Abstract
Background
Multiparameter flow cytometric analysis of bone marrow and peripheral blood cells has proven to be of help in the diagnostic workup of myelodysplastic syndromes. However, the usefulness of flow cytometry for the detection of megakaryocytic and platelet dysplasia has not yet been investigated. The aim of this pilot study was to evaluate by flow cytometry the diagnostic and prognostic value of platelet dysplasia in myelodysplastic syndromes.
Design and Methods
We investigated the pattern of expression of distinct surface glycoproteins on peripheral blood platelets from a series of 44 myelodysplastic syndrome patients, 20 healthy subjects and 19 patients with platelet alterations associated to disease conditions other than myelodysplastic syndromes. Quantitative expression of CD31, CD34, CD36, CD41a, CD41b, CD42a, CD42b and CD61 glycoproteins together with the PAC-1, CD62-P, fibrinogen and CD63 platelet activation-associated markers and platelet light scatter properties were systematically evaluated.
Results
Overall, flow cytometry identified multiple immunophenotypic abnormalities on platelets of myelodysplastic syndrome patients, including altered light scatter characteristics, over-and under expression of specific platelet glycoproteins and asynchronous expression of CD34; decreased expression of CD36 (n=5), CD42a (n=1) and CD61 (n=2), together with reactivity for CD34 (n=1) were only observed among myelodysplastic syndrome cases, while other alterations were also found in other platelet disorders. Based on the overall platelet alterations detected for each patient, an immunophenotypic score was built which identified a subgroup of myelodysplastic syndrome patients with a high rate of moderate to severe alterations (score>1.5; n=16) who more frequently showed thrombocytopenia, megakaryocytic dysplasia and high-risk disease, together with a shorter overall survival.
Conclusions
Our results show the presence of altered phenotypes by flow cytometry on platelets from around half of the myelodysplastic syndrome patients studied. If confirmed in larger series of patients, these findings may help refine the diagnostic and prognostic assessment of this group of disorders.
Keywords: myelodysplastic syndromes, megakaryocytic dysplasia, platelets, flow cytometry, immunophenotyping
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal hematopoietic stem cell disorders characterized by bone marrow (BM) failure and increased risk of transformation to acute myeloid leukemia (AML).1 Usually, MDS patients are elderly and they already show anemia, leukopenia and/or thrombocytopenia at presentation.2 Conventional diagnosis of MDS is based on a combination of morphology and cytogenetics, besides exclusion of other disorders as primary cause for cytopenia/dysplasia.3 However, in some cytopenic patients, diagnosis of MDS remains a challenge. Because of this, more refined definitions and criteria for the diagnosis of MDS have been recently proposed;4 among other tests (diagnostic co-criteria), multiparameter flow cytometry immunophenotyping has been reported to be a useful additional diagnostic tool.
Over the past two decades, a great number of immunophenotypic alterations have been described in both BM and peripheral blood (PB) cells from MDS patients through the use of multiparameter flow cytometry (MFC) immunophenotyping.4,5 Immunophenotypic alterations most frequently involve myeloblasts, CD34+ B-cell precursors as well as the CD34− precursors,6–9 e.g. maturing neutrophil, monocytic and erythroid cells.10–16 On the other hand, few studies have been reported in which the BM megakaryocytic compartment is evaluated and, to the best of our knowledge, no attempts have been made to investigate the potential presence of phenotypic alterations in PB platelets by MFC. For this reason, an analysis of megakaryocytic lineage by MFC is usually excluded from consensus recommendations on MFC immunophenotyping of MDS due to the limited data available.17
Both the immunophenotype and DNA ploidy status of BM megakaryocytic precursors from healthy subjects and MDS patients have been previously investigated by MFC.18 However, due to their large size, low frequency (<0.05%) in BM, increased fragility and adhesion properties with a tendency to aggregate,19,20 analysis of BM megakaryocytes typically requires screening of large volumes of sample, and complex sample preparation procedures have been used.18,21,22 Consequently, immunophenotyping of megakaryocytic BM cells by MFC has never become routine in MDS.
Despite all of this, it could be hypothesized that a potentially useful alternative cellular target to evaluate the presence of megakaryocytic dysplasia in MDS patients by MFC, could be PB platelets through the assessment of the immunophenotypic pattern of expression of multiple surface glycoproteins (GP) in combination or not with other functional properties of platelets.
In this pilot study, we evaluated the pattern of expression of multiple surface GP on PB platelets from a series of 44 MDS patients versus normal PB platelets, in order to identify potential alterations that could contribute to the diagnosis of megakaryocytic dysplasia in MDS and evaluation of its prognostic impact on overall patient survival.
Design and Methods
Patients, controls and samples
PB samples from 44 MDS patients were collected into Vacutainer™ tubes containing sodium citrate (Becton-Dickinson, New Jersey, USA). The study was approved by the local institutional review boards. All patients and control subjects gave their informed consent prior to entering the study, in line with the guidelines of the local ethics committees and the Declaration of Helsinki. During PB collection, the first 2 mL were systematically discharged. None of the subjects was taking aspirin or other platelet-activating medication, had received blood transfusions during the preceding two weeks, or had been treated with hematopoietic growth factors or chemotherapy for the previous six months.
Diagnosis of MDS was established according to the World Health Organization (WHO) criteria based on clinical data, morphological analysis of PB and BM smears, and cytogenetic studies, after excluding other diseases which could potentially contribute to BM dysplasia and/or cytopenias.3 The distribution of the MDS patients according to the WHO classification was: refractory cytopenia with unilineage dysplasia (n=12), refractory anemia (RA) (n=9), and refractory thrombocytopenia (RT) (n=3); RA with ringed sideroblasts (RARS) (n=9); refractory cytopenia with multilineage dysplasia (RCMD) (n=14); RA with excess of blasts type 1 (RAEB-1) (n=2); RAEB-2 (n=5), and 5q-syndrome (n=2). Mean age was 74±11 years (median 76 years, range 45–90 years). There were 14 males and 30 females.
According to the International Prognostic Scoring System (IPSS),23 18 patients were classified as low risk, 16 as intermediate-1, 4 as intermediate-2, and one as high risk MDS. Regarding the WHO-based Prognostic Score System (WPSS),24 MDS patients were distributed as follows: very low risk (n=11), low risk (n=17), intermediate risk (n=6), high risk (n=4), and very high risk (n=1). In the remaining 5 patients, metaphases could not be obtained from cytogenetic cultures. All 44 were untreated MDS patients and 22 of them were newly diagnosed cases seen at the MDS outpatient clinic at the Universidade Federal de São Paulo (UNIFESP, São Paulo, Brazil).
At the moment of closing this study, the median follow-up time of the whole patient series was 38 months (range 4–127 months). Overall, 20 of 44 MDS cases were transfusion dependent.
In parallel, 20 PB samples from an age and sex-matched group of adult healthy volunteers were studied as normal controls. In addition, in order to evaluate the specificity of the immunophenotypic abnormalities observed among MDS patients, PB platelets from 19 patients with different disease conditions other than MDS were also analyzed: 4 myeloproliferative disorders (2 chronic myeloid leukemia, one essential thrombocytemia and one myelofibrosis), 6 idiopathic thrombocytopenic purpura patients and 9 reactive cytopenias (associated with solid tumors in 2 cases, with sepsis in another 2, and with portal hypertension, nutritional deficiency, drug administration, lupus and chronic kidney disease in one case each).
Cytomorphological studies
PB and BM films were stained with May-Grünwald-Giemsa (MGG) and cell morphology was independently evaluated with an optical microscope by 2 expert cytomorphologists; 500 or over nucleated cells and more than 20 megakaryocytes were counted in each BM smear. Erythroid and neutrophil dysplasia was defined by the presence of more than 10% BM cells with morphological abnormalities as per WHO criteria;3 presence of more than 15% ringed sideroblasts was also considered a diagnostic sign for erythroid dysplasia. Megakaryocytic dysplasia was characterized by the presence of abnormalities (e.g. micromegakaryocytes, nuclear hypolobulation and large mononuclear cells) involving more than 40% of all megakaryocytic cells.
Multiparameter flow cytometry immunophenotypic studies
MFC immunophenotypic studies were systematically performed at room temperature (RT) on fresh PB samples, within less than 3 h after the sample had been obtained. Expression of platelet-associated GP was evaluated using a FACSCalibur flow cytometer (Becton-Dickinson, San José, CA, USA) and the following combinations of monoclonal antibodies (MAb) were systematically used: fluorescein isothiocyanate (FITC)/phycoerythrin (PE)/peridinin chlorophyll protein (PerCP)−: CD42a/CD31/−; CD41b/−/−; CD36/CD41a/−; CD63/42b/−; PAC-1/CD62P/CD61; anti-fibrinogen/−/−; and CD61/CD34/−. Reagents were purchased from 1) Becton-Dickinson: CD31-PE (clone L133.1), CD34-PE (clone 8G12), CD41b-FITC (clone HIP2), CD42a-FITC (clone Beb1), CD61-PerCP (clone RUU-PL 7F12), CD62P-PE (clone AC1.2) and PAC-1-FITC; 2) Immunotech (IOT, Marseille, France): CD36-FITC (clone FA6.152), CD41a-PE (clone P2), CD42b-PE (clone SZ2), CD61-FITC (clone SZ21) and CD63-FITC (clone CLBGran/12). In addition, an anti-fibrinogen polyclonal antiserum conjugated with FITC (DAKO, Glostrup, Denmark) was also used to stain platelets. Non-stained PB was used in all experiments as a negative control.
Briefly, 5 μL of whole PB was pipetted into multiple 12 x 75 mm polystyrene tubes containing 100 μL of phosphate buffered saline (PBS) each (pH=7.4), and stained with saturating amounts (0.5–1 μg) of the different combinations of MAb reagents. After gentle mixing, samples were incubated for 15 min in darkness (RT) and washed once in 2 mL PBS (5 min at 2800 rpm). The cell pellet was then resuspended in 500 μL of PBS. No fixative or lysing solution was used during the staining procedure.
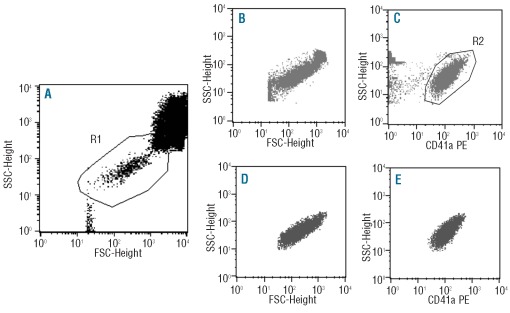
Data acquisition was performed in two sequential steps immediately after sample preparation, using the CellQUEST software program (BD). In a first step, information on a total of 2×104 events corresponding to the whole PB cellularity was acquired. In a second step, information was specifically collected for 2×104 or more platelets through an electronic live gate set in a forward (FSC) and sideward light scatter (SSC) dot plot (Figure 1). Prior to data acquisition, instrument settings were optimized for the flow cytometric analysis of platelets. The Paint-a-Gate PRO software program (BD) was used for data analysis.
Figure 1.
Example of the two-step acquisition procedure and the gating strategy used for the immunophenotypic identification and characterization of platelets in stained whole PB samples. After initial acquisition of a total of 2x104 events (A), an electronic live gate (R1) was drawn around the platelets based on their unique FSC/SSC characteristics. Then, in a second step, information about ≥ 2x104 platelets was acquired through this electronic live gate (B and C). Finally, platelets (gray events) were identified as those events being CD41+ (R2 in panel C) (see C-E).
Platelets were specifically identified on the basis of their unique FSC/SSC characteristics (expressed in arbitrary mean channel linear unit values scaled from 0 to 1024) and expression of pan-platelet GP (Figure 1). For each specific GP, results were expressed as mean fluorescence intensity values (MFI; relative linear units scaled from 0 to 104). Aberrant phenotypes were defined when the patients MFI and FSC/SSC values were greater or lower than the mean value±2 standard deviations (SD) of the mean MFI obtained for the same GP in PB platelets from adult healthy individuals.
Immunophenotypic score
For each of the 15 platelet immunophenotypic variables analyzed, an immunophenotypic score for mild to severe alterations was established similar to that previously described by Matarraz et al.8 First, a value of 0, 0.5, 1 or 2 was given when the MFI value obtained for each of the phenotypic variables evaluated was within the mean±2 SD, between the mean±2 SD and the mean±3 SD, between the mean±3 SD and the mean±4 SD and, over the mean±4 SD of those values found for that parameter among normal PB samples, respectively. The immunophenotypic score was then calculated for each patient by adding up the score obtained for that patient for each of the 15 immunophenotypic parameters evaluated.
Statistical analysis
Mean value and SD, median and range of all parameters under study were calculated using the SPSS 13.0 software program (SPSS, Chicago, IL, USA). Student’s t-test and either the Mann-Whitney U or the Kruskal-Wallis tests (two-sided) were used to calculate the statistical significance of differences observed between groups for parametric and non-parametric data, respectively. Survival curves were plotted according to the Kaplan-Meyer method and compared with the log rank test. Duration of survival was defined as the time from diagnosis of MDS until death or the last follow-up visit. Cox’s regression model was used for multivariate analyses. P<0.05 was considered statistically significant.
Results
Cytomorphological features of BM megakaryocytic cells from myelodysplastic syndrome patients
In 33 of 44 MDS patients, enough megakaryocytes were detected on BM films to allow morphological assessment. Megakaryocytic dysplasia was found in 17 of 33 cases (52%) in between 50 and 98% of the cells. The most frequent alterations were: hypolobulated megakaryocytes (n=18), mononuclear large megakaryocytic cells (n=12), megakaryocytes with widely-separated nucleus (n=8), and presence of micromegakaryocytes (n=4).
Immunophenotypic characteristics of PB platelets in myelodysplastic syndromes
The overall median number of PB platelets in MDS cases was 185×109/L (range 4–890×109/L). Twenty-six cases (59%) had platelet counts over 100×109/L, 8 (18%) between 50 and 100×109/L and 10 showed less than 50×109 platelets/L in PB.
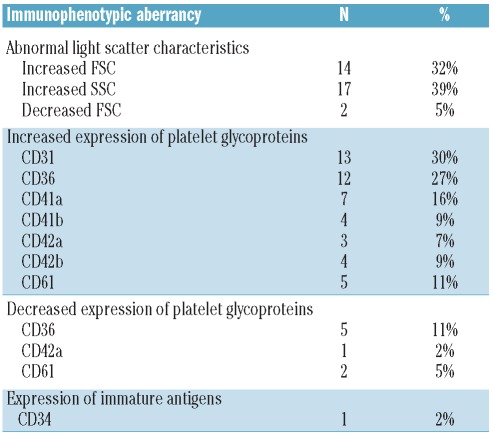
An analysis of immunophenotypic characteristics showed platelets from MDS patients with multiple alterations compared to PB platelets from healthy adults. These included altered light scatter characteristics (FSC 32%, SSC 39%), increased and decreased expression of platelet glycoproteins, as well as aberrant expression of the CD34 antigen in one case (Table 1). Interestingly, no statistically significant difference was found between MDS patients and normal controls regarding the expression of any of the platelet activation-associated proteins investigated (e.g. CD62P, CD63, PAC-1 and anti-fibrinogen).
Table 1.
Type and frequency of immunophenotypic alterations detected on PB platelets from MDS patients (n = 44) versus normal control subjects (n=20).
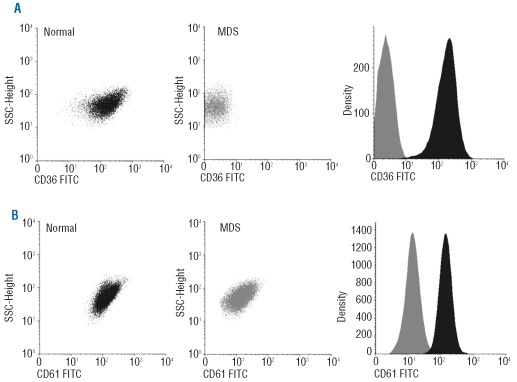
Nevertheless, immunophenotypic analysis of PB platelets from patients with disease conditions other than MDS showed that increased light scatter and increased CD36 and CD31 expression were not specific for MDS, as they were also found among non-MDS cases. In contrast, decreased expression of CD36 (11%), CD61 (5%) (Figure 2) and CD42a (2%), aberrant expression of CD34 (2%) and decreased FSC values (5%) were only detected among MDS patients. CD36 deficiency was found in 5 cases: 2 RCMD, 2 RAEB-1 and one refractory thrombocytopenia. Most of them (4 of 5) also presented thrombocytopenia with megakaryocytic dysplasia on morphological grounds. In turn, CD61 deficiency was seen in 2 MDS cases (one RCMD and one RA) that had normal platelet counts. Megakaryocytic dysplasia was observed in one of these 2 patients. In turn, decreased expression of CD42a was identified in one case (RARS) in association with normal platelet counts and megakaryocytic morphology. CD34 was expressed on platelets from a RAEB-2 patient who had thrombocytopenia and megakaryocytic dysplasia, whereas decreased FSC values were found in 2 RA cases.
Figure 2.
Bivariate dot plots and single-parameter histograms illustrating some of the immunophenotypic alterations present on PB platelets from MDS patients. (A) CD36 expression on platelets from a normal control (black) and an MDS patient (gray). (B) CD61 expression by normal (black) versus MDS (gray) platelets.
Immunophenotypic score
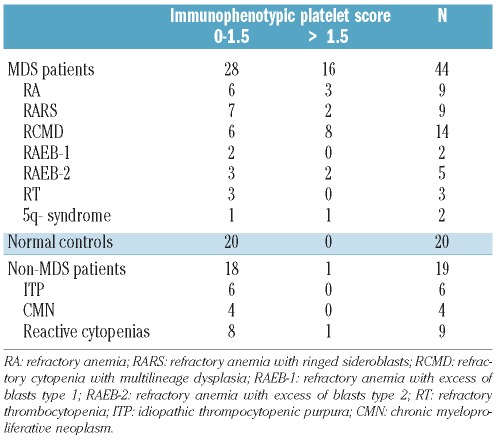
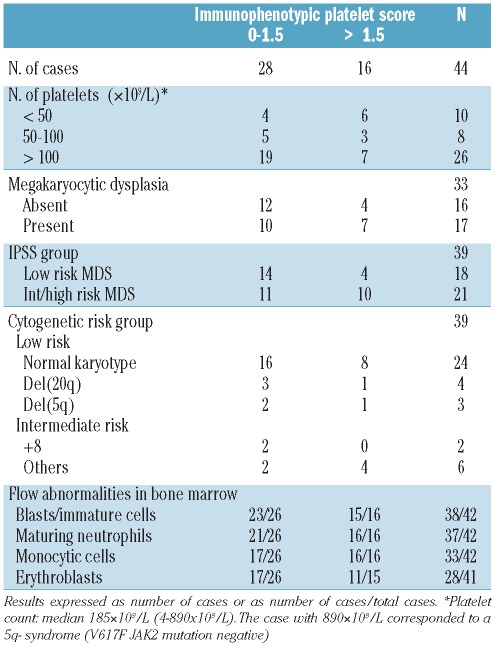
The overall immunophenotypic score of PB platelets from MDS patients, ranged from 0 to 10.5 (mean 2.3±2.9), while all controls had a score of 1.5 or under except for one patient with cytopenias and portal hypertension who had a score of 2. According to this score, three groups of MDS patients could be identified: i) cases showing a normal platelet phenotype with a score of between 0 and 1.5 (n=28, 64%); ii) patients with a score of between 2 and 3 (n=6, 14%); and iii) cases with severe platelet dysplasia and a score of over 3 (n=10, 23%).
It is worthy of note that high immunophenotypic scores (>1.5) were observed in all WHO subtypes of MDS except RAEB-1, the higher frequencies corresponding to RAEB-2 (n=2 of 5, 40%) and RCMD cases (n=8 of 14,57%) (Table 2). As could be expected, a high number of patients with platelet counts over 100×109 platelets/L (73 vs. 40% for cases with less than 50×109 platelets/L), normal megakaryocytic morphology (75 vs. 59%) and low-risk MDS (78 vs. 52%) had a score of 1.5 or under, although these differences did not reach statistical significance (Table 3). Interestingly, all cases who showed MDS-specific immunophenotypic alterations (decreased CD36, CD61 and/or CD42a expression and positivity for CD34) displayed an immunophenotypic score of under 3 and all 3 refractory thrombocytopenia cases presented a score of between 0 and 1.5 (Table 2).
Table 2.
Distribution of MDS patients, healthy adults and non-MDS patients according to the MFC immunophenotypic score of peripheral blood platelets.
Table 3.
Distribution of MDS patients according to the MFC immunophenotypic score of platelets versus platelet counts, megakaryocytic dysplasia, IPSS subtype of the disease, cytogenetic risk group and bone marrow flow cytometric abnormalities.
Survival of myelodysplastic syndrome cases according to the presence of thrombocytopenia, megakaryocytic dysplasia and an altered platelet phenotype by MFC
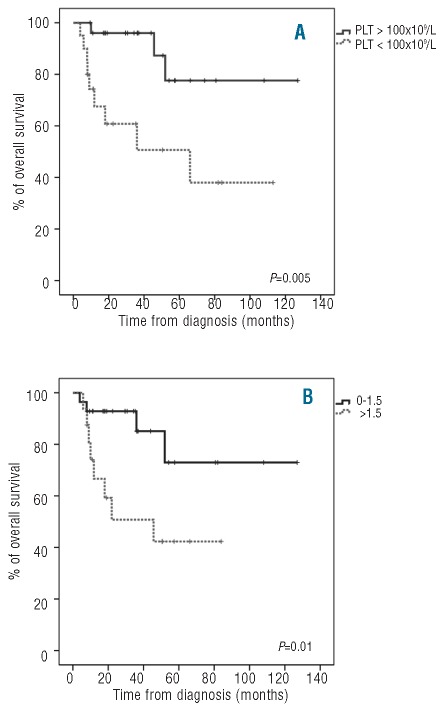
The estimated median overall survival (OS) of patients with megakaryocytic dysplasia by morphology was slightly lower than those cases without dysplasia (80±12 vs. 108±11; P>0.05). In turn, cases with platelet counts under 100×109/L showed a significantly shorter OS than those with more than 100×109 platelets/L (59±12 vs. 108±10 months, respectively; P=0.005) (Figure 3A). None of the individual immunophenotypic abnormalities identified on its own showed a significant impact on OS of MDS patients. Nevertheless, a high overall platelet immunophenotypic score (score >1.5) was associated with a shorter OS (mean 45±9 vs. 102±11 months, respectively; P=0.01) (Figure 3B).
Figure 3.
Overall survival of MDS patients according to PB platelet (PLT) counts (A) and the platelet immunophenotypic score (B).
Multivariate analysis for OS showed that a high immunophenotypic score (P=0.04) together with a high (Int-2/high) IPSS (P=0.01) were the only independent adverse prognostic factors for overall patient survival.
Discussion
MDS comprise a heterogeneous spectrum of disorders characterized by a clonal dysregulation of hematopoiesis, which is responsible for markedly altered multilineage morphological patterns of BM cells associated with PB cytopenias.2 Such abnormalities are associated with multiple and frequently complex cytogenetic changes25 which also translate into aberrant antigen expression profiles during differentiation and maturation of hematopoietic cells.7,26,27 Despite the increasing evidence in support of the occurrence of a great variety of immunophenotypic aberrations on CD34+ precursors,6,8,9,28 as well as maturing neutrophil, monocytic and erythroid cells10,11,13,14,29 which may contribute to a more refined diagnosis and prognostic assessment of MDS, so far only a few studies have been reported in which megakaryocytic lineage cells from MDS patients are analyzed by MFC; in addition, in one of these reports10 no altered MFC profiles for megakaryocytic cells in MDS were reported.
Megakaryocytic dysplasia can be found by morphology in around 40% of all MDS cases30 and it contains key diagnostic information. At the same time it is associated with a poor prognosis of the disease.30,31 Similarly, multiple reports continue to show that thrombocytopenia is also associated with both a worse outcome and decreased survival in MDS,8,32–34 even among low-risk MDS cases.35 In our series, megakaryocytic dysplasia was not seen to have a significant impact on the outcome of MDS patients. In contrast, patients with less than 100×109 platelets/L had a worse outcome. Interestingly, analysis of BM films does not always provide enough megakaryocytic-lineage cells to correctly identify megakaryocytic dysplasia,30 as was the case in approximately 25% of our patients, which limits the applicability of this measurement.
Detection of immunophenotypic megakaryocytic dysplasia has proven to be difficult, especially because of the complexity of the ex vivo analysis of megakaryocytes and megakaryoblasts by flow cytometry. Typically, megakaryocytic cells are large frail cells with propensity to aggregate, which are present at low frequencies in the BM (<0.05%).19,20 Because of this, immunophenotypic studies of megakaryocytic lineage cells require large volumes of highly cellular BM samples and complex methods for specific purification/selection of megakaryocytic cells for further analyses.1.8,21,22 Alternatively, the MFC study of PB platelets may offer many advantages as regards the detection of megakaryocytic dysplasia: it is a non-invasive method that requires small volumes of PB even in severe thrombocytopenic patients, and it is methodologically feasible, fast and reproducible.36
Therefore, in the present study we investigated the immunophenotypic profiles of PB platelets from MDS patients compared with healthy subjects. Overall, multiple distinct flow cytometric abnormalities were found on platelets from MDS patients. These included: altered light scatter properties, over and underexpression of platelet associated GP and asynchronous antigen expression. Of all the different alterations identified, decreased expression of CD36, CD42a and CD61, together with asynchronous CD34 expression, were the only alterations that were observed exclusively among MDS cases. Despite the fact that the other aberrations were not specific for MDS, and that they were also present in platelets from other (different) pathological conditions, MDS cases frequently showed a higher number and severity of abnormalities, as reflected by a higher immunophenotypic score. Importantly, all healthy controls and non-MDS cases except one (score of 2), showed a low immunophenotypic score. Because of this, those cases with scores of 2 or under should be considered with caution, in order to keep false positive and false negative results to a minimum. Overall, it should also be emphasized that a combination of both specific and/or multiple alterations is required to increase the specificity for MDS of the platelet abnormalities described here, in line with previous reports of MDS-associated phenotypic alterations of other hematopoietic cell compartments.8,11,16,17
Two of our MDS cases showed abnormally decreased expression of CD61, in association with normal levels of CD41a (GPIIb-α) and CD41b (GPIIb-β) and normal PB platelet counts. In turn, reduced levels of CD42b and/or CD42a were found in only one patient. Acquired deficiency of platelet GP in patients with hematologic neoplasms has sometimes been reported. Berndt et al.37 reported a case of juvenile MDS with loss of CD42a on platelets (similar to what is seen in patients with Bernard-Soulier’s syndrome). Also, acquired Glanzmann thrombasthenia due to anti-GPIIb/IIIa auto-antibodies is usually associated with autoimmune conditions38 and has been reported in both non-Hodgkin’s38 (NHL) and Hodgkin’s lymphomas.39 In turn, decreased levels of CD42a have also been described in platelets from AML40 and MDS patients.41 In this latter study,41 reduced amounts of CD42b were reported to be a common finding in MDS cases, although no patient showed complete lack or decreased expression of CD36.
The CD36 GP (GP IV) expressed on the platelet surface, acts as a receptor for thrombospondin and collagen; it is expressed by normal platelets, monocytes, nucleated red cell precursors and endothelial cells.42 CD36 deficiency was first described in 1989 by Ikeda et al.43 in a case that was refractory to platelet transfusion due to the presence of a new anti-platelet antibody, named anti-Naka. Yamamoto et al.44 later showed that the Naka epitope is part of the CD36 molecular structure and that in the patients mentioned above this protein was not well expressed. Congenital CD36 deficiency is not directly associated with either quantitative or qualitative platelet disorders. However, when in contact with this antigen (e.g. through transfusion and/or pregnancy), CD36-deficient patients may develop allo-antibodies against platelets leading to transfusion refractoriness, posttransfusional purpura and neonatal alloimune thrombocytopenia.42,45 Two types of CD36 deficiency have been described so far: type I cases show reduced CD36 expression on platelets and monocytes and type II subjects show CD36 deficiency restricted to platelets.46 Association of CD36 deficiency and a clonal hematologic malignancy has been described in only one case of NHL (type I deficiency).47 In contrast, here we show that CD36 deficiency is a relatively common finding in MDS, being associated in most cases with both thrombocytopenia and megakaryocytic dysplasia. From our CD36-deficient MDS patients, only one case presented type I deficiency, as both monocytes and NRBC erythrocytes were CD36−. The precise current prevalence of CD36 deficiency in the Brazilian and the Spanish populations is not known. Despite this, the frequency of CD36 deficiency observed among our MDS patients was by far superior to that reported in the literature for both congenital CD36 deficiency44,48,49 and MDS,41 independently of ethnic origin. The reasons for these differences require further investigation.
Based on the overall immunophenotypic profile of PB platelets, variable immunophenotypic scores were found among MDS patients, with a significant proportion of cases showing immunophenotypic scores clearly higher than those observed among normal and reactive PB platelets. Interestingly, some patients with unilineage dysplasia (RA and RARS) presented high (>1.5) immunophenotypic scores or deficient GP expression profiles, supporting the existence of multilineage involvement already among these subgroups of MDS patients. This may imply that the study of platelets by MFC, in addition to cytomorphology, may contribute to a more robust distinction between a true unilineage versus multilineage dysplasia, as is also suggested by others.16,17,50 In addition, the immunophenotypic platelet score showed an important prognostic impact on the outcome of MDS patients, independently of the WHO and IPSS subtypes, the presence of megakaryocytic dysplasia and the PB platelet count, cases with high immunophenotypic scores showing a shorter overall survival. Interestingly, although an association between thrombocytopenia and poor patient outcome has been described in large series of MDS patients,33,34 in our study approximately 26% (7 of 26 cases) of those patients showing normal platelet counts had a high immunophenotypic score, reinforcing the potential added value of immunophenotyping to the number of platelets in PB.
In contrast, some cases with morphological megakaryocytic dysplasia and thrombocytopenia showed low immunophenotypic scores. Apoptosis of dysplastic megakaryocytes and shortened platelet life expectancy in the BM,51,52 together with a decreased platelet release by the dysplastic megakaryocytic precursors53 reported in MDS, might contribute to explain the absence of an altered platelet immunophenotype in these cases, because under these conditions a normal immunophenotypic score may result from the immunophenotypic analysis of residual normal, rather than dysplastic, PB platelets.
There did not seem to be a significant difference in baseline platelet activation status as assessed by flow cytometry (e.g. CD62P levels) between MDS and healthy subjects. Despite this and the fact that no bleeding complications were detected among our patients, correlation of the presence and type of platelet imunophenotypic alterations with the functionality of the platelets by other tests, such as bleeding time, PFA-100 and platelet aggregation, may provide further insight into the potential direct functional impact of the phenotypic alterations observed here on MDS platelets.
In summary, the present study shows the presence of altered platelet immunophenotypes by MFC in almost half of all MDS cases. Such alterations are highly specific of MDS because they are not observed in other conditions (e.g. deficiency of CD36, CD61, CD42a and expression of CD34+) or they reflect the alteration of multiple markers that translate into high immunophenotypic scores. From a clinical point of view, assessment of aberrant antigen expression profiles associated with a high immunophenotypic platelet score may also help to better identify high-risk MDS cases. However, due to the relatively limited number of cases analyzed in this pilot study, further analyses of larger series of patients are needed to confirm our preliminary observations.
Footnotes
Funding: this work was supported by a grant from FAPESP (proc n.05/57792-0) and FADA (UNIFESP) and Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid, Spain; Grant number: RTICC RD06/0020/0035-FONDOS FEDER. AS was supported by CNPq (proc n.142968/2006-4) and CAPES (proc. n. PDEE BEX 1025/05-8).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Greenberg PL, Young NS, Gattermann N. Myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2002:136–61. doi: 10.1182/asheducation-2002.1.136. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872–85. doi: 10.1056/NEJMra0902908. [DOI] [PubMed] [Google Scholar]
- 3.Brunning RD, Orazi A, Germing U, Le Beau MM, Porwit A, Baumann I, et al. Chapter 5, Myelodysplastic syndromes/neoplasms overview. Lyon: IARC; 2008. WHO classification of tumours of haematopoietic and lymphoid tissues; pp. 88–93. [Google Scholar]
- 4.Valent P, Horny HP, Bennett JM, Fonatsch C, Germing U, Greenberg P, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leuk Res. 2007;31(6):727–36. doi: 10.1016/j.leukres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Loken MR, van de Loosdrecht A, Ogata K, Orfao A, Wells DA. Flow cytometry in myelodysplastic syndromes: report from a working conference. Leuk Res. 2008;32(1):5–17. doi: 10.1016/j.leukres.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, et al. Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood. 2002;100(12):3887–96. doi: 10.1182/blood-2002-01-0222. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Holgado N, Arroyo JL, Pata C, Villaron E, Sanchez GF, Martin A, et al. Analysis of hematopoietic progenitor cells in patients with myelodysplastic syndromes according to their cytogenetic abnormalities. Leuk Res. 2004;28(11):1181–87. doi: 10.1016/j.leukres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Matarraz S, Lopez A, Barrena S, Fernandez C, Jensen E, Flores J, et al. The immunophenotype of different immature, myeloid and B-cell lineage-committed CD34+ hematopoietic cells allows discrimination between normal/reactive and myelodysplastic syndrome precursors. Leukemia. 2008;22(6):1175–83. doi: 10.1038/leu.2008.49. [DOI] [PubMed] [Google Scholar]
- 9.Ogata K, Kishikawa Y, Satoh C, Tamura H, Dan K, Hayashi A. Diagnostic application of flow cytometric characteristics of CD34+ cells in low-grade myelodysplastic syndromes. Blood. 2006;108(3):1037–44. doi: 10.1182/blood-2005-12-4916. [DOI] [PubMed] [Google Scholar]
- 10.Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, Molldrem J, Barrett AJ, et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood. 2001;98(4):979–87. doi: 10.1182/blood.v98.4.979. [DOI] [PubMed] [Google Scholar]
- 11.Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102(1):394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- 12.del Canizo MC, Fernandez ME, Lopez A, Vidriales B, Villaron E, Arroyo JL, et al. Immunophenotypic analysis of myelodysplastic syndromes. Haematologica. 2003;88(4):402–7. [PubMed] [Google Scholar]
- 13.Della Porta MG, Malcovati L, Invernizzi R, Travaglino E, Pascutto C, Maffioli M, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006;20(4):549–55. doi: 10.1038/sj.leu.2404142. [DOI] [PubMed] [Google Scholar]
- 14.Stachurski D, Smith BR, Pozdnyakova O, Andersen M, Xiao Z, Raza A, et al. Flow cytometric analysis of myelomonocytic cells by a pattern recognition approach is sensitive and specific in diagnosing myelodysplastic syndrome and related marrow diseases: emphasis on a global evaluation and recognition of diagnostic pitfalls. Leuk Res. 2008;32(2):215–24. doi: 10.1016/j.leukres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Lorand-Metze I, Ribeiro E, Lima CS, Batista LS, Metze K. Detection of hematopoietic maturation abnormalities by flow cytometry in myelodysplastic syndromes and its utility for the differential diagnosis with non-clonal disorders. Leuk Res. 2007;31(2):147–55. doi: 10.1016/j.leukres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Matarraz S, Lopez A, Barrena S, Fernandez C, Jensen E, Flores-Montero J, et al. Bone marrow cells from myelodysplastic syndromes show altered immunophenotypic profiles that may contribute to the diagnosis and prognostic stratification of the disease: a pilot study on a series of 56 patients. Cytometry B Clin Cytom. 2010;78(3):154–68. doi: 10.1002/cyto.b.20513. [DOI] [PubMed] [Google Scholar]
- 17.van de Loosdrecht AA, Alhan C, Bene MC, Della Porta MG, Drager AM, Feuillard J, et al. Standardization of flow cytometry in myelodysplastic syndromes: report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica. 2009;94(8):1124–34. doi: 10.3324/haematol.2009.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomer A. Human marrow megakaryocyte differentiation: multiparameter correlative analysis identifies von Willebrand factor as a sensitive and distinctive marker for early (2N and 4N) megakaryocytes. Blood. 2004;104(9):2722–7. doi: 10.1182/blood-2004-02-0769. [DOI] [PubMed] [Google Scholar]
- 19.Levine RF, Hazzard KC, Lamberg JD. The significance of megakaryocyte size. Blood. 1982;60(5):1122–31. [PubMed] [Google Scholar]
- 20.Rabellino EM, Levene RB, Leung LL, Nachman RL. Human megakaryocytes. II. Expression of platelet proteins in early marrow megakaryocytes. J Exp Med. 1981;154(1):88–100. doi: 10.1084/jem.154.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomer A, Harker LA, Burstein SA. Flow cytometric analysis of normal human megakaryocytes. Blood. 1988;71(5):1244–52. [PubMed] [Google Scholar]
- 22.Tomer A, Friese P, Conklin R, Bales W, Archer L, Harker LA, et al. Flow cytometric analysis of megakaryocytes from patients with abnormal platelet counts. Blood. 1989;74(2):594–601. [PubMed] [Google Scholar]
- 23.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 24.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 25.Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 26.Arroyo JL, Fernandez ME, Hernandez JM, Orfao A, San Miguel JF, del Canizo MC. Impact of immunophenotype on prognosis of patients with myelodysplastic syndromes. Its value in patients without karyotypic abnormalities. Hematol J. 2004;5(3):227–33. doi: 10.1038/sj.thj.6200370. [DOI] [PubMed] [Google Scholar]
- 27.Cutler JA, van de Loosdrecht AA, de Baca ME, Kalnoski M, Zehentner BK, Eidenschink L, et al. Phenotypic abnormalities strongly reflect genotype in patients with unexplained cytopenias. Cytometry B Clin Cytom. 2011;80(3):150–7. doi: 10.1002/cyto.b.20582. [DOI] [PubMed] [Google Scholar]
- 28.Ogata K. Diagnostic flow cytometry for low-grade myelodysplastic syndromes. Hematol Oncol. 2008;26(4):193–8. doi: 10.1002/hon.857. [DOI] [PubMed] [Google Scholar]
- 29.Scott BL, Wells DA, Loken MR, Myerson D, Leisenring WM, Deeg HJ. Validation of a flow cytometric scoring system as a prognostic indicator for posttransplantation outcome in patients with myelodysplastic syndrome. Blood. 2008;112(7):2681–86. doi: 10.1182/blood-2008-05-153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verburgh E, Achten A, Louw VJ, Brusselmans C, Delforge M, Boogaerts M, et al. A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia. 2007;21(4):668–77. doi: 10.1038/sj.leu.2404564. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda A, Germing U, Jinnai I, Iwanaga M, Misumi M, Kuendgen A, et al. Improvement of criteria for refractory cytopenia with multilineage dysplasia according to the WHO classification based on prognostic significance of morphological features in patients with refractory anemia according to the FAB classification. Leukemia. 2007;21(4):678–86. doi: 10.1038/sj.leu.2404571. [DOI] [PubMed] [Google Scholar]
- 32.Lorand-Metze I, Califani SM, Ribeiro E, Lima CS, Metze K. The prognostic value of maturation-associated phenotypic abnormalities in myelodysplastic syndromes. Leuk Res. 2008;32(2):211–3. doi: 10.1016/j.leukres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Neukirchen J, Blum S, Kuendgen A, Strupp C, Aivado M, Haas R, et al. Platelet counts and haemorragic diathesis in patients with myelodysplastic syndromes. Eur J Haematol. 2009;83:477–82. doi: 10.1111/j.1600-0609.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 34.Al Ameri A, Jabbour E, Garcia-Manero G, O’Brien S, Faderl S, Ravandi F, et al. Significance of thrombocytopenia in myelodysplastic syndromes: associations and prognostic implications. Clin Lymphoma Myeloma Leuk. 2011;11(2):237–41. doi: 10.1016/j.clml.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538–43. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 36.Orfao A, Ruiz-Arguelles A, Lacombe F, Ault K, Basso G, Danova M. Flow cytometry: its applications in hematology. Haematologica. 1995;80(1):69–81. [PubMed] [Google Scholar]
- 37.Berndt MC, Kabral A, Grimsley P, Watson N, Robertson TI, Bradstock KF. An acquired Bernard-Soulier-like platelet defect associated with juvenile myelodysplastic syndrome. Br J Haematol. 1988;68(1):97–101. doi: 10.1111/j.1365-2141.1988.tb04185.x. [DOI] [PubMed] [Google Scholar]
- 38.Giannini S, Mezzasoma AM, Guglielmini G, Rossi R, Falcinelli E, Gresele P. A new case of acquired Glanzmann’s thrombasthenia: diagnostic value of flow cytometry. Cytometry B Clin Cytom. 2008;74(3):194–9. doi: 10.1002/cyto.b.20396. [DOI] [PubMed] [Google Scholar]
- 39.Malik U, Dutcher JP, Oleksowicz L. Acquired Glanzmann’s thrombasthenia associated with Hodgkin’s lymphoma: a case report and review of the literature. Cancer. 1998;82(9):1764–8. doi: 10.1002/(sici)1097-0142(19980501)82:9<1769::aid-cncr25>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Aziz KA. An acquired form of Bernard Soulier syndrome associated with acute myeloid leukemia. Saudi Med J. 2005;26(7):1095–8. [PubMed] [Google Scholar]
- 41.Seidl C, Siehl J, Ganser A, Hofmann WK, Fischer M, Kirchmaier CM, et al. Platelet glycoprotein expression in patients with myelodysplastic syndrome. Thromb Res. 2000;100(1):27–34. doi: 10.1016/s0049-3848(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 42.Curtis BR, Ali S, Glazier AM, Ebert DD, Aitman TJ, Aster RH. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42(9):1173–9. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda H, Mitani T, Ohnuma M, Haga H, Ohtzuka S, Kato T, et al. A new platelet-specific antigen, Naka, involved in the refractoriness of HLA-matched platelet transfusion. Vox Sang. 1989;57(3):213–7. doi: 10.1111/j.1423-0410.1989.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto N, Ikeda H, Tandon NN, Herman J, Tomiyama Y, Mitani T, et al. A platelet membrane glycoprotein (GP) deficiency in healthy blood donors: Naka-platelets lack detectable GPIV (CD36) Blood. 1990;76(9):1698–703. [PubMed] [Google Scholar]
- 45.Bierling P, Godeau B, Fromont P, Bettaieb A, Debili N, el Kassar N, et al. Posttransfusion purpura-like syndrome associated with CD36 (Naka) isoimmunization. Transfusion. 1995;35(9):777–82. doi: 10.1046/j.1537-2995.1995.35996029165.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Akamatsu N, Sakuraba H, Yamazaki H, Tanoue K. Platelet glycoprotein IV (CD36) deficiency is associated with the absence (type I) or the presence (type II) of glycoprotein IV on monocytes. Blood. 1994;83(2):392–7. [PubMed] [Google Scholar]
- 47.Aota Y, Sumi M, Iguchi T, Okabe S, Gotoh A, Ohyashiki K. Type I CD36 deficiency in hematologic disorder. Haematologica. 2004;89(8):EIM17. [PubMed] [Google Scholar]
- 48.Lee K, Godeau B, Fromont P, Plonquet A, Debili N, Bachir D, et al. CD36 deficiency is frequent and can cause platelet immunization in Africans. Transfusion. 1999;3(8):873–9. doi: 10.1046/j.1537-2995.1999.39080873.x. [DOI] [PubMed] [Google Scholar]
- 49.Curtis BR, Aster RH. Incidence of the Nak(a)-negative platelet phenotype in African Americans is similar to that of Asians. Transfusion. 1996;36(4):331–4. doi: 10.1046/j.1537-2995.1996.36496226147.x. [DOI] [PubMed] [Google Scholar]
- 50.van de Loosdrecht AA, Westers TM, Westra AH, Dräger AM, van der Velden VH, Ossenkoppele GJ. Identification of distinct prognostic subgroups in low-and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood. 2008;111(3):1067–77. doi: 10.1182/blood-2007-07-098764. [DOI] [PubMed] [Google Scholar]
- 51.Houwerzijl EJ, Blom NR, van der Want JJ, Louwes H, Esselink MT, Smit JW, et al. Increased peripheral platelet destruction and caspase-3-independent programmed cell death of bone maroow megakaryocytes in myelodysplastic patients. Blood. 2005;105(9):3472–79. doi: 10.1182/blood-2004-06-2108. [DOI] [PubMed] [Google Scholar]
- 52.Braun T, Carvalho G, Grosjean J, Ades L, Fabre C, Boehrer S, et al. Differentiating megakaryocytes in myelodysplastic syndromes succumb to mitochondrial derangement without caspase activation. Apoptosis. 2007;12(6):1101–08. doi: 10.1007/s10495-006-0030-z. [DOI] [PubMed] [Google Scholar]
- 53.Houwerzijl EJ, Blom NR, van der Want JJ, Vellenga E, de Wolf JT. Megakaryocytic dysfunction in myelodysplastic syndromes and idiopathic thrombocytopenic purpura is in part due to different forms of cell death. Leukemia. 2006;20(11):1937–42. doi: 10.1038/sj.leu.2404385. [DOI] [PubMed] [Google Scholar]