Abstract
Background
Fc gamma receptor polymorphisms were linked to outcome in follicular lymphoma patients treated with single-agent rituximab, an anti-CD20 monoclonal antibody. In particular, 158F/F genotype of Fc gamma receptor 3A and 131R/R genotype of Fc gamma receptor 2A correlated with worse outcome compared to high-affinity 158V/V and 131H/H, respectively. We examined this association in the context of anti-CD20 monoclonal antibody combined with chemotherapy, as compared to chemotherapy alone, in follicular lymphoma patients treated on SWOG clinical trials.
Design and Methods
Tissue from 142 SWOG patients treated with chemotherapy alone (protocol S8809, n=70) or combined chemotherapy and anti-CD20 monoclonal antibody (rituximab and Iodine I-131 tositumomab on protocols S9800 and S9911, n=30 and 42, respectively) was analyzed. DNA was extracted and assayed for Fc gamma receptor 3A V158F and 2A R131H polymorphisms using a TaqMan SNP assay. Stratified Cox’s regression was used to assess association with overall survival.
Results
For Fc gamma receptor 3A, there was an association with overall survival in the combination therapy trials but not in the chemotherapy-only trial. Having at least one Fc gamma receptor 3A V allele was associated with improved overall survival versus F/F (HR=0.33, 95% CI, 0.11, 0.96, P=0.042). For overall survival, there was evidence of a statistical interaction between the use of mAb and the number of V alleles (0, 1, or 2) (P=0.006). There was no such association for Fc gamma receptor 2A.
Conclusions
Fc gamma receptor 3A polymorphism status may be predictive of survival in follicular lymphoma patients receiving treatments containing an anti-CD20 antibody but not treatment with chemotherapy alone. Thus, Fc gamma receptor 3A polymorphisms may be important to consider in designing new follicular lymphoma trials and new anti-CD20 monoclonal antibodies.
Keywords: follicular lymphoma, SWOG, FCGR, combined monoclonal antibody, chemotherapy
Introduction
Rituximab is a monoclonal IgG1 antibody against CD20 with human gamma1 and kappa constant regions and murine variable domains, approved for use by the FDA in non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), and rheumatoid arthritis.1 The exact mechanism of rituximab activity is not completely understood. Possible mechanisms of action include apoptosis, complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), opsonization-phagocytosis, and a ‘vaccinal’ effect.2 ADCC is thought to be a particularly prominent mechanism of action of rituximab in follicular lymphoma (FL),3 the most common indolent NHL type.
ADCC requires activation of a receptor for the Fc portion of IgG (Fc gamma receptor, FCGR) on a leukocyte such as an NK cell or macrophage. There are three classes of FCGR (1, 2 and 3) with polymorphisms that impart different IgG-binding properties. In particular, FCGR3A (CD16), expressed on NK cells and macrophages, has a polymorphism encoding phenylalanine (F) or valine (V) at aminoacid position 158 (V158F). Position 158 on FCGR3A interacts with the lower hinge region of IgG1,4 and IgG1 binds strongest to NK cells with 158V/V.5 It is therefore plausible that having 158V/V may result in stronger NK activation and increased benefit from rituximab.
Indeed, patients with FCGR3A 158V/V, and to a lesser degree with 158V/F, genotype have been reported to have better overall response rate (ORR), progression-free survival (PFS), or event-free survival (EFS) when treated with single agent rituximab for follicular lymphoma.3,6,7 However, other studies failed to confirm the association between FCGR polymorphisms and outcome when FL patients were treated with rituximab and CHOP chemotherapy.8–10 However, these studies did not compare outcomes with chemotherapy alone which would have helped to delineate the contribution of anti-CD20 monoclonal therapy, since any effects of FcR polymorphisms on outcome could be overwhelmed by the effect of chemotherapy. Furthermore, it is not known if there is an impact of FcR polymorphisms on overall survival (OS) which would be the outcome of greatest importance.
Therefore, we investigated the association between FCGR3A polymorphism and survival outcome in FL patients treated with a combination of chemotherapy and an anti-CD20 monoclonal antibody (mAb) in the context of large multi-institutional trials conducted by the Southwest Oncology Group (SWOG). In particular, we tested whether the 158V-containing genotype was predictive of survival following all treatment regimens, or just regimens containing mAb. In addition to FCGR3A V158F, we also assessed the prognostic importance of FCGR2A R131H polymorphism, since 131 H/H (histidine) binds human IgG2 stronger than 131R/R, and was correlated with a more favorable outcome in a previous study of FL by Weng and Levy.3
Design and Methods
Patients
All patients had previously untreated advanced-stage (bulky II, III or IV) follicular lymphoma (grades 1, 2 or 3). The patients were enrolled on one of three trials: prednisone, methotrexate, doxorubicin, cyclophosphamide, and etoposide (ProMACE) plus mechlorethamine, vincristine, procarbazine, and prednisone (MOPP), with randomization of responders to interferon maintenance (SWOG 8809: 1988–1994); CHOP followed by 4 doses of rituximab (SWOG 9800: 1998) and CHOP followed by tositumomab and iodine I 131 tositumomab (BEXXAR therapeutic regimen) (SWOG 9911: 1999–2000). Specimens had to be excisional biopsies with sufficient remaining tissue. All patients gave informed consent for the use of remaining tissue for research purposes in accordance with the Declaration of Helsinki, after approval by a local Human Investigations Committee and in accordance with an assurance filed with and approved by the Department of Health and Human Services, where appropriate.
Polymorphism determination
Paraffin blocks were faced to reveal complete tissue sections and then 3 cuts at 5 microns each were taken and put into Ambion (Austin, TX, USA) RNAase free 2 mL nucleic acid tubes. DNA was extracted using the QIAamp DNA FFPE mini kit from Qiagen (Valencia, CA, USA). The resulting DNA was eluted using 50 microliters of the ATE buffer, reapplying the 50 microliters and eluting a second time. The DNA concentration was determined using a NanoDrop ND-1000 (Wilmington, DE, USA).
The R131H polymorphism (rs1801274) in FCGR2A and the V158F polymorphism (rs396991) in FCGR3A were determined in a blinded fashion, using a TaqMan SNP assay (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Prognostic factor data were collected prospectively at registration. Not all FLIPI risk factors were available for the patients on the S9800 trial. We used χ2 tests to assess differences in distributions of prognostic factors and polymorphism frequencies between the two treatment groups. OS and PFS were calculated from the date of registration until death from any cause (OS), or until disease progression or death from any cause (PFS). Patients from S8809 with small lymphocytic lymphoma (working formulation A) were excluded from this analysis, as were patients from S9800 and S9911 who did not have antibody therapy documented in their charts. All other eligible patients were included. Potential bias due to differential length of follow up between studies was addressed by truncating follow up at ten years. Stratified analyses on study were also conducted to address any differences in baseline overall survival between older and newer studies. OS and PFS were estimated using the Kaplan-Meier method,11 and CIs were calculated. Stratified Cox’s regression12 was used to assess marker associations with OS as the primary endpoint and PFS as the secondary endpoint.
Results
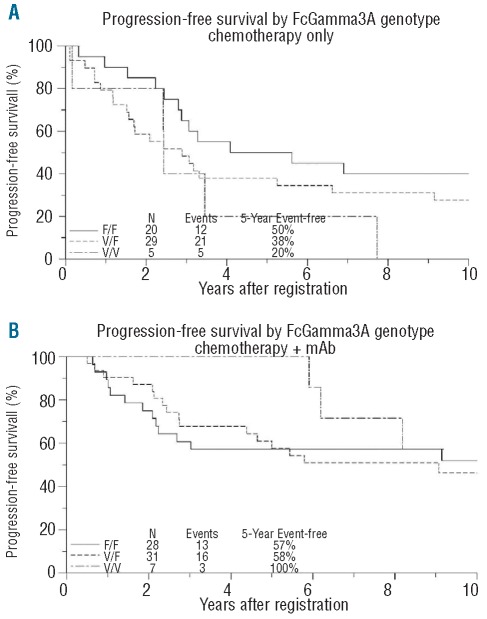
Sufficient tissue was available on 70 of 442 patients with FL patients from the chemotherapy only trial (S8809), and 72 patients from the chemo + mAb trials (S9800, CHOP + rituximab, n=30 (of 87 who received mAb); S9911, CHOP + Iodine I 131 tositumomab, n=42 (of 87 who received mAb)). Patients’ clinical characteristics are shown in Table 1. There were no statistically significant differences between the clinical characteristics of patients with available tissue and those of the study population as a whole. There were also no statistically significant differences between the clinical characteristics of patients on different trials.
Table 1.
Patient’s characteristics across clinical trials.
The frequency of FCGR3A genotypes was 10% V/V, 50% V/F and 40% F/F. The frequency of FCGR2A genotypes was 29% H/H, 56% R/H and 15% R/R. These frequencies were similar to those published in prior studies with predominantly Caucasian populations.3,6,7,9,13,14 The genotype could not be determined in 22 cases for FCGR3A (16 of them in S8809), and 4 cases for FCGR2A (all in S8809) due to technical difficulties. There were no differences in genotype frequencies between trials. The favorable genotypes for FCGR3A and FCGR2A appeared to be associated (χ2 P=0.022), with 158V/V patients more likely to be 131H/H than the overall population.
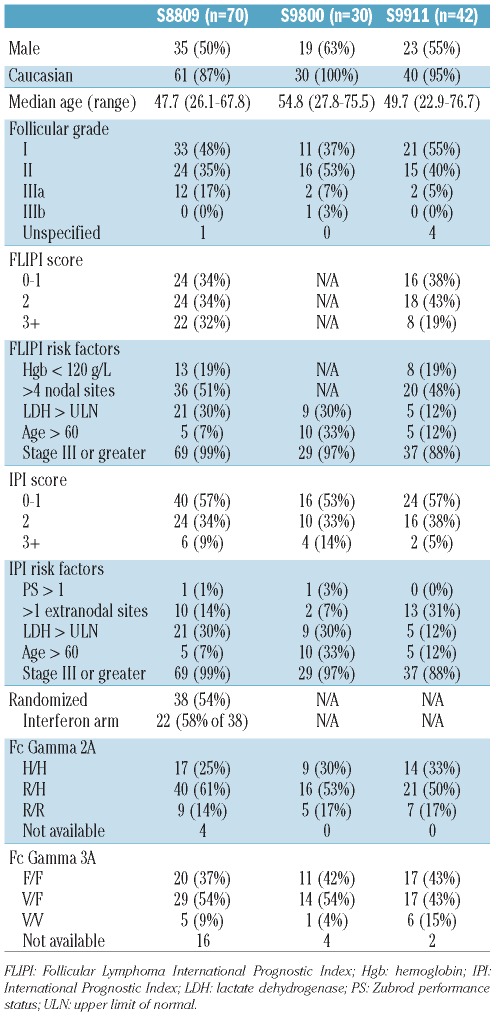
For FCGR3A, as expected, there was no statistically significant association with OS in the chemotherapy only trial (S8809) (HR=1.40, 95% CI, 0.58–3.41, P=0.46), with 5-year OS of 60%, 79% and 75% for V/V, V/F and F/F, respectively. However, there was evidence of an association with OS in the chemotherapy + mAb trials (S9800 and S9911). In both S9800 and S9911, patients with at least 1 FCGR3A V allele had better 5-year OS than F/F (93% vs. 82% in S9800, 100% vs. 71% in S9911). Since the association between genotype and OS was not found to differ between S9800 and S9911, these study populations were pooled in this analysis. Having at least one FCGR3A V allele was associated with improved OS vs. F/F (HR=0.33, 95% CI, 0.11–0.96, P=0.042) (Figure 1A and B), with 5-year OS of 100%, 97% and 75% for V/V, V/F and F/F, respectively. Regarding OS, there was evidence of a statistical interaction between the use of mAb and the number of V alleles (0, 1 or 2) (P=0.006), suggesting that an increasing number of V alleles predicted a greater improvement in OS when treated with chemotherapy + mAb, while making no difference in OS when treated with chemotherapy alone.
Figure 1.
(A) Overall survival (OS) by FCGR3A genotype for the chemotherapy only trial, S8809. (B) Overall survival (OS) by FCGR3A genotype for the chemotherapy + mAb trials, S9800 and S9911 (B). Kaplan-Meier curves, with follow up truncated at ten years. 158 F/F (unfavorable) genotype denoted by solid line; 158 V/F genotype denoted by dotted line; 158 V/V genotype denoted by dashed line.
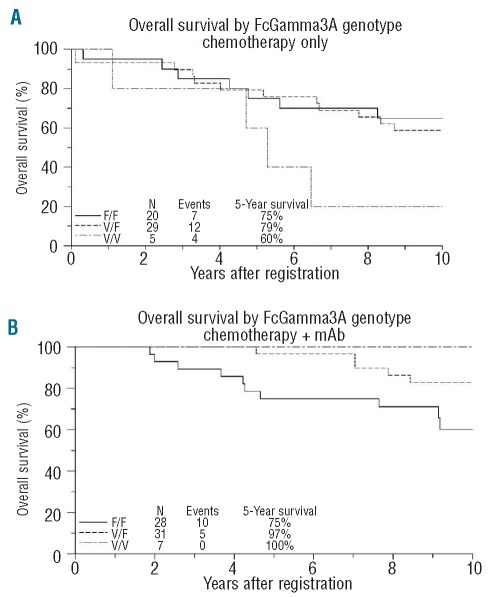
There was no association between FCGR3A genotype and PFS in either the chemotherapy-only trial (HR=1.66, 95% CI, 0.84–3.30, P=0.15) or in the chemotherapy + mAb trials (HR=1.03, 95% CI, 0.51–2.09, P=0.94) (Figure 2A-and B). In the chemotherapy-only trial, 5-year PFS was 20%, 38% and 50% for V/V, V/F and F/F, respectively; while in the chemotherapy + mAb trials, 5-year PFS was 100%, 58% and 57% for V/V, V/F and F/F, respectively. Within S9800, 5-year PFS in patients with at least one V allele was 40% vs. 55% for F/F; in S9911, 5-year PFS in these subgroups was 83% and 59%, respectively. Although these results suggest that the relationship between FCGR3A genotype and PFS may have differed between these 2 study populations, no significant association was found within either S9800 (HR=1.97, 95% CI, 0.69–5.61, P=0.21) or S9911 (HR=0.52, 95% CI, 0.19–1.43, P=0.20).There was only a weak suggestion of a statistical interaction between mAb-containing treatment and the number of V alleles (P=0.12).
Figure 2.
(A) Progression-free survival (PFS) by FCGR3A 158 genotype for the chemotherapy only trial, S8809. (B) Progression-free survival (PFS) by FCGR3A 158 genotype for the chemotherapy + mAb trials, S9800 and S9911. Kaplan-Meier curves, with follow up truncated at ten years. 158 F/F (unfavorable) genotype denoted by solid line; 158 V/F genotype denoted by dotted line; 158 V/V genotype denoted by dashed line.
For FCGR2A, there were no associations between genotype and either OS or PFS, either in the chemotherapy only or chemotherapy + mAb trials (either pooled or separately). There was also no evidence for interaction between mAb-containing treatment and the number of H alleles. Conclusions regarding genotype survival associations remained unchanged after adjustment for IPI. It was not possible to adjust analyses for FLIPI due to missing values for some factors in S9800.
Discussion
The importance of Fc gamma receptor polymorphisms for outcome in lymphoid malignancies may differ by disease type, treatment, and by the way the outcome is measured. In this study, sequential SWOG trials in newly diagnosed follicular lymphoma (FL) suggest that patients with favorable FCGR3A genotype (158 V-containing) derive a greater benefit from addition of an anti-CD20 monoclonal antibody therapy to chemotherapy than the FCGR3A 158F/F genotype, as opposed to having no such advantage when treated with chemotherapy alone, when measured by 5-year OS. This would imply that patients with the 158 V-containing genotype do not in general have a better outcome, but only in the context of mAb-containing treatment.
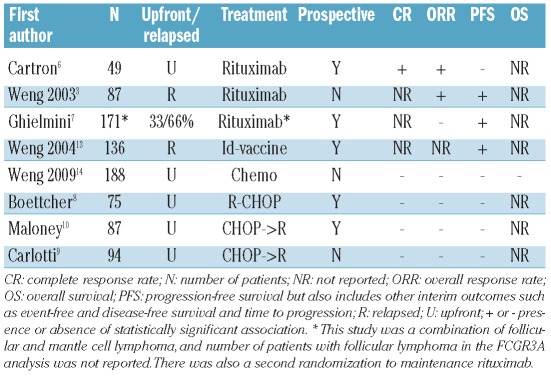
Several studies examined the impact of FCGR3A on outcome in FL, but only one looked at overall survival as an outcome measure (Table 2). A recent study by Weng and Levy14 showed that FCGR polymorphisms did not correlate with OS in FL patients treated with chemotherapy alone; a conclusion that our study confirms. Earlier reports by the same authors demonstrated improved overall response rate (ORR) and PFS with rituximab in relapsed FL,3 and improved PFS with an anti-idiotype vaccine in relapsed FL.13 Cartron and colleagues showed the impact of FCGR3A upon complete response (CR) and ORR but not on PFS when rituximab was given for previously untreated FL.6 On the other hand, three studies using rituximab with chemotherapy reported no impact of FCGR3A on ORR, time to treatment failure, EFS or PFS, including a separate analysis of SWOG 9800, one of the three studies reported here.8–10 Indeed, both in the overall analysis and in all subgroup analyses, our study also shows only a weak (not statistically significant) association between FCGR3A genotype and PFS, in agreement with those reports. However, it may be possible to impact OS without showing significant impact on PFS in first-line therapy. Since with subsequent treatments patients typically undergo repeated exposure to anti-CD20 mAbs, we hypothesize that patients with favorable FCGR3A genotypes continue to enjoy an advantage compared to the FCGR3A 158F/F genotype, eventually resulting in improved OS; unfortunately we cannot confirm this in a setting of multiple cooperative group trials. This is supported by circumstantial evidence that patients with favorable FCGR3A genotypes preferentially benefit from rituximab maintenance given after initial rituximab cycle.15 Finally, FCGR polymorphisms may affect non-ADCC immune functions, including antigen uptake and presentation enhancement,16 and stronger NK cell activation17 which may only become apparent with long-term follow up.
Table 2.
Fc gamma receptor 3A polymorphism studies in follicular lymphoma.
Our study found no association between the FCGR2A genotype and outcome in chemo + mAb trials. In contrast, Weng and Levy reported an association between FCGR2A 131H/H and improved ORR and PFS in relapsed FL treated with rituximab,3 but not chemotherapy,14 while Carlotti et al. did not find any such association in FL patients treated with CHOP followed by rituximab.9 Curiously, we did find an association between FCGR2A 131H/H and favorable FCGR3A 158V/V, and this is in line with studies by Treon et al.18 and other investigators19 suggesting linkage disequilibrium between the 131H/H and 158V/V genotypes.
The impact of FCGR polymorphisms on treatment with radioimmunoconjugates has not, to our knowledge, been previously reported. With tositumomab and iodine I 131 tositumomab (BEXXAR therapeutic regimen), both unlabeled and radio-isotope (I-131) labeled anti-B1 antibodies are given. While radio-isotope is likely responsible for part of the activity, at least 2 studies have shown that unlabeled anti-B1 antibody has substantial anti-lymphoma activity, which does take advantage of ADCC mechanism. In a study by Buchsbaum et al.,20 the activity of radio-isotope labeled and unlabeled anti-B1 antibody were similar, while in the study by Cardarelli et al. unlabeled anti-B1 antibody had nearly identical ADCC as rituximab.21 Overall survival with CHOP followed by tositumomab and iodine I 131 tositumomab were similar to those with CHOP followed by rituximab with respect to FCGR3A and FCGR2A, so the groups were pooled. Although there may have been some difference between these 2 study populations in terms of the association between FCGR3A and progression-free survival, in neither one was this association significant.
Tositumomab is a murine IgG2a lambda anti-CD20 monoclonal antibody, while rituximab is a chimeric murine/human IgG1 kappa anti-CD20 monoclonal antibody. However, FCGR-binding residues in murine IgG2a are conserved and are similar to human IgGs (except E269D), consistent with the observation that murine mAbs can serve as ligands for human FCGRs.22
Genotype could not be determined in 22 cases for FCGR3A (16 of them from the earliest trial, S8809), and 4 cases for FCGR2A (all in S8809). This was most likely due to DNA degradation in older tissue specimens. In prior studies the source of DNA varied. DNA was extracted from tissue, tumor cells, peripheral blood mononuclear cells, or bone marrow, without reports of different detection rates or genotyping results depending on DNA origin. A variety of techniques are in common usage, including Single-Strand Conformation Polymorphism (SSCP) and Polymerase Chain Reaction (PCR) based techniques, including the Taqman SNP assay. Again, there is no clear evidence that one technique is superior to the others, although SSCP has largely been abandoned due to variability in sensitivity of detection imposed by changes in temperature, pH, and fragment length. In this study, imbalance in detection rates between FCGR3A and FCGR2A genotypes may be due to the FCGR3A assay being technically more difficult due to close similarity with the FCGR3B assay. However, there is no reason to suspect that a particular FCGR3A genotype has a higher detection rate, which potentially could have biased the study.
A factor that limits the power to detect a difference in outcomes by genotype is the low frequency of the 158V/V genotype in studies of Caucasian patients. This genotype is generally present in 10–20% of Caucasians, as opposed to 47% of Koreans23 and 49% of Chinese.24 This low frequency of the favorable genotype has contributed to the design of new anti-CD20 antibodies which have a higher affinity for FCGR3A 158F/F, such as GA-101, AME-133v, and rhuMAb v114, and thus may overcome the survival disadvantage of 158F/F genotype.
In conclusion, we found that in sequential SWOG trials of previously untreated follicular lymphoma, patients with the FCGR3A 158V/V or V/F genotypes had a better 5-year OS (100% and 97%, respectively) with addition of anti-CD20 monoclonal therapy to chemotherapy than patients with the 158 F/F genotype (5-year OS 75%). In contrast, the 5-year OS was similar across the genotypes in protocols where chemotherapy alone was used. These findings suggest that the genetic constitution of patients with follicular lymphoma can influence outcomes according to the therapeutic modality selected. Future studies should focus on assessment of a larger cohort of patients, with more uniform treatment, comparing different technologies and specimen types, as well as on examining the importance of other polymorphisms. FCGR3A polymorphism status may be an important factor to consider in designing new FL trials with mAb-containing regimens, as well as in designing new anti-CD20 antibodies with better affinity for 158F/F genotype.
Acknowledgments
The authors would like to thank Ellen Chase, Southwest Oncology Group Chair’s Office and Operative Office for their support. We are indebted to our patients for participating in SWOG trials.
Footnotes
Funding: this work was supported by PHS Cooperative Agreement grants awarded by the National Cancer Institute, DHHS: CA32102, CA38926 and CA114748; and by Genentech and GlaxoSmithKline.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.http://www.gene.com/gene/products/information/pdf/rituxan-prescribing.pdf.
- 2.Hilchey SP, Hyrien O, Mosmann TR, Livingstone AM, Friedberg JW, Young F, et al. Rituximab immunotherapy results in the induction of a lymphoma idiotype-specific T-cell response in patients with follicular lymphoma: support for a “vaccinal effect” of rituximab. Blood. 2009;113(16):3809–12. doi: 10.1182/blood-2008-10-185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406(6793):267–73. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 5.Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–14. [PubMed] [Google Scholar]
- 6.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 7.Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Leger-Falandry C, Cogliatti S, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2005;16(10):1675–82. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- 8.Boettcher S, Pott C, Ritgen M, Hiddemann W, Unterhalt M, Kneba M. Evidence for Fcγ receptor IIIA-independent rituximab effector mechanisms in patients with follicular lymphoma treated with combined immuno-chemotherapy. Blood (ASH Annual Meeting Abstracts) 2004;104 Abstract 590. [Google Scholar]
- 9.Carlotti E, Palumbo GA, Oldani E, Tibullo D, Salmoiraghi S, Rossi A, et al. FcgammaRIIIA and FcgammaRIIA polymorphisms do not predict clinical outcome of follicular non-Hodgkin’s lymphoma patients treated with sequential CHOP and rituximab. Haematologica. 2007;92(8):1127–30. doi: 10.3324/haematol.11288. [DOI] [PubMed] [Google Scholar]
- 10.Maloney D, Pender-Smith B, Unger J, Braziel R, Radich J, Press O, et al. Fcγ receptor polymorphisms do not influence progression free survival (PFS) of follicular NHL patients treated with CHOP followed by rituximab (SWOG 9800) Blood (ASH Annual Meeting Abstracts) 2004;104 Abstract 589. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958:53457–81. [Google Scholar]
- 12.Cox DR. Regression models and life-tables. JR Stat Soc [B] 1972:34187–220. [Google Scholar]
- 13.Weng WK, Czerwinski D, Timmerman J, Hsu FJ, Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22(23):4717–24. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Weng WK, Levy R. Immunoglobulin G Fc receptor polymorphisms do not correlate with response to chemotherapy or clinical course in patients with follicular lymphoma. Leuk Lymphoma. 2009;50(9):1494–500. doi: 10.1080/10428190903128660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierz KA, Gu S, Lewis ME, Hsu S, Falandry C, Salles GA, et al. Predictive value of FCGR3A genotype on response to rituximab induction and maintenance therapy (MT) in follicular non-Hodgkin’s lymphoma (NHL) J Clin Oncol. 2010;28:15s. suppl; asbtr 8065. [Google Scholar]
- 16.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28(28):4390–9. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood. 2011;118(12):3347–9. doi: 10.1182/blood-2011-05-351411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatjiharissi E, Hansen M, Santos DD, Xu L, Leleu X, Dimmock EW, et al. Genetic linkage of Fc gamma RIIa and Fc gamma RIIIa and implications for their use in predicting clinical responses to CD20-directed monoclonal antibody therapy. Clin Lymphoma Myeloma. 2007;7(4):286–90. doi: 10.3816/clm.2007.n.004. [DOI] [PubMed] [Google Scholar]
- 19.Lejeune J, Thibault G, Ternant D, Cartron G, Watier H, Ohresser M. Evidence for linkage disequilibrium between Fcgamma RIIIa-V158F and Fcgamma RIIa-H131R polymorphisms in white patients, and for an Fcgamma RIIIa-restricted influence on the response to therapeutic antibodies. J Clin Oncol. 2008;26(33):5489–91. doi: 10.1200/JCO.2008.19.4118. [DOI] [PubMed] [Google Scholar]
- 20.Buchsbaum DJ, Wahl RL, Normolle DP, Kaminski MS. Therapy with unlabeled and 131I-labeled pan-B-cell monoclonal anti-bodies in nude mice bearing Raji Burkitt’s lymphoma xenografts. Cancer Res. 1992;52(23):6476–81. [PubMed] [Google Scholar]
- 21.Cardarelli PM, Quinn M, Buckman D, Fang Y, Colcher D, King DJ, et al. Binding to CD20 by anti-B1 antibody or F(ab')(2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51(1):15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276(19):16469–77. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Jung HD, Kim JG, Lee JJ, Yang DH, Park YH, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;108(8):2720–5. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Feng J, Zhang L, Hu Y, Luan B, Yue W, et al. Distribution of variant genotypes of Fc gamma receptor IIIa in healthy Chinese population of Zhengzhou City. J Huazhong Univ Sci Technolog Med Sci. 2003;23(3):239–41. doi: 10.1007/BF02829502. [DOI] [PubMed] [Google Scholar]