Marketing approval of cancer drugs is granted centrally in Europe, but pricing and reimbursement levels are decided by individual countries. Most drug development research in hemato-oncology is carried out in industry-led trials intended to meet regulatory requirements. Most comparative data are produced in cooperative group trials after a drug has entered the market. These investigator-led studies are currently hindered by the lack of access to new drugs before licensing, limited availability of independent funding, and a high administrative burden. Regulations do not take into account the lower risk to participants associated with investigator-initiated observational or interventional studies. Thus, early optimistic results from industry-led randomized controlled trials before a clear characterization of adverse effects and long-term efficacy can lead to inappropriately high pricing in some European countries and a refusal to put the drug on the market in others. Patients are at risk of being treated inappropriately, at an excessive price, or not at all. Industry, the independent research community and health care authorities all need to rethink their approach to research and find ways to work alongside a wider clinical and public health perspective.
Traditionally, developing new cancer treatments has been a collaborative process between industry and academic researchers. An initial industry-led and industry-financed research process led to licensing, followed by an investigator- or cooperative group-led treatment optimization phase. This process substantially improved therapeutic options for many cancer patients and is probably best illustrated in the field of hematologic malignancies. In recent years, this process has been put at risk. There has been a substantial reduction in the number of investigator-initiated and independent group studies, partly due to the high cost of trials and difficulties in obtaining funding, but also due to regulatory complexities and the fact that new drugs cannot be integrated into clinical trials before they are licensed.
The Independent Clinical Research in Oncology Bergamo (Italy) meeting was held on 14–15 October 2011 to discuss issues surrounding hemato-oncology drug development and approval. We discuss here some of the key issues identified and suggest ways to address the future of investigator-driven clinical trials in Europe.
Marketing legislation in Europe
European Union licensing of drugs for acquired immune deficiency syndrome, cancer, neurodegenerative conditions, diabetes and orphan diseases is complex. Initial assessment of scientific data for new treatments is made by the Committee for Proprietary Medicinal Products for the European Medicines Agency (EMA).1 If their conclusions are positive, the European Commission decides whether to grant an EU-wide marketing authorization. Approved products enter a national licensing, pricing and reimbursement process. Companies must apply to each individual country’s Health Technology Assessment (HTA) agencies to market their product.2 HTA agencies base their decisions on the individual country’s public health needs and budgetary limitations, but they all have different ways of doing this. As a consequence, many similar drugs with a minimal difference in benefit between them can be available in different countries but with sometimes the same drug sold at different prices. This process does not stimulate competition nor does it offer any benefit to HTA agencies or patients. It only creates unnecessary delays in the time needed to proceed from marketing submission to drug availability.
The ‘normal’ centralized process should be completed within 210 days, but ‘clock-stop’ time to obtain further scientific data is unlimited. The ‘accelerated’ process should be completed within 150 days with a maximum of 30 clock-stop days, but this is rarely applied.3 The national-level approval and pricing decisions are also frequently subject to delay. Decisions concerning access to the market are supposed to be completed within 180 days,4 but this deadline has seldom been met: the average additional delay varies from country to country from a few months to well over one year.5 These varying national approval time lines are a huge obstacle to multinational clinical trials. It is hoped that establishment of the EUnetHTA (http://www.eunethta.eu/) will soon improve efficiency and coordination of the process.
In Europe, clinical research must adhere to the Clinical Trials Directive (2001/20/EC)6 introduced in May 2004 and the Good Clinical Practice Guidelines Directive introduced in 2005.7 Both directives aim to harmonize administrative processes across EU member states, to protect patients, and to optimize the reliability of results. Both take into account the perspective of large industry-led randomized clinical trials (RCTs) but neglect the vast experience in observational and interventional trials of cooperative groups. In reality, therefore, the administrative burden for all studies has increased enormously. As a consequence, the cost of conducting cancer trials, including insurance, increased prohibitively,8 as did the time from concept to trial initiation. There are as yet no data showing that the directives have increased patient safety.
Many researchers9 consider the administrative burden to be unnecessary and the progress of independent research in Europe seems to have been hindered. The European Organization for Research and Treatment of Cancer reported a fall in the number of trials and in numbers of patients enrolled after its implementation.10 The effect was particularly detrimental on small, independently funded, investigator-led trials, especially those investigating already licensed drugs. The quality criteria of the Good Clinical Practice Directive are not questioned, but they should not act as a barrier to non-profit clinical trials. The insurance policy of any European hospital should be sufficient to cover studies of drugs or treatment approaches with market authorization. Clearly, the issues of insurance and administrative requirements need to be revisited.
Funding
Global spending on cancer research is estimated to be around 14 billion euro of which at least 6 billion euro comes from direct private funding. This accounts for approximately a quarter of total global research expenditure, provides around 70% of funding for all clinical cancer drug trials, and goes mainly to the USA and Europe.11–13 Industry-led studies are primarily financed by industry. Independent investigator-led studies might be funded at least partly by industry, but are more frequently funded by other sources, such as government agencies and philanthropic, non-governmental and charitable organizations. Nearly 45% of non-industry funding comes from indirect sources, such as academic institutions and hospitals.14 Hence a substantial amount of money for cancer research is available. Discrepancies exist both in concrete terms and in the perception of research groups. Notable disparities exist between member states in the amounts of funding raised from governmental and nongovernmental sources and in the amount spent per capita or as a percentage of GDP. Most money is raised in the UK, Germany, France and Italy; the highest spending for cancer research is seen in the UK, Sweden and the Netherlands.14 These gaps in funding might prevent independent groups carrying out research in countries where it is urgently needed. For example, the focus in some of the lower-income EU member states is placed on public-health awareness and prevention programs.
The gap might further increase with the new emerging trend of industry investment in early-phase studies and in translational studies using new technologies, such as genomics and proteomics.15 For too long now, there has been a lack of pre-clinical and molecular studies for cancer drugs, leading to high failure rates15 and resulting in a near unchanged number of new drugs coming onto the market in the past 30 years.16 Mechanistic studies, known as ‘personalized’ medicine, are attractive to industry if there is a link between a ‘marker’ and a ‘targeted’ drug, for example, as shown by the antibody treatment for breast cancer with Herceptin® (Genentech). In the absence of such markers, research and post-marketing investigations of drugs in specific patient subgroups or for orphan diseases will be left to independent researchers.
Investigator-driven studies can lead to substantial progress being made independently from industry; hematopoietic stem cell transplantation (HSCT) is a prime example. HSCT is now an accepted therapy for many congenital or acquired severe disorders of the hematopoietic system and holds promise as a treatment for multiple cancers. Because many of the cancers for which it may be used are classified as rare cancers (www.rarecare.eu), HSCT would probably not have been developed by biomedical companies. Its success is primarily based on observational studies. Interventional studies are generally small, cover a vast geographical area, and rarely lead on to phase III studies. Roughly only one in 100 investigational trials contributes substantial data to the pool of evidence supporting the use of this technique.
Universities and funding agencies are in part responsible for the decline in investigator-led interventional or observational studies. Basic research in biology and genetics has been given priority. Funds are rarely made available for these confirmatory studies, irrespective of the substantial need. Participation in national or international cooperative trials is no longer given sufficient academic credit and young investigators have been discouraged from joining such groups. Additionally, HTA has largely been left to bureaucratic institutions with little or no academic input or supervision.
Study design directly impacts on study outcome and the desired results. Industry-led RCTs address safety and efficacy and have to meet regulatory requirements. Study populations in industry-led trials are carefully selected. Extensive inclusion and exclusion criteria are applied to test a very specific hypothesis; they can be used to maximize the chances of protocol compliance. Frequently, the age of trial populations differs substantially from that in the general patient population and it might become impossible to form generalized conclusions.
The primary end point of a phase III study should establish a valid and reliable assessment of the clinical benefit to risk ratio.17 The definition of benefit is open to interpretation. For cancer trials, improvement in overall survival compared with no treatment or a known therapy assessed in a large cohort over a long duration represents the gold standard.18 Crossover designs can obscure survival benefits. Industry-led RCTs, therefore, tend to select disease activity end points, such as response rate, progression-free survival, and time to progression or surrogate end points, such as biomarkers. These outcomes can be assessed in smaller and shorter trials but the results are at risk of being confounded by investigator bias. They do little to quantify risk and benefit for patients in the real world18,19 and are not always validated before use. Their relationship with overall survival is also frequently unclear. Interim analyses can falsify study results. Long-term efficacy and adverse effect profiles might remain poorly characterized, early benefits might be over-valued compared with late detrimental effects and, even worse, late benefits may be missed if there are early disadvantages.20–21
An intention-to-treat analysis is generally viewed as the most appropriate way to assess the primary end point in large RCTs as it shows the results for all patients, irrespective of whether they did or did not complete treatment. The quality and consistency of intention-to-treat analysis has been questioned as its definition has been modified. Modifications are not standard across trials and are seldom clearly explained. Missing data and deviations from protocol can affect outcomes and make interpretation difficult.22
As part of the marketing approval process, new drugs must be compared with available treatments to assess relative risks and benefits. Comparative studies should help regulatory authorities to safeguard patients from inferior and unsafe treatments, to ensure that HTAs and consumers make sound decisions about cost and payment, and to aid clinicians’ and patients’ understanding of how the therapies work and fit into the treatment pathway. Unfortunately, the comparators and their selection processes vary between countries and there are no standards for reporting. Finland and Sweden require products to be compared with three well-defined comparators, the UK asks for data on all relevant comparators and then the HTA decides, while in Switzerland comparison data for all treatments in the same therapeutic group is requested. Where similar drugs exist, they are tested with varying doses and units by different groups, in different patient populations and with a variety of different end points. These differences make it difficult, if not impossible, to compare findings, and minimum standards for research and reporting are needed to help physicians interpret the data for clinical practice.22 More realistically designed trials, including observational trials as part of the pre-approval process, would undoubtedly be useful.
New approaches to cancer research
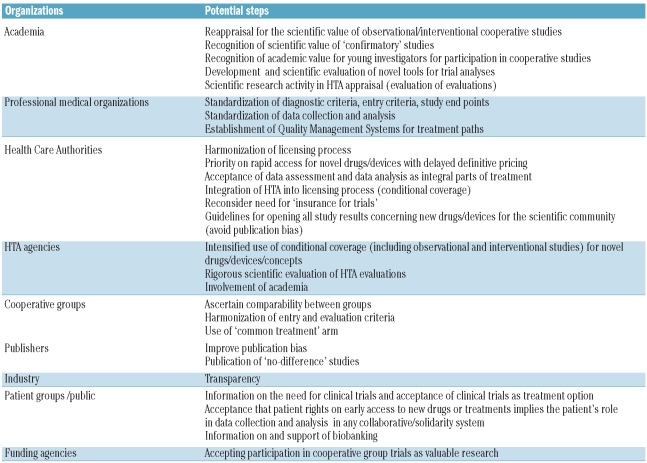
The key aim of the Independent Clinical Research in Oncology meeting in Bergamo (Italy) was to propose ways to close the widening gap between industry-led and investigator-led studies in cancer research (Table 1).
Table 1.
Ways to improve clinical research activity in Europe.
A two-tier model in which industry-led ‘commercial’ studies are aimed purely at regulatory requirements and investigator-led ‘non-commercial’ studies to define the value of the treatment in the general population could ease the marketing authorization and subsequent HTA process. The value of investigator-led trials may then be seen to be of a similar value to those carried out by industry. Regulatory requirements would then need to be adapted to reflect the risk associated with the study and not with its commercial or non-commercial objective.
Efforts are needed to improve collaboration between regulatory agencies, cooperative groups, academic institutions, industry, and reimbursement agencies to enhance research. The advances in genomics and proteomic technologies will increase research and treatment costs, but it will also avoid the inefficient use of expensive drugs. If entry points and end points are standardized, data can be pooled for analysis across study groups; a prerequisite for rare cancers or for patients with underlying or concomitant disorders. HSCT or cirrhosis and addiction in Hepato Cellular Carcinoma are good examples. The funds for such observational studies should be provided by the reimbursement agencies under a conditional coverage scheme.
New approaches to study design should address thresholds set for clinically worthwhile effects, integrating toxic effects and overall costs including surgery, radiotherapy, drug therapies and supportive care. Design should be based on pre-trial evidence from pre-clinical and phase II studies in the same cancer and/or cancers with similar features. Combining phase I and phase II studies, preferentially randomized, might help to increase industry interest in pre-clinical research, lower the drug failure rate, and save time and money.
Registries and databases should be more widely used. They offer large pools of prospectively gathered data. They can show meaningful differences between groups of patients and are ideally suited to assess long-term risk, especially in chronic diseases in which risk changes over time. This approach can be supplemented by conditional coverage requirements for HTAs, ideally within the context of a quality management system. Data from HSCT studies show that such a strategy can directly improve outcomes.23 Comprehensive databases, systematic reviews or meta-analyses require little funding but can help determine the direction future studies should take. As a prerequisite, efforts should be made to work towards uniformity in surveillance protocols and staging, diagnostic and other relevant research criteria. The professional societies, such as the European Hematology Association, European Leukemia Net, the European Society for Medical Oncology or the International Society on Thrombosis and Haemostasis, are challenged to do so. Universities and granting agencies are challenged to recognize the academic value of international collaboration in observational studies and HTA.
Transparency must be improved. Not all the study reports submitted to the EMA as part of the approval process are published. The European Public Assessment Report, written by the EMA in agreement with the industry summarizes the documentation provided by the manufacturers. Access to these unpublished positive and negative reports should be made available to investigators.22
Last but not least, patients need a voice in the research process, as they frequently complain of a mismatch between what clinical researchers do and they themselves need. Patient advocacy groups provide a lot of money to support research; they expect better care to be promoted. A research governance strategy that brings together patients, physicians, academic experts and other stakeholders could help.24
Conclusions
Industry-led trials designed with marketing approval in mind dominate the drug development process. Regulatory agencies are allowing drugs to be sold according to a biased perspective of performance. A culture of high costs for limited benefits leaves local health systems with already stretched budgets to bear the brunt of this financial burden.
Independent researchers often join industry studies to access new drugs and to obtain funding for large clinical trials but they then have little or no influence over the design of the study and the interpretation of the data. Their expert knowledge of patient populations and clinical experience is frequently lost.
Industry, regulatory agencies and the independent research community need to rethink the approach to research and find ways to cooperate within a wider clinical and public health perspective. Research before approval of new drugs should assess how they fit into the treatment pathway or what degree of benefit they yield as well as safety and efficacy. Companies should be encouraged to investigate new regimens for existing drugs and to develop new drugs for orphan diseases.
The availability of funding for investigator-led independent studies must improve. Integration of observational studies into an HTA process, with a conditional coverage approach and funding by the reimbursement agencies, could be a way to foster the development of innovative drugs or technologies.
Academics needs to rethink their unfavorable attitude toward observational studies and collaboration, and professional societies should evaluate their roles and obligations in the standardization of end points. Without change, the outlook is bleak: local health systems will be crippled by rising costs, funding will be lost, desperately needed new drugs will not get onto the market, and patients will not receive the treatment they need.
Acknowledgments
This Perspective represents the views of the authors, but also reflects the discussion at a 2-day symposium entitled “Independent Clinical Research in Oncology”, promoted by the Menarini International Foundation (Florence, Italy), and organized by the Research Foundation (FROM) of Ospedali Riuniti, Bergamo, in Bergamo, Italy, on 14–15 October 2011. It was attended by people from a variety of different backgrounds, such as public health and government institutions, clinicians, nurses, and clinical epidemiologists. We are indebted to them all for their contribution to the formulation of the new approaches to cancer research studies. The list of contributors can be found on www.fondazione-menarini.it.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Commission of the European Communities. Regulation (EC) n. 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. 2009. [accessed Dec 9, 2011]. http://ec.europa.eu/health/files/eudralex/vol-1/reg_2004_726_cons/reg_2004_726_cons_en.pdf.
- 2.Council of the European Communities. Council Directive 89/105/EEC of 21 December 1988 relating to the transparency of measures regulating the pricing of medicinal products for human use and their inclusion in the scope of national health insurance systems. Official Journal of the European Union. 1989;L040:8–11. [Google Scholar]
- 3.Ridley DB. Introduction of European priority review vouchers to encourage development of new medicines for neglected diseases. Lancet. 2010;376(9744):922–7. doi: 10.1016/S0140-6736(10)60669-1. [DOI] [PubMed] [Google Scholar]
- 4.Zentner A, Velasco-Garrido M, Busse R. Methods for the comparative evaluation of pharmaceuticals. GMS Health Technol Assess. 2005;1 Doc 09. [PMC free article] [PubMed] [Google Scholar]
- 5.European Federation of Pharmaceutical Industries and Associations. Patients W.A.I.T. indicator: 2010 report – based on EFPIA’s database (first EU marketing authorisation in the period 2007-’09) [accessed Dec 9, 2011]. http://www.efpia.eu/content/default.asp?PageID=559&DocID=10200.
- 6.European Parliament and the Council of the European Union Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Union. 2001;L121:34p–44. [PubMed] [Google Scholar]
- 7.Commission of the European Communities. Commission Directive 2005/28/EC of 8 April 2005 laying down principles and detailed guidelines for good clinical practice as regards investigational medicinal products for human use, as well as the requirements for authorisation of the manufacturing or importation of such products. Official Journal of the European Union. 2005;L91:13–9. [Google Scholar]
- 8.van Vyve D. Facing the Challenges of the European Clinical Trials Directive: the European Organisation for Research and Treatment of Cancer perspective. Eur Oncol. 2008;4:1. [Google Scholar]
- 9.Frewer LJ, Coles D, van der Lans IA, Schroeder D, Champion K, Apperley JF. Impact of the European Clinical Trials Directive on prospective academic clinical trials associated with BMT. Bone Marrow Transplant. 2011;46(3):443–7. doi: 10.1038/bmt.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemminki A, Kellokumpu-Lehtinen P-L. harmful impact of EU clinical trials directive. BMJ. 2006;332(7540):501–2. doi: 10.1136/bmj.332.7540.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckhouse S, Lewison G, Sullivan R. Trends in the global funding and activity of cancer research. Mol Oncol. 2008;2(1):20–32. doi: 10.1016/j.molonc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth CM, Cescon DW, Wang L, Tannock IF, Kryzankowska MK. Evolution of the randomized control trial in oncology over three decades. J Clin Oncol. 2008;26(33):558–64. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 14.Kanavos P, Sullivan R, Lewison G, Schurer W, Eckhouse S, Vlachopioti Z European Cancer Research Managers Forum. The role of funding and policies on innovation in cancer drug development. London: London School of Economics and Political Science; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Cancer: fact sheet n. 297. [last accessed Dec 6, 2011]. http://www.who.int/mediacentre/factsheets/fs297/en/
- 16.McCabe C, Bergmann L, Bosanquet N, Ellis M, Enzmann H, von Euler M, Jönsson B, Kallen KJ, Newling D, Nüssler V, Paschen B, de Wilde R, Wilking N, Teale C, Zwierzina H Biotherapy Development Association. Market and patient access to new oncology products in Europe: a current, multidisciplinary perspective. Ann Oncol. 2009;20(3):403–12. doi: 10.1093/annonc/mdn603. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Guideline on the evaluation of anticancer medicinal products in Man. 2005. [accessed Dec 9, 2011]. http://www.emea.europa.eu/pdfs/human/ewp/020595en.pdf.
- 18.Schilsky RL. End points in cancer clinical trials and the drug approval process. Clin Cancer Res. 2002;8(4):935–8. [PubMed] [Google Scholar]
- 19.Park JW, Kerbel RS, Kelloff GJ, Barrett JC, Chabner BA, Parkinson DR, et al. Rationale of the biomarkers and surrogate end points in mechanism-driven oncology drug development. Clin Cancer Res. 2004;10(11):3885–96. doi: 10.1158/1078-0432.CCR-03-0785. [DOI] [PubMed] [Google Scholar]
- 20.Montedori A, Bonacini MI, Casazza G, Luchetta ML, Duca P, Cozzolino F, Abraha I. Modified versus standard intention-to-treat reporting: are there differences in methodological quality, sponsorship, and findings in randomized trials? A cross-sectional study. Trials. 2011;12:58. doi: 10.1186/1745-6215-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorenson C, Naci H, Cylus J, Mossialos E. Evidence of comparative efficacy should have a formal role in European drug approvals. BMJ. 2011;343:d4849. doi: 10.1136/bmj.d4849. [DOI] [PubMed] [Google Scholar]
- 22.Barbui C, Baschirotto C, Cipriani A. EMA must improve the quality of its clinical trial reports. BMJ. 2011;342:d2291. doi: 10.1136/bmj.d2291. [DOI] [PubMed] [Google Scholar]
- 23.Gratwohl A, Brand R, Niederwieser D, Baldomero H, Chabannon C, Cornelissen J, de Witte T, Ljungman P, McDonald F, McGrath E, Passweg J, Peters C, Rocha V, Slaper-Cortenbach I, Sureda A, Tichelli A, Apperley J. Introduction of a quality management system and out-come after hematopoietic stem-cell transplantation. J Clin Oncol. 2011;29(15):1980–6. doi: 10.1200/JCO.2010.30.4121. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A. Need to realign patient-oriented and commercial and academic research. Lancet. 2011;378:1777–8. doi: 10.1016/S0140-6736(11)61772-8. [DOI] [PubMed] [Google Scholar]