Abstract
Background
The value of performing post-therapy routine surveillance imaging in patients with Hodgkin lymphoma is controversial. This study evaluates the utility of positron emission tomography/computed tomography using 2-[18F]fluoro-2-deoxyglucose for this purpose and in situations with suspected lymphoma relapse.
Design and Methods
We conducted a multicenter retrospective study. Patients with newly diagnosed Hodgkin lymphoma achieving at least a partial remission on first-line therapy were eligible if they received positron emission tomography/computed tomography surveillance during follow-up. Two types of imaging surveillance were analyzed: “routine” when patients showed no signs of relapse at referral to positron emission tomography/computed tomography, and “clinically indicated” when recurrence was suspected.
Results
A total of 211 routine and 88 clinically indicated positron emission tomography/computed tomography studies were performed in 161 patients. In ten of 22 patients with recurrence of Hodgkin lymphoma, routine imaging surveillance was the primary tool for the diagnosis of the relapse. Extranodal disease, interim positron emission tomography-positive lesions and positron emission tomography activity at response evaluation were all associated with a positron emission tomography/computed tomography-diagnosed preclinical relapse. The true positive rates of routine and clinically indicated imaging were 5% and 13%, respectively (P=0.02). The overall positive predictive value and negative predictive value of positron emission tomography/computed tomography were 28% and 100%, respectively. The estimated cost per routine imaging diagnosed relapse was US$ 50,778.
Conclusions
Negative positron emission tomography/computed tomography reliably rules out a relapse. The high false positive rate is, however, an important limitation and a confirmatory biopsy is mandatory for the diagnosis of a relapse. With no proven survival benefit for patients with a pre-clinically diagnosed relapse, the high costs and low positive predictive value make positron emission tomography/computed tomography unsuitable for routine surveillance of patients with Hodgkin lymphoma.
Keywords: Hodgkin lymphoma, follow-up, PET/CT, positron emission tomography, relapse, health care costs
Introduction
The introduction of multi-agent chemotherapy has profoundly changed the prognosis of patients with Hodgkin lymphoma (HL) who can now achieve long-term survival even when they present with advanced stage disease.1 Nevertheless, with current first-line therapies, 20–35% of patients with advanced stage disease and 10–15% of patients with early stage disease relapse after initial therapy.2–4 The report generated at the Cotswolds meeting in 1989 encourages frequent follow-up visits during the initial years of follow-up, but does not provide specific guidelines for the use of routine surveillance imaging.5
Conclusions from previous studies support a restrictive use of routine surveillance imaging, as the majority of relapses are preceded by symptoms or abnormalities on clinical examination, but these studies mainly used conventional radiograms and not whole-body computed tomography (CT), which is now more commonly performed.6,7 Routine CT surveillance, however, also appears to have limited value; in a study by Dryver et al., only 9% of HL relapses were diagnosed by CT.8 Besides, CT surveillance is probably not cost-effective and could decrease quality of life mainly by increasing patients’ anxiety.9,10 Thus, the extensive use of routine surveillance imaging for HL patients remains controversial, with no international consensus.
Positron emission tomography using 2-[18F] fluoro-2-deoxyglucose (PET) visualizes patterns of glucose metabolism in the body and enhances discrimination between active lymphoma and fibrotic residual masses.11,12 The discriminating power could be important during follow-up as a residual mass often persists after therapy.13 The image resolution of stand-alone PET is, however, limited and differentiating pathological from physiological glucose metabolism can be difficult.14 This problem has been partly overcome by integrating PET and the anatomic details of CT in a single procedure (PET/CT).15,16 At present, PET/CT is widely used in the pre-therapy staging and response evaluation of HL, but its role during post-therapy disease surveillance has not been established.17
The aim of this retrospective study was to investigate the value of PET/CT surveillance during the follow-up of HL patients in first remission.
Design and Methods
Patients
This was a multicenter, retrospective study. The medical records of HL patients diagnosed at Rigshospitalet during the period 2000–2010, Odense University Hospital during the period 2003–2009 and Aalborg Hospital during the period 2003–2010 were reviewed. Patients who were previously untreated, had achieved at least a partial remission on first-line therapy and in whom PET/CT surveillance had been used during follow-up were eligible for inclusion. Information on the patients’ characteristics, disease features, relapse details and PET/CT indications were obtained from medical records. PET/CT results were obtained from radiology/nuclear medicine files.
Positron emission tomography/computed tomography classification, interpretation and protocol
Surveillance PET/CT were categorized according to indication as “routine PET/CT” when patients were considered to be in remission at the time of referral and “clinically indicated PET/CT” when the referring physician suspected a relapse due to reported symptoms, palpation findings, abnormalities on other imaging investigations or blood test results.
During 2006–2010, a subgroup of 43 consecutive HL patients from Aalborg Hospital was scheduled for three or four routine PET/CT surveys during the first 2 years of follow-up as this was local practice. The remaining patients underwent PET/CT surveillance at the treating doctor’s discretion.
The PET/CT scans had either been performed as PET with low-dose CT occasionally followed by a separate high quality diagnostic CT, or as PET/CT with enhanced, high quality diagnostic CT included in the scanning protocol. This study is based on a review of the PET/CT reports and no central review of PET/CT images was performed. However, all participating centers are serviced by PET/CT readers highly experienced in lymphoma imaging. PET/CT studies had been assessed visually with PET-activity in the mediastinal blood pool as the reference.
True positive PET/CT were defined by biopsy verified HL relapse. PET/CT suggesting HL relapse, but disproved by biopsy or by repeated imaging or follow-up were considered false positive. True and false negative PET/CT were defined as continuous remission 2 months after a negative PET/CT, and relapse within 2 months after a negative PET/CT, respectively. PET/CT lesions were categorized by localization as follows: “area infiltrated by HL” if lesions were localized in a previously HL infiltrated area in contrast to “area unaffected by HL”. In addition, PET/CT with concordant PET and CT pathology were recorded.
Statistical analysis
The primary end-points of this study were the predictive values of surveillance PET/CT. We report median values and range for continuous variables and percentages for categorical values. Follow-up time was defined from therapy response assessment until relapse, death, or last follow-up, whichever came first. Differences between categorical values were tested with Fisher’s exact test. The health care cost calculations were based on the tariffs in the Danish Diagnosis Related Grouping System (DRG).18 The 2011 cost of a PET/CT was estimated to be US$ 2,418.
All data analyses were performed using the statistical software package PASW 18.0 (SPSS Inc., Chicago, IL, USA). The study was approved by the Danish Data Protection Authority.
Results
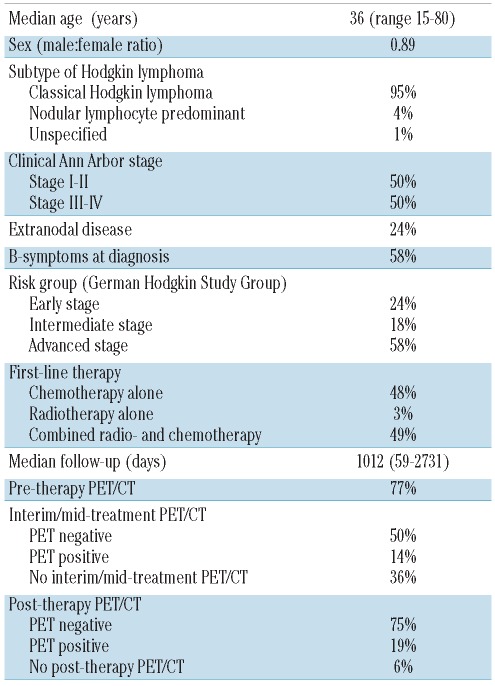
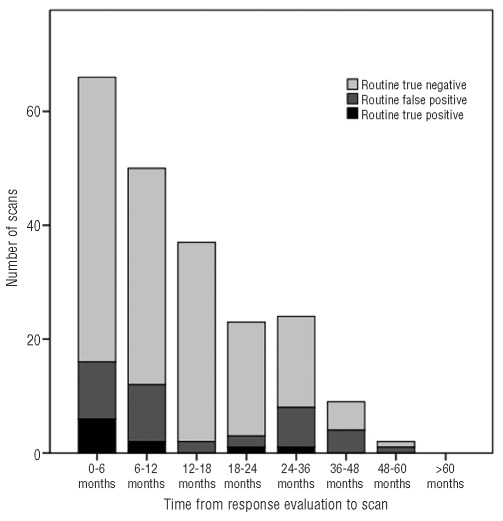
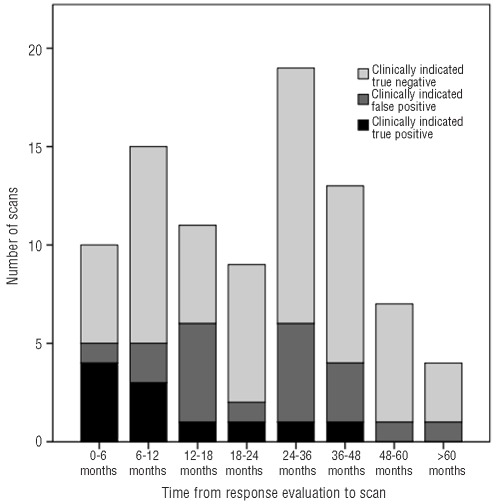
A total of 161 patients were included (Table 1) and 299 surveillance PET/CT were performed for an average of 1.9 PET/CT per patient. The PET/CT were performed during the first year of follow-up (47%), the second year (27%), the third year (14%) and the fourth year or later (12%). PET/CT results as a function of time after response evaluation are shown in Figures 1 and 2.
Table 1.
Patients’ characteristics.
Figure 1.
Results of “routine” PET/CT as a function of time from response evaluation.
Figure 2.
Results of “clinically indicated” PET/CT as a function of time from response evaluation.
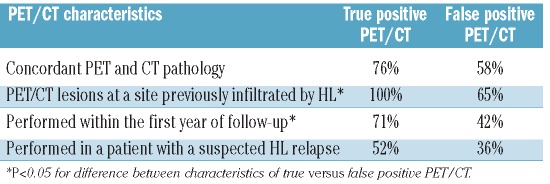
There were 21 true positive PET/CT and 55 false positive PET/CT. The characteristics of true positive versus false positive PET/CT are shown in Table 2. PET/CT surveillance diagnosed malignant disease other than HL in two patients (gastric adenocarcinoma and enteropathy-associated T-cell lymphoma).
Table 2.
Characteristics of true positive versus false positive PET/CT.
Twenty-two patients relapsed during a median follow-up of 34 months (range, 2–96 months). The primary methods for diagnosing relapse were routine PET/CT (n=10), physical examination/reported symptoms/blood tests (n=9), other routine imaging (n=2) and autopsy (n=1).
Routine positron emission tomography/computed tomography
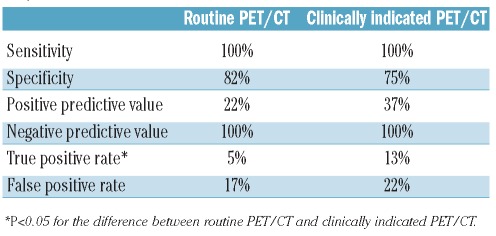
A total of 211 PET/CT were performed as “routine PET/CT” of which 165 showed continuous remission and 46 showed possible HL relapse. Biopsy confirmed the HL relapse in ten patients, all of whom subsequently received second-line therapy. Thus, the true positive rate of routine PET/CT was 5%. In the remaining 36 positive routine PET/CT, relapse was disproved by biopsy (n=11), other/repeated imaging (n=12) or clinical course (n=13): thus, the false positive rate of routine PET/CT was 17%. The sensitivity and specificity of routine PET/CT surveillance was 100% and 82%, respectively. The positive predictive value (PPV) of routine PET/CT surveillance was 22%, while its negative predictive value (NPV) was 100% (Table 3). The false positive rate of routine PET/CT was higher (45%) in patients with the nodular lymphocyte predominant subtype of HL than in patients with other subtypes (P=0.02).
Table 3.
Characteristics of routine PET/CT versus clinically indicated PET/CT.
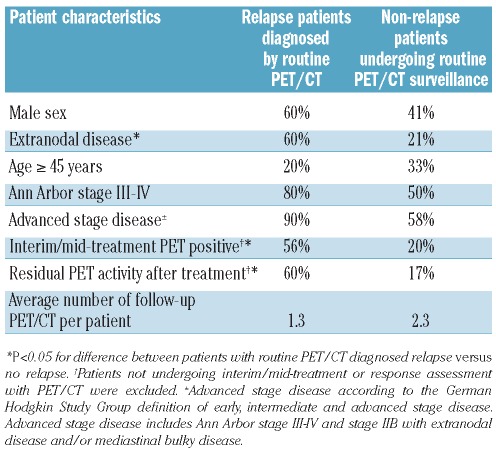
Patients who had a relapse diagnosed as a result of routine PET/CT were more likely to have the following characteristics at primary disease: extranodal disease, PET-positive interim/mid-treatment scans and residual PET activity after completing treatment (partial remission) compared to the remaining routine PET/CT scanned patients (Table 4). Forty-six of the routine PET/CT-scanned patients had one or more of these risk factors and underwent a total of 77 routine PET/CT (average 1.7 per patient), of which nine scans were true positive and 16 scans were false positive, i.e. a PPV of 36%. For the remaining patients without these risk factors the PPV of routine PET/CT was 5% (P=0.01 for the difference).
Table 4.
Characteristics of patients with a routine PET/CT-diagnosed relapse.
Four relapses occurred in the cohort of 43 patients from Aalborg Hospital who underwent systematic routine PET/CT surveillance. All relapses were diagnosed by routine PET/CT within 7 months of response assessment in patients with advanced stage disease. Two of these patients were PET-positive at the interim/mid-treatment survey, with one of them continuing to have residual PET activity at response assessment. However, three of the four patients who relapsed were PET-negative at the end of therapy.
Clinically indicated positron emission tomography/computed tomography
A total of 88 “clinically indicated” PET/CT were performed due to reported symptoms (n=38), abnormalities on clinical examination (n=14), abnormal blood tests (n=7), abnormalities on other imaging studies (n=20) or a combination (n=9). Fifty-eight PET/CT showed continuous remission, while 30 were reported as possible HL relapse. HL recurrences were diagnosed in 11 patients, for a true positive rate of 13%. In the remaining 19 instances of positive PET/CT, relapses were disproven by biopsy (n=10), other/repeated imaging (n=3) or clinical course (n=6), for a false positive rate of 22%. The sensitivity and specificity of clinically indicated PET/CT were 100% and 75%, respectively, while the PPV and NPV of clinically indicated PET/CT were 37% and 100%, respectively (Table 3).
Health-care costs of positron emission tomography/computed tomography surveillance
The estimated price per routine PET/CT-diagnosed relapse was US$ 50,778 (21 PET/CT per relapse), but the price was reduced to US$ 21,762 if routine PET/CT surveillance had been restricted to patients with extranodal disease at presentation, PET-positive interim/mid-treatment scan and/or residual PET activity after completing treatment (9 PET/CT per relapse). The price per PET/CT-confirmed relapse in patients with suspected HL relapse was US$ 19,344 (8 PET/CT per relapse).
Discussion
This retrospective study investigated the value of PET/CT surveillance in 161 HL patients in first remission. Detailed information was available from medical records and only for five patients were we unable to retrieve the medical records. Important limitations of the study are its retrospective design and the absence of uniform PET/CT surveillance guidelines among the three participating hematology centers. High-risk patients could have been more likely to undergo repeated routine PET/CT which would increase the chance of diagnosing an imminent relapse with surveillance imaging. However, the average numbers of PET/CT per patient with advanced stage disease and patients with early/intermediate stage disease were almost balanced (1.9 versus 1.7). As the vast majority of included PET/CT scans were performed as “routine” surveys, this study mainly evaluates the efficacy of routine PET/CT surveillance in HL.
Routine PET/CT surveillance diagnosed 45% of relapses at an asymptomatic stage in our cohort and all relapses in the subgroup of 43 patients undergoing systematic PET/CT surveillance were diagnosed by PET/CT. Thus, the yield of routine PET/CT surveillance was higher than expected from previous results based on non-PET imaging, but still consistent with other studies including PET or PET/CT surveillance.19–24 The ability of PET/CT to visualize the inflammatory component of HL could at least partially explain the larger relapse fraction diagnosed by surveillance imaging in PET or PET/CT studies.
FDG-avid lesions were present in all patients diagnosed with HL relapse alive, which resulted in a sensitivity of 100%. Similar sensitivities between 95–100% have been reported by other authors.24–26 The combination of high sensitivity and a NPV of 99 –100% makes PET or PET/CT ideal for studying a suspected HL relapse as it seems to effectively disprove a relapse and to make further investigations unnecessary.25,26
A major limitation of PET/CT surveillance in our study was the high false positive rate and an accordingly low PPV of 22–37% (depending on the PET/CT indication). The PPV in other PET or PET/CT follow-up studies ranged from 11% to 98 % with the highest value reported by Petraush et al.19–20,23–26 High PPV are obtainable by a high threshold for referral to PET/CT or by restrictive reporting of positive PET/CT. Accordingly, Zinzani et al. and Levine et al. used a distinct category for equivocal/inconclusive positive PET or PET/CT.21,25 In daily clinical practice, however, the value of such an indeterminate category is uncertain, as it would possibly generate further investigations and anxiety in patients, like “unequivocally” positive PET/CT. The higher false positive rate of routine PET/CT studies in patients with the nodular lymphocyte predominant subtype of HL might reflect the potentially waxing and waning course of this subtype of HL without any of the patients with nodular lymphocyte predominant HL experiencing a clinical relapse during follow-up.
Besides hazardous biopsy procedures and expensive supplementary imaging, false positive PET/CT are accompanied by periods of anxiety for affected patients. A cost-benefit analysis by Guadagnolo et al. postulated that surveillance CT could decrease the quality of life for some HL patients due to false positive results.9 Furthermore, it was shown by Thomson et al. that the period around surveillance imaging exacerbated anxiety symptoms and fear of recurrence in survivors of aggressive lymphoma.10 Thus, surveillance imaging could actually increase uncertainty rather than have a reassuring effect.
Identifying characteristics of true positive PET/CT, whether routine or clinically indicated, could help to overcome the problem of false positive reporting. Lee et al. reported that lesions in previously involved HL sites as well as concordant PET and CT pathology were more likely to represent active HL.20 Inspired by this, we performed a similar analysis and consistently found that patients with true positive PET/CT always had lesions located in areas previously infiltrated by HL, whereas this was only the case for 65% of the false positive PET/CT. It is likely, however, that some PET-positive findings located in sites previously unaffected by HL would have been questioned in a regular clinical situation in which correlation between PET/CT results and present time clinical information is possible (signs of infection, etc.). No association between concordant PET/CT pathology and HL was observed, but this could be due to the lower number of events in our study. In agreement with Lee et al., we observed that true positive PET/CT were more frequent in the first year of follow-up, which is probably a reflection of the high risk of relapse in this period.
Considering predictive factors for a routine PET/CT-diagnosed HL relapse, Zinzani et al. reported that PET-positive interim scans were predictive of later PET-diagnosed relapses, while Petrausch et al. found that a residual mass predicted PET/CT-diagnosed relapse.19,21 Not surprisingly, we found extranodal disease, interim PET positivity and residual PET positivity after treatment (partial remission) to be associated with routine PET/CT-diagnosed relapses in our study. Confining routine PET/CT surveillance to the population of high-risk patients would have increased the PPV to 36%.
HL is characterized by a peak onset in young age and high cure rates compared to other cancers. Thus, the risk of radiation-induced secondary malignancies from repetitive surveillance imaging needs to be considered although the carcinogenic effect of low-dose radiation is still controversial.27 The CT components of the PET/CT surveys included in our study consisted of both contrast-enhanced and low-dose depending on the local PET/CT protocols. Interestingly, a recent publication by Nakamoto et al. suggests that PET/CT without contrast-enhanced CT is sufficient for lymphoma surveillance and therefore the use of PET/CT instead of CT surveillance could in fact reduce the overall exposure to ionizing radiation in HL survivors.28
Attention to the health care costs of PET/CT surveillance is commanded in a period in which massive budget problems in public health care systems are obvious across most of Europe. The price per relapse diagnosed by routine PET/CT was US$ 50,778, which was slightly more than the US$ 38,736 – 48,757 reported by Lee et al.20 The total costs of PET/CT surveillance are probably even higher because of the numerous supplementary investigations performed due to false positive PET/CT results. The indiscriminate use of surveillance PET or PET/CT for all HL patients is not, therefore, likely to be cost-effective. Restricting routine PET/CT surveillance to patients with a high risk of having a relapsed diagnosed by routine PET/CT would, however, have reduced the costs substantially.
This study adds to the existing evidence in showing that PET/CT surveillance, both routine and clinically indicated, is associated with low PPV and that routine PET/CT surveillance has unacceptably high costs. However, the results do indicate that routine PET/CT surveillance could be more effective in diagnosing early relapse compared to previously used surveillance imaging. Indiscriminate use of routine PET/CT surveillance is not efficacious and, if used at all, it should be reserved for high-risk patients for a limited follow-up period. Finally, the use of expensive surveillance imaging can only be justified if an early, preclinical relapse diagnosis actually improves a patient’s outcome. This issue remains to be resolved in prospective trials.
Footnotes
Funding: this work was supported by a grant from the Speciallaege H. Kopps foundation.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Seam P, Janik JE, Longo DL, Devita VT., Jr Role of chemotherapy in Hodgkin’s lymphoma. Cancer J. 2009;15(2):150–4. doi: 10.1097/PPO.0b013e3181a27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engert A, Diehl V, Franklin J, Lohri A, Dörken B, Ludwig WD, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–54. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 3.Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640–52. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 4.Eich HT, Diehl V, Görgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28(27):4199–206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 5.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 6.Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin’s disease: role of routine follow-up studies. J Clin Oncol. 1997;15(3):1123–30. doi: 10.1200/JCO.1997.15.3.1123. [DOI] [PubMed] [Google Scholar]
- 7.Radford JA, Eardley A, Woodman C, Crowther D. Follow up policy after treatment for Hodgkin’s disease: too many clinic visits and routine tests? A review of hospital records. BMJ. 1997;314(7077):343–6. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dryver ET, Jernström H, Tompkins K, Buckstein R, Imrie KR. Follow-up of patients with Hodgkin’s disease following curative treatment: the routine CT scan is of little value. Br J Cancer. 2003;89(3):482–6. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guadagnolo BA, Punglia RS, Kuntz KM, Mauch PM, Ng AK. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24(25):4116–22. doi: 10.1200/JCO.2006.07.0409. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CA, Charlson ME, Schenkein E, Wells MT, Furman RR, Elstrom R, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21(11):2262–6. doi: 10.1093/annonc/mdq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit M, Bumann D, Beyer W, Herbst K, Clausen M, Hossfeld DK. Whole-body positron emission tomography (PET) for diagnosis of residual mass in patients with lymphoma. Ann Oncol. 1997;8(suppl1):s57–s60. [PubMed] [Google Scholar]
- 12.Mikhaeel NG, Timothy AR, Hain SF, O’Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol. 2000;11(suppl1):147–50. [PubMed] [Google Scholar]
- 13.Jochelson M, Mauch P, Balikian J, Rosenthal D, Canellos G. The significance of the residual mediastinal mass in treated Hodgkin’s disease. J Clin Oncol. 1985;3(5):637–40. doi: 10.1200/JCO.1985.3.5.637. [DOI] [PubMed] [Google Scholar]
- 14.Cherry SR. The 2006 Henry N. Wagner Lecture: of mice and men (and positrons)--advances in PET imaging technology. J Nucl Med. 2006;47(11):1735–45. [PubMed] [Google Scholar]
- 15.Allen-Auerbach M, Quon A, Weber WA, Obrzut S, Crawford T, Silverman DH, et al. Comparison between 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and positron emission tomography/computed tomography hardware fusion for staging of patients with lymphoma. Mol Imaging Biol. 2004;6(6):411–6. doi: 10.1016/j.mibio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44(8):1200–9. [PubMed] [Google Scholar]
- 17.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29(14):1844–54. doi: 10.1200/JCO.2010.32.5225. [DOI] [PubMed] [Google Scholar]
- 18.DRG tariffs [Internet] Copenhagen: Ministry of Interior and Health, Denmark; 2011. [Cited 2011, May 29th]. Available from: http://www.im.dk/Sundhed/DRG-systemet/Takster/2011.aspx. [Google Scholar]
- 19.Petrausch U, Samaras P, Veit-Haibach P, Tschopp A, Soyka JD, Knuth A, et al. Hodgkin’s lymphoma in remission after first-line therapy: which patients need FDG-PET/CT for follow-up? Ann Oncol. 2010;21(5):1053–7. doi: 10.1093/annonc/mdp519. [DOI] [PubMed] [Google Scholar]
- 20.Lee AI, Zuckerman DS, Van den Abbeele AD, Aquino SL, Crowley D, Toomey C, Lacasce AS, et al. Surveillance imaging of Hodgkin lymphoma patients in first remission: a clinical and economic analysis. Cancer. 2010;116(16):3835–42. doi: 10.1002/cncr.25240. [DOI] [PubMed] [Google Scholar]
- 21.Zinzani PL, Stefoni V, Tani M, Fanti S, Musuraca G, Castellucci P, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol. 2009;27(11):1781–7. doi: 10.1200/JCO.2008.16.1513. [DOI] [PubMed] [Google Scholar]
- 22.Goldschmidt N, Or O, Klein M, Savitsky B, Paltiel O. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann Hematol. 2011;90(2):165–71. doi: 10.1007/s00277-010-1044-8. [DOI] [PubMed] [Google Scholar]
- 23.Mocikova H, Obrtlikova P, Vackova B, Trneny M. Positron emission tomography at the end of first-line therapy and during follow-up in patients with Hodgkin lymphoma: a retrospective study. Ann Oncol. 2010;21(6):1222–7. doi: 10.1093/annonc/mdp522. [DOI] [PubMed] [Google Scholar]
- 24.Jerusalem G, Beguin Y, Fassotte MF, Belhocine T, Hustinx R, Rigo P, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol. 2003;14(1):123–30. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 25.Levine JM, Weiner M, Kelly KM. Routine use of PET scans after completion of therapy in pediatric Hodgkin disease results in a high false positive rate. J Pediatr Hematol Oncol. 2006;28(11):711–4. doi: 10.1097/01.mph.0000243648.66734.eb. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes MM, Delbeke D, Whitlock JA, Martin W, Kuttesch JF, Frangoul HA, et al. Utility of FDG-PET/CT in follow-up of children treated for Hodgkin and non-Hodgkin lymphoma. J Pediatr Hematol Oncol. 2006;28(5):300–6. doi: 10.1097/01.mph.0000212912.37512.b1. [DOI] [PubMed] [Google Scholar]
- 27.Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251(1):13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamoto Y, Nogami M, Sugihara R, Sugimura K, Senda M, Togashi K. Is contrast material needed after treatment of malignant lymphoma in positron emission tomography/computed tomography? Ann Nucl Med. 2011;25(2):93–9. doi: 10.1007/s12149-010-0429-z. [DOI] [PubMed] [Google Scholar]