Abstract
Background
Mobilization of hematopoietic stem/progenitor cells from the bone marrow to the peripheral blood by granulocyte colony-stimulating factor is the primary means to acquire stem cell grafts for hematopoietic cell transplantation. Since hematopoietic stem/progenitor cells represent a minority of all blood cells mobilized by granulocyte colony-stimulating factor, the underlying mechanisms need to be understood in order to develop selective drugs.
Design and Methods
We analyzed phenotypic, biochemical and genetic changes in bone marrow cell populations from granulocyte colony-stimulating factor-mobilized and control mice, and linked such changes to effective mobilization of hematopoietic stem/progenitor cells.
Results
We show that granulocyte colony-stimulating factor indirectly reduces expression of surface vascular cell adhesion molecule 1 on bone marrow hematopoietic stem/progenitor cells, stromal cells and endothelial cells by promoting the accumulation of microRNA-126 (miR126)-containing microvescicles in the bone marrow extracellular compartment. We found that hematopoietic stem/progenitor cells, stromal cells and endothelial cells readily incorporate these miR126-loaded microvescicles, and that miR126 represses vascular cell adhesion molecule 1 expression on bone marrow hematopoietic stem/progenitor cells, stromal cells and endothelial cells. In line with this, miR126-null mice displayed a reduced mobilization response to granulocyte colony-stimulating factor.
Conclusions
Our results implicate miR126 in the regulation of hematopoietic stem/progenitor cell trafficking between the bone marrow and peripheral sites, clarify the role of vascular cell adhesion molecule 1 in granulocyte colony-stimulating factor-mediated mobilization, and have important implications for improved approaches to selective mobilization of hematopoietic stem/progenitor cells.
Keywords: hematopoietic stem and progenitor cells, miR126, VCAM-1, G-CSF, mobilization
Introduction
Hematopoietic stem/progenitor cells (HSPC) reside in bone marrow niches that support their survival and function.1 A variety of compounds can induce mobilization of HSPC from the bone marrow to the peripheral circulation. In the clinical setting, granulocyte colony-stimulating factor (G-CSF) is the most commonly used inducer of HSPC mobilization, which has become the preferred source of HSPC for autologous and allogeneic hematopoietic reconstitution.2,3 Although it is effective and safe, G-CSF mobilizes committed myeloid cells in vast excess of HSPC, and these cells are mobilized only after 5 to 7 days of treatment.4 Thus, a better understanding of the mechanisms of action of G-CSF could provide a means to pursue a more selective and rapid mobilization of HSPC.
Mechanistic studies demonstrated that expression of G-CSF receptor (R) in hematopoietic cells is required for G-CSF-induced HSPC mobilization.5 However, hematopoietic stem cells do not generally express G-CSFR,6 and transplant studies demonstrated that G-CSF mobilizes HSPC that do or do not express G-CSFR equally effectively,5 suggesting that G-CSF induces HSPC mobilization indirectly. Cell depletion and other genetic experiments provided evidence that neutrophils and/or monocytes are key intermediate regulators of HSPC mobilization by G-CSF.7,8
Compelling evidence established that CXCR4 and its unique ligand SDF1/CXCL12 are essential for the retention of granulocytes and other myeloid-lineage cells in the bone marrow, and that disruption of CXCR4 signaling is sufficient for mobilization of neutrophils and other myeloid-lineage cells to the peripheral circulation.9–12 Patients with WHIM (warts, hypogammaglobulinemia, infections, myelokathexis) are neutropenic because of reduced neutrophil mobilization attributable, in most cases, to “gain-of-function” mutations of CXCR4.13,14 Selective deletion of CXCR4 in myeloid cells causes a redistribution of neutrophils from the bone marrow to the blood resulting in neutrophilia.10 AMD3100, a synthetic inhibitor of SDF1 binding to CXCR4, promotes neutrophil mobilization when injected into mice and humans.11
G-CSF reduces CXCR4 expression in myeloid cells,15,16 and SDF1 expression in bone marrow stromal cells, osteoblasts and endothelial cells, which are the main source of SDF1 at this site.12,17–19 G-CSF also promotes the release of neutrophil proteases, including neutrophil elastase, cathepsin-G and MMP-9, generating a proteolytic environment in the bone marrow.7,20 Yet, the linkage between those changes induced directly by G-CSF in neutrophils, monocyte/macrophages and potentially other cells, and the indirect HSPC mobilizing effect of G-CSF is unclear. Some studies suggested that CXCR4 is critical to mobilization of hematopoietic progenitors by G-CSF,17 but other mechanisms have also been proposed and conclusive evidence is lacking.2,7,20
Based on the observation that neutrophils and monocytes are critical to HSPC mobilization by G-CSF, we hypothesized that at least some of the changes induced by G-CSF on these cells may serve to promote the accompanying HSPC mobilization. We further considered the possibility that agents which more selectively promote the mobilization of HSPC in preference to neutrophils, such as BIO5192 a small molecule inhibitor of very latent antigen 4 (VLA4; also known as α4β1 integrin) binding to vascular cell adhesion molecule 1 (VCAM1)21 and antibodies to VLA4 or VCAM1,22–26 may provide valuable mechanistic insights into G-CSF-induced HSPC mobilization.
Design and Methods
Mice
All mice (C57BL/6; 4–8 weeks of age) were housed in animal facilities at the National Institutes of Health; animal studies approved by the NCI Institutional Animal Care and Use Committee were conducted in accordance with institutional guidelines. G-CSF mobilization was induced as described previously.27
Cells and microvesicles preparation
Bone marrow cells were obtained by flushing femora and tibiae; Lin− cells were derived with Mouse Lineage Cell Depletion Kit (Miltenyi Biotech) as described by the manufacturer. Progenitor cells were derived from Lin− cells by positive sorting of live (DAPI-negative or with LIVE/DEAD kit, Invitrogen/Molecular Probes) APC-labeled Sca-1brightAPCeFluor 780-labeled cKitbright cells, gating out cells expressing CD45, TER119, 7–4, CD11b, CD19, Ly-6G/C and CD5 (all biotin-labeled antibodies followed by FITC-streptavin). Lin−Ter115−CD45− cells were sorted (FACSVantage SE; BD Biosciences) from Lin− cells after staining with CD45 and TER119 biotin-labeled antibodies and FITC-streptavin. VE-cadherin+ cells were obtained from Lin− cells by positive sorting of live, APC-labeled VE-cadherin+ cells (all antibodies and FITC-streptavidin from BD Pharmingen). Microvesicles were prepared essentially as described elsewhere28 from bone marrow extracellular fluid. Details on microvescicle preparation and cell culture are provided in the Online Supplementary Design and Methods.
Transfection, RNA isolation and real-time polymerase chain reaction
A precursor to miR126 (premiR126, Ambion PM12841), a precursor control (Ambion 17110) or a CyTM3-labeled premiR control (AM17120) was transfected into freshly derived Lin− bone marrow cells (5×106 cells/transfection) or MS-5 cells (after trypsinization/washing 5×106 cells/transfection) using the Amaxa system (4DNucleofectorTM protocol for mouse T cells). After 18 h culture (bone marrow cells: 2×106 cells/mL, in DMEM supplemented with 10% serum, 25 ng/mL stem cell factor, 25 ng/mL cKit ligand and 10 ng/mL interleukin-3; MS-5 cells: 1×106 cells/mL in αMEM supplemented with 10% fetal bovine serum), transfection efficiency was assessed by CyTM3 (premiR control) and DAPI fluorescence detected by flow cytometry. We isolated total RNA using TRIsol reagent (Invitrogen). Real-time polymerase chain reactions (PCR) were performed using Assay-on-Demand Taqman probes for mouse CXCR4, CXCR2, VCAM1, and GAPDH (Applied Biosystems). We used a miRVANA isolation kit to isolate miR, a Taqman microRNA reverse transcription kit for amplification, and miR126 and miR16 Taqman probes (all from Applied Biosystems) for the real-time PCR.
Flow cytometry, immunocytochemistry and immunoblotting
Cells were stained with panels of antibodies, analyzed by FACSCalibur cytofluorometer (BD Biosciences) and the results acquired from 104 live cells analyzed with CELLQuest software (BD Biosciences). Details on flow cytometry are provided in the Online Supplementary Design and Methods.
Fluorescence and bright-field images were acquired through a Nikon Eclipse E600 microscope equipped with Plan Apo 40X/0.95 DIC M, 60X/1.40 oil DIC H, and 100X/1.40 oil DIC H lenses, and photographed with a digital camera (Retiga 1300, Qimaging). Images obtained with IPLab for Windows software (Scanalytics) were imported into Adobe Photoshop.
Protein extracts prepared in NP-40 lysis buffer with protease inhibitor cocktail setIII (Calbiochem), 50 mM NaF, and 1 mM sodium orthovanadate were resolved through 4–12% Bis Tris gels (Invitrogen). After protein transfer, the nitrocellulose membranes were immunoblotted with specific rat anti-mouse antibodies to VCAM1/CD106 (R&D Systems) and re-probed with goat IgG anti-actin antibodies (Santa Cruz Biotechnology).
Statistical analysis
Group differences were evaluated by the non-parametric Mann-Whitney U test for sample sizes ≥5 and the two-tailed Student’s t test for sample sizes ≤5. P values less than 0.05 were considered statistically significant.
Results
Granulocyte colony-stimulating factor modulates surface vascular cell adhesion molecule 1 in bone marrow hematopoietic stem/progenitor cells and non-hematopoietic stromal and endothelial cells
Administration of G-CSF induces characteristic changes in the expression of CXCR4 and CXCR2 in bone marrow myeloid cells.15,29 We now examined whether such changes are also observed in HSPC, which account for <0.5% of bone marrow cells and include cells with hematopoietic reconstitution potential. Administration of G-CSF significantly reduced (P<0.05) surface CXCR4 levels in unselected bone marrow cells (“All cells”), in Gr1lo (immature neutrophils and monocytes) and in Gr1hi (mature neutrophils; do not stain for the monocyte marker CD115/M-CSFR) (Online Supplementary Figure S1A). By contrast, G-CSF administration was not associated with a significant change in either the percentage or mean fluorescence intensity (MFI, data not shown) of surface CXCR4+ cells within the Lin−cKithiSca-1hi cell population (Online Supplementary Figure S1B). In addition, surface expression of CXCR7, the alternative SDF1 receptor, was low and minimally changed in Lin−cKithiSca-1hi cells after G-CSF administration (data not shown). Consistently, we found that Lin−cKithiSca-1hi cells from control and G-CSF-mobilized mice responded similarly to SDF-1 in trans-well migration assays over a wide range (1–100 ng/mL) of chemokine concentrations (Online Supplementary Figure S1C).
G-CSF administration induced a significant increase in surface CXCR2 expression in “All” marrow cells, including the Gr1lo and Gr1hi cell populations (P<0.05 all comparisons) (Online Supplementary Figure S1D), but not within the Lin−cKithiSca-1hi cell population (Online Supplementary Figure S1E). By real-time PCR, we found that levels of CXCR4 and CXCR2 mRNA were similar in Lin−cKithiSca-1hi cells sorted from untreated and G-CSF-mobilized bone marrows, but they differed markedly in the unsorted bone marrows (Online Supplementary Figure S2). These results indicated that administration of G-CSF is not typically associated with changes in surface CXCR4 and CXCR2 levels in bone marrow HSPC, and suggested that changes in surface CXCR4 and CXCR2 expression are not required for HSPC mobilization by G-CSF.
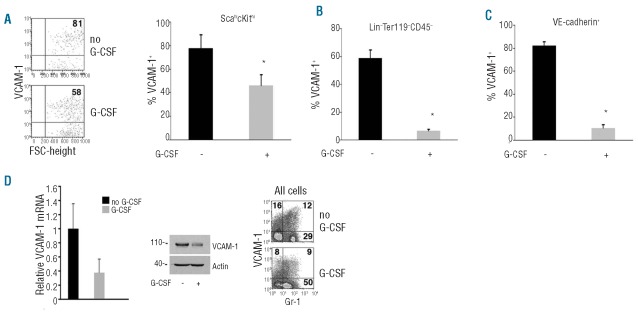
We looked for potential changes induced by G-CSF in HSPC, focusing on integrin α4 and VCAM1. Surface levels of integrin α4 expression were similar in Lin−cKithiSca-1hi cells from untreated and G-CSF-treated mice; virtually all Lin−cKithiSca-1hi cells expressed surface integrin α4 with similar MFI within bone marrows from control and G-CSF-treated mice (data not shown). By contrast, we found that G-CSF mobilization was associated with a statistically significant (P<0.05) decrease in the proportion of surface VCAM1-positive Lin−cKithiSca-1hi cells (Figure 1A). VCAM1 MFI was also reduced on Lin−cKithiSca-1hi cells from a mean of 572 units to a mean of 304 units. However, we found no difference in the levels of VCAM1 mRNA in sorted Lin−cKithiSca-1hi cells from non-mobilized and G-CSF-mobilized mice (Online Supplementary Figure S3). The reduction in the proportion of surface VCAM1-positive HSPC was comparable in magnitude to the reduction in bone marrow HSPC induced by G-CSF.30
Figure 1.
G-CSF administration is associated with decreased surface VCAM1 expression on HSPC, Lin−Ter119−CD45− and VE-cadherin+ cells from the bone marrow. Cell surface VCAM1 in (A) Lin−cKithiSca-1hi cells (representative results, left; group results, right); (B) Lin−Ter119−CD45− and (C) VE-cadherin+ cells in bone marrow from mice mobilized with G-CSF or left untreated (5–10/group). Bar graphs reflect the means ± SD. (D) Relative levels of VCAM1 mRNA (left; measured by quantitative PCR; n=6), cell-associated protein (middle; immunoblotting results from 3 bone marrows combined) and cell surface (right; representative flow cytometry profile) in bone marrow cells from mice mobilized with G-CSF or left untreated. *denotes P<0.05.
We also found a marked reduction in the proportion of VCAM+ cells within the non-hematopoietic bone marrow Lin−Ter11−CD45− cells (which include stromal cells) (Figure 1B) and the Lin−VE-cadherin+ cells (likely endothelial cells) (Figure 1C).
A global reduction of VCAM1 expression induced by G-CSF was evident by analysis of mRNA, protein and surface expression in unselected bone marrow cells from G-CSF-mobilized and non-mobilized bone marrows (Figure 1D). These results raise the possibility that modulation of surface VCAM1 contributes to HSPC mobilization by G-CSF.
miR126 regulates vascular cell adhesion molecule 1 expression in hematopoietic and non-hematopoietic cells
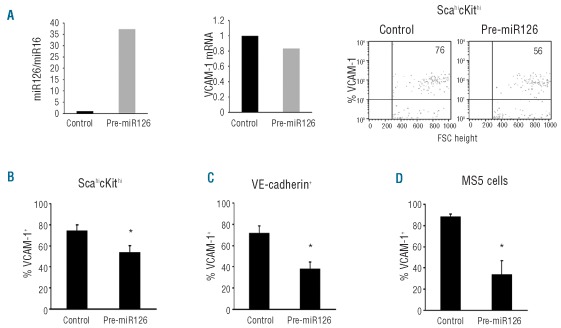
In silico analysis identified VCAM1 3′-UTR mRNA as a target of miR126; experimental studies documented that miR126 inhibits VCAM1 expression in endothelial cells.31 We examined whether miR126 can regulate VCAM1 expression in HSPC, in VE-cadherin+ bone marrow cells, and in the MS-5 bone marrow stromal cell line. We transfected Lin− bone marrow cells and MS-5 cells with a precursor RNA to miR126, a precursor control or with a CyTM3-labeled pre-miR control. After 18 h, 36–96% of the bone marrow cells and >62% of MS-5 cells transfected with the fluorescent pre-miR control were fluorescent (data not shown). In three experiments, pre-miR126 transfection of primary Lin− bone marrow cells increased levels of miR126 (relative to miR16 used as a control) by >35-fold in comparison to control (representative results, Figure 2A, left).
Figure 2.
miR126 regulates VCAM1 expression in bone marrow cells. (A) Transfection of pre-miR126 or a pre-miR control in Lin− bone marrow cells (representative experiment of 3): relative levels of miR126 (mir126/miR16; left), VCAM1 mRNA (middle), and surface VCAM1 (right) were measured 18 h after transfection. Surface levels of VCAM1 on Lin−cKithiSca-1hi (B), VE-cadherin+ (C) and MS5 cells (D) 18 h after transfection with pre-miR126 or control. The results are expressed as % means (n=3 ± SD). *denotes P<0.05.
Forced expression of miR126 in the Lin− bone marrow cells induced minimal change in the relative levels of VCAM1 mRNA (Figure 2A, middle), but caused a decrease in the proportion of surface VCAM1-positive Sca−1hicKithi cells within this population (Figure 2A, right). We confirmed that forced expression of miR126 in sorted Sca−1hicKithi cells (>22-fold increase in miR126 levels) reduced the percentage of Sca−1hicKithi cells that expressed surface VCAM1 (Figure 2B), and additionally found that forced expression of miR126 in VE-cadherin+ bone marrow cells (>28-fold increase in miR126 levels) reduced the percent surface VCAM1+ cells in bone marrow VE-cadherin-expressing cells compared to the control (Figure 2C). Similar effects were observed in MS-5 cells transfected (>25-fold increase in miR126 levels) with the miR126 precursor (Figure 2D). Thus, miR126 can reduce VCAM1 protein expression in HSPC and non-hematopoietic bone marrow cells without significantly affecting VCAM1 mRNA levels, similar to its previously reported effects in endothelial cells.31
Regulation of miR126 in bone marrow following granulocyte colony-stimulating factor mobilization
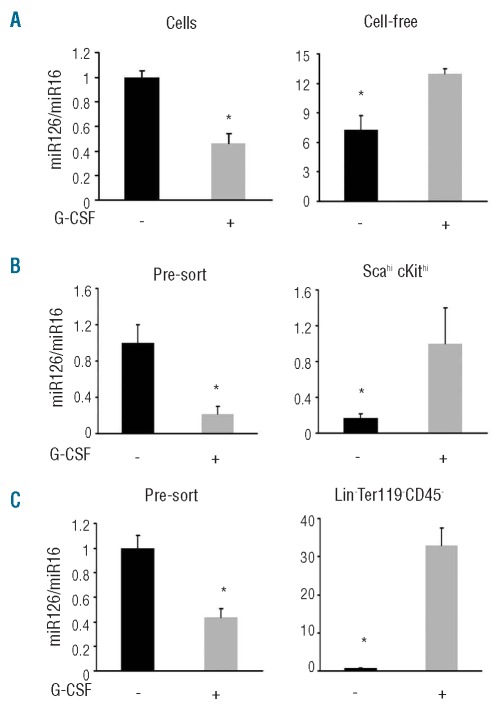
We examined whether G-CSF modulates levels of miR126 in bone marrow. GCSF mobilization significantly reduced levels of miR126 in cells (Figure 3A left), but increased levels of miR126 in the cell-free fraction of flushed bone marrows (obtained after removal of cells, filtration through 0.45 μm filters, ultracentrifugation and pellet suspension in 1/10th the original volume) (Figure 3A right). Similarly, addition of G-CSF in culture decreased somewhat the levels of miR126 in bone marrow cells and promoted the accumulation of miR126 in the culture supernatant after 24 h of culture (Online Supplementary Figure S4).
Figure 3.
G-CSF administration regulates miR126 distribution in the bone marrow. (A) G-CSF administration reduced relative levels of miR126 in bone marrow cells (left) and increased these levels in the cell-free fraction (right) (mean ± SD; 5–8 mice/group untreated and G-CSF-treated). (B) Relative levels of miR126 (miR126/miR16) in unfractionated bone marrow cells (Pre-sort) and in Lin−Sca-1hicKithi cells sorted from these cells. (C) Relative levels of miR126 in bone marrow cells and in Lin−Ter119−CD45− cells sorted from these cells. The results in B and C reflect relative mean (± SD) miR126 levels measured in nine untreated and nine G-CSF-mobilized bone marrows (3 each combined prior to sorting). *denotes P<0.05.
We measured the levels of miR126 in bone marrow HSPC and non-hematopoietic cells. The Lin−cKithiSca-1hi cells sorted from G-CSF-mobilized bone marrows (n=9; 3 bone marrows combined for 3 sortings) contained significantly more miR126 compared to the Lin−cKithiSca-1hi cells from untreated mice (n=9) (Figure 3B). Similarly, the Lin−Ter119−CD45− cells sorted from G-CSF-mobilized bone marrows (n=9; 3 bone marrows combined for 3 sortings) contained significantly more miR126 than the Lin−Ter119−CD45− cells from untreated mice (n=9) (Figure 3C). We measured VCAM1 mRNA levels in these cell populations. The Lin−cKithiSca-1hi and the Lin−Ter119−CD45− cells sorted from G-CSF-mobilized or untreated bone marrows contained similar levels of VCAM1 mRNA (Online Supplementary Figure S5). Thus, G-CSF administration is associated with a complex redistribution of miR126 in the bone marrow: an overall reduction in cells with a relative increase in HSPC and Lin−Ter119−CD45− cells; and an increase in the extracellular bone marrow compartment.
Microvesicle production and transfer to hematopoietic stem/progenitor cells, and non-hematopoietic cell subsets
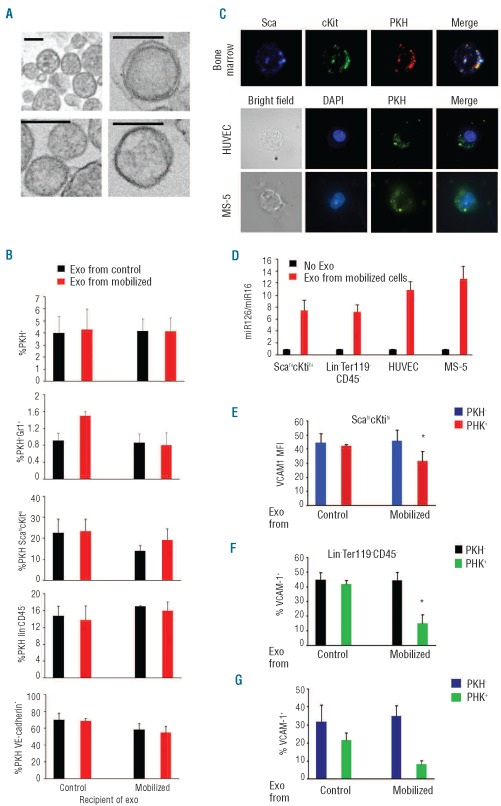
miRNA are found intracellularly and are released into the extracellular compartment through “exosomes” and apop-totic bodies, which play various roles in intracellular communication.32,33 Electron microscopy of the cell-free fraction from G-CSF-mobilized bone marrows revealed large numbers of vesicles resembling exosomes (Figure 4A) and others (not shown) morphologically similar to neutrophil-derived apoptotic bodies described in the bone marrow.34 Similar, but fewer, particles were visualized in the cell-free fraction of non-mobilized bone marrows (not shown).
Figure 4.
Microvesicle uptake by bone marrow cells and their effects on surface VCAM1 levels. (A) Electron microscopy images of microvesicles from the cell-free fraction of a G-CSF-mobilized bone marrow (scale bars=100 nm). (B) Uptake of PKH-labeled exosome preparations (Exo) from bone marrow of G-CSF-mobilized or untreated mice (5 bone marrows each) by (from the top): unfractionated, Gr1+, Sca-1hicKithi, Lin−CD45− and VE-cadherin+ bone marrow cell populations. The results are means ± SD. (C) Microscopy images of bone marrow cells co-expressing Sca-1 (blue), cKit (green) and PKH (red) after incubation with PKH-labeled exosomes (top); endothelial cells (HUVEC middle) and bone marrow stromal cells (MS-5 bottom) unstained (captured by the bright field) or stained for (blue) DAPI and (green) PKH (captured by fluorescent microscopy); original magnification (100x). (D) Relative levels of miR126 in bone marrow cell populations, HUVEC and MS-5 cells after incubation in medium only or with microvesicle preparations from G-CSF-mobilized bone marrows (means ± SD; 3 independent experiments). (E) VCAM1 MFI was measured on Sca-1hicKithi cells from bone marrows incubated with PKH-labeled exosome preparations from untreated or GCSF-mobilized bone marrows. VCAM1 MFI was measured on Sca-1hicKithi cells with or without microvesicle-derived fluorescence (means ± SD of 3 independent experiments). The % VCAM1+ cells was measured in Lin−Ter119−CD45− (F) and VE-cadherin+ (G) bone marrow cells that had either acquired or not acquired microvesicle-derived PKH fluorescence after 18 h of incubation. Microvescicles were from untreated and G-CSF-mobilized bone marrows. The results reflect the means ± SD of three independent experiments. *denotes P<0.05.
We examined whether miR126, detected in the purified microvesicles (Online Supplementary Figure S6), might be delivered by microvesicles to bone marrow HSPC and to subsets of non-hematopoietic cells. We labeled microvesicles (purified following protocols used for exosome preparation) from G-CSF-mobilized and non-mobilized bone marrow cells with a red (PKH26) or green (PKH67) fluorescent lipid dye, and after washing we incubated them (for 18 h) with cells from control or G-CSF-mobilized bone marrows (Figure 4B). A low proportion (2.8%-6.7%) of all bone marrow cells acquired red fluorescence, suggesting low-level microvesicle uptake by bone marrow cells. Only a minority (0.5%–1.6%) of Gr1+ cells picked up the dye, regardless of the source of the “exosome” preparations (bone marrow from G-CSF-treated or untreated mice) or the derivation of the Gr1+ cells from G-CSF-mobilized or not mobilized marrows (Figure 4B). By contrast, we found that a greater proportion of bone marrow Sca−1hicKithi HSPC (14%–24%), Lin−CD45− cells (14%–17%) and VE-cadherin+ cells (55%-70%; likely endothelial) acquired the microvesicle fluorescence in comparison to the Gr1+ cells, indicative of preferential uptake (Figure 4B). The source of the “exosome” preparations (from G-CSF mobilized or non-mobilized bone marrows) and the source of cells incubated with the microvesicles affected the magnitude of uptake minimally (Figure 4B). After incubation with PKH-labeled “exosome” preparations from G-CSF-mobilized bone marrows and imaging, we found that a proportion of bone marrow Sca−1hicKithi cells, primary human umbilical vein endothelial cells (HUVEC) and the mouse MS-5 cells exhibited a dot-like cytoplasmic fluorescence attributable to endocytosis of the labeled exosomes (Figure 4C). Consistent with exosome-mediated transfer of miR126, we found that the relative content of miR126 was increased in Sca−1hicKithi and in Lin−Ter119−CD45− bone marrow cells, and in HUVEC and MS-5 cells that had been incubated for 18 h with exosome preparations from G-CSF-mobilized bone arrows (Figure 4D).
We examined whether microvesicles from G-CSF-mobilized bone marrows, which contain more miR126 than those from non-mobilized bone marrows (Online Supplementary Figure S6), reduce VCAM1 cell surface expression. Gating on Sca-1hicKithi bone marrow progenitors that had incorporated the fluorescent microvesicles, we found that VCAM1 mean fluorescence intensity (MFI) was significantly reduced when the exosomes were derived from G-CSF-mobilized bone marrows (Figure 4E). When gating instead on the non-fluorescent Sca-1hicKithi cells, we found that VCAM1 MFI values changed minimally after incubation with the microvesicles (Figure 4E).
Gating on the Lin−Ter119−CD45− cells that had acquired the fluorescent microvescicles, we found a significant reduction in the percentage of VCAM1+ cells after incubation with microvesicles from G-CSF-mobilized in comparison to non-mobilized bone marrows (Figure 4F). No such VCAM1 reduction was observed in the Lin−Ter119−CD45−cells that had not acquired the fluorescent microvescicles (Figure 4F). A similar reduction of surface VCAM1 was observed in VE-cadherin+ bone marrow cells that had acquired the fluorescent microvesicles (Figure 4G). Thus, miR126-containing microvesicles from G-CSF-mobilized bone marrows can reduce levels of VCAM1 surface expression in HSPC, Lin−Ter119−CD45− and VE-cadherin+ cells.
Defective mobilization of hematopoietic stem/progenitor cells by granulocyte colony-stimulating factor in miR126-deficient mice
If miR126 is important for HSPC mobilization by G-CSF, miR126-deficient mice may be defective at mobilizing HSPC with G-CSF. Previous studies showed that miR126-null mice have a distinctive vascular phenotype with leaky vessels and hemorrhages; approximately 40–50% of mice die in utero or perinatally.35,36 We mobilized with G-CSF groups of 13-week old miR126+/+ and miR126−/− littermates and compared them to non-mobilized mice.
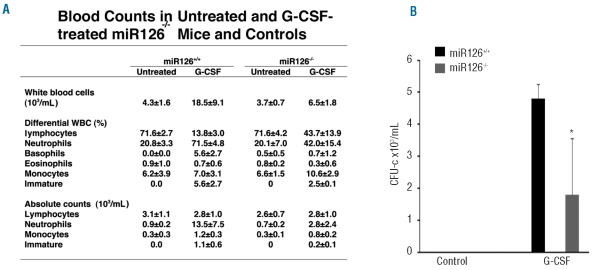
Blood cell counts showed that G-CSF treatment induced an increase in circulating white blood cells and neutrophils in miR126−/− mice, albeit lower than that displayed by their miR126+/+ littermates (Figure 5A). Colony assays showed that blood from the G-CSF-mobilized miR126−/− mice contained significantly fewer CFU-c compared to the miR126+/+ controls, whereas blood from untreated miR126+/+ and miR126−/− mice contained no colony precursors (Figure 5B). We detected some variability within the mobilized miR126−/− mice, consistent with the variability of other defective phenotypes in these mice.35 Thus, these results indicate that miR126-null mice are defective at mobilizing HSPC after G-CSF treatment.
Figure 5.
Defective HSPC mobilization in G-CSF-treated miR126-deficient mice. (A) Control miR126+/+ and miR126−/− littermates were mobilized with G-CSF or left untreated. Neutrophils, lymphocytes, monocytes and immature bone marrow cells were counted; the results reflect the group means ± SD (n=3). (B) CFU-c were measured in the peripheral blood of control miR126+/+ and miR126−/− littermates untreated or mobilized with G-CSF. The results represent group means ± SD (n=3) for the mobilized mice; no colonies developed from blood of untreated mice. *denotes P<0.05.
Discussion
In the present study, we show that administration of G-CSF is associated with reduced expression of VCAM1 in bone marrow HSPC, stromal and endothelial cells, and provide evidence that miR126 contributes to this reduction. Consistently, we found that miR126-deficient mice mobilize poorly in response to G-CSF. Thus, these results identify miR126 as a regulator of HSPC trafficking from the bone marrow to the peripheral blood, and clarify the role of VCAM1 in G-CSF mobilization. A schematic representation of this process is shown in Online Supplementary Figure S7.
Previous studies have established a critical role of VCAM1/VLA4 interactions in the retention and mobilization of HSPC from the bone marrow. Conditional deletion of VCAM1 or α4-integrin in hematopoietic, stromal and endothelial cells by a Tie2-driven cre transgene increased the release of hematopoietic progenitors from the bone marrow into the circulation.37,38 Antibodies to VCAM1, to α4 integrin,22,24,25 and BIO5192, a small molecule inhibitor of VLA4 (α4β1 integrin) binding to VCAM1,21 promoted the mobilization of HSPC in mice and/or humans, providing strong evidence that disruption of an interaction between VLA4 and VCAM1 promotes HSPC mobilization.37 However, a link between G-CSF mobilization of HSPC and modulation of VCAM1/VLA4 in the bone marrow has previously been missing. G-CSF was reported to lower VCAM1 expression in bone marrow stromal cells,20 and it was suggested that enzymatic cleavage of VCAM1 on these stromal cells removes a critical retention ligand for HSPC, which express VLA4.20,39,40 However, since enzyme-deficient mice mobilized HSPC normally with G-CSF, and since VCAM1-deficient HSPC mobilized normally when transplanted into normal recipients, it was concluded that a reduction in VCAM1 in bone marrow stromal cells is not required for the mobilization of HSPC by G-CSF.40,41 In contrast to this conclusion, mice lacking VCAM1 in hematopoietic, endothelial and stromal cells mobilized HSPC poorly in response to G-CSF, in spite of having a normal number of bone marrow progenitors.37
Apoptotic bodies and exosomes have emerged as important conduits for intercellular communication as they can transfer microRNA, mRNA and proteins to other cells and have, therefore, been implicated in many cell functions.33 Exosomes are found in many tissues, including the bone marrow and blood,42 and apoptotic bodies are abundant in the bone marrow as they are largely a physiological product of neutrophil death in this site.43 Interestingly, in the setting of atherosclerotic lesions, endothelium-derived apoptotic bodies were found to be enriched in miR126, and to trigger the incorporation of Sca1+ progenitors into the atherosclerotic plaques, in part through stimulation of SDF1 expression.33 In addition, human CD34+ peripheral blood HSPC mobilized by G-CSF contained significantly higher levels of miR126 compared to peripheral blood mononuclear cells.44
G-CSF regulation of CXCR4 has gained much attention as a critical pathway for HSPC release in response to G-CSF. While the role of CXCR4 in the mobilization of myeloid cells, particularly with a contribution of CXCR2, is well-documented,7,8,10,13–16,45 its role in the mobilization of HSPC is not well documented. Previous studies showed that G-CSF administration in man induces a gradual increase in the expression of CXCR4 on bone marrow progenitors (CD34+ or CD38+ cells) over 5 days of treatment.18 Here we found that CXCR4 and CXCR2 did not change significantly on bone marrow HSPC after G-CSF administration. Several studies have documented that CXCR4 antagonists promote HSPC mobilization.2,18,46 Based on the observation that G-CSF was ineffective at enhancing HSPC mobilization to blood and spleen of mice with CXCR4−/− bone marrows, but that an antagonist of VLA4/VCAM1 induced a 2-fold increase in HSPC mobilization,17 it was suggested that G-CSF is ineffective at mobilizing CXCR4-null HSPC. An alternative explanation, supported by the current results, is that the absence of CXCR4 on myeloid lineage cells prevents G-CSF-induced neutrophil/monocyte mobilization, which is indirectly required for HSPC mobilization by G-CSF as these cells are a source of miR-containing microvescicles. Recent studies have linked miR126 to the regulation of SDF1 expression in endothelial cells.33,47 Since G-CSF reduces the SDF1 content in the bone marrow,12,17–19 the role of miR126 in the regulation of bone marrow SDF1 is potentially important.
We found that G-CSF promotes the accumulation of miR126-containing microvescicles in the bone marrow extracellular compartment, and that miR126 delivered by these microvescicles reduces surface VCAM1 expression in bone marrow HSPC and other non-hematopoietic cells. VCAM1 is critical to the retention of HSPC in the bone marrow. Thus, our study provides new insights for understanding the pathways of HSPC mobilization and identifies miR126 as a mobilizing agent for HSPC.
Great progress has been made in the understanding of miR function and development of delivery technologies offering the possibility for miR to emerge as a new class of potentially important therapeutics.
Acknowledgments
We thank Drs. Doug Lowy, Linda Wolff, Robert Yarchoan, Lily Huang, the NIH Clinical Pathology Laboratory of the Clinical Center and the Lab Animal Science Program for helping in different aspects of this work.
Footnotes
Funding: Intramural Research Program at NIH, NCI, Center for Cancer Research and the Division of Intramural Research, National Institutes of Health, USA.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25(6):862–4. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25(2):211–7. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 3.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22(3):466–73. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan MA, Nattamai KJ, Xing E, Schleimer D, Daria D, Sengupta A, et al. Pharmacological inhibition of EGFR signaling enhances G-CSF-induced hematopoietic stem cell mobilization. Nat Med. 2010;16(10):1141–6. doi: 10.1038/nm.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Poursine-Laurent J, Link DC. Expression of the G-CSF receptor on hematopoietic progenitor cells is not required for their mobilization by G-CSF. Blood. 2000;95(10):3025–31. [PubMed] [Google Scholar]
- 6.Ebihara Y, Xu MJ, Manabe A, Kikuchi A, Tanaka R, Kato S, et al. Exclusive expression of G-CSF receptor on myeloid progenitors in bone marrow CD34+ cells. Br J Haematol. 2000;109(1):153–61. doi: 10.1046/j.1365-2141.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 7.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103(1):110–9. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 8.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–60. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105(6):2449–57. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 10.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113(19):4711–9. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 12.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91(5):368–76. [PubMed] [Google Scholar]
- 14.McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One. 2009;4(12):e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108(3):812–20. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114(7):1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7):687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 19.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106(11):1331–9. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001 Sep;98(5):1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, DiPersio JF. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114(7):1340–3. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993;90(20):9374–8. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA. 1995;92(21):9647–51. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zohren F, Toutzaris D, Klarner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111(7):3893–5. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 25.Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111(7):3439–41. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92(3):894–900. [PubMed] [Google Scholar]
- 27.De La Luz Sierra M, Gasperini P, McCormick PJ, Zhu J, Tosato G. Transcription factor Gfi-1 induced by G-CSF is a negative regulator of CXCR4 in myeloid cells. Blood. 2007;110(7):2276–85. doi: 10.1182/blood-2007-03-081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama T, Mutsuga N, Tosato G. Effect of fibroblast growth factor 2 on stromal cell-derived factor 1 production by bone marrow stromal cells and hematopoiesis. J Natl Cancer Inst. 2007;99(3):223–35. doi: 10.1093/jnci/djk031. [DOI] [PubMed] [Google Scholar]
- 30.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120(7):2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B, Tsukamoto A. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997;89(2):465–72. [PubMed] [Google Scholar]
- 32.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105(5):1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 34.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 35.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183(7):1187–91. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22(9):3111–9. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135(24):3989–93. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 39.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106(1):86–94. doi: 10.1182/blood-2004-09-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109(1):109–11. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simmons PJ, Masinovsky B, Longenecker BM, Berenson R, Torok-Storb B, Gallatin WM. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992;80(2):388–95. [PubMed] [Google Scholar]
- 42.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104(1):65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 43.Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp Hematol. 2007;35(4):565–71. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76(10):1503–10. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 46.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 47.Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010;88(2):241–51. doi: 10.1189/jlb.0210112. [DOI] [PubMed] [Google Scholar]