Combination chemotherapy with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine1 is considered the gold standard for Hodgkin’s lymphoma (HL), but a small fraction of patients fail to achieve long-term disease control for either resistant or relapsing disease.2 The most promising intensive regimen BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) has been proposed to promote a better control of the disease, despite immediate and long-term side effects.2 Therefore, affordable tools that can identify risk-adapted strategies are an emerging need in order to limit toxic treatments to high-risk patients, since the current International Prognostic Score is not sufficient to do this.3,4
The two most promising biomarkers recently under investigation are the interim 2-(1)fluoro-2-deoxy-D-glucose positron emission tomography evaluation after two cycles (PET-2) of chemotherapy5 and the amount of macrophage infiltrate in the lymphonode at diagnosis,6,7 but both suffer from a lack of reproducibility in different series.8 Recently, in this journal, Porrata and colleagues reported that the evaluation of the ALC/AMC-DX ratio, obtained by dividing the absolute lymphocyte count (ALC) over the absolute monocyte count (AMC) from the complete blood count, is an additional independent marker with prognostic value in HL.9 ALC and AMC have been used as surrogates of host immune homeostasis and tumor-associated macrophages, respectively. Therefore, we tested the validity of the ALC/AMC-DX ratio in a series of patients treated upfront with the ABVD scheme and a risk-adapted strategy based on PET-2 findings. Patients with positive PET-2 continued treatment with the BEACOPP protocol for a further four courses.
From September 2008 to January 2012, 115 consecutive patients with classical HL were evaluated. The study was conducted in accordance with the Declaration of Helsinki. All patients were treated with standard ABVD therapy followed by consolidation radiotherapy in case of bulky presentation or residual tumor mass. Both baseline and PET-2 were performed using standard techniques.5 Progression, relapse, disease-free survival (DFS) and progression-free survival (PFS) were defined according to criteria established by the International Harmonization Project on Lymphoma.10
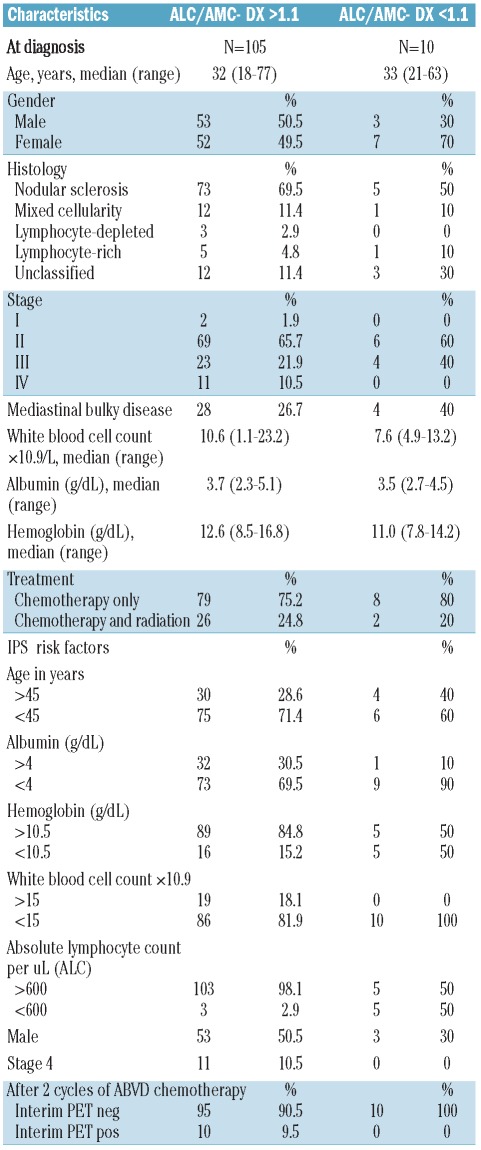
The distribution of baseline characteristics, including stage, IPS and PET-2, is summarized in Table 1 according to whether patients presented with an ALC/AMC-DX of 1.1 or more versus less than 1.1 (the cut-off point indicated by Porrata et al.9)
Table 1.
Characteristics of the patients divided according to ALC/AMC-DX ratio ≥ 1.1 versus < 1.1.
After a median follow up of 31.4 months (range 5.4–84.4 months), 97 patients (84.3%) were in continued complete remission (cCR), 2 patients (1.7%) progressed during therapy or immediately after (during the first six months), and 16 relapsed (13.9%) after a median of 16 months.
The largest number of patients showed an ALC/AMC-DX ratio greater or equal to 1.1 (105 of 115). ALC/AMC-DX ratio of 1.1 or more had sensitivity for predicting 2-year PFS of 93.8% (95% CI, 69.8–99.8%), but a low specificity of 9.1% (95% CI, 4.2–16.5%). All PET-2 positive patients showed ALC/AMC-DX greater or equal to 1.1.
Ten patients (8.7%) were PET-2 positive and 105 patients (91.3%) were PET-2 negative, similar to other large previously reported series.5 Of 10 PET-2–positive patients, 7 were switched to the BEACOPP scheme, one was treated with IGEV and autologous transplantation, and 2 proceeded on ABVD. Despite this risk-adapted strategy, 6 patients (60%) showed treatment failure (progression/relapse), whereas 3 were in PR (and still on treatment), and one in cCR at the latest follow up. PET-2 had sensitivity for predicting 2-year PFS of 37.5% (95% CI%, 15.2–64.6%) and a specificity of 95.9% (95% CI, 89.9–98.9%).
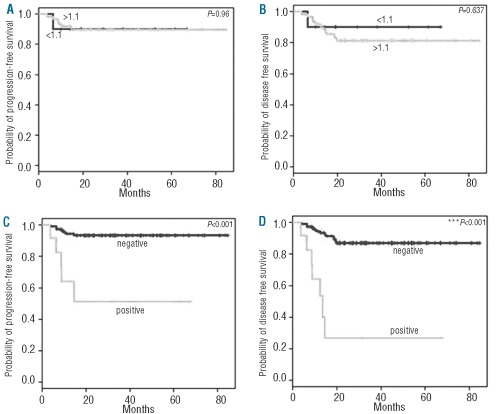
There was no difference in PFS (Figure 1A) or DFS (Figure 1B) between patients with an ALC/AMC-DX of 1.1 or more and those patients with an ALC/AMC-DX less than 1.1: median PFS and DFS were not reached in either. In contrast, patients with positive PET-2 had inferior PFS (Figure 1C) and DFS (Figure 1D) compared with patients with a negative PET-2: median PFS not reached versus 13.7 months; median DFS not reached versus 18.5 months.
Figure 1.
Kaplan-Meier plot showing the (A) PFS and (B) DFS according to ALC/AMC-DX ratio and (C) PFS and (D) DFS according to PET-2 findings after two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine)
In contrast to the cohort of Porrata et al.9 in which 58% of patients showed an ALC/AMC-DX ratio greater or equal to 1.1, in our series this value reached 91%. This suggests that, in different series, a different cut-off value should perhaps be calculated. In addition, although we confirmed that a low ALC/AMC-DX ratio correlates with a good prognosis, we were not able to confirm the high specificity of this marker and its predicting value was certainly inferior to PET-2. Despite the therapy switching in case of PET-2 positivity, in our series, PET-2 still maintained high sensitivity and specificity for predicting 2-year PFS and DFS.
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Santoro A, Bonadonna G. Prolonged disease-free survival in MOPP-resistant Hodgkin’s disease after treatment with adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) Cancer Chemother Pharmacol. 1979;2(2):101–5. doi: 10.1007/BF00254081. [DOI] [PubMed] [Google Scholar]
- 2.Engert A, Diehl V, Franklin J, Lohri A, Dörken B, Ludwig WD, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–54. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 3.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 4.Hasenclever D. The disappearance of prognostic factors in Hodgkin’s disease. Ann Oncol. 2002;13(Suppl 1):75–8. doi: 10.1093/annonc/13.s1.75. [DOI] [PubMed] [Google Scholar]
- 5.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 6.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris JA, Jain S, Ren Q, Zarineh A, Liu C, Ibrahim S. CD163 Versus CD68 in Tumor Associated Macrophages of Classical Hodgkin Lymphoma. Diagn Pathol. 2012;7(1):12. doi: 10.1186/1746-1596-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azambuja D, Natkunam Y, Biasoli I, Lossos IS, Anderson MW, Morais JC, et al. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin's lymphoma. Ann Oncol. 2012;23(3):736–42. doi: 10.1093/annonc/mdr157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma. Haematologica. 2012;97(2):262–9. doi: 10.3324/haematol.2011.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21(5):841–54. doi: 10.1016/j.hoc.2007.06.011. [DOI] [PubMed] [Google Scholar]