infection (25), antihypertensive therapy (26), and lipid-lowering therapy (26, 27), so this observation is not surprising. It is therefore imperative that clinicians emphasize antiviral adherence throughout the course of HCV therapy and not only at treatment initiation.
Our study also examined risk factors for low adherence to anti-HCV medications. Patients prescribed growth factors in response to leukopenia or anemia as well as either thyroid hormone replacement or anti-thyroid medications due to on-treatment thyroid dysfunction had slightly higher mean adherence to interferon and ribavirin. Use of these therapies during HCV therapy might require more frequent follow-up visits and laboratory monitoring that could promote antiviral adherence. Although patients with a history of bipolar disorder/depression and schizophrenia might be expected to be at risk for non-adherence, our results show that antiviral adherence is not lower among these subgroups. Clinicians should therefore not be reluctant to initiate treatment in these patients because of a perceived risk of non-adherence, particularly since evidence suggests that clinicians’ predictions are poor measures of their patients’ likely adherence (28). Additional risk factors for non-adherence to HCV therapy should be examined to determine the subgroups that might be at increased risk for poor adherence and in whom interventions to improve adherence should be targeted.
Our results emphasize the need to evaluate adherence to the new direct acting antiviral therapies for HCV (e.g., telaprevir and bocepravir). These drugs may require more frequent dosing (e.g., three times daily) and are expected to be used with pegylated interferon and ribavirin (29–31), further increasing the complexity of HCV therapy. Additionally, reduced adherence to these antivirals could select for drug-resistant mutations that could result in virological breakthrough (32, 33). As new antiviral regimens for chronic HCV are introduced, antiviral adherence will need to be emphasized to achieve viral suppression and prevent antiviral resistance.
This study has several limitations. First, it provides no evidence that pharmacy refill data reflect the actual number of pills taken correctly by a patient. Pharmacy refills might underestimate adherence if patients acquire their medications from non-VA sources. However, the majority of VA patients receive their medications from VA pharmacies (34, 35). Refills might overstate adherence if patients fill prescriptions but do not take the medication. Yet, numerous studies have shown the validity of VA refill data as a surrogate of adherence (20, 21, 36, 37). An additional limitation of refill data is that patients who had their ribavirin dosage reduced but who had no prescription rewritten to decrease the quantity dispensed might have been misclassified as having reduced ribavirin adherence. However, subanalyses among patients prescribed recommended dosages of antivirals and not “at risk” for ribavirin dosage reduction showed results that were similar to the overall cohort.
Second, by including only persons who had a follow-up viral load, we might have selected individuals who were more likely to be adherent to antiviral therapy. However, the characteristics of patients excluded from the study were similar to those included, so we do not believe that these exclusions alter our conclusions.
Third, the retrospective study design did not permit HCV RNA testing to be performed at the same time during treatment for all patients. However, we used standard definitions for early and sustained virologic response and required week 12 viral loads to be obtained in a narrow window to reduce early virologic response misclassification.
Fourth, the small sample of genotype 2 and 3 patients receiving antiviral therapy from 12–24 weeks of treatment may have limited our ability to find an association between antiviral adherence during this interval and sustained response.
Finally, our study sample was predominantly male and excluded patients with HIV co-infection, potentially limiting the generalizability of our results.
In summary, this analysis demonstrated that higher levels of adherence to pegylated interferon and ribavirin were associated with higher rates of early and sustained virologic response. Adherence to both antiviral medications declined over time, but more so for ribavirin. Future studies should evaluate interventions to maximize adherence to HCV therapy.
Supplementary Material
Acknowledgments
Primary Funding Sources: National Institutes of Health, Agency for Healthcare Research and Quality, and Department of Veterans Affairs.
Financial support: National Institutes of Health research grant K01 AI070001 [V.L.R.], an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (grant #HS10399) [V.L.R., A.R.L., and R.G.], and a Department of Veterans Affairs Competitive Pilot Project Fund grant [V.K.A., V.L.R.].
This material is based upon work supported in part by the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Grant Support: National Institutes of Health research grant K01 AI070001 [V.L.R.], Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (grant #HS10399) [V.L.R., A.R.L., and R.G.], and Department of Veterans Affairs Competitive Pilot Project Fund grant [V.K.A., V.L.R.].
Abbreviations
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus infection
- VA
Veterans Affairs
- EVR
early virologic response
- SVR
sustained virologic response
Footnotes
Potential conflicts of interest: None to report.
Portions of this study were presented at the 26th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Brighton, United Kingdom, 19–22 August 2010 and the International Conference on Viral Hepatitis 2011, Baltimore, Maryland, 11–12 April 2011.
Protocol: Available by request from Dr. Lo Re (vincentl@mail.med.upenn.edu)
Statistical Code: Available by request from Dr. Lo Re (vincentl@mail.med.upenn.edu)
Data: The data in the National VA Hepatitis C Clinical Case Registry available to VA researchers only and can be requested from the Department of Veterans Affairs (http://vaww.publichealth.va.gov/docs/surveillance/backus_clinical_case_registries.pdf)
Disclaimer: This material is based upon work supported in part by the Department of Veterans Affairs. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 5.Yee HS, Currie SL, Darling JM, Wright TL. Management and treatment of hepatitis C viral infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center program and the National Hepatitis C Program office. Am J Gastroenterol. 2006;101(10):2360–78. doi: 10.1111/j.1572-0241.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 6.Coverdale SA, Khan MH, Byth K, et al. Effects of interferon treatment response on liver complications of chronic hepatitis C: 9-year follow-up study. Am J Gastroenterol. 2004;99(4):636–44. doi: 10.1111/j.1572-0241.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- 7.Shiratori Y, Ito Y, Yokosuka O, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142(2):105–14. doi: 10.7326/0003-4819-142-2-200501180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 9.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147(10):677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–17. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 11.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 12.Weiss JJ, Brau N, Stivala A, Swan T, Fishbein D. Adherence to medication for chronic hepatitis C - building on the model of human immunodeficiency virus antiretroviral adherence research. Aliment Pharmacol Ther. 2009;30(1):14–27. doi: 10.1111/j.1365-2036.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123(4):1061–9. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 14.Raptopoulou M, Tsantoulas D, Vafiadi I, et al. The effect of adherence to therapy on sustained response in daily or three times a week interferon alpha-2b plus ribavirin treatment of naive and nonresponder chronic hepatitis C patients. J Viral Hepat. 2005;12(1):91–5. doi: 10.1111/j.1365-2893.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Reddy KR, Shiffman ML, Morgan TR, et al. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon alfa-2a/ribavirin treatment. Clin Gastroenterol Hepatol. 2007;5(1):124–9. doi: 10.1016/j.cgh.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132(1):103–12. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Weiss JJ, Bhatti L, Dieterich DT, et al. Hepatitis C patients’ self-reported adherence to treatment with pegylated interferon and ribavirin. Alimentary Pharmacology & Therapeutics. 2008;28:289–93. doi: 10.1111/j.1365-2036.2008.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Re V, 3rd, Amorosa VK, Localio AR, et al. Adherence to hepatitis C virus therapy and early virologic outcomes. Clin Infect Dis. 2009;48(2):186–93. doi: 10.1086/595685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775–83. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57(10):1107–10. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 22.Fitzmaurice GM, MLN, Ware JH. Applied Longitudinal Analysis. New York: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 23.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia: Lippincott Williams and Wilkins; 1998. [Google Scholar]
- 24.Berlin JA, Kimmel SE, Ten Have TR, Sammel MD. An empirical comparison of several clustered data approaches under confounding due to cluster effects in the analysis of complications of coronary angioplasty. Biometrics. 1999;55(2):470–6. doi: 10.1111/j.0006-341x.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 25.Lima VD, Harrigan R, Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–36. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman RH, Benner JS, Petrilla AA, et al. Predictors of adherence with antihypertensive and lipid-lowering therapy. Arch Intern Med. 2005;165(10):1147–52. doi: 10.1001/archinte.165.10.1147. [DOI] [PubMed] [Google Scholar]
- 27.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 28.Gross R, Bilker WB, Friedman HM, Coyne JC, Strom BL. Provider inaccuracy in assessing adherence and outcomes with newly initiated antiretroviral therapy. AIDS. 2002;16(13):1835–7. doi: 10.1097/00002030-200209060-00021. [DOI] [PubMed] [Google Scholar]
- 29.Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–50. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 30.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 376(9742):705–16. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 31.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 32.Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology. 2010;138(2):447–62. doi: 10.1053/j.gastro.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 33.Thompson AJ, McHutchison JG. Antiviral resistance and specifically targeted therapy for HCV (STAT-C) J Viral Hepat. 2009;16(6):377–87. doi: 10.1111/j.1365-2893.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 34.Elixhauser A, Eisen SA, Romeis JC, Homan SM. The effects of monitoring and feedback on compliance. Med Care. 1990;28(10):882–93. doi: 10.1097/00005650-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814–23. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21(12):1579–89. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When more is not better: treatment intensification among hypertensive patients with poor medication adherence. Circulation. 2008;117(22):2884–92. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.