Abstract
Background
Nilotinib is a second-generation tyrosine kinase inhibitor with significant efficacy as first- or second-line treatment in patients with chronic myeloid leukemia. Despite preclinical evidence indicating a risk of prolongation of the QT interval, which was confirmed in clinical trials, detailed information on nilotinib’s cardiac safety profile is lacking.
Design and Methods
Here, we retrospectively assessed cardiovascular risk factors in 81 patients who were being or had previously been treated with nilotinib therapy and evaluated cardiovascular parameters by longitudinal monitoring of the QT interval and left ventricular ejection fraction. Detailed information on the occurrence and management of defined cardiac adverse events was extracted.
Results
The median duration of nilotinib therapy was 26 months (range, 1–72). The median QT interval at baseline was 413 msec (range, 368–499 msec). During follow-up, the median QT was not significantly different from the baseline value at any time-point. Sixteen of 81 patients (20%) had new electrocardiographic changes. Cardiac function, as assessed by measurement of left ventricular ejection fraction, did not change significantly from baseline at any time-point. During a median follow-up of 44 months (range, 2–73), seven patients (9%), all of whom had received prior imatinib therapy, developed 11 clinical cardiac adverse events requiring treatment. The median time from the start of nilotinib therapy to an event was 14.5 months (range, 2–68). Five of seven patients were able to continue nilotinib therapy with only one brief interruption.
Conclusions
Whereas new electrocardiographic abnormalities were recorded in 20% of all patients and some of them developed severe or even life-threatening coronary artery disease, QT prolongation, changes in left ventricular ejection fraction, and clinical cardiac adverse events were uncommon in patients treated with nilotinib.
Keywords: nilotinib, cardiac function, safety, heart, chronic myeloid leukemia
Introduction
The pathogenetic role of the BCR-ABLp210 tyrosine kinase resulting from the reciprocal translocation t(9;22)(q34;q11) and its gene product BCR-ABL1 in chronic myeloid leukemia (CML) led to the development of imatinib mesylate1 (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA). The first small molecule tyrosine kinase inhibitor (TKI) to be approved, imatinib mesylate additionally targets c-Kit and PDGFR-α and -β and is now the standard of care in patients with CML,2 a disease which naturally progresses from a chronic phase to an accelerated phase and ultimately to a fatal blast crisis.3 However, because of problems of intolerance and resistance, the latter resulting from specific point mutations in the kinase domain of ABL,4 several second-generation TKI with increased efficacy and specificity have been developed.5
Nilotinib (Novartis Pharmaceuticals Corporation), formerly AMN107, is an orally active aminopyrimidine-derivative based on imatinib mesylate with increased selectivity and inhibitory potency against wild-type BCR-ABL1 and most imatinib-resistance conferring mutations.6 Considerable clinical efficacy in imatinib-intolerant or –resistant patients led to nilotinib’s approval for use after the failure of imatinib treatment.7,8 Subsequently, the ENESTnd trial showed that nilotinib is superior to imatinib in terms of cytogenetic and molecular remission rates and rates of disease progression in newly diagnosed patients.9 Nilotinib has, therefore, additionally been approved as first-line therapy for patients with CML in chronic phase by the Food and Drug Administration and the European Medicines Agency.
However, preclinical safety studies indicated that nilo-tinib may have cardiac side effects.10 In vitro, cardiac electro-physiological investigations utilizing the hERG channel and the isolated rabbit heart assay revealed preclinical signs of QT prolongation10 but without evidence of cytotoxicity in neonatal rat ventricular myocytes in vitro or overt cardiovascular pathology or heart failure in vivo.11 In an exploratory analysis of patients treated within a phase I trial, the corrected QT interval by Fridericia’s formula (QTcF) increased by 5 to 15 msec.12 Among the 119 patients, two had adverse cardiac events associated with nilotinib, including one who developed a pericardial effusion and atrial fibrillation. In addition, one unexplained sudden death was reported. Nilotinib carries a Food and Drug Association boxed warning on possible life-threatening heart problems that may lead to an irregular heartbeat and possible sudden death. Therefore, caution in patients with significant cardiac disease or at risk of having or developing prolongation of QTcF, correction of low potassium or magnesium levels and careful monitoring for cardiac events are advised.
As the clinical significance of cardiac side effects of nilo-tinib is unknown and this may prohibit access of a number of patients to a highly active drug, we retrospectively analyzed subclinical and clinical cardiac parameters in patients treated with nilotinib.
Design and Methods
Patients
All patients at the Department of Hematology and Oncology of the Charité - Universitätsmedizin Berlin, Campus Virchow-Klinikum, who were being or had previously been treated with nilotinib between November 2004 and June 2011 were retrospectively studied for this safety analysis. Nilotinib was administered as therapy for CML (n=77), for acute lymphoblastic leukemia (n=2), hypereosinophilic syndrome (n=1) or systemic mastocytosis (n=1) at a starting daily dose of 400 mg BID orally. As per prescription, nilotinib had to be taken after at least 2 hours of fasting. Most patients were treated within clinical trials (Clinicaltrials.gov: NCT00109707, NCT00471497, and NCT00905593). All trials were conducted in accordance with the applicable regulatory requirements. Patients gave their written informed consent to participation in a clinical trial or retrospective analysis of their data. All procedures were followed in accordance with the Helsinki Declaration and were approved by the local ethics committee. Patients were excluded from the trial if they had known uncon-trolled or medically significant cardiac disease, a left ventricular ejection fraction (LVEF) <45%, or a QTcF interval >450 msec.
Evaluation of patients
Patients were followed regularly at intervals of 3 to 6 months, as clinically indicated. Cardiovascular risk factors, body mass index, and World Health Organization/Eastern Cooperative Oncology Group (WHO/ECOG), New York Heart Association and Canadian Cardiovascular Society scores were determined retrospectively from the patients’ charts and case report forms of clinical trials.
Cardiovascular evaluation
In addition to standard biochemical and hematologic parameters, a baseline 12-lead electrocardiogram and echocardiography were performed in all patients. A LVEF ≥55% was considered normal. LVEF values of 41–54% and 26–40% defined mild and moderate left ventricular dysfunction, respectively. Specific cardiovascular biochemical parameters (creatine kinase-MB or troponin T) were analyzed only in patients with cardiac events. Every 3 to 6 months, longitudinal cardiac monitoring was conducted for QT prolongation via electrocardiograms and for heart failure/systolic dysfunction via echocardiograms. Assessment of prolongation of the QTc interval was based on the LQTS diagnostic score.13 The QTc interval was calculated using different methods including Bazett’s formula and Fridericia’s formula, but the latter was used to ensure compatibility with previous studies. The 12-lead electrocardiograms and echocardiograms were performed, analyzed or supervised in-house by board-certified cardiologists.
Definition of cardiac events
A cardiac adverse event was defined as the occurrence of a symptomatic arrhythmia that required treatment, syncope, new left ventricular dysfunction, acute coronary syndrome including angina pectoris and myocardial infarction, and sudden death. In patients who developed cardiac symptoms during nilotinib therapy, changes in electrocardiograms were analyzed, and the patients underwent echocardiography. The initiation and type of cardiac treatment were based on the clinical findings. Coronary angiography was performed when indicated, according to the guidelines of the European Society of Cardiology.14,15 Electrocardiograms, echocardiograms and chest X-rays were conducted routinely before treatment and as clinically indicated during the follow-up.
Statistical analysis
A descriptive statistical analysis was performed using the one-way ANOVA-test with Dunnett’s post-test when appropriate. Overall survival was measured from the start of nilotinib therapy to death, and event-free survival (EFS) was measured from the start of nilotinib therapy to any of the following events while on therapy: death from any cause, cardiac adverse event, discontinuation of therapy because of toxicity or lack of efficacy, loss of complete hematologic response, loss of complete cytogenetic response, or progression to accelerated or blast phase disease. A P value less than 0.05 was considered statistically significant. All tests were two-sided.
Results
Patients’ characteristics
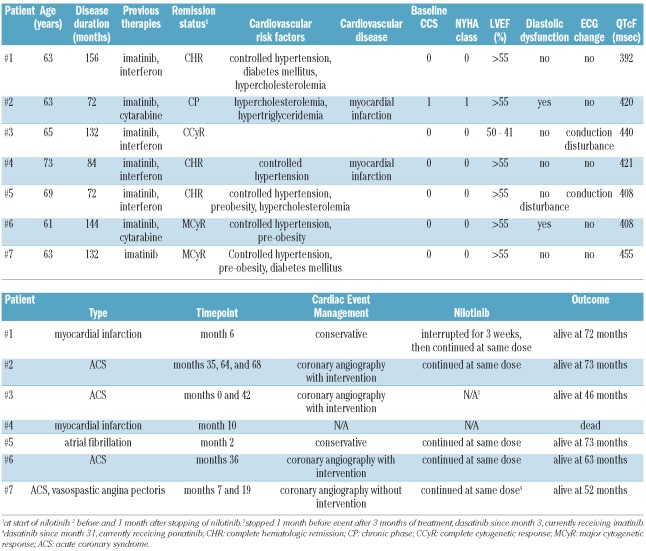
Eighty-one patients [44 males (54.3%)], with a median age of 57 years (range, 25–85) were studied and followed up for a median of 44 months (range, 2–73). The median disease duration was 36 months (range, 1–180). Patients received nilotinib either as part of clinical trials (n=50, 62%) or as per prescription (n=31, 38%). All patients had a WHO/ECOG score of 2 or better and 61 patients (75%) had a WHO/ECOG score of 0. Previous therapies included primarily imatinib mesylate in 66 patients (81%), and interfer-on-α in 22 (27%).
At baseline, the documented cardiovascular risk factors in the 81 patients included type 2 diabetes mellitus in 11 patients (14%), untreated hypertension in two (2%), controlled hypertension in 26 (32%), current or former nicotine abuse in three (4%), grade 1 obesity in seven (9%), and grade 2 obesity in two (2%). Overall, 41 of 81 patients (51%) had one or more cardiovascular risk factors. A medical history of cardiovascular disease, including coronary artery disease and arrhythmia, was each present in six (7%) patients. Four of six patients with coronary artery disease had had a myocardial infarction prior to the nilotinib therapy. Clinically, only one patient had a Canadian Cardiovascular Society score of 1, and one patient each had New York Heart Association (NYHA) grade 1 or 2 heart failure. Systolic and diastolic dysfunction were present in three (4%) and 32 (40%) patients, respectively.
Patients treated within clinical trials had a significantly shorter median disease duration (15 months), but longer treatment with nilotinib (37 months) than patients who received nilotinib as per prescription (60 and 8 months, respectively). No other baseline characteristic differed significantly between these two groups (data not shown).
Cardiovascular monitoring
At baseline, 26 patients (32%) had electrocardiographic changes including rhythm abnormalities in six (7%), conduction disturbances in 12 (15%), ST segment changes in two (2%), T wave changes in four (5%), and QTc prolongation in four (5%) patients. Of note, two patients had conduction disturbances and QTc prolongation concomitantly. Under treatment, 12 of these 26 patients had resolution of their electrocardiographic changes and 16 of all 81 patients (20%) developed new changes including rhythm abnormalities in 4 (5%), conduction disturbances in one (1%), ST segment changes in two (2%), T wave changes in two (2%), and QTc prolongation in nine (11%) patients.
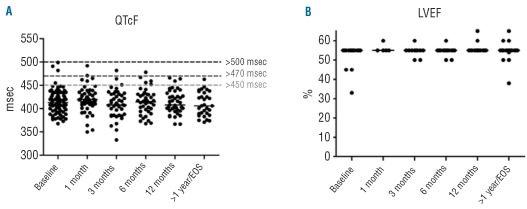
Overall, the median QTcF interval at baseline was 413 msec (range, 368–499 msec). An increase of >30 msec or >60 msec occurred in 18 (22%) and two (2%) patients, respectively. No patients had an absolute QTcF interval >500 msec at any time during the observational period. During follow-up, the median QTcF was never higher than 420 msec (range, 406–420 msec) (Figure 1A).
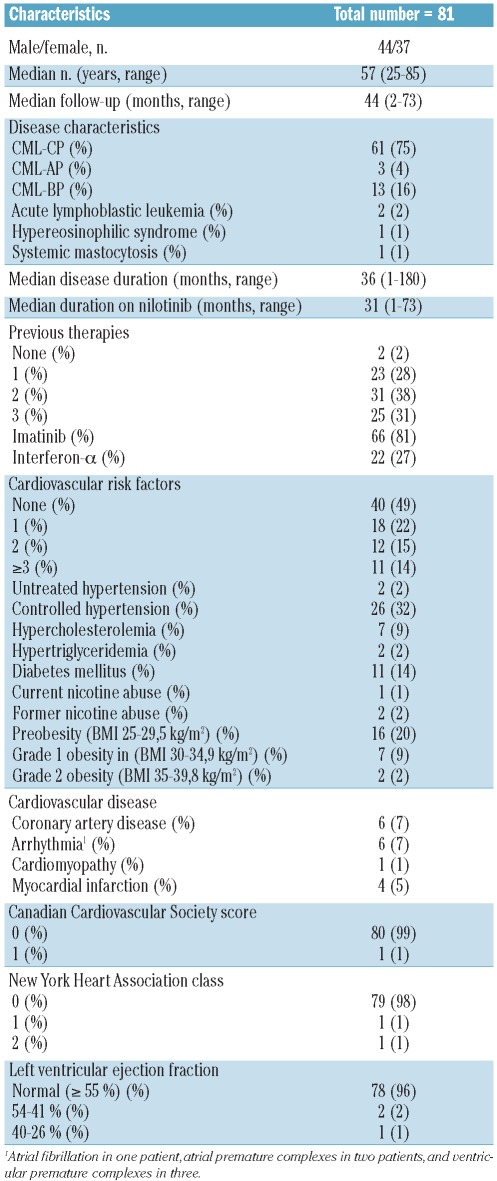
Figure 1.
(A) Duration of QTcF intervals and (B) quantitative left ventricular ejection fractions (LVEF) at baseline, after 1, 3, 6, and 12 months and more than 1 year or at the end of study (EOS).
At least one LVEF measurement was available during follow-up for 46 of the 81 patients (57%). The median baseline LVEF was 55%. After 1, 3, 6 and 12 months of follow-up and more than 1 year later or at end of study there were no significant differences from baseline (Figure 1B). LVEF did not decrease below 50% in any patient, but seven patients had echocardiographic evidence of new diastolic dysfunction without clinical symptoms.
Table 1.
Patients’ characteristics.
Clinical cardiac events and management
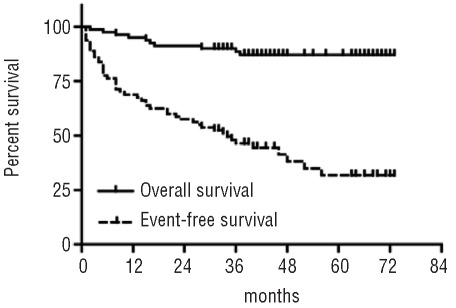
Overall, seven patients (9%) developed 11 clinical cardiac adverse events that required treatment (Table 2), of whom three received nilotinib as per prescription. One event occurred immediately prior to the start of nilotinib. The median time from the start of nilotinib therapy to the ten events that occurred during or after nilotinib exposure was 14.5 months (range, 2–68).
Table 2.
Individual results of patients who experienced a cardiac event.
The median age of the seven patients who had cardiac events was 63 years (range, 61–73), and their median disease duration was 132 months (range, 72–156). None, one, two, or three cardiovascular risk factors were present in one, one, two, and three patients, respectively. Two patients had had a myocardial infarction prior to nilotinib treatment and one patient had a reduced LVEF, whereas four of six patients with a previous medical history of coronary artery disease had no cardiac events during follow-up. All patients had previously received imatinib therapy.
Six patients had an acute coronary syndrome including two with a myocardial infarction, of which one was lethal, and one had newly developed atrial fibrillation. Management included coronary angiography with intervention in three patients. With the exception of one patient who developed an acute coronary syndrome after cessation of nilotinib and one patient who died of myocardial infarction, all other patients were able to continue nilotinib therapy with only one brief interruption for 3 weeks.
Clinical outcome
The median duration of nilotinib therapy in the entire population was 26 months (range, 1–72) for a total exposure of 187 patient-years. There were 48 events, including disease progression (n=12), cardiac adverse events (n=7), and deaths (n=10), including one due to myocardial infarction in a 73-year old male patient, but no confirmed cases of sudden death. There was no evidence of cumulative toxicity, i.e. an increase of adverse events with longer exposure. Event-free survival rates at 3 and 5 years were 46% and 32%, respectively; the overall survival rates at 3 and 5 years were 88% and 87%, respectively.
Figure 2.
Event-free and overall survival for the entire cohort of patients receiving nilotinib.
Discussion
It is well-known that some anticancer drugs are car-diotoxic and the importance of this is being increasingly recognized.16–18 In addition to sharing risk factors, the aging of the general population in developed countries makes it more probable that a patient has both cancer and cardiovascular disease, which may present a considerable therapeutic challenge and has a significant impact on the overall prognosis and survival of cancer patients. Manifestations range from asymptomatic QT prolongation to reduction in LVEF, symptomatic congestive heart failure, acute coronary syndromes with myocardial infarction and sudden death. However, there are currently no standardized definitions, which has precluded direct comparisons of various studies and the establishment of universally accepted guidelines or recommendations. Mechanisms of toxicity to the cardiovascular system include direct damage to cardiomyocytes or inflammation of the pericardium, promotion of blood clotting in the vessels, and through induction of hypertension.16
TKI, although originally considered to be less toxic and better tolerated than other drugs, may also induce cardiac side effects.19–21 Toxicity may result from inhibition of the intended target that is expressed in the heart and the vasculature, e.g. on-target toxicity, or inhibition of alternative targets through limited selectivity, e.g. off-target toxicity.22 Previous reports described cardiotoxicity resulting from therapy with sunitinib,23,24 sorafinib,24 and imatinib mesylate.25 Although the clinical occurrence and/or significance of the latter are being disputed,26–33 reengineering efforts have been undertaken to alleviate the problem.34 More recently, QTc prolongation and myocardial infarction related to dasatinib therapy have been reported.35 The generally low incidence of cardiotoxicity from TKI therapy argues against a class effect. However, with increasing therapeutic success long-term follow-up needs to confirm the lack of late detrimental effects. Recent data related to possible increases in peripheral arterial occlusive disease under treatment with nilotinib36–38 and pulmonary arterial hypertension under treatment with dasatinib39 have heightened awareness on the need to monitor carefully for relatively rare toxicities after approval of a new drug. Our experience has, so far, raised the possibility of an increase of severe vascular events in patients with CML receiving nilotinib therapy,38 necessitating the prospective evaluation of this risk.
Targeted therapies have a well-described effect on the QT interval40 with QT prolongation known to increase the risk of malignant cardiac arrhythmias, such as torsade de pointes, and sudden cardiac death.41 Among patients with long QT syndrome, the risk of a first cardiac event increases with a QTc interval >447 msec (RR 1.6 compared to QTc <446 msec) and >470 msec (RR 5.3). The highest risk is found when QTc is prolonged to more than 498 msec (RR 8.36 compared to QTc <498 msec).42,43 There is preclinical evidence of a concentration-dependent effect of nilotinib on cardial ventricular repolarization.10 In clinical studies, changes of the QTcF interval from baseline were in the range of 5 to 15 msec, between 2.5 and 4% of patients had increases of QTcF of more than 60 msec, and in only study was an incidence of 1.2% of QTcF intervals >500 msec reported.7–9,12 In line with these studies, in our cohort no patient had an absolute QTcF interval >500 msec and 2% of patients had an increase of QTcF of more than 60 msec. With a median follow-up of 44 months there were no significant changes in QTcF intervals from baseline. However, 20% of patients developed new electrocardiographic changes that were asymptomatic and clinically irrelevant with the exception atrial fibrillation in one patient.
Measurement of the LVEF is the most common radiological method used to screen for toxic effects on the heart.44 However, its low sensitivity for early prediction of cardiomyopathy limits its usefulness and diastolic measurements, including the E/A ratio (peak early atrial velocity divided by peak late atrial velocity) and more recent techniques such as tissue velocity imaging of early diastole, strain, and strain rate, may be more sensitive to early changes in cardiac function. In our cohort, there was no significant decline in LVEF at any time-point, excluding cases of overt cardiotoxicity. However, new diastolic dysfunction was seen in seven patients indicating potential subtle cardiac changes. Since the diagnosis is examiner-dependent and there are no standardized tests, the clinical significance remains unclear.
Seven patients had cardiac events that occurred at a median of 14.5 months after initiation of nilotinib therapy. With the exception of one case of fatal myocardial infarction, all events could be managed as clinically indicated without necessitating significant changes to the continued nilotinib therapy. In addition, nilotinib does not seem to have a negative long-term impact. With several confounding factors involved in these patients, a direct relationship between the cardiac events and nilotinib cannot be either proven or excluded. It should be noted that these seven patients were older and had a longer disease duration compared to the entire study population. The occurrence of cardiac events may, therefore, merely reflect cardiovascular disease of the elderly population and may not be a direct effect of TKI therapy which would be supported by the lack of overt cardiotoxicity in vitro and in vivo.11 However, nilotinib may add to established cardiovascular risk factors or other comorbidities either by direct vascular effects, e.g. through interaction with discoidin domain receptor 1 (DDR1), or by metabolic effects, e.g. through increased fasting glucose levels, as has been described for the development of peripheral arterial occlusive disease.36,45
There are several strategies to prevent or address clinically relevant cardiovascular toxicity. Only a subgroup of treated patients will develop relevant cardiac damage and screening may allow the early identification of those at increased risk.44 A cardiovascular examination, together with a thorough personal and family history, can evaluate the patient at baseline, whereas careful monitoring allows detection of changes after initiation of treatment. However, the optimal method has not been established. Echocardiography, electrocardiography, and the determination of biomarkers, notably, cardiac troponins and natriuretic peptides46 have distinct advantages and disadvantages.44 Susceptibility to cardiotoxicity is clearly multifactorial and given the low incidence of cardiovascular events, the determination of risk factors was not possible. In addition, there is no information about disease-related or -unrelated cardiac morbidity in patients with CML. The influence of previous therapies, e.g. interferon-α or TKI, on cardiotoxicity is hard to discern, although it should be noted that all patients with an event had previously received imatinib mesylate. These patients may benefit particularly from cardiovascular screening before initiation with nilotinib, although the anticipated wider use of second-generation TKI as first-line treatment may lead to a decrease of this population. Currently, prophylactic medication based on treatment with nilotinib alone does not seem to be justified. As comorbidities may significantly influence the occurrence of cardiac and vascular adverse events under second-generation TKI, including nilotinib,36,45 a high degree of awareness is mandatory during the follow-up, since comorbidities may not be present at the onset of nilotinib therapy and may only develop over time with long-term treatment. As for the presence of specific BCR-ABL mutations, knowledge about substance-specific side effects may influence treatment decisions substantially.45,47
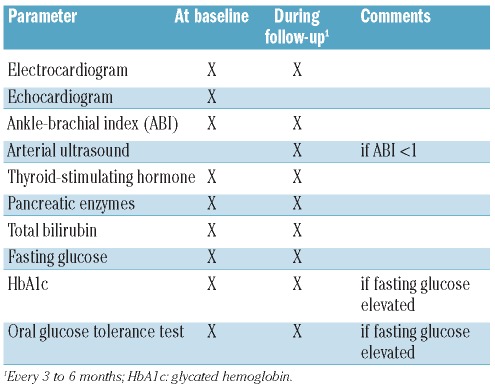
Based on our own experience and published data, Table 3 presents a list of parameters that can be reasonably monitored during the follow-up of patients under nilotinib.
Table 3.
Recommendations for the assessment of relevant comorbidities and non-hematologic adverse events at baseline and during the follow-up of patients treated with nilotinib. In addition, all parameters should be evaluated if clinically indicated.
In conclusion, whereas new electrocardiographic abnormalities were recorded in 20% of all patients and some of them developed severe or even life-threatening coronary artery disease, QT prolongation, changes in LVEF, and clinical cardiac adverse events were uncommon in patients treated with nilotinib and seldom led to treatment discontinuation. There was no cumulative effect of nilotinib exposure indicating the cardiac safety of this TKI. Therefore, the data indicate the need to adhere to current practice guidelines for monitoring patients with CML who are receiving TKI therapy. Important future questions are whether TKI therapy can be discontinued in patients with CML48,49 and whether the period of TKI therapy prior to discontinuation can be sufficiently brief to avoid or reduce cardiac or other vascular adverse events, especially in those with risk factors.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112(13):4808–17. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 4.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(11):1018–29. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 5.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–56. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–41. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Giles FJ, Bhalla KN, Pinilla-Ibarz JA, Larson RA, Gattermann N, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase following imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–5. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.le Coutre P, Ottmann OG, Giles F, Kim DW, Cortes J, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111(4):1834–9. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 9.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 10.Center for Drug Evaluation and Research. Nilotinib Pharmacology/Toxicology Review and Evaluation. 2007. [Google Scholar]
- 11.Wolf A, Couttet P, Dong M, Grenet O, Heron M, Junker U, et al. Preclinical evaluation of potential nilotinib cardiotoxicity. Leuk Res. 2011;35(5):631–7. doi: 10.1016/j.leukres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–51. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88(2):782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 14.Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Bassand JP, Hamm CW, Ardissino D, Boersma E, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28(13):1598–660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 15.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 16.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monsuez JJ, Charniot JC, Vignat N, Artigou JY. Cardiac side-effects of cancer chemotherapy. Int J Cardiol. 2010;144(1):3–15. doi: 10.1016/j.ijcard.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106(1):21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- 20.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 21.Orphanos GS, Ioannidis GN, Ardavanis AG. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncol. 2009;48(7):964–70. doi: 10.1080/02841860903229124. [DOI] [PubMed] [Google Scholar]
- 22.Hasinoff BB, Patel D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol Appl Pharmacol. 2010;249(2):132–9. doi: 10.1016/j.taap.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 25.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12(8):908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 26.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110(4):1233–7. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 27.Estabragh ZR, Knight K, Watmough SJ, Lane S, Vinjamuri S, Hart G, et al. A prospective evaluation of cardiac function in patients with chronic myeloid leukaemia treated with imatinib. Leuk Res. 2011;35(1):49–51. doi: 10.1016/j.leukres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Marcolino MS, Ribeiro AL, Clementino NC, Nunes Mdo C, Barbosa MM, Silva MH, et al. The use of imatinib mesylate has no adverse effects on the heart function. Results of a pilot study in patients with chronic myeloid leukemia. Leuk Res. 2011;35(3):317–22. doi: 10.1016/j.leukres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Perik PJ, Rikhof B, de Jong FA, Verweij J, Gietema JA, van der Graaf WT. Results of plasma N-terminal pro B-type natriuretic peptide and cardiac troponin monitoring in GIST patients do not support the existence of imatinib-induced cardiotoxicity. Ann Oncol. 2008;19(2):359–61. doi: 10.1093/annonc/mdm468. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro AL, Marcolino MS, Bittencourt HN, Barbosa MM, Nunes Mdo C, Xavier VF, et al. An evaluation of the cardiotoxicity of imatinib mesylate. Leuk Res. 2008;32(12):1809–14. doi: 10.1016/j.leukres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Tiribelli M, Colatutto A, Marin L, Barbina G, Qualizza U, Damiani D, et al. Brain natriuretic peptide level as marker of cardiac function in imatinib--treated chronic myeloid leukemia patients: no evidence of cardiotoxicity of imatinib therapy. Am J Hematol. 2008;83(6):517–8. doi: 10.1002/ajh.21157. [DOI] [PubMed] [Google Scholar]
- 32.Turrisi G, Montagnani F, Grotti S, Marinozzi C, Bolognese L, Fiorentini G. Congestive heart failure during imatinib mesylate treatment. Int J Cardiol. 2010;145(1):148–50. doi: 10.1016/j.ijcard.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Verweij J, Casali PG, Kotasek D, Le Cesne A, Reichard P, Judson IR, et al. Imatinib does not induce cardiac left ventricular failure in gastrointestinal stromal tumours patients: analysis of EORTC-ISG-AGITG study 62005. Eur J Cancer. 2007;43(6):974–8. doi: 10.1016/j.ejca.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez A, Sanguino A, Peng Z, Ozturk E, Chen J, Crespo A, et al. An anticancer C-Kit kinase inhibitor is reengineered to make it more active and less cardiotoxic. J Clin Invest. 2007;117(12):4044–54. doi: 10.1172/JCI32373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 36.Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber C, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilo-tinib therapy in CML. Am J Hematol. 2011;86(7):533–9. doi: 10.1002/ajh.22037. [DOI] [PubMed] [Google Scholar]
- 37.Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–51. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 38.Le Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, et al. Severe peripheral arterial disease during nilo-tinib therapy. J Natl Cancer Inst. 2011;103(17):1347–8. doi: 10.1093/jnci/djr292. [DOI] [PubMed] [Google Scholar]
- 39.NeLM news service: Healthcare professional communication regarding association of dasatinib (Sprycel®) with pulmonary arterial hypertension. 2011. [Google Scholar]
- 40.Strevel EL, Ing DJ, Siu LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25(22):3362–71. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 41.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proar-rhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21(15):1216–31. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 42.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348(19):1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 43.Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49(3):329–37. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 44.Altena R, Perik PJ, van Veldhuisen DJ, de Vries EG, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol. 2009;10(4):391–9. doi: 10.1016/S1470-2045(09)70042-7. [DOI] [PubMed] [Google Scholar]
- 45.Valent P. Severe adverse events associated with the use of second-line BCR/ABL tyro-sine kinase inhibitors: preferential occurrence in patients with comorbidities. Haematologica. 2011;96(10):1395–7. doi: 10.3324/haematol.2011.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130(5):688–95. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 47.Jabbour E, Hochhaus A, Cortes J, La Rosee P, Kantarjian HM. Choosing the best treatment strategy for chronic myeloid leukemia patients resistant to imatinib: weighing the efficacy and safety of individual drugs with BCR-ABL mutations and patient history. Leukemia. 2010;24(1):6–12. doi: 10.1038/leu.2009.193. [DOI] [PubMed] [Google Scholar]
- 48.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 49.Rea D, Rousselot P, Nicolini FE, Legros L, Tulliez M, Giraudier S, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia (CML) patients (pts) with stable undetectable Bcr-Abl transcripts: results from the French CML Group (FILMC) ASH Annual Meeting Abstracts. 2011;118(21):604. [Google Scholar]