Abstract
Therapy-related acute myeloid leukemia is an unfortunate complication of cancer treatment, particularly for patients with highly curable primary malignancies and favorable life expectancy. The risk of developing therapy-related acute myeloid leukemia also applies to patients with non-malignant conditions, such as autoimmune diseases treated with cytotoxic and/or immunosuppressive agents. There is considerable evidence to suggest that there is an increased occurrence of hematologic malignancies in patients with autoimmune diseases compared to the general population, with a further increase in risk after exposure to cytotoxic therapies. Unfortunately, studies have failed to reveal a clear correlation between leukemia development and exposure to individual agents used for the treatment of autoimmune diseases. Given the dismal outcome of secondary acute myeloid leukemia and the wide range of available agents for treatment of autoimmune diseases, an increased awareness of this risk and further investigation into the pathogenetic mechanisms of acute leukemia in autoimmune disease patients are warranted.
This article will review the data available on the development of acute myeloid leukemia in patients with autoimmune diseases. Possible leukemogeneic mechanisms in these patients, as well as evidence supporting the association of their primary immunosuppressive status and their exposure to specific therapies, will also be reviewed. This review also supports the idea that it may be misleading to label leukemias that develop in patients with autoimmune diseases who are exposed to cytotoxic agents as ‘therapy-related leukemias’. A better understanding of the molecular defects in autoimmune disease patients who develop acute leukemia will lead to a better understanding of the association between these two diseases entities.
Keywords: autoimmune disease, secondary leukemia, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, systemic lupus erythematosus
Introduction
The term ‘secondary leukemia’ is used to describe leukemia that did not develop as a primary or de novo process.1 Secondary leukemia generally refers to leukemia developing in patients who have a history of exposure to various therapeutic agents as well as a history of antecedent myeloid stem cell disorders, such as myelodysplasia (MDS). Therapy-related acute myeloid leukemia (t-AML) is a term used to describe the former.1 According to the World Health Organization (WHO), t-AML, therapy-related myelodysplastic syndrome (t-MDS), and myelodysplastic syndrome/myeloproliferative neoplasms (t-MDS/MPN) developing in patients who are exposed to cytotoxic agents are collectively classified as therapy-related myeloid neoplasms (t-MN).2
t-AML, which accounts for about 10% of all AML diagnoses, is a well-recognized complication in patients with primary malignant or non-malignant conditions after exposure to chemotherapy, radiotherapy, or immunosuppressive therapies.3 Autoimmune diseases (ADs) are the most common non-malignant conditions associated with t-AML, accounting for 1.5% of t-AML cases.4 The reported latency period between exposure to treatment for ADs and the development of t-AML is variable, ranging from a few months to several years and depends on the type of therapy, dose schedule, and cumulative dose, as well as patient-related factors.3 The genetic and host risk factors potentially predisposing to the development of t-AML in ADs are currently the subject of intense research. Tumor cells need to evade the immune system to develop overt disease, thus defects in the immune system are recognized risk factors for cancer development.5 In turn, patients diagnosed with diseases involving a defective immune system, or those receiving immunosuppressive therapies, are believed to be at high risk of developing acute leukemia.6
Literature search method
Patients with secondary acute leukemia most commonly develop AML rather than ALL.7 Our research is, therefore, focused on AML cases. Since AML is a relatively rare disease, most studies available in the literature estimate AML risk in autoimmune diseases within a wider group of hematologic malignancies (e.g. acute and chronic myeloid leukemia, myeloproliferative diseases, and acute myeloid and lymphoid leukemia). All cases diagnosed as secondary acute leukemia associated with ADs, were identified by a Medline search, in bibliographies of articles, case reports, and meeting abstracts. All cases with available clinical data were collected and analyzed.
Autoimmune diseases
With the exception of rheumatoid arthritis and autoimmune thyroiditis, ADs are relatively uncommon, with a low incidence (90 per 100,000 person years) and low prevalence rates (3–7%) in Western countries.8,9 Most ADs affect middle-aged women more than men, with considerable morbidity and mortality. Large, well-designed population-based case-control studies in patients with rheumatoid arthritis10 and systemic lupus erythematosus (SLE) suggest that a number of factors might be implicated in the development of ADs. These include cigarette smoking, infection, hair treatment, hormonal treatment, occupational exposures, drugs and psychosocial stressors.11–13 Together with reports on families and twins, these studies indicate that ADs may also result from the interaction of genetic and environmental factors.14–19 Clinically, ADs are classified into systemic and localized organ specific forms. Systemic ADs include rheumatoid arthritis, SLE, systemic sclerosis, Sjogren’s syndrome, scleroderma and dermatomyositis/polymyositis, while multiple sclerosis and inflammatory bowel diseases affect specific body organs. Autoimmune diseases exhibit variable courses with clinical remissions and exacerbations. Advanced cases have progressive courses with high morbidity and mortality. In the past decade, important progress has been made in the therapeutic approach to ADs, particularly with the development of biological drugs.20 However, these diseases remain in most cases incurable.8,9
Autoimmune diseases and the risk of myeloid leukemia
Anderson et al. explored the association of myeloid malignancies with ADs by comparing 13,486 patients aged over 67 years with myeloid malignancies to 160,086 population-based matched controls using the SEER-Medicare database of Hematopoietic Malignancy Risk Traits (SMAHRT). The authors found that having an AD is associated with a significant increase in the risk of AML with odds ratios (OR) of 1.29, (95% CI, 1.2–1.39) and myelodysplastic syndrome (MDS) with OR of 1.50, (CI, 1.35–1.66). AML risk was significantly associated with rheumatoid arthritis (OR 1.28), SLE (OR 1.92), polymyalgia rheumatica (OR 1.73), autoimmune hemolytic anemia (OR 3.74), systemic vasculitis (OR 6.23), ulcerative colitis (OR 1.72) and pernicious anemia (OR 1.57).21 This was confirmed in a recent study by Kristinsson et al. that included 9,219 patients with AML, 1,662 patients with MDS, and 42,878 matched controls from population-based central registries in Sweden. The authors of this study observed that a previous history of any autoimmune disease was associated with a 1.7-fold (95% CI, 1.5–1.9) increased risk of AML and 2.1-fold (95% CI, 1.7–2.6) increased risk of MDS.22
The association between rheumatoid arthritis and myeloid leukemia
Non-steroidal anti-inflammatory drugs (NSAIDs) are used as single agents in RA for mild, well-controlled disease. Disease modifying anti-rheumatic drugs (DMARDs), used in the treatment of rheumatoid arthritis, include corticosteroids, and immune response modulators (leflunomide, abatacept, and anakinra and TNF blockers). DMARDs are further divided into biological (anti-TNF agents) and non-biological subclasses. TNF-α is one of the most important regulators of inflammatory processes, thus a chimeric or humanized TNF-α inhibitor is a key anti-inflammatory targeted therapy in these diseases. The biological DMARDs include anti-TNF agents, such as etanercept, infliximab and adalimumab. Non-biological DMARDs include methotrexate (MTX), leflunomide, sulfasalazine, antimalarial (hydroxychloroquine), gold injections, d-penicillamine, minocycline, azathioprine (AZA) and cyclophosphamide (CTX). Non-biological DMARDs are used in early disease stages and combination therapy is recommended for more severe cases with progressive courses.20
Early case reports (in the 1970s) and small case series for AML and MDS developing in rheumatoid arthritis patients raised concerns about leukemia risk in these patients. Rheumatoid arthritis patients were exposed to azathioprine,23,24 methotrexate,25–27 alkylating agents (cyclophosphamide and chlorambucil)28 and biological agents such as anti-TNF drugs.29 Rosenthal and Farhi identified 8 cases of MDS/AML in the pathology records of the University of Cleveland and Emory University for autoimmune disease patients: 5 cases treated with methotrexate for rheumatoid arthritis, 2 cases treated with chlorambucil for Behcet's disease, and one case treated with cyclophosphamide for systemic lupus erythematosus. Exposure periods were between six months and over ten years, and cytogenetic data were available for 3 patients. One patient had t(8;21), one had complex karyotype and the third had inv(16) after methotrexate, chlorambucil and cyclophosphamide exposure, respectively. Outcome information was reported for 5 patients: 3 AML patients remained in remission for some years after bone marrow transplantation, one MDS patient died from infectious complications and the other MDS patient had evolution into AML.30 A significant excess of leukemia risk in rheumatoid arthritis patients was described in early cohorts of cancer registries in Finland,31 Sweden,32 Denmark33 and Birmingham.34 This significant risk was further confirmed in a prospective cohort of 862 rheumatoid arthritis patients diagnosed between 1966 and 1974, and followed up for a mean of 17.4 years (P=0.027). Only 2 of 12 cases had been exposed to cyclophosphamide alone or in combination with azathioprine.35
The main data of large population-based studies exploring the risk of leukemia in rheumatoid arthritis are summarized in Table 1. The standardized incidence ratio is calculated by comparing the number of observed cases to what is expected in the general population as a control.
Table 1.
Calculated risk of leukemia developing in rheumatoid arthritis (RA) patients from population based studies.
Four large population-based studies suggest an association between the risk of leukemia and rheumatoid arthritis in patients treated with non-biological DMARDs. In one study, the risk of leukemia was assessed in three cohorts of rheumatoid arthritis patients retrieved from the Swedish Cancer Registry. The study included three cohorts, one of early arthritis patients (recruited within one year of rheumatoid arthritis onset), one of hospitalized patients with advanced disease and a third of patients treated with TNF blockers. A significant association between rheumatoid arthritis and the risk of AML was observed in the inpatient prevalent and early-arthritis incident cohorts.36 In the second study, the rheumatoid arthritis cohort, derived from California’s statewide discharge data set, both inpatient men and women with rheumatoid arthritis patients had a significant risk of being diagnosed with leukemia, with males at a substantially greater risk37 compared to the general population. The Swedish Cancer registry was used in the third study which examined 42,262 hospitalized rheumatoid arthritis patients between 1980 and 2004. There was an overall increase in AML with further risk in patients diagnosed after 1999 and those under the age of 50 years. AML was also seen more during the first year after diagnosis with a standardized incidence ratio (SIR) of 4.88 for less than one year and 2.03 for 1–4 years.38
The fourth study analyzed the potential associations between individual DMARDs and the risk of developing hematologic neoplasms. This was a cohort of elderly patients (mean age 61.7 years) linked to the population data base of Quebec. The incidence rate of hematologic malignancies was 391.6 cases per 100,000 person-years in a cohort of 23,733 rheumatoid arthritis patients followed for a mean of 6.7 years. The risk of hematologic malignancy was significant in patients exposed to azacytidine and cyclophosphamide and borderline to methotrextae in an un-adjusted rate ratio in a case control design. Only with cyclophosphamide, the risk remained significant when the adjusted rate ratio was assessed.39 Cyclophosphamide, a potent immunosuppressive agent whose use is restricted to severe cases of refractory rheumatoid arthritis, such as vasculitis, is known to be leukemogenic.43
Three additional population based studies examined leukemia/cancer risk, in patients treated with anti-TNF agents.40–42 The risk of hematologic cancers in patients treated with anti-TNF therapy was compared to patients treated with any non-biological disease modifying agent in one study41 and to methotrexate-treated patients in another.42 Both studies observed no substantial increase in the risk of hematologic malignancies related to anti-TNF therapy. The short follow-up time (~2.5 year) in the anti-TNF cohorts was a limitation of both studies. Askling et al. reported one of the largest population-based studies on TNF blocker-associated cancer risk. Patients treated with anti-TNF (adalimumab, etanercept, and infliximab) were compared to biological-naive, a methotrexate, and a disease modifying combination therapy cohorts, as well as to the general population. The relative risk of cancer in anti-TNF treated patients was higher than in the general population that was statistically significant in the following 2–4 years with SIR of 1.25 (CI 1.01–1.56). However, the overall cancer risk including hematologic malignancies in the anti-TNF therapy cohort was no different from the other treatment groups. In addition, this risk was the same regardless of the duration of active anti-TNF therapy during the 6-year follow-up period.40
There is insufficient data linking secondary hematologic malignancies to higher mortality in rheumatoid arthritis patients. Two important studies attempted to answer this question. In the 2003 study by Wolfe et al., the mortality due to leukemia/lymphoma was high in 3,501 rheumatoid arthritis patients.44 The ratio between the observed mortality for leukemia/lymphoma to that expected was 1.78 and the rate per 100 patient deaths was 1.92 (95% CI 1.16–3.09). However, such a rise was not reproduced in a randomly selected cohort of 789 rheumatoid arthritis patients.45 In this study, although there was an increased risk of hematopoietic cancers in rheumatoid arthritis patients, the overall mortality due to cancer was no greater than expected.
The association between inflammatory bowel diseases and myeloid leukemia
Inflammatory bowel diseases include Crohn’s disease and ulcerative colitis. The incidence and prevalence of inflammatory bowel diseases have increased over the past decade. They affect adults under 30 years of age, with Crohn’s disease affecting younger patients.46–48 Ulcerative colitis affects men and women equally and is restricted to the colon, while Crohn’s disease tends to affect women more than men and may affect any part of the gastrointestinal tract. The current medications used in the treatment of inflammatory bowel disease include a large number of immunosuppressive agents, non-biological and step-wise treatments, such as 5-aminosalycilic acid (5-ASA), and corticosteroids, azathioprine, 6-mercaptopurine or methotrexate. TNF blockers were approved for inflammatory bowel diseases in the late 1990s and have recently been used not only for advanced stage disease but also as front-line therapy in early disease.47,49
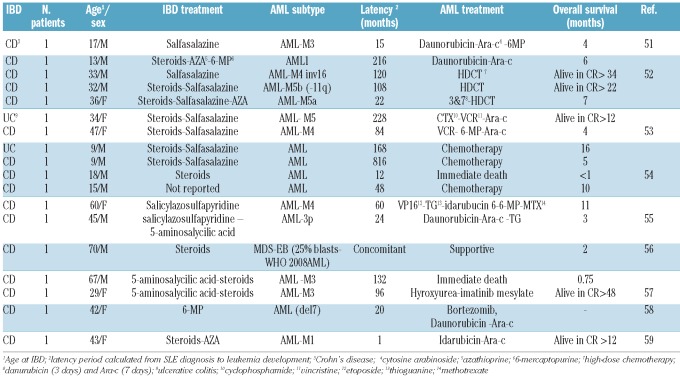
The first description of secondary AML in patients with inflammatory bowel disease was published in 1980.50 Fabry et al. observed 5 AML cases among 400 ulcerative colitis patients treated over an 8-year period. This was followed by a number of case reports and case series that suggested an association between inflammatory bowel disease and hematologic malignancies, in particular Crohn’s disease to lymphoma and ulcerative colitis to leukemia,51–59 as summarized in Table 2. There are 18 case reports of AML developing in patients with inflammatory bowel diseases. Patients were treated with steroids (12 cases), salfasalazine (8 cases), azathioprine (3 cases), 5-aminosalycilic acid (3 cases) and 6-mercaptopurine (2 cases). The reported median survival was seven months (range 3 weeks-4 years). Survival exceeded two years in patients treated with intensive chemotherapy.
Table 2.
Literature review of acute myeloid leukemia in inflammatory bowel disease (IBD) patients.
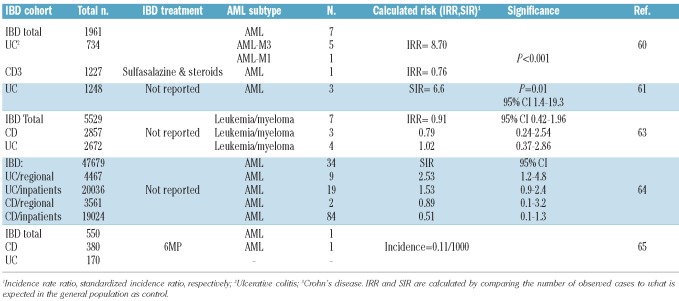
Large case series and population-based studies exploring the risk of leukemia in inflammatory bowel disease treated with different agents are summarized in Table 3.60–65 For patients treated with non-biological agents, there are five large case series and population based studies that observed an association between hematologic malignancies and inflammatory bowel diseases, particularly ulcerative colitis.60–64 The association was statistically significant in three of the studies.60,61,64 AML frequency was 8.7 times higher than expected in a series of 734 ulcerative colitis patients. Again, the AML subtypes were skewed more towards AML-M3 (5 of 7) in this series who were treated mainly with sulfasalazine and steroids.60 AML-M3 is a rare AML subtype and is fortunately associated with a high cure rate. Likewise in the second study, a significant approximately 7-fold increase in AML risk was observed among 1,248 ulcerative colitis patients who were followed for a mean of 18 years.61 These early observations were subsequently confirmed in a population based study of 47,679 patients with inflammatory bowel disease. Patients were linked to 3 regional and a nationwide inpatient Swedish registry and were followed for up to 40 years. A significant excess in AML occurred in ulcerative colitis patients specifically in regional cohorts. Thirty-four cases of AML were observed within five years of follow up. Male sex and the period between the sixth and tenth year of follow up were associated with the highest risk of leukemia.64
Table 3.
Calculated risk of leukemia developing in inflammatory bowel disease (IBD) patients from large case series and population based studies.
For patients exposed to biological agents, such as anti-TNF agents, there are no large case series or population based studies estimating the associated leukemia risk in inflammatory bowel disease. However, in the 2006 post-marketing worldwide safety report for all patients exposed to TNF blockers, 74 cases of leukemia were identified: 23 after exposure to infliximab, 39 after etanercept, and 12 rheumatoid arthritis or Crohn’s disease patients after adalimumab. Unfortunately, information about the total number of patients exposed to these agents as a denominator is lacking.66 It is worth noting that in 2009, the FDA reviewed 147 post-marketing reports of leukemia in all patients using TNF blockers. Of these, acute myeloid leukemia was seen in 44 cases. Of the total 30 deaths, 26 were due to leukemia. The average time to onset of secondary leukemia was within the first 1–2 years of therapy. The FDA added a warning to the current information regarding prescription of TNF blockers about malignancies in general, but this does not specifically mention leukemia.67
With regards to the leukemia related mortality rate in patients with inflammatory bowel disease, four studies reported increased mortality rate in ulcerative colitis patients, two of which reached statistical significance. Additionally, a 5-fold increase in mortality rate due to AML was observed among 1,248 ulcerative colitis patients compared to the general population with standardized mortality rate (SMR) of 5.3 (95% CI 1.7–12.3).68 Similarly, in an Italian study of 2,066 ulcerative colitis patients, a statistically significant excess death rate from hematologic malignancies, mainly lymphoma and myeloma, was reported with SMR of 2.8 (95% CI 1.0–6.1).69 However, in two ulcerative colitis cohorts, the significance of such an increased risk could not be confirmed with SMR of 1.43 (95% CI 0.02–7.9), one death from leukemia out of 689 in the first cohort, and SMR of 3 (95% CI 0.4–11), 2 deaths due to leukemia out of 62 in the second study.70,71 Details of leukemia subtypes, treatments or causes of death are beyond the scope of these studies.
The association between multiple sclerosis and myeloid leukemia
Multiple sclerosis is a chronic autoimmune demyelinating disease of the central nervous system characterized by variable periods of relapse and remission of neurological symptoms with progressive disability over time. Patients whose disease fails to respond to first-line therapy using disease-modifying treatment, like interferon beta (IFN-β) and/or glatiramer acetate (GA), may be considered for second-line treatments, such as mitoxantrone or natalizumab.72,73 Mitoxantrone was the first immunosuppressive drug approved in the US and Europe as a single agent for the treatment of aggressive relapsing–remitting or progressive multiple sclerosis.74 Mitoxantrone is the topoisomerase II (topo-II) inhibitor most frequently associated with development of t-AML.75,76
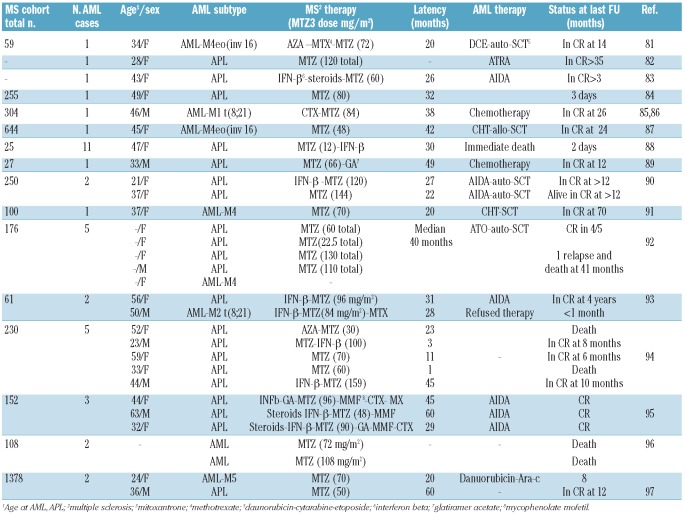
Acute myeloid leukemia is a well-known sequel of multiple sclerosis patients, a in particular those treated with mitoxantrone.75,76 While few secondary AML cases in multiple sclerosis were mainly exposed to chorambucil, azathioprine or INF-beta(77–79), the majority occurred after exposure to mitoxantrone. The first case report of t-AML (M3) in a multiple sclerosis patient was published in 1998.80 Subsequently, a number of case reports and case series were published on t-AML in mitoxantrone treated patients,81–96 as summarized in Table 4.
Table 4.
Literature review of acute myeloid leukemia in multiple sclerosis (MS) patients from case series and population based studies.
Larger series of patients with multiple sclerosis treated with mitoxantrone consistently show an excess in AML risk, particularly of the promyelocytic subtype (M3). Acute promyelocytic leukemia is a highly curable disease, yet it is associated with high rates of early hemorrhagic death if not promptly diagnosed and treated. Therefore, early recognition of AML-M3 in patients at risk and proper management are critical. In 2002, Ghalie et al. reviewed 1,378 MS patients treated with mitoxantrone. Patients were exposed to a mean cumulative mitoxantrone dose of 60 mg/m2 and were followed up for a mean of 36 months. The authors found the t-AML rate was found to be relatively low (0.15%).97 Meanwhile, in a post-marketing report for mitoxantrone-treated multiple sclerosis patients from 2003 through 2007, 39 cases of acute leukemia were identified. The mean age of leukemia patients was 48.2 years and 28 years for men and women, respectively. The mean cumulative dose of mitoxantrone was 83.2 mg/m2 (range 48–135). Acute promyelocytic leukemia represented 33.3% of French American British Classification (FAB) subtypes. The outcome of multiple sclerosis patients was worse when DNA-damaging antineoplastic agents, cytotoxic drugs, or escalating doses of anthracyclines were added to the therapeutic regimens. Therefore, caution should be used while prescribing these agents to multiple sclerosis patients.98 In a 2009 preliminary report of 35 Italian centers, 21 cases of acute leukemia were identified among a cohort of 2,854 multiple sclerosis patients. Mitoxantrone dose-dependent risk was observed with an incidence rate ratio of 1.84 at cumulative doses below 60 mg/m2 and 2.74 at doses over 82.4 mg/m2. The main AML subtype in this study was myelomoncytic (M4) (19.0%). The clinical outcome of leukemia patients in this series was again not encouraging.99
Pascual et al. prospectively studied the rate of AML in two Spanish cohorts, from Valencia (n=142) and from Catalonia (n=88), of mitoxantrone treated multiple sclerosis patients. The cummulative incidence and incidence density of AML for the Valencian cohort were 2.82 and 0.62% respectively; and 2.27 and 0.44% for the Catalonian cohort. The latency period between treatment discontinuation and AML ranged from one to 45 months in both cohorts. There was no association between AML occurrence and dose, age at the beginning of the disease or at beginning of the treatment, disease duration, gender or concomitant medications. The authors further compared the cumulative incidence and incidence density of this cohort with the 32 t-AML cases from nine previously reported series. Latency period range was 1–60 months. The calculated incidence varied from 0.15 to 0.80% and main AML subtype was promyelocytic (57.1%). Furthermore, the incidence of acute leukemia did not vary significantly in the years since 2001, with 2 to 5 cases reported per year.94 Ellis et al. reported an acute leukemia incidence of 0.33% (risk of 1:333) in multiple sclerosis patients. This frequency is 100 times higher than expected for de novo leukemia in a healthy population (0.03% by 70 years of age). However, this rate is still lower than that reported for patients who are treated for primary cancer with combination chemotherapy (2–12%).100 Again APL was the most common subtype representing 46.4% of cases. About 80% of these cases were exposed to mitoxantrone at acumulative doses exceeding 60 mg/m2.
In 2010, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology re-evaluated and reviewed incidence rates for acute leukemia occurring in multiple sclerosis patients. The overall incidence of acute leukemia in patients treated with mitoxantrone was found to be 33 of 4,076 (0.81%), ranging from 0.15 to 2.8%. The majority of cases was APL and occurred within three years of mitoxantrone treatment.101 The previous studies and reports collectively showed a high incidence of AML in multiple sclerosis and of AML-M3 after mitoxantrone exposure. Long and close follow up for hematologic changes in multiple sclerosis patients, particularly for those exposed to cytotoxic agents, should be part of their management.
Recent reports found that the outcome of AML in multiple sclerosis patients was not inferior compared to de novo cases. The reported overall mortality of acute myeloid leukemia developing in mitoxantrone exposed multiple sclerosis patients was 24%, similar to that of de novo AML.102 Our group recently reported the clinical features and treatment outcome of 33 patients with multiple sclerosis who developed AML-M3, the most frequently reported subtype in these patients. Thirty patients had been previously exposed to mitoxantrone. Twenty-nine (90%) patients achieved hematologic remission after all-trans retinoic acid (ATRA) and chemotherapy or arsenic trioxide and ATRA. The 5-year cumulative incidence of relapse and overall survival were 23 and 68%, respectively,103 i.e. comparable to that of de novo APL.82,104 However, the availability of alternative therapies to mitoxantrone associated with less severe toxicities (e.g. interferon-alpha and glatiramer acetate) should lead physicians to weigh the benefit against the potential harm of mitoxantrone for each individual patient with multiple sclerosis.101
The association between systemic lupus erythematosus and myeloid malignancies
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that may affect any body organ. The disease affects about 250,000 in the USA and is more common in women of childbearing age. It has a course of remission and exacerbations with a 10-year survival rate exceeding 90%.105 Treatment recommendations depend on the severity of the disease. In milder disease forms, NSAIDs, hydroxychloroquine or short steroid courses are effective. In severe forms, more aggressive immunosuppression is recommended, including methotrexate, cyclophosphamide, azathioprine and mycophenolate.106
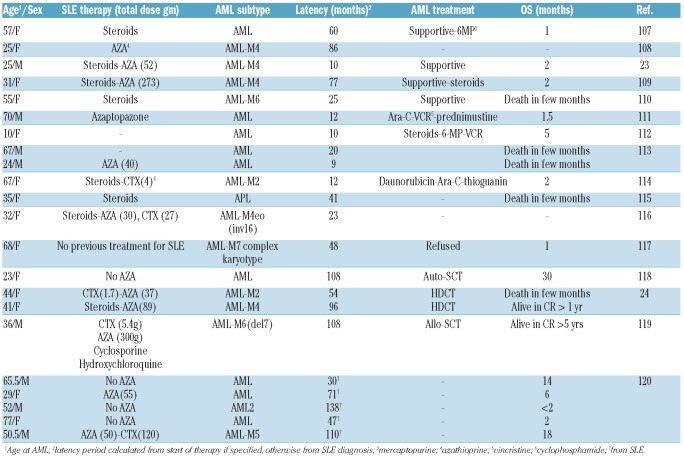
Similar to other ADs, the association between systemic lupus erythematosus and myeloid malignancies was initially suggested from case reports23,24,107–120 and small case series which are summarized in Tables 5 and 6. On reviewing the literature, we identified 22 cases of AML developing in systemic lupus patients reported between 1967 and 2009. Median age was 42.5 years (range 10–77 years) and females made up 14 of 22 cases. AML was classified as AML-M4 in 4 cases, AML-M2 in 3 cases, AML-M6 in 2 cases and AML-M3, AML-M5 and AML-M7 were diagnosed in one case each. The remaining 9 cases did not have a defined subtype. The main exposure was to azathioprine in 11 patients while exposure to cyclophosphamide was noted in 5 patients. The median latency period to AML was 44 months (range 9–138). The majority of AML patients received only supportive care with poor outcome. With high-dose chemotherapy and stem cell transplantation, some patients achieved long remissions (Table 5).
Table 5.
Literature review of acute myeloid leukemia in systemic lupus (SLE) patients.
Table 6.
Calculated risk of leukemia developing in systemic lupus erythematosus (SLE) patients from large case series and population based studies.
There are three large population based studies estimating the risk of malignancies in systemic lupus erythematosus patients (Table 6). Bernatsky et al. reported an increased risk of all hematologic malignancies among an international cohort of 9,547 SLE patients. Patients were followed for eight years and the calculated leukemia standardized incidence ratio was 1.89 (95% CI 0.76–3.88).121
Tarr et al. observed that 13.5% of 860 SLE patients (771 women and 89 men) developed hematologic cancer within ten years of diagnosis. Hematologic cancers were observed at a higher number than expected (SIR of 1.31, 95% CI 0.424–3.071). Median age was 33 years (range 16–64 years) and 47 years (range 20–73 years) at time of lupus and cancer diagnoses, respectively. The principle exposure was to cyclophosphamide (7 cases), azathioprine (8 cases), cyclophosphamide and azathioprine (4 cases), methotrexate (one case) cyclosporine-A (one case), chloroquine (6 cases) and corticosteroids alone (9 cases). No association between exposure to a specific therapy and the development of malignancy was observed in this study.122
In the third study, risk factors for developing myeloid leukemia in SLE were studied in 5,715 hospitalized patients from the Swedish national registry and followed from 1964 through 1995. Except for preceding leukopenia, factors such as age, sex, disease duration and other SLE features were not significantly associated with leukemia development. Furthermore, the possibility that AML developing in these patients was therapy-related is unlikely since the study did not find any difference in the frequency of cytotoxic exposure between cases and control cohorts.120
In a retrospective analysis of death causes in 300 SLE patients registered at the Bloomsbury (Rheumatology-Unit SLE) clinic between 1978 and 2000, the most common cause of death was malignancy (20%).123 Leukemia or hematologic cancer associated mortality rates were not estimated. However, a significant increase in hematologic cancer related mortality was reported in 9,547 SLE patients observed for an average of 8.1 years. Standardized mortality rate for hematologic malignancies (excluding NHL) was 2.1 (95% CI 1.2–3.4), thus double the risk in the general population.124 Again, the overall mortality ratio was 19% and cancer-related mortality ratio was 2% among 860 SLE patients (771 women and 89 men). In this series, hematologic malignancies were third in frequency after breast and gastrointestinal cancers.122 These studies were not specifically designed to estimate the outcome of SLE patients who developed acute leukemia. Therefore, it is difficult to draw any conclusions about secondary leukemia related mortality in SLE patients.
Systemic sclerosis and myeloid malignancies
Systemic sclerosis is a chronic multisystem disorder characterized by collagen accumulation in skin and visceral organs of unknown etiology. Few cases of hematologic malignancies have been reported in these patients. Some case reports were described as concomitant to systemic sclerosis. Hematologic malignancies were in the form of CML,125–127 CLL128,129 large granular lymphocyte leukemia,130 hairy cell leukemia,131–133 multiple myeloma,134,135 lymphoma,136–138 ALL139 and AML.140,141 The main reported medication in these cases was D-penicillamine, in particular with CLL.142,143 Since these are sporadic case reports, the apparent associations with any therapy remain unproven.
Mechanisms underlying autoimmune diseases in common with cancer pathogenesis
Nearly all cancers develop due to mutations in genes responsible for the regulation of cell growth, differentiation, apoptosis and repair. Defects in repair and apoptosis are also established mechanisms in ADs.144–146 Mutations in the tumor suppressor gene p53 are frequent in autoimmune diseases, such as rheumatoid arthritis and inflammatory bowel disease.147 Fas, a death receptor belonging to the tumor necrosis factor receptor superfamily, is defective in ADs and is also linked to cancer susceptibility.144,148,149 The phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B,PKB)/mammalian Target Of Rapamycin (mTOR) signaling pathway, known to be altered in leukemia,150,151 is also involved in the pathogenesis of some ADs.152–156 The mitogen-activated protein kinases (MAPKs) pathway mediates signal transduction in response to various stimuli, including stress and inflammation. Three groups of MAPKs include the extracellular-regulated kinases (ERKs) and the stress-activated protein kinases (SAPKs) p38 and c-Jun NH2-terminal kinase (JNK).157 They regulate control gene expression, cell division, cell survival, apoptosis, metabolism, differentiation as well as inflammation.158 Deregulated Ras/Raf/mitogen-activated protein kinase (MEK)/ERK pathway is involved in AML158 and immune disorders such as IBD.159 Moreover, leukemia associated with mutations in this pathway are resistant to inhibitors specific to this pathway, as well as to inhibitors of other pathways like the PI3K/PTEN/Akt/mTOR.151
Both specific and non-specific immune responses play a major role in controlling the growth of malignant cells (tumor surveillance).160,161 Any disruption of this system by a primary pathological or iatrogenic process will provide the opportunity for abnormal cells to evade surveillance and progress into malignancy. In addition, the interaction between tumor cells and the host microenvironment of stromal cells and inflammatory/immune cells contributes to a complex inflammatory signaling process that enhances tumor progression.162,163 For example, NF-kappaB a major player in the pathogenesis of autoimmune diseases, such as rheumatoid arthritis, is involved in cancer and leukemia development.164 Furthermore, chemokines and cytokines produced by inflammatory cells have a powerful pro-tumor activity.165 Early in the neoplastic process, inflammatory cells facilitate genomic instability, promote angiogenesis and create an attractive environment for tumor growth. Successively, tumor cells direct inflammatory mechanisms which favor the dissemination of neoplastic elements via lymphatics and capillaries, evading host defence. This is achieved by remodelling the extra-cellular matrix and regulating selectin–ligand interactions, as well as metalloproteinase production and chemokine functions and receptors.165
T-cell responses are implicated in the pathogenesis of lupus, rheumatoid arthritis and other autoimmune diseases.166 Interestingly, Young suggested a role for cytotoxic lymphocyte attack in individuals with defective immune system in triggering apoptosis of hematopoietic stem cells. In some patients, a few cells may survive cytotoxic lymphocyte attack and retain a residual DNA injury.167 Permanent genomic alterations, such as point mutations, deletions, or rearrangements, may accumulate over years ultimately resulting in clonal outgrowth and leukemia.
The carcinogenic potentials of immunosuppressive and chemotherapeutic agents may act synergistically. However, with the exception of azathioprine, cyclophosphamide and mitoxantrone, there are insufficient data on the carcinogenic potential of other immunosuppressive agents such as steroids and anti-lymphocyte globulin.168–171 Recent studies from our group have shown that DNA breakpoints (hotspots) are tightly clustered in an 8-bp region within PML intron 6 in MS patients who developed APL after mitoxantrone. In vitro cleavage experiments showed that this region contains a preferential site of mitoxantrone-induced cleavage by topoisomerase II.76,172 Individual genetic variations in drug metabolism and response to DNA damage3,173 play major roles in cancer development after cytotoxic exposure. Individual genetic variations in resistance to DNA damage by means of repair and/or apoptosis are just as critical to leukemogenesis. For example, increased susceptibility to develop promyelocytic leukemia in patients with multiple sclerosis receiving mitoxantrone was found to be linked to genetic variants in DNA repair and drug-metabolizing enzymes that result in impaired detoxification of chemotherapy or inefficient repair of drug-induced genetic damage.75
Conclusions
Although the evidence regarding the risk of hematologic malignancies, and in particular lymphoma in Ads has been growing, few studies have focused on AML. Therefore, the importance of this complication in patients with ADs is not well-known. This is the first systematic review of published data regarding the risk of AML in individual autoimmune diseases. Most studies investigating the association between hematologic malignancies and ADs revealed an excess AML risk in these patients. However, this risk did not reach statistical significance in some of the studies because AML is a rare disease and the studies were not designed to assess AML risk. For rheumatoid arthritis, the risk of AML was significantly higher than in the general population in two large population based studies.36,38 The lack of treatment details in these studies, however, limited our ability to reach any conclusion about their leukemogenic role. For inflammatory bowel disease, AML risk was reported to be significantly high in ulcerative colitis patients in two studies.36,64 Again, the evidence currently available means it is not possible to attribute such cases to therapy.
Multiple sclerosis patients are known to be at risk of developing APL, particularly those treated with mitoxantrone. However, not all multiple sclerosis patients treated with mitoxantrone develop secondary leukemia while others develop leukemia without mitoxantrone exposure.71 Therefore, patient (host) related factors seem to play a fundamental role in the pathogenesis as described previously. The risk of myeloid leukemia was found to double in an SLE cohort.120 However, studies addressing the leukemogenic potential of SLE therapy gave conflicting results. While patients were observed to develop malignancy without previous exposure to cytotoxic or immunosuppressive agents,174 others reported an increased risk after exposure to immunosuppressive drugs.175,176 With the exception of multiple sclerosis, no studies have investigated the mechanism underlying leukemogensis in autoimmune diseases.
The fact that patients with various ADs as well as those given immunosuppressive therapy for other reasons, such as those receiving organ transplants, are at very high risk of cancer development, supports the hypothesis that the primary defect is related to the host. Additionally, not all cancer patients exposed to cytotoxic agents develop leukemia, and leukemia develops after cancer in patients with no exposure.
This review also supports the idea that there is insufficient evidence to label leukemias that develop in patients with ADs who are exposed to cytotoxic agents as ‘therapy-related leukemias’. Hence, one should exercise caution when using the term ‘therapy-related leukemia’ as it can bias our attitudes when trying to understand the association between these two types of diseases. Our focus should be on investigating the molecular defects in the autoimmune diseases, including defects in immunity, DNA repair, and apoptosis in these patients rather than studying only drug mechanisms that lead to leukemogenesis. In conclusion, the precise pathophysiology underlying ADs and its link to cancer development remains unclear. Finally, the risk of AML in AD patients warrants more attention, as it provides a model for investigating the role of defective immunological mechanisms in leukemogenesis.
Acknowledgments
The authors gratefully acknowledge the generous support of Dr. Isabel Cunningham, for her intellectual input and editorial assistance. The authors would also like to thank Dr. Gabriele Mazzitelli, Tor-Vergata Digital Library, for his prompt response in providing the original full text of the reviewed literature. Prof. F. Lo-Coco acknowledges the support from the AIL and AIRC.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Rowe JM. Therapy of secondary leukemia. Leukemia. 2002;16(4):748–50. doi: 10.1038/sj.leu.2402456. [DOI] [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–29. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser S, Dohner K, Krauter J, Kohne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 117(7):2137–45. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 5.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–64. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104(3):822–8. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 7.Montesinos P, Gonzalez JD, Gonzalez J, Rayon C, de Lisa E, Amigo ML, et al. Therapy-related myeloid neoplasms in patients with acute promyelocytic leukemia treated with all-trans-retinoic Acid and anthracycline-based chemotherapy. J Clin Oncol. 2010;28(24):3872–9. doi: 10.1200/JCO.2010.29.2268. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 9.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3–4):197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svendsen AJ, Holm NV, Kyvik K, Petersen PH, Junker P. Relative importance of genetic effects in rheumatoid arthritis: historical cohort study of Danish nationwide twin population. BMJ. 2002;324(7332):264–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Parks CG, Walitt BT, Pettinger M, Chen JC, de Roos AJ, Hunt J, et al. Insecticide use and risk of rheumatoid arthritis and systemic lupus erythematosus in the Women's Health Initiative Observational Study. Arthritis Care Res (Hoboken) 2011;63(2):184–94. doi: 10.1002/acr.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper GS, Parks CG, Schur PS, Fraser PA. Occupational and environmental associations with antinuclear antibodies in a general population sample. J Toxicol Environ Health A. 2006;69(23):2063–9. doi: 10.1080/15287390600746165. [DOI] [PubMed] [Google Scholar]
- 13.Cooper GS, Parks CG. Occupational and environmental exposures as risk factors for systemic lupus erythematosus. Curr Rheumatol Rep. 2004;6(5):367–74. doi: 10.1007/s11926-004-0011-6. [DOI] [PubMed] [Google Scholar]
- 14.Invernizzi P, Pasini S, Podda M. X chromosome in autoimmune diseases. Expert Rev Clin Immunol. 2008;4(5):591–7. doi: 10.1586/1744666X.4.5.591. [DOI] [PubMed] [Google Scholar]
- 15.Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity. 2008;41(4):272–7. doi: 10.1080/08916930802024574. [DOI] [PubMed] [Google Scholar]
- 16.Kira J. [Epidemiology of multiple sclerosis: environmental factors versus genetic factors] Nippon Rinsho. 2003;61(8):1300–10. [PubMed] [Google Scholar]
- 17.Leslie RD, Hawa M. Twin studies in auto-immune disease. Acta Genet Med Gemellol (Roma) 1994;43(1–2):71–81. doi: 10.1017/s000156600000297x. [DOI] [PubMed] [Google Scholar]
- 18.Vinuesa CG, Cook MC. Genetic analysis of systemic autoimmunity. Novartis Found Symp. 2007;281:103–20. doi: 10.1002/9780470062128.ch10. discussion 120–8. [DOI] [PubMed] [Google Scholar]
- 19.Zenewicz LA, Abraham C, Flavell RA, Cho JH. Unraveling the genetics of autoimmunity. Cell. 2010;140(6):791–7. doi: 10.1016/j.cell.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 21.Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009;100(5):822–8. doi: 10.1038/sj.bjc.6604935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 29(21):2897–903. doi: 10.1200/JCO.2011.34.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexson E, Brandt KD. Acute leukemia after azathioprine treatment of connective tissue disease. Am J Med Sci. 1977;273(3):335–40. [PubMed] [Google Scholar]
- 24.Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with -7/7q. Cancer Genet Cytogenet. 1998;104(2):94–7. doi: 10.1016/s0165-4608(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 25.Espinosa G, Font J, Munoz-Rodriguez FJ, Cervera R, Ingelmo M. Myelodysplastic and myeloproliferative syndromes associated with giant cell arteritis and polymyalgia rheumatica: a coincidental coexistence or a causal relationship? Clin Rheumatol. 2002;21(4):309–13. doi: 10.1007/s100670200081. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto H, Teramura M, Kamatani N. Myelodysplastic syndrome associated with low-dose methotrexate in rheumatoid arthritis. Ann Pharmacother. 2004;38(1):172–3. doi: 10.1345/aph.1D117. [DOI] [PubMed] [Google Scholar]
- 27.Choi BR, Ahn MJ, Lee WS, Kim TH, Bae SC, Jun JB. Acute erythroleukemia in a rheumatoid arthritis patient during low-dose methotrexate therapy. Rheumatol Int. 2005;25(4):311–3. doi: 10.1007/s00296-004-0502-9. [DOI] [PubMed] [Google Scholar]
- 28.Balakrishnan C, Pathan E, Khodaiji S, Dasgupta A, Mangat G, Joshi VR. Myelodysplasia and acute myeloid leukaemia in a case of rheumatoid arthritis with secondary amyloidosis treated with chlorambucil. J Assoc Physicians India. 2004;52:423–5. [PubMed] [Google Scholar]
- 29.Saba NS, Kosseifi SG, Charaf EA, Hammad AN. Adalimumab-induced acute myelogenic leukemia. South Med J. 2008;101(12):1261–2. doi: 10.1097/SMJ.0b013e318188950a. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal NS, Farhi DC. Myelodysplastic syndromes and acute myeloid leukemia in connective tissue disease after single-agent chemotherapy. Am J Clin Pathol. 1996;106(5):676–9. doi: 10.1093/ajcp/106.5.676. [DOI] [PubMed] [Google Scholar]
- 31.Isomaki HA, Hakulinen T, Joutsenlahti U. Excess risk of lymphomas, leukemia and myeloma in patients with rheumatoid arthritis. J Chronic Dis. 1978;31(11):691–6. doi: 10.1016/0021-9681(78)90071-1. [DOI] [PubMed] [Google Scholar]
- 32.Gridley G, McLaughlin JK, Ekbom A, Klareskog L, Adami HO, Hacker DG, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85(4):307–11. doi: 10.1093/jnci/85.4.307. [DOI] [PubMed] [Google Scholar]
- 33.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A(10):1753–7. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 34.Prior P. Cancer and rheumatoid arthritis: epidemiologic considerations. Am J Med. 1985;78(1A):15–21. doi: 10.1016/0002-9343(85)90240-2. [DOI] [PubMed] [Google Scholar]
- 35.Cibere J, Sibley J, Haga M. Rheumatoid arthritis and the risk of malignancy. Arthritis Rheum. 1997;40(9):1580–6. doi: 10.1002/art.1780400906. [DOI] [PubMed] [Google Scholar]
- 36.Askling J, Fored CM, Baecklund E, Brandt L, Backlin C, Ekbom A, et al. Haematopoietic malignancies in rheumatoid arthritis: lymphoma risk and characteristics after exposure to tumour necrosis factor antagonists. Ann Rheum Dis. 2005;64(10):1414–20. doi: 10.1136/ard.2004.033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh-Patel A, White RH, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001–10. doi: 10.1007/s10552-009-9298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemminki K, Li X, Sundquist K, Sundquist J. Cancer risk in hospitalized rheumatoid arthritis patients. Rheumatology (Oxford) 2008;47(5):698–701. doi: 10.1093/rheumatology/ken130. [DOI] [PubMed] [Google Scholar]
- 39.Bernatsky S, Clarke AE, Suissa S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Intern Med. 2008;168(4):378–81. doi: 10.1001/archinternmed.2007.107. [DOI] [PubMed] [Google Scholar]
- 40.Askling J, Baecklund E, Granath F, Geborek P, Fored M, Backlin C, et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann Rheum Dis. 2009;68(5):648–53. doi: 10.1136/ard.2007.085852. [DOI] [PubMed] [Google Scholar]
- 41.Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, et al. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis. 2005;64(5):699–703. doi: 10.1136/ard.2004.030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setoguchi S, Solomon DH, Weinblatt ME, Katz JN, Avorn J, Glynn RJ, et al. Tumor necrosis factor alpha antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2757–64. doi: 10.1002/art.22056. [DOI] [PubMed] [Google Scholar]
- 43.Keyszer G, Keysser C, Keysser M. Efficacy and safety of a combination therapy of methotrexate, chloroquine and cyclophosphamide in patients with refractory rheumatoid arthritis: results of an observational study with matched-pair analysis. Clin Rheumatol. 1999;18(2):145–51. doi: 10.1007/s100670050073. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe F, Fries JF. Rate of death due to leukemia/lymphoma in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(9):2694–5. doi: 10.1002/art.11242. [DOI] [PubMed] [Google Scholar]
- 45.Abasolo L, Judez E, Descalzo MA, Gonzalez-Alvaro I, Jover JA, Carmona L. Cancer in rheumatoid arthritis: occurrence, mortality, and associated factors in a South European population. Semin Arthritis Rheum. 2008;37(6):388–97. doi: 10.1016/j.semarthrit.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Colombel JF, Loftus EV, Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126(1):19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 47.Hanauer SB, Present DH, Rubin DT. Emerging issues in ulcerative colitis and ulcerative proctitis: individualizing treatment to maximize outcomes. Gastroenterol Hepatol (NY) 2009;5(6 Suppl):4–16. [PMC free article] [PubMed] [Google Scholar]
- 48.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 49.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabry TL, Sachar DB, Janowitz HD. Acute myelogenous leukemia in patients with ulcerative colitis. J Clin Gastroenterol. 1980;2(3):225–7. doi: 10.1097/00004836-198009000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Orii S, Sugai T, Nakano O, Yoshinari H, Sato S. Acute promyelocytic leukemia in Crohn's disease. Case report and review of the literature. J Clin Gastroenterol. 1991;13(3):325–7. [PubMed] [Google Scholar]
- 52.Dombret H, Marolleau JP. De novo acute myeloid leukemia in patients with Crohn's disease. Nouv Rev Fr Hematol. 1995;37(3):193–6. [PubMed] [Google Scholar]
- 53.Hanauer SB, Wong KK, Frank PH, Sweet DL, Kirsner JB. Acute leukemia following inflammatory bowel disease. Dig Dis Sci. 1982;27(6):545–8. doi: 10.1007/BF01296735. [DOI] [PubMed] [Google Scholar]
- 54.Cohn EM, Pearlstine B. Inflammatory bowel disease and leukemia. J Clin Gastroenterol. 1984;6(1):33–5. [PubMed] [Google Scholar]
- 55.Halme L, von Knorring J, Elonen E. Development of acute myelocytic leukemia in patients with Crohn's disease. Dig Dis Sci. 1990;35(12):1553–6. doi: 10.1007/BF01540575. [DOI] [PubMed] [Google Scholar]
- 56.Boberg KM, Brinch L, Vatn M. Crohn disease and the myelodysplastic syndrome. Ann Intern Med. 1995;122(5):395. doi: 10.7326/0003-4819-122-5-199503010-00023. [DOI] [PubMed] [Google Scholar]
- 57.Crispino P, Pica R, Angelucci E, Consolazio A, Rivera M, Cassieri C, et al. Hematological malignancies in chronic inflammatory bowel diseases: report of five cases and review of the literature. Int J Colorectal Dis. 2007;22(5):553–8. doi: 10.1007/s00384-006-0202-x. [DOI] [PubMed] [Google Scholar]
- 58.Das KK, Nishino HT, Chan AT. Treatment-associated acute myeloid leukemia in a patient with Crohn's disease on 6-mercaptopurine. Inflamm Bowel Dis. 2010;16(9):1454–6. doi: 10.1002/ibd.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kallel L, Naijaa N, Fekih M, Frikha I, Boubaker J, Bellaaj H, et al. Acute myeloid leukemia after one month of azathioprine therapy in a Crohn's disease patient. J Clin Gastroenterol. 2010;44(9):660. doi: 10.1097/MCG.0b013e3181d6b52e. [DOI] [PubMed] [Google Scholar]
- 60.Greenstein AJ, Gennuso R, Sachar DB, Heimann T, Smith H, Janowitz HD, et al. Extraintestinal cancers in inflammatory bowel disease. Cancer. 1985;56(12):2914–21. doi: 10.1002/1097-0142(19851215)56:12<2914::aid-cncr2820561232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 61.Mir Madjlessi SH, Farmer RG, Weick JK. Inflammatory bowel disease and leukemia. A report of seven cases of leukemia in ulcerative colitis and Crohn's disease and review of the literature. Dig Dis Sci. 1986;31(10):1025–31. doi: 10.1007/BF01300254. [DOI] [PubMed] [Google Scholar]
- 62.Karlen P, Lofberg R, Brostrom O, Leijonmarck CE, Hellers G, Persson PG. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94(4):1047–52. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 63.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 64.Askling J, Brandt L, Lapidus A, Karlen P, Bjorkholm M, Lofberg R, et al. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54(5):617–22. doi: 10.1136/gut.2004.051771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korelitz BI, Mirsky FJ, Fleisher MR, Warman JI, Wisch N, Gleim GW. Malignant neoplasms subsequent to treatment of inflammatory bowel disease with 6-mercaptopurine. Am J Gastroenterol. 1999;94(11):3248–53. doi: 10.1111/j.1572-0241.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10(42):iii–iv. xi–xiii, 1–229. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- 67.ALERT F. Tumor Necrosis Factor (TNF) Blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) [cited September 2011]; Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174474.htm.
- 68.Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer. 1986;58(7):1569–74. doi: 10.1002/1097-0142(19861001)58:7<1569::aid-cncr2820580731>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 69.Viscido A, Bagnardi V, Sturniolo GC, Annese V, Frieri G, D'Arienzo A, et al. Survival and causes of death in Italian patients with ulcerative colitis. A GISC nationwide study. Dig Liver Dis. 2001;33(8):686–92. doi: 10.1016/s1590-8658(01)80046-3. [DOI] [PubMed] [Google Scholar]
- 70.Palli D, Trallori G, Saieva C, Tarantino O, Edili E, D'Albasio G, et al. General and cancer specific mortality of a population based cohort of patients with inflammatory bowel disease: the Florence Study. Gut. 1998;42(2):175–9. doi: 10.1136/gut.42.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Intestinal and extra-intestinal cancer in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther. 2004;19(3):287–93. doi: 10.1111/j.1365-2036.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 72.Stuart WH. Clinical management of multiple sclerosis: the treatment paradigm and issues of patient management. J Manag Care Pharm. 2004;10(3 Suppl B):S19–25. [PubMed] [Google Scholar]
- 73.Rieckmann P, Traboulsee A, Devonshire V, Oger J. Escalating immunotherapy of multiple sclerosis. Ther Adv Neurol Disord. 2008;1(3):181–92. doi: 10.1177/1756285608098359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodin DS, Arnason BG, Coyle PK, Frohman EM, Paty DW. The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;61(10):1332–8. doi: 10.1212/01.wnl.0000095425.84407.39. [DOI] [PubMed] [Google Scholar]
- 75.Hasan SK, Buttari F, Ottone T, Voso MT, Hohaus S, Marasco E, et al. Risk of acute promyelocytic leukemia in multiple sclerosis: coding variants of DNA repair genes. Neurology. 76(12):1059–65. doi: 10.1212/WNL.0b013e318211c3c8. [DOI] [PubMed] [Google Scholar]
- 76.Hasan SK, Mays AN, Ottone T, Ledda A, La Nasa G, Cattaneo C, et al. Molecular analysis of t(15;17) genomic breakpoints in secondary acute promyelocytic leukemia arising after treatment of multiple sclerosis. Blood. 2008;112(8):3383–90. doi: 10.1182/blood-2007-10-115600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tchernia G, Mielot F, Subtil E, Parmentier C. Acute myeloblastic leukemia after immunodepressive therapy for primary nonmalignant disease. Nouv Rev Fr Hematol Blood Cells. 1976;17(1–2):67–80. doi: 10.1007/978-3-642-66312-3_5. [DOI] [PubMed] [Google Scholar]
- 78.Aymard JP, Witz F, Conroy T, Lederlin P, Humbert JC, Barroche G, et al. [Acute leukemia secondary to the treatment of multiple sclerosis with chlorambucil] Rev Neurol (Paris) 1985;141(2):152–4. [PubMed] [Google Scholar]
- 79.Then Bergh F, Niklas A, Strauss A, von Ahsen N, Niederwieser D, Schwarz J, et al. Rapid progression of Myelodysplastic syndrome to acute myeloid leukemia on sequential azathioprine, IFN-beta and copolymer-1 in a patient with multiple sclerosis. Acta Haematol. 2006;116(3):207–10. doi: 10.1159/000094683. [DOI] [PubMed] [Google Scholar]
- 80.Vicari AM, Ciceri F, Folli F, Lanzi R, Colombo B, Comi G, et al. Acute promyelocytic leukemia following mitoxantrone as single agent for the treatment of multiple sclerosis. Leukemia. 1998;12(3):441–2. doi: 10.1038/sj.leu.2400915. [DOI] [PubMed] [Google Scholar]
- 81.Heesen C, Bruegmann M, Gbdamosi J, Koch E, Monch A, Buhmann C. Therapy-related acute myelogenous leukaemia (t-AML) in a patient with multiple sclerosis treated with mitoxantrone. Mult Scler. 2003;9(2):213–4. doi: 10.1191/1352458503ms891xx. [DOI] [PubMed] [Google Scholar]
- 82.Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21(11):2123–37. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 83.Novoselac AV, Reddy S, Sanmugarajah J. Acute promyelocytic leukemia in a patient with multiple sclerosis following treatment with mitoxantrone. Leukemia. 2004;18(9):1561–2. doi: 10.1038/sj.leu.2403417. [DOI] [PubMed] [Google Scholar]
- 84.Delisse B, de Seze J, Mackowiak A, N'Kendjuo JB, Verier A, Derepeer O, et al. Therapy related acute myeloblastic leukaemia after mitoxantrone treatment in a patient with multiple sclerosis. Mult Scler. 2004;10(1):92. doi: 10.1191/1352458504ms977xx. [DOI] [PubMed] [Google Scholar]
- 85.Debouverie M, Taillandier L, Pittion-Vouyovitch S, Louis S, Vespignani H. Clinical follow-up of 304 patients with multiple sclerosis three years after mitoxantrone treatment. Mult Scler. 2007;13(5):626–31. doi: 10.1177/1352458506072543. [DOI] [PubMed] [Google Scholar]
- 86.Tanasescu R, Debouverie M, Pittion S, Anxionnat R, Vespignani H. Acute myeloid leukaemia induced by mitoxantrone in a multiple sclerosis patient. J Neurol. 2004;251(6):762–3. doi: 10.1007/s00415-004-0439-7. [DOI] [PubMed] [Google Scholar]
- 87.Voltz R, Starck M, Zingler V, Strupp M, Kolb HJ. Mitoxantrone therapy in multiple sclerosis and acute leukaemia: a case report out of 644 treated patients. Mult Scler. 2004;10(4):472–4. doi: 10.1191/1352458504ms1047cr. [DOI] [PubMed] [Google Scholar]
- 88.Arruda WO, Montu MB, de Oliveira Mde S, Ramina R. Acute myeloid leukaemia induced by mitoxantrone: case report. Arq Neuropsiquiatr. 2005;63(2A):327–9. doi: 10.1590/s0004-282x2005000200024. [DOI] [PubMed] [Google Scholar]
- 89.Ramtahal J, Jacob A, Das K, Boggild M. Sequential maintenance treatment with glatiramer acetate after mitoxantrone is safe and can limit exposure to immunosuppression in very active, relapsing remitting multiple sclerosis. J Neurol. 2006;253(9):1160–4. doi: 10.1007/s00415-006-0178-z. [DOI] [PubMed] [Google Scholar]
- 90.Ledda A, Caocci G, Spinicci G, Cocco E, Mamusa E, La Nasa G. Two new cases of acute promyelocytic leukemia following mitoxantrone treatment in patients with multiple sclerosis. Leukemia. 2006;20(12):2217–8. doi: 10.1038/sj.leu.2404443. [DOI] [PubMed] [Google Scholar]
- 91.Le Page E, Leray E, Taurin G, Coustans M, Chaperon J, Edan G. [Mitoxantrone as induction therapy in aggressive relapsing remitting multiple sclerosis: a descriptive analysis of 100 consecutive patients] Rev Neurol (Paris) 2006;162(2):185–94. doi: 10.1016/s0035-3787(06)74998-0. [DOI] [PubMed] [Google Scholar]
- 92.Cordioli C, Cattaneo C, Capra R. Analysis of incidence, risk factors and prognosis of acute promyelocytic leukaemia related to mitoxantrone therapy in multiple sclerosis. Neurology. 2007;68(Suppl 1):A276. [Google Scholar]
- 93.Pielen A, Goffette S, Van Pesch V, Gille M, Sindic CJ. Mitoxantrone-related acute leukemia in two MS patients. Acta Neurol Belg. 2008;108(3):99–102. [PubMed] [Google Scholar]
- 94.Pascual AM, Tellez N, Bosca I, Mallada J, Belenguer A, Abellan I, et al. Revision of the risk of secondary leukaemia after mitoxantrone in multiple sclerosis populations is required. Mult Scler. 2009;15(11):1303–10. doi: 10.1177/1352458509107015. [DOI] [PubMed] [Google Scholar]
- 95.Woo DA, Collins RH, Rossman HS, Stüve O, Frohman EM. Mitoxantrone-associated leukemia in multiple sclerosis: case studies. Int J MS Care. 2008;10:41–6. [Google Scholar]
- 96.Hum S, Lapierre Y. A clinical retrospective report on mitoxantrone treatment in active multiple sclerosis patients. Neurology. 2009;72(Suppl 3):A237. [Google Scholar]
- 97.Ghalie RG, Mauch E, Edan G, Hartung HP, Gonsette RE, Eisenmann S, et al. A study of therapy-related acute leukaemia after mitoxantrone therapy for multiple sclerosis. Mult Scler. 2002;8(5):441–5. doi: 10.1191/1352458502ms836oa. [DOI] [PubMed] [Google Scholar]
- 98.Rammohan K, Kita M, Lynn D, Dawson D, Bennett R, AL-Sabbagh A, et al. Post-marketing reports of acute leukemia in mitoxantrone-treated multiple sclerosis patients. Multiple Sclerosis. 2008;497 [Google Scholar]
- 99.Martinelli V, Radaelli M, Straffi L, Rodegher M, Comi G. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci. 2009;30(Suppl 2):S167–70. doi: 10.1007/s10072-009-0142-7. [DOI] [PubMed] [Google Scholar]
- 100.Vickers M, Jackson G, Taylor P. The incidence of acute promyelocytic leukemia appears constant over most of a human lifespan, implying only one rate limiting mutation. Leukemia. 2000;14(4):722–6. doi: 10.1038/sj.leu.2401722. [DOI] [PubMed] [Google Scholar]
- 101.Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 74(18):1463–70. doi: 10.1212/WNL.0b013e3181dc1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellis R, Boggild M. Therapy-related acute leukaemia with Mitoxantrone: what is the risk and can we minimise it? Mult Scler. 2009;15(4):505–8. doi: 10.1177/1352458508100967. [DOI] [PubMed] [Google Scholar]
- 103.Ammatuna E, Montesinos P, Hasan SK, Ramadan SM, Esteve J, Hubmann M, et al. Presenting features and treatment outcome of acute promyelocytic leukemia arising after multiple sclerosis. Haematologica. 96(4):621–5. doi: 10.3324/haematol.2010.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pulsoni A, Pagano L, Lo Coco F, Avvisati G, Mele L, Di Bona E, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100(6):1972–6. doi: 10.1182/blood-2001-12-0312. [DOI] [PubMed] [Google Scholar]
- 105.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 106.Bertsias G, Gordon C, Boumpas DT. Clinical trials in systemic lupus erythematosus (SLE): lessons from the past as we proceed to the future--the EULAR recommendations for the management of SLE and the use of endpoints in clinical trials. Lupus. 2008;17(5):437–42. doi: 10.1177/0961203308090031. [DOI] [PubMed] [Google Scholar]
- 107.Deaton JG, Levin WC. Systemic lupus erythematosus and acute myeloblastic leukemia. Report of their coexistence and a survey of possible associating features. Arch Intern Med. 1967;120(3):345–8. [PubMed] [Google Scholar]
- 108.Rosner F, Grunwald H. Multiple myeloma terminating in acute leukemia. Report of 12 cases and review of the literature. Am J Med. 1974;57(6):927–39. doi: 10.1016/0002-9343(74)90171-5. [DOI] [PubMed] [Google Scholar]
- 109.Vismans JJ, Briet E, Meijer K, den Ottolander GJ. Azathioprine and subacute myelomonocytic leukemia. Acta Med Scand. 1980;207(4):315–9. doi: 10.1111/j.0954-6820.1980.tb09727.x. [DOI] [PubMed] [Google Scholar]
- 110.Ng HS, Ng HW, Sinniah R, Feng PH. A case of systemic lupus erythematosus with sider-oblastic anaemia terminating in erythroleukaemia. Ann Rheum Dis. 1981;40(4):422–6. doi: 10.1136/ard.40.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saxne T, Turesson I, Wollheim FA. Preleukemic syndrome simulating SLE. A case report. Acta Med Scand. 1982;212(6):421–4. doi: 10.1111/j.0954-6820.1982.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 112.Tsunematsu Y, Koide R, Sasaki M, Takahashi H. Acute myeloid leukemia with preceding systemic lupus erythematosus and autoimmune hemolytic anemia. Jpn J Clin Oncol. 1984;14(1):107–13. [PubMed] [Google Scholar]
- 113.Paolozzi FP, Goldberg J. Acute granulocytic leukemia following systemic lupus erythematosus. Am J Med Sci. 1985;290(1):32–5. doi: 10.1097/00000441-198507000-00006. [DOI] [PubMed] [Google Scholar]
- 114.Gibbons RB, Westerman E. Acute nonlymphocytic leukemia following short-term, intermittent, intravenous cyclophosphamide treatment of lupus nephritis. Arthritis Rheum. 1988;31(12):1552–4. doi: 10.1002/art.1780311212. [DOI] [PubMed] [Google Scholar]
- 115.Taguchi F, Miyoshi T, Nakajima N, Nishimura J, Nawata H. [Acute promyelocytic leukemia developed in the course of systemic lupus erythematosus: a case report] Rinsho Ketsueki. 1990;31(12):1965–6. [PubMed] [Google Scholar]
- 116.Vasquez S, Kavanaugh AF, Schneider NR, Wacholtz MC, Lipsky PE. Acute nonlymphocytic leukemia after treatment of systemic lupus erythematosus with immunosuppressive agents. J Rheumatol. 1992;19(10):1625–7. [PubMed] [Google Scholar]
- 117.Colovic M, Jankovic G, Lazarevic V, Novak A. Acute megakaryoblastic leukaemia in a patient with systemic lupus erythematosus. Med Oncol. 1997;14(1):31–4. doi: 10.1007/BF02990942. [DOI] [PubMed] [Google Scholar]
- 118.Meloni G, Capria S, Vignetti M, Mandelli F, Modena V. Blast crisis of chronic myelogenous leukemia in long-lasting systemic lupus erythematosus: regression of both diseases after autologous bone marrow transplantation. Blood. 1997;89(12):4659. [PubMed] [Google Scholar]
- 119.Eilertsen GO, Nossent JC. Erythroleukaemia complicating ANA-negative systemic lupus erythematosus. Scand J Rheumatol. 2007;36(6):478–80. doi: 10.1080/03009740701483063. [DOI] [PubMed] [Google Scholar]
- 120.Lofstrom B, Backlin C, Sundstrom C, Hellstrom-Lindberg E, Ekbom A, Lundberg IE. Myeloid leukaemia in systemic lupus erythematosus--a nested case-control study based on Swedish registers. Rheumatology (Oxford) 2009;48(10):1222–6. doi: 10.1093/rheumatology/kep204. [DOI] [PubMed] [Google Scholar]
- 121.Bernatsky S, Ramsey-Goldman R, Clarke A. Exploring the links between systemic lupus erythematosus and cancer. Rheum Dis Clin North Am. 2005;31(2):387–402. viii–ix. doi: 10.1016/j.rdc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Tarr T, Gyorfy B, Szekanecz E, Bhattoa HP, Zeher M, Szegedi G, et al. Occurrence of malignancies in Hungarian patients with systemic lupus erythematosus: results from a single center. Ann NY Acad Sci. 2007;1108:76–82. doi: 10.1196/annals.1422.008. [DOI] [PubMed] [Google Scholar]
- 123.Moss KE, Ioannou Y, Sultan SM, Haq I, Isenberg DA. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis. 2002;61(5):409–13. doi: 10.1136/ard.61.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernatsky S, Clarke A, Gladman DD, Urowitz M, Fortin PR, Barr SG, et al. Mortality related to cerebrovascular disease in systemic lupus erythematosus. Lupus. 2006;15(12):835–9. doi: 10.1177/0961203306073133. [DOI] [PubMed] [Google Scholar]
- 125.Kasifoglu T, Korkmaz C, Yasar S, Gulbas Z. Scleroderma and chronic myeloid leukemia: a sheer coincidence, a consequence of long lasting D-penicillamine therapy or a plausible relationship of both diseases? Rheumatol Int. 2006;27(2):175–7. doi: 10.1007/s00296-006-0167-7. [DOI] [PubMed] [Google Scholar]
- 126.Senel S, Kaya E, Aydogdu I, Erkurt MA, Kuku I. Rheumatic diseases and chronic myelogenous leukemia, presentation of four cases and review of the literature. Rheumatol Int. 2006;26(9):857–61. doi: 10.1007/s00296-005-0100-5. [DOI] [PubMed] [Google Scholar]
- 127.Watanabe S, Sugihara T, Takahashi M, Ata K, Kanzaki A, Yamada O, et al. [Concordant improvement of progressive systemic sclerosis and chronic myelogenous leukemia with interferon-alpha treatment] Rinsho Ketsueki. 1994;35(9):895–7. [PubMed] [Google Scholar]
- 128.Sidi Y, Fadilah R, Pinkhas J, Prokocimer M. Systemic sclerosis and chronic lymphocytic leukaemia. Postgrad Med J. 1990;66(782):1071–2. doi: 10.1136/pgmj.66.782.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Angeli C, Lacour JP, Taillan B, Ortonne JP. [Chronic lymphoid leukemia in a case of systemic scleroderma] Presse Med. 1991;20(9):426. [PubMed] [Google Scholar]
- 130.Charlanne H, Lambert M, Hachulla E, Launay D, Queyrel V, Hatron PY, et al. Large granular lymphocyte leukaemia associated with systemic sclerosis. Rheumatology (Oxford) 2004;43(9):1197–8. doi: 10.1093/rheumatology/keh293. [DOI] [PubMed] [Google Scholar]
- 131.Blanche P, Bachmeyer C, Mikdame M, Dreyfus F, Sicard D. Scleroderma, polymyositis, and hairy cell leukemia. J Rheumatol. 1995;22(7):1384–5. [PubMed] [Google Scholar]
- 132.Cavallero GB, Bonferroni M, Gallamini A, Grasso M, Carbone A. Scleroderma and hairy-cell leukemia. Eur J Haematol. 1994;52(3):189–90. doi: 10.1111/j.1600-0609.1994.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 133.Constans J, Cadenne M, Schaeverbeke T, Leman C, Aupy M, Broustet A, et al. [Systemic scleroderma and hairy cell leukemia. A new case] Rev Rhum Ed Fr. 1993;60(5):371–2. [PubMed] [Google Scholar]
- 134.Nakanishi H, Takehara K, Soma Y, Ishibashi Y. Atypical scleroderma associated with multiple myeloma. Dermatologica. 1989;178(3):176–8. doi: 10.1159/000248420. [DOI] [PubMed] [Google Scholar]
- 135.Gouveris H, Hansen T, Franke K. Solitary extramedullary plasmacytoma and granulomatous sialadenitis of the parotid gland preceding a B-cell non-Hodgkin's lymphoma. Mund Kiefer Gesichtschir. 2006;10(2):122–5. doi: 10.1007/s10006-006-0673-5. [DOI] [PubMed] [Google Scholar]
- 136.Pulik M, Teillet-Thiebaud F, Mahe A, Teillet F. [Non-Hodgkin's lymphoma associated with scleroderma] Presse Med. 1991;20(31):1513–4. [PubMed] [Google Scholar]
- 137.Duggal L, Gupta S, Aggarwal PK, Sachar VP, Bhalla S. Hodgkin's disease and scleroderma. J Assoc Physicians India. 2002;50:1186–8. [PubMed] [Google Scholar]
- 138.Szekanecz E, Szamosi S, Gergely L, Keszthelyi P, Szekanecz Z, Szucs G. Incidence of lymphoma in systemic sclerosis: a retrospective analysis of 218 Hungarian patients with systemic sclerosis. Clin Rheumatol. 2008;27(9):1163–6. doi: 10.1007/s10067-008-0925-x. [DOI] [PubMed] [Google Scholar]
- 139.Golovanova OE, Pechorina EG. [Association of systemic scleroderma with lymphocytic leukemia] Ter Arkh. 1978;50(6):131–4. [PubMed] [Google Scholar]
- 140.Barnard RD, Appel A. Scleroderma associated with malignant estrapenic leukoblastosis ("leukemia"); case report illustrating therapeutic response to orally administered crude "B" fermentation concentrates. Urol Cutaneous Rev. 1950;54(6):345–8. [PubMed] [Google Scholar]
- 141.Dupond JL, Humbert P, Fest T, de Wazieres B. [Scleroderma, Gougerot-Sjogren syndrome and myelomonocytic leukemia] Rev Rhum Mal Osteoartic. 1989;56(5):425–6. [PubMed] [Google Scholar]
- 142.Gilman PA, Holtzman NA. Acute lymphoblastic leukemia in a patient receiving penicillamine for Wilson's disease. JAMA. 1982;248(4):467–8. [PubMed] [Google Scholar]
- 143.Clausen JE, Arndal JC, Gram L, Kudahl SB. Chronic lymphocytic leukaemia after treatment with penicillamine. Lancet. 1978;2(8081):152. doi: 10.1016/s0140-6736(78)91532-5. [DOI] [PubMed] [Google Scholar]
- 144.Ramenghi U, Bonissoni S, Migliaretti G, DeFranco S, Bottarel F, Gambaruto C, et al. Deficiency of the Fas apoptosis pathway without Fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood. 2000;95(10):3176–82. [PubMed] [Google Scholar]
- 145.Koike T. The new era of autoimmune disease research. Arthritis Res Ther. 2011;13(3):113. doi: 10.1186/ar3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7(4):321–8. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 147.Mullauer L, Gruber P, Sebinger D, Buch J, Wohlfart S, Chott A. Mutations in apoptosis genes: a pathogenetic factor for human disease. Mutat Res. 2001;488(3):211–31. doi: 10.1016/s1383-5742(01)00057-6. [DOI] [PubMed] [Google Scholar]
- 148.Cerutti E, Campagnoli MF, Ferretti M, Garelli E, Crescenzio N, Rosolen A, et al. Co-inherited mutations of Fas and caspase-10 in development of the autoimmune lymphoproliferative syndrome. BMC Immunol. 2007;8:28. doi: 10.1186/1471-2172-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Telegina E, Reshetnyak T, Moshnikova A, Proussakova O, Zhukova A, Kuznetsova A, et al. A possible role of Fas-ligand-mediated "reverse signaling" in pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Immunol Lett. 2009;122(1):12–7. doi: 10.1016/j.imlet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 150.Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14(19):2009–23. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- 151.Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Basecke J, et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25(7):1080–94. doi: 10.1038/leu.2011.66. [DOI] [PubMed] [Google Scholar]
- 152.Patel RK, Mohan C. PI3K/AKT signaling and systemic autoimmunity. Immunol Res. 2005;31(1):47–55. doi: 10.1385/IR:31:1:47. [DOI] [PubMed] [Google Scholar]
- 153.Venable JD, Ameriks MK, Blevitt JM, Thurmond RL, Fung-Leung WP. Phosphoinositide 3-kinase gamma (PI3Kgamma) inhibitors for the treatment of inflammation and autoimmune disease. Recent Pat Inflamm Allergy Drug Discov. 4(1):1–15. doi: 10.2174/187221310789895603. [DOI] [PubMed] [Google Scholar]
- 154.Tong XM, Wang JC, Shen Y, Xie JJ, Zhang JY, Jin J. Inhibition of inflammatory mediators and related signaling pathways by macrophage-stimulating protein in rheumatoid arthritis synovial fibroblasts. Inflamm Res. 2011;60(9):823–9. doi: 10.1007/s00011-011-0338-1. [DOI] [PubMed] [Google Scholar]
- 155.Besliu AN, Pistol G, Marica CM, Banica LM, Chitonu C, Ionescu R, et al. PI3K/Akt signaling in peripheral T lymphocytes from systemic lupus erythematosus patients. Roum Arch Microbiol Immunol. 2009;68(2):69–79. [PubMed] [Google Scholar]
- 156.Wu T, Mohan C. The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets. 2009;9(2):145–50. doi: 10.2174/187153009788452417. [DOI] [PubMed] [Google Scholar]
- 157.Kostenko S, Dumitriu G, Laegreid KJ, Moens U. Physiological roles of mitogen-activated-protein-kinase-activated p38-regulated/activated protein kinase. World J Biol Chem. 2011;2(5):73–89. doi: 10.4331/wjbc.v2.i5.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86(2):237–50. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 159.Scaldaferri F, Correale C, Gasbarrini A, Danese S. Molecular signaling blockade as a new approach to inhibit leukocyte-endothelial interactions for inflammatory bowel disease treatment. Cell Adh Migr. 2009;3(3):296–9. doi: 10.4161/cam.3.3.9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arum CJ, Anderssen E, Viset T, Kodama Y, Lundgren S, Chen D, et al. Cancer immunoediting from immunosurveillance to tumor escape in microvillus-formed niche: a study of syngeneic orthotopic rat bladder cancer model in comparison with human bladder cancer. Neoplasia. 2010;12(6):434–42. doi: 10.1593/neo.91824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659(1–2):15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 163.de Visser KE, Jonkers J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr Pharm Des. 2009;15(16):1844–53. doi: 10.2174/138161209788453239. [DOI] [PubMed] [Google Scholar]
- 164.Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, et al. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25(51):6781–99. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 165.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun. 2009;10(5):509–16. doi: 10.1038/gene.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Young NS. The problem of clonality in aplastic anemia: Dr Dameshek's riddle, restated. Blood. 1992;79(6):1385–92. [PubMed] [Google Scholar]
- 168.Hattan D, Cerilli GJ. Spontaneous reticulum cell sarcomas developing in C3H-HeJ mice on prolonged immunosuppressive therapy. Transplantation. 1971;11(6):580–1. [PubMed] [Google Scholar]
- 169.Koranda FC, Loeffler RT, Koranda DM, Penn I. Accelerated induction of skin cancers by ultraviolet radiation in hairless mice treated with immunosuppressive agents. Surg Forum. 1975;26:145–6. [PubMed] [Google Scholar]
- 170.Nehlsen SL. Immunosuppression, virus and oncogenesis in mice. Transplant Proc. 1971;3(1):811–3. [PubMed] [Google Scholar]
- 171.Chalastanis A, Penard-Lacronique V, Svrcek M, Defaweux V, Antoine N, Buhard O, et al. Azathioprine-induced carcinogenesis in mice according to Msh2 genotype. J Natl Cancer Inst. 2010;102(22):1731–40. doi: 10.1093/jnci/djq389. [DOI] [PubMed] [Google Scholar]
- 172.Hasan SK, Ottone T, Schlenk RF, Xiao Y, Wiemels JL, Mitra ME, et al. Analysis of t(15;17) chromosomal breakpoint sequences in therapy-related versus de novo acute promyelocytic leukemia: association of DNA breaks with specific DNA motifs at PML and RARA loci. Genes Chromosomes Cancer. 2010;49(8):726–32. doi: 10.1002/gcc.20783. [DOI] [PubMed] [Google Scholar]
- 173.Leone G, Pagano L, Ben-Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92(10):1389–98. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 174.Kiran S, Cocco P, Mannetje A, Satta G, D'Andrea I, Becker N, et al. Occupational Exposure to Ethylene Oxide and Risk of Lymphoma. Epidemiology. 2010;21(6):905–10. doi: 10.1097/EDE.0b013e3181f4cc0f. [DOI] [PubMed] [Google Scholar]
- 175.Sweeney DM, Manzi S, Janosky J, Selvaggi KJ, Ferri W, Medsger TA, Jr, et al. Risk of malignancy in women with systemic lupus erythematosus. J Rheumatol. 1995;22(8):1478–82. [PubMed] [Google Scholar]
- 176.Menon S, Snaith ML, Isenberg DA. The association of malignancy with SLE: an analysis of 150 patients under long-term review. Lupus. 1993;2(3):177–81. doi: 10.1177/096120339300200309. [DOI] [PubMed] [Google Scholar]