Abstract
Background
Allogeneic hematopoietic stem cell transplantation is associated with profound changes in levels of various cytokines. Emphasis has been placed on conditioning-associated mucosal damage and neutropenia and associated bacterial translocation as the initiating conditions predisposing to acute graft-versus-host disease. The post-transplant period is, however, also associated with increases in certain homeostatic cytokines. It is unclear how much the homeostatic drive to lymphocyte recovery and the production of cytokines from the engrafting donor immune system determine cytokine fluctuations in the peri- and immediate post-transplant period. The aim of this study was to examine the contributions of the conditioning regimen, donor engraftment, infections, and graft-versus-host disease to fluctuations in cytokines involved in homeostasis and inflammation.
Design and Methods
We examined the levels of 33 cytokines in relation to peri- and post-transplant events such as conditioning regimen, chimerism, and acute graft-versus-host disease in myeloablative, non-T cell-replete HLA-identical sibling donor stem cell transplantation for hematologic malignancies.
Results
We identified two cytokine storms. The first occurred following conditioning and reached peak levels when all the leukocytes were at their lowest concentrations. The second cytokine storm occurred concurrently with hematopoietic reconstitution and subsided with the achievement of full donor lymphocyte chimerism.
Conclusions
Our results indicate that both recipient-related and donor-related factors contribute to the changes in cytokine levels in the recipient following allogeneic hematopoietic stem cell transplantation. The study reported here was performed using plasma samples drawn from patients enrolled in the ClinicalTrials.gov-registered trials NCT00467961 and NCT00378534
Keywords: cytokines, post-transplantation, conditioning, allogeneic stem cell transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is associated with events which perturb the normal steady state of circulating cytokines. Animal studies suggest that immunosuppressive and myeloablative conditioning regimens can increase inflammatory cytokines such as interleukin (IL)-1 and tumor necrosis factor-α (TNFα) and establish a cycle of lymphocyte activation which contributes to the subsequent development of graft-versus-host disease (GvHD).1 In man the profound neutropenia and mucosal damage associated with myeloablative conditioning has been assumed to cause similar cytokine fluctuations triggered by lipopolysaccharides derived from gut bacteria. Studies of the post-transplant humoral cytokine milieu also identified homeostatic increases in some cytokines such as granulocyte colony-stimulating factor (G-CSF),2 IL-15,3,4 and IL-74,5 during the period of cytopenia as well as cytokine patterns associated with the GvHD alloresponse.4,6 While emphasis has been placed on the role of mucosal damage from the conditioning regimen in determining early cytokine fluctuations it is unclear how much contribution to the cytokine changes that favor GvHD comes from the homeostatic drive to lymphocyte recovery and the production of cytokines from the engrafting donor immune system. Here we studied the peri- and post-transplant cytokine dynamics in patients undergoing myeloablative conditioning and T-cell-depleted or T-cell-manipulated SCT to explore the contribution of the conditioning regimen, donor engraftment, infections, and GvHD to fluctuations in 33 cytokines (homeostatic, inflammatory and anti-inflammatory cytokines, and chemokines).
Design and Methods
Study population
All patients and donors studied participated in Institutional Review Board-approved protocols 07-H-0136 and 06-H-0248 (ClinicalTrials.gov identifiers NCT00467961 and NCT00378534, respectively). Patients received a 7-day conditioning regimen consisting of fludarabine, 25 mg/m2 daily on days −8 to −4, fractionated total body irradiation at a dose of 150 cGy x 8 fractions on days −7 to −4, and cyclophosphamide 60 mg/kg on days −3 and −2. On day 0 the patients received a CD34 cell-selected HSCT from an HLA identical sibling donor; the graft was depleted of T lymphocytes (residual CD3+ cell dose, 5×104/kg) (Miltenyi, Inc.). GvHD prophylaxis consisted of low dose cyclosporine A (plasma levels 100–200 μg/mL), and any GvHD that did develop was treated with steroids, and, in some patients, with methotrexate, budesonide, and/or rituximab. Standard prophylaxis with sulfamethoxazole/trimethoprim and valacyclovir was given and pre-emptive treatment with valganciclovir was given immediately if cytomegalovirus antigenemia was detected. Plasma samples were collected twice weekly from day −8 until 100 days post-transplantation and stored at −80°C until analysis. Establishment of donor hematopoiesis was routinely assessed at days 14, 30, 45, 60, 90 and later by short tandem repeat polymerase chain reaction analysis of chimerism in CD3+ and CD14/15+ cells.7
Sample preparation and analysis
Plasma samples from 20 patients and eight transplant donors were collected into heparin. Samples were stored as 300 μL aliquots in cryovials at −80°C until analysis. Levels of 33 hematopoietic and non-hematopoietic cell-derived cytokines (Online Supplementary Table S1) with known involvement in inflammatory, anti-inflammatory, homeostatic, chemotactic, and tissue repair processes were determined in duplicate using the Luminex multiplexing platform (Austin, TX, USA) with detection reagents from Bio-Rad (Hercules, CA, USA). Samples with cytokine levels that were below the assay’s lower limit of detection were assigned the values of the midpoint between the lower limit of detection and zero.
Statistical methods
Log-transformed leukocyte counts, C-reactive protein and cytokine levels were used to reduce the variability of individual measurements and improve normality of the data. For the analysis of the conditioning effect pre-transplant, a linear mixed-effects model was used to estimate the mean fold change of each variable from 1 week prior to HSCT (day −7) to the time of transplantation (day 0).8 An estimate of a positive mean slope indicates an increase of the marker level pre-transplant following the conditioning, whereas a negative mean slope indicates a decrease of the marker level. To estimate the mean curve and subject-specific trajectory of each variable over time, a non-parametric mixed-effects regression spline model was used.8,9 Cytokines without significant fluctuations during the study were identified by examining the 95% confidence bands of their estimated mean curves. To describe the correlation between leukocytes and cytokines or between cytokines, the non-parametric Spearman’s rank correlation was used. The resampling-subject bootstrap was used to compute the P value accounting for correlation among repeated measurements from each patient.9 Cox proportional hazards regression models were used to examine the association between the maximum cytokine levels within 2 weeks post-transplant and development of each of the three outcomes (full donor chimerism, acute GvHD and cytomegalovirus reactivation). Statistical significance was set at a two-sided P value less than 0.05. Analyses were performed using the R statistical programming software (www.r-project.org).
Results
Patients studied and transplant outcome
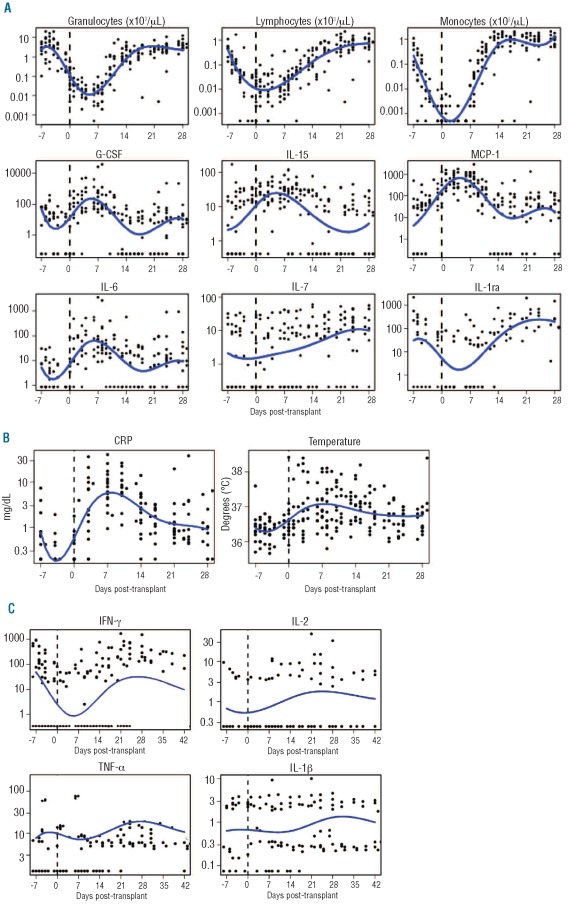
The characteristics and major outcomes of the 20 patients studied are summarized in Table 1. Seven patients over the age of 55 years received reduced dose conditioning of 400 cGy. Seven patients received a T-cell-depleted graft (protocol 06-H-0248) and 13 received a selectively depleted graft (protocol 07-H-0136).10 During conditioning, lymphocyte and monocyte counts fell rapidly whereas granulocytes showed a median 3-fold (range, 1.5–6.3) increase over 2 days (related to the dexamethasone administered during the radiation treatment) followed by a rapid decline to zero over the subsequent 5 days (Figure 1A). All patients established donor myeloid chimerism by day 14, and all 20 patients achieved greater than 95% donor T-cell chimerism at a median of 30 days (range, 14–270 days). The median time to neutrophil recovery was 12 days (range, 9–17 days). The median lymphocyte count on day 30 post-HSCT was 781/μL (range, 384–1805/μL). Low-grade (I-II) acute GvHD developed in 17 patients at a median of 35 days (range, 21–108 days), predominantly with skin involvement (Table 1). Cytomegalovirus reactivation occurred in 11 patients on one or two occasions between days 20–55 (median, day 34) after transplantation. Most patients developed a fever with concomitant rise of C-reactive protein following the transplant (Figure 1B), which became undetectable again within 2–4 weeks.
Table 1.
Patients’ characteristics.
Figure 1.
Fluctuations of C-reactive protein (CRP), temperature, leukocytes, and cytokines around the time of transplant (indicated as a broken vertical line at day 0) show two separate patterns. (A) Dynamics of the main leukocyte subsets and homeostatic cytokines relative to the time of transplant. Each variable is plotted in log10 scale. Ranges within days −7 to +21 are: granulocytes, 0.002–20.18; lymphocytes, 0.002–2.307; monocytes, 0.001–2.084; G-CSF, 0.12–38310; IL-15, 0.84–170.2; MCP-1, 6.34–2918; IL-6, 1.72–3524; IL-7, 0.41–60.97; IL-1ra, 3.94–2170. Observations below the detection limit were replaced with the midpoint between 0 and the detection limit. (B) Individual measurements and mean curve over time for CRP and temperature. The inflammation indicator CRP and temperature both reach a maximum a week after infusion of the T cell-depleted transplant. CRP is plotted in log10 scale. Time is plotted in days relative to the time of transplantation. (C) Dynamics of inflammatory cytokines around the time of transplant shows a pattern directly correlated with leukocyte counts. The ranges within days −7 to 42 are: IFN-γ, 0.34–1677 pg/mL; IL-2, 0.24–48.77 pg/mL; TNF-α, 1.26–186.6 pg/mL; IL-1β, 0.08–10.01 pg/mL. Observations below the detection limit were replaced with the midpoint between 0 and the detection limit. Patients with all measurements below the detection limits were excluded.
Plasma collection
The cytokine profile of a total of 329 plasma samples obtained every 3–4 days from the start of conditioning was studied (Online Supplementary Table S1).
Categorization of cytokine changes
Five cytokine patterns were observed (Figure 1): (i) Cytokines detected in less than 15% of samples or detected below the median level of 15 pg/mL: IL-1β, IL-2, IL-4, IL-5, IL-7, IL-8, IL-10, IL-13, IL-12p70 and macrophage inflammatory protein (MIP)-1α; (ii) cytokines detectable in more than 90% of samples at normal levels without significant fluctuations: stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), IL-17, and MIP-1β; (iii) cytokines positively correlated with leukocyte counts and showing the lowest levels at the time of transplant: IL-1ra and RANTES (regulated upon activation normal T-cell expressed and secreted); (iv) cytokines inversely correlated with leukocyte count reaching a peak within 1 week after transplantation: monocyte chemotactic protein-1 (MCP-1), G-CSF, IL-6 and IL-15; (v) cytokines showing a gradual increase from transplant until day 35: hepatocyte growth factor (HGF), stem cell growth factor beta (SCGFβ), interferon-inducible γ-induced protein-10 (IP10), IL-18, platelet-derived growth factor-BB (PDGF-BB), IL-9, eotaxin, IL-1ra, leukemia inhibitory factor (LIF), and RANTES.
Cytokine fluctuations during the conditioning regimen
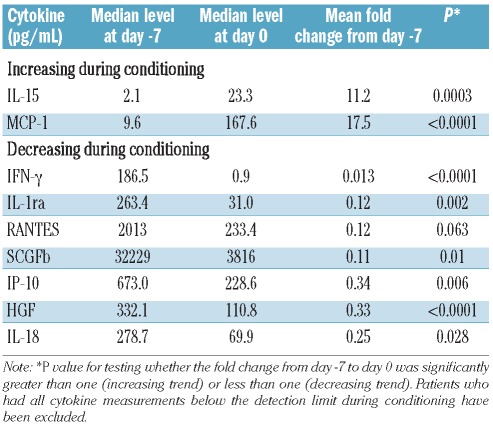
We tested the hypothesis that the conditioning regimen induces a surge in inflammatory cytokines such as tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), and IL-1β.11 We took a global approach to determine whether these and other cytokines with homeostatic, inflammatory, and anti-inflammatory functions showed any change from the start of conditioning to the day of transplantation. The results are shown in Table 2 and Figure 1. Three cytokine patterns were observed in this pre-transplant period: (i) no change during conditioning: TNFα, IL-1β, IL-12p70, IFN-α2, IL-2, and IL-7; (ii) decreased during conditioning: IFNγ, IL-18, IL-1ra, and C-reactive protein levels; (iii) increased more than 10-fold during conditioning: MCP-1 and IL-15. IL-10 was undetectable during this period. G-CSF and IL-6 levels did not change significantly between day −7 and day 0; however, further analysis revealed a nonlinear trend during this period (Figure 1A). Thus, the conditioning regimen was associated with a steady-state or a decrease in inflammatory cytokines.
Table 2.
Estimated cytokine levels and fold changes during conditioning.
Peri-transplant leukopenia is associated with a cytokine storm
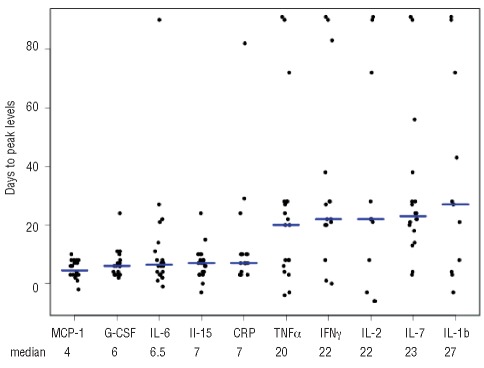
The leukopenic phase from conditioning until day 14 after HSCT was characterized by significant transient increases in the neutrophil homeostatic cytokines IL-6 and G-CSF, peaking at week 1 post-transplant and declining as granulocytes returned (Figure 1A). There was an inverse correlation between neutrophil numbers and G-CSF and IL-6 (neutrophils and G-CSF, Spearman’s correlation r = −0.57, P<0.001; neutrophils and IL-6 r = −0.58; P<0.001). A similar inverse correlation was observed between lymphocyte count and IL-15 (r = −0.32; P=0.01), a cytokine which is required for NK-cell and T-cell recovery (Figure 1A).3,12–15 Peak levels of these homeostatic cytokines occurred within 7 days of transplantation, suggesting a close homeostatic relationship between the cytokine and its target (Figure 2).
Figure 2.
Days to peak levels of C-reactive protein (CRP) and cytokines. Patients with all measurements below detection limits have been excluded.
In contrast, levels of the lymphopoietic cytokine IL-7 correlated directly with leukocyte numbers (r = 0.39; P=0.005), suggesting that IL-7-producing cells were contained within the graft. C-reactive protein levels increased after transplantation and peaked at a median of 7 days after HSCT (range, 3–82 days), correlating significantly with IL-6 levels (r = 0.74; P<0.001), and confirming a regulatory role of IL-6 in determining C-reactive protein levels. Furthermore, peak body temperature appeared at a median of 8.5 days (range, 0–62 days), following closely after the IL-6 peak.
Cytokine changes associated with donor lymphocyte engraftment and later post-transplant events
In a univariate Cox regression analysis of cytokine peak levels occurring within 2 weeks post-transplantation and the occurrence of chimerism, cytomegalovirus reactivation, and acute GvHD, we found that peak IP-10 levels were significantly associated with early full donor chimerism. A trend was noted for eotaxin, PDGF-BB, IL-1β, LIF and IL-17 (all 0.05< P <0.1). None of the cytokines was significantly correlated with the onset of acute GvHD or cytomegalovirus reactivation (all P>0.1). In contrast to the uniform fluctuations in homeostatic cytokine peak levels, inflammatory cytokines displayed wide patient-dependent variation, suggesting that other frequent post-transplant events not monitored here (including changes in hepatic and renal function, transfusions, polypharmacy, infections, and their treatment) contributed to individual day-to-day cytokine changes.
Discussion
Allogeneic HSCT profoundly disrupts various homeostatic mechanisms and inflicts damage upon organs, simultaneously eliciting homeostatic, repair, and immune responses. Some of the “cytokine fingerprints” of these processes have been studied here, including cytokines driving cell-mediated immunity (IL-1β; IL-12p70; IL-18; LIF; TNFα; IFNγ; IL-17; IL-9) and humoral immunity (IL-2; IL-4; IL-5; IL-10; IL-13); chemokines (RANTES; eotaxin; MIP-1α and -β; MCP-1; IL-8) and cytokines with inflammatory and chemotactic functions (IL-17); and homeostatic and tissue repair cytokines (SCF; SCGF; HGF; FGF-basic; IL-7; IL-15; G-CSF; IL-6; GM-CSF; VEGF; HGF). We correlated their levels and dynamics with clinical (GvHD; fever) and clinical laboratory (C-reactive protein; leukocyte counts) data. Our main findings are: (i) the conditioning regimen caused a decrease rather than increase in inflammatory cytokine levels; (ii) two cytokine surges were identified (Figure 2), the first coinciding with pancytopenia and the second with leukocyte reconstitution. The first cytokine storm involved predominantly hematopoietic and lymphopoietic homeostatic cytokines, whereas the second involved inflammatory and anti-inflammatory cytokines as well as chemokines. Predictably, therefore, we found an inverse correlation not only between granulocyte numbers and G-CSF levels, but also between granulocyte numbers and IL-6 levels, suggesting the latter’s involvement in granulocyte reconstitution following myeloablation;2 (iii) importantly, we found that the switch to donor chimerism and not the occurrence of GvHD correlated most closely with a surge in IP-10.
The transplant conditioning regimen causes widespread damage to healthy tissues, including the gastrointestinal tract with its diverse commensal microbiome.16,17 In a pre-clinical T-cell-replete HSCT model Ferrara and co-workers gave fractionated total body irradiation to mice, followed within a few hours by a bone marrow and spleen T-cell-replete MHC-mismatched transplant.18 Infused donor T cells caused GvHD, but only at the higher dose of total body irradiation which correlated with higher levels of lipopolysaccharides in the circulation. Transplantation of conditioned mice with a graft from lipopolysaccharide-resistant or -sensitive donors showed that mice receiving lipopolysaccharide-resistant donor cells developed significantly less GvHD,19 pointing toward a role for lipopolysaccharides in the induction of GvHD. Further studies showed that blocking lipopolysaccharides could prevent GvHD,20 leading to the conclusion that conditioning regimen-induced damage to the gastrointestinal tract was primarily responsible for experimental GvHD.
Unlike the murine transplant model, our patients received a conditioning regimen consisting of both total body irradiation and chemotherapeutics, spread out over 7 days, which causes a slow decline of leukocyte numbers (Figure 1) until the day of transplantation when the T-cell-depleted or allo-depleted transplant is infused from an MHC matched donor. Under these conditions, immune cells responsive to bacteria-derived danger molecules were at a nadir at the time of transplantation (Figure 1). Furthermore, patients received cyclosporine for GvHD prophylaxis, which could blunt the T-cell response.21 These differences may explain the lack of increase in inflammatory cytokines during conditioning and contrast with findings in murine studies in which total body irradiation followed immediately by infusion of MHC-mismatched immunocompetent cells correlated with rises in IL-1 and IL-6. It, therefore, appears that the radiation-induced myeloablation we used did not cause as extensive tissue damage as is generally assumed, although bacterial invasion across a neutropenic mucosa remains a potential mechanism for bacterial lipopolysaccharide-driven cytokine surges during conditioning. It was not possible in this small study to relate peri-transplant changes of cytokines with the subsequent development of GvHD. Furthermore, our findings do not exclude the possibility that irradiation activates tissue resident antigen-presenting cells,22 subsequently triggering GvHD. It should be borne in mind that cytokine changes are likely to vary considerably according to the regimen and the type of transplant (for example, a reduced intensity regimen was shown to induce increased TNFα levels compared to mye-loablative therapy23). Our findings concern T-cell-depleted or manipulated myeloablative transplants without heavy immunosuppression. Furthermore, T-cell depletion was achieved by selecting CD34+ cells, thus removing NK cells, B cells, monocytes, and other peripheral blood cells in addition to the T cells, all of which may have affected the quality of the cytokine dynamics post-HSCT. It will be necessary to study other transplant regimens including reduced intensity stem cell transplantation, autologous stem cell transplantation and T-cell-replete transplants to better characterize the contribution of alloreactive T cells, and regimen intensity to peri-transplant cytokine fluctuations.
Many studies have sought to relate levels of cytokines and soluble receptors with GvHD in the T-replete HSCT setting but currently there is no consensus.24–35 The wide discrepancies may be attributable to the diversity of the diseases transplanted, the transplant conditioning (notably inclusion of antithymocyte globulin);23 genetic diversity, including cytokine (receptor) gene polymorphisms,36–40 infection, veno-occlusive disease and lymphocytopenia in the case of transient IL-7 and IL-15 increases.3–6,29,41,42 However, the achievement of full lymphoid chimerism has so far not been identified as a significant moment for cytokine changes. Nevertheless, the strong correlation of the acquisition of full donor T-cell chimerism with subsequent graft-versus-host and graft-versus-leukemia reactions would make it likely that cytokine fluctuations might be detected at this time.
It was surprising that in this study IL-7 levels did not follow the same pattern as the other homeostatic cytokines IL-15, G-CSF, and IL-6. IL-7 is produced by non-hematopoietic stromal and epithelial cells in the thymus, bone marrow, intestine, and lymphoid tissue,43 but also by dendritic cells44,45 and B cells.46 Others have found an inverse correlation between IL-7 and lymphocyte numbers post-HSCT.4,5 Our data suggest that the cells that produced this important lymphopoietic cytokine were contained within the graft, and that the non-hematopoietic tissues were not a significant source of IL-7 in our patients.
To conclude, the absence of a cytokine storm during the conditioning regimen in these T-cell-depleted myeloablative HSCT recipients suggests a revision of the concept that total body irradiation-based regimens cause an important release of inflammatory cytokines. Rather, HSCT is characterized by two cytokine storms, the first occurring during pancytopenia, and the second with immune reconstitution, possibly coinciding with the replacement of the patient’s immune system by that of the donor. Our findings support the idea that immune reconstitution might benefit from cytokine therapy with IL-15 which could improve both early NK-cell and T-cell recovery – factors which we have found to be beneficial to the outcome of HSCT.47,48
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–9. [PubMed] [Google Scholar]
- 2.Lieschke G, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–46. [PubMed] [Google Scholar]
- 3.Boyiadzis M, Memon S, Carson J, Allen K, Szczepanski MJ, Vance BA, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. 2008;14(3):290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 4.Thiant S, Yakoub-Agha I, Magro L, Trauet J, Coiteux V, Jouet JP, et al. Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse. Bone Marrow Transplant. 2010;45(10):1546–52. doi: 10.1038/bmt.2010.13. [DOI] [PubMed] [Google Scholar]
- 5.Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26(35):5735–41. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, et al. An unusual CD56brightCD16low NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181(3):2227–37. doi: 10.4049/jimmunol.181.3.2227. [DOI] [PubMed] [Google Scholar]
- 7.Childs R, Clave E, Contentin N, Jayasekera D, Hensel N, Leitman S, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94(9):3234–41. [PubMed] [Google Scholar]
- 8.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. [Google Scholar]
- 9.Huang JZ, Wu CO, Zhou L. Varying-coefficient models and basis function approximations for the analysis of repeated measurements. Biometrika. 2002;89:111–28. [Google Scholar]
- 10.McIver ZA, Melenhorst JJ, Grim A, Naguib N, Weber G, Fellowes V, et al. Immune Reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol Blood Marrow Transplant. 2011;17(12):1846–54. doi: 10.1016/j.bbmt.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porrata LF, Inwards DJ, Micallef IN, Johnston PB, Ansell SM, Hogan WJ, et al. Interleukin-15 affects patient survival through natural killer cell recovery after autologous hematopoietic stem cell trans-plantation for non-Hodgkin lymphomas. Clin Dev Immunol. 2010;2010:914945. doi: 10.1155/2010/914945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 16.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–870. doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–51. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–13. [PubMed] [Google Scholar]
- 19.Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J, Jr, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102(10):1882–91. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107(12):1581–9. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–60. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 22.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remberger M, Sundberg B. Cytokine production during myeloablative and reduced intensity therapy before allogeneic stem cell transplantation. Haematologica. 2004;89(6):710–6. [PubMed] [Google Scholar]
- 24.Imamura M, Hashino S, Kobayashi H, Kubayashi S, Hirano S, Minagawa T, et al. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-gamma, and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant. 1994;13(6):745–51. [PubMed] [Google Scholar]
- 25.Liem LM, van Houwelingen HC, Goulmy E. Serum cytokine levels after HLA-identical bone marrow transplantation. Transplantation. 1998;66(7):863–71. doi: 10.1097/00007890-199810150-00009. [DOI] [PubMed] [Google Scholar]
- 26.Rowbottom AW, Riches PG, Downie C, Hobbs JR. Monitoring cytokine production in peripheral blood during acute graft-versus-host disease following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1993;12(6):635–41. [PubMed] [Google Scholar]
- 27.Imoto S, Oomoto Y, Murata K, Das H, Murayama T, Kajimoto K, et al. Kinetics of serum cytokines after allogeneic bone marrow transplantation: interleukin-5 as a potential marker of acute graft-versus-host disease. Int J Hematol. 2000;72(1):92–7. [PubMed] [Google Scholar]
- 28.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110(12):3827–32. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 29.Sakata N, Yasui M, Okamura T, Inoue M, Yumura-Yagi K, Kawa K. Kinetics of plasma cytokines after hematopoietic stem cell transplantation from unrelated donors: the ratio of plasma IL-10/sTNFR level as a potential prognostic marker in severe acute graft-versus-host disease. Bone Marrow Transplant. 2001;27(11):1153–61. doi: 10.1038/sj.bmt.1703060. [DOI] [PubMed] [Google Scholar]
- 30.Kumaki S, Minegishi M, Fujie H, Sasahara Y, Ohashi Y, Tsuchiya S, et al. Prolonged secretion of IL-15 in patients with severe forms of acute graft-versus-host disease after allo-geneic bone marrow transplantation in children. Int J Hematol. 1998;67(3):307–12. doi: 10.1016/s0925-5710(97)00117-5. [DOI] [PubMed] [Google Scholar]
- 31.Min CK, Lee WY, Min DJ, Lee DG, Kim YJ, Park YH, et al. The kinetics of circulating cytokines including IL-6, TNF-alpha, IL-8 and IL-10 following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28(10):935–40. doi: 10.1038/sj.bmt.1703258. [DOI] [PubMed] [Google Scholar]
- 32.Hori T, Naishiro Y, Sohma H, Suzuki N, Hatakeyama N, Yamamoto M, et al. CCL8 is a potential molecular candidate for the diagnosis of graft-versus-host disease. Blood. 2008;111(8):4403–12. doi: 10.1182/blood-2007-06-097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura H, Komatsu K, Ayaki M, Kawamoto S, Murakami M, Uoshima N, et al. Serum levels of soluble IL-2 receptor, IL-12, IL-18, and IFN-gamma in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Allergy Clin Immunol. 2000;106(1 Pt 2):S45–50. doi: 10.1067/mai.2000.106774. [DOI] [PubMed] [Google Scholar]
- 34.Yabe M, Yabe H, Hattori K, Shimizu T, Matsumoto M, Morimoto T, et al. Role of interleukin-12 in the development of acute graft-versus-host disease in bone marrow transplant patients. Bone Marrow Transplant. 1999;24(1):29–34. doi: 10.1038/sj.bmt.1701819. [DOI] [PubMed] [Google Scholar]
- 35.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75(4):1011–6. [PubMed] [Google Scholar]
- 36.Cavet J, Middleton PG, Segall M, Noreen H, Davies SM, Dickinson AM. Recipient tumor necrosis factor-alpha and inter-leukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood. 1999;94(11):3941–6. [PubMed] [Google Scholar]
- 37.Middleton PG, Taylor PR, Jackson G, Proctor SJ, Dickinson AM. Cytokine gene polymorphisms associating with severe acute graft-versus-host disease in HLA-identical sibling transplants. Blood. 1998;92(10):3943–8. [PubMed] [Google Scholar]
- 38.Stark GL, Dickinson AM, Jackson GH, Taylor PR, Proctor SJ, Middleton PG. Tumour necrosis factor receptor type II 196M/R genotype correlates with circulating soluble receptor levels in normal subjects and with graft-versus-host disease after sibling allogeneic bone marrow transplantation. Transplantation. 2003;76(12):1742–9. doi: 10.1097/01.TP.0000092496.05951.D5. [DOI] [PubMed] [Google Scholar]
- 39.Cullup H, Dickinson AM, Jackson GH, Taylor PR, Cavet J, Middleton PG. Donor interleukin 1 receptor antagonist genotype associated with acute graft-versus-host dis-ease in human leucocyte antigen-matched sibling allogeneic transplants. Br J Haematol. 2001;113(3):807–13. doi: 10.1046/j.1365-2141.2001.02811.x. [DOI] [PubMed] [Google Scholar]
- 40.Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98(5):1594–600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Ghosh A, Goldman S, Slone V, van de Ven C, Suen Y, Murphy L, et al. Immunological reconstitution and correlation of circulating serum inflammatory mediators/cytokines with the incidence of acute graft-versus-host disease during the first 100 days following unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 1999;24(5):535–44. doi: 10.1038/sj.bmt.1701921. [DOI] [PubMed] [Google Scholar]
- 42.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23(8):783–8. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 43.Lee SK, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev. 2005;208:169–80. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 44.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666–76. [PubMed] [Google Scholar]
- 45.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198(5):514–26. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin D, Sharma V, Knobloch T, Armitage R, Dayton M, Goodwin R. B cell IL-7. Human B cell lines constitutively secrete IL-7 and express IL-7 receptors. J Immunol. 1994;152(10):4749–57. [PubMed] [Google Scholar]
- 47.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107(4):1688–95. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21(10):2145–52. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]