Abstract
Berberine (BBR) has recently been shown to improve insulin sensitivity in rodent models of insulin resistance. Although this effect was explained partly through an observed activation of AMP-activated protein kinase (AMPK), the upstream and downstream mediators of this phenotype were not explored. Here, we show that BBR supplementation reverts mitochondrial dysfunction induced by High Fat Diet (HFD) and hyperglycemia in skeletal muscle, in part due to an increase in mitochondrial biogenesis. Furthermore, we observe that the prevention of mitochondrial dysfunction by BBR, the increase in mitochondrial biogenesis, as well as BBR-induced AMPK activation, are blocked in cells in which SIRT1 has been knocked-down. Taken together, these data reveal an important role for SIRT1 and mitochondrial biogenesis in the preventive effects of BBR on diet-induced insulin resistance.
Keywords: Metabolic syndrome, Berberine, Mitochondria, SIRT1, AMPK, NAMPT
1. Introduction
The global emergence of obesity as an epidemic has made it a worldwide public health problem, promoted by a sedentary lifestyle and a diet rich in fats and sugar [1,2]. Indeed, obesity has been linked to numerous health-related pathologies. Visceral obesity is associated with insulin resistance, dyslipidemia, hypertension and increased risk of atherosclerosis, a condition known as metabolic syndrome [3]. Metabolic syndrome results from a positive energy balance, in which caloric intake exceeds oxidation, leading to a disregulation of glucose and lipid metabolism [4].
Skeletal muscle plays an important role in the development of the metabolic syndrome [3,5,6]. Since the oxidative capacity of skeletal muscle is predominately dependent on mitochondria, there is growing evidence suggesting that mitochondrial dysfunction, and the associated impairment of fatty acid oxidation, may directly cause or accelerate insulin resistance [7,8]. This has been shown in patients with insulin resistance and type 2 diabetes [9–12], as well as in several animal models [13,14]. In skeletal muscle, stimulation of AMPK and SIRT1 has been shown to increase the expression and activity of peroxisome proliferator activated receptor gamma (PPARγ) coactivator 1-alpha (PGC-1alpha), an essential cofactor involved in mitochondrial biogenesis [15,16].
The mammalian sirtuins (SIRT1–SIRT7) have been implicated in a number of cellular and physiological processes including gene silencing, apoptosis, mitochondrial function, energy homeostasis, and longevity [17]. SIRT1 has emerged as a key regulator of mammalian metabolism, and has been shown to deacetylate and activate PGC-1apha [16,18]. Furthermore, several SIRT1 activators were recently demonstrated to improve key features of the metabolic syndrome. The beneficial effects of SIRT1 activation are related with metabolic changes similar to those triggered by caloric restriction, including improvement of mitochondrial function in skeletal muscle [19–21].
Berberine (BBR), [18,5,6-dyhydro-9,10-dimethoxybenzo(g)-1,3benzodioxolo(5.6-a) quinolizinium], is an isoquinoline alkaloid derived from the Berberidacea plant family, which has been used in traditional Chinese medicine for centuries. Multiple pharmacologic effects of BBR have been reported including anti-inflammatory [22], anti-hypertensive [23], and anti-proliferative actions [24]. Moreover, beneficial effects of BBR on insulin sensitivity and glucose tolerance have shown promise in the treatment of metabolic disorders such as hyperglycemia and hyperlipidemia [25–28]. These effects are related, in part, to the ability of berberine to activate AMPK [25,27,29] and to suppress gluconeogenesis [30].
Since SIRT1 activity is reported to be regulated through AMPK [31] and SIRT1 can also regulate AMPK activity [32], it is tempting to speculate that the beneficial effects of BBR on metabolism may be mediated in part by SIRT1. Here, we demonstrate that BBR supplementation increases skeletal muscle mitochondrial biogenesis and improves mitochondrial function in a rodent model of diet induced obesity. Furthermore, we show that these effects are SIRT1-dependent. These effects are associated with significant reductions in adiposity and improvements in overall insulin sensitivity.
2. Materials and methods
2.1. Animals, diets and treatments
Male Sprague Dawley rats aged 6–8 weeks were housed under a 12–12 h light/dark cycle at 22 °C and given free access to water and standard chow (Control group) or high fat diet (HFD) for 12 weeks. After the 12 weeks a third group of rats was maintained on HFD with a supplement of berberine (100 mg/kg/day) in the drinking water for 4 more weeks (HFD+BBR). Berberine intake was monitored over the course of the study. The diets were purchased from Research Diets, Inc (New Jersey, USA). The diet used to induce obesity (HFD) has 60% kcal from fat, whereas the control diet (Ctl) has 10% kcal from fat. All experimental procedures respected the guidelines of the European Directive 86/609/CEE and present in the Portuguese law.
2.2. Surgical procedures, body composition and glucose tolerance test
Chronic indwelling catheters were implanted and the animals were monitored daily and the catheters flushed with saline. All animals were allowed to recover for 5 days. Experiments were performed on conscious, unrestrained animals fasted for 24 h. A saline solution enriched to 70% with 2H2O was administered via the jugular catheter for 225 min to measure total body water and fat-free mass (FFM). 2H-enrichments of plasma water and infusate precursor were quantified using 2H NMR as describe before [33,34]. For determining 2H-body water enrichment, a blood sample was taken at 225 min, or 45 min after the infusion was terminated. The body water pool (ml) for each animal was quantified from the ratio of infusate to plasma body water 2H-enrichment of the 225 minute sample multiplied by the total volume of infused 2H2O. Body water mass (grams) was assumed to be equal to body water volume. FFM was calculated by dividing the body water mass by 0.73 [33]. Fat mass was calculated by subtracting FFM from the total body weight. To determine insulin sensitivity and glucose tolerance a glucose tolerance test was performed, by oral gavage of 1.5 g glucose/kg body weight. Blood samples were taken from the tail vein at several time points after the bolus injection and the concentration of glucose for each sample was measured.
2.3. Glucose, triglycerides and hormone levels
Plasma glucose was determined by a glucometer (Accu-cheK Aviva — Roche, Portugal) and by a glucose microplate assay kit (Invitrogen, Spain). Plasma insulin levels were determined by ELISA (EZRMI-13K — Millipore, Portugal). Plasma leptin and adiponectin levels were measured using a commercially available kit (Invitrogen, Spain). Triglycerides were extracted using isopropanol and quantified using a colorimetric based commercial available kit (Cromatest, Spain).
2.4. C2C12 cell cultures and treatments
C2C12 cell line (ATCC) was cultured in low glucose Dulbecco’s modified eagle medium (DMEM) (Invitrogen) supplemented with 10% FBS (Invitrogen) and mix of antibiotic and antimycotic (Invitrogen). Differentiation of C2C12 myoblasts to myotubes was achieved by allowing the cells to reach confluence and then replacing the FBS with 2% Horse Serum (Invitrogen) for 6 days. C2C12 cells were cultured in high glucose media (25 mM) for 96 h to mimic hyperglycemic conditions or with 500 μM of Palmitate and Oleate in a ratio of 1:1 (FFAs) [35] to mimic hyperlipidemic conditions. For experiments performed with FFAs, the cells were cultured in medium containing 0.5% of BSA (Sigma-Aldrich) [35]. Cells treated with 25 mM glucose or FFAs were also treated with the vehicle (0.001% DMSO) or 5 μM of Berberine chloride (BBR) for the entire period of treatment. To investigate the intracellular pathways involved in BBR effects on mitochondrial function and mitochondrial biogenesis, C2C12 myotubes were treated with the vehicle (0.001% DMSO), 1 μM of EX-527, or 10 μM DCHC during the same period of treatment with hyperglycemia or fatty acids. To investigate the short term effects of BBR on mitochondrial function, C2C12 cells were cultured in low glucose DMEM with the vehicle (0.001% DMSO), BBR 5 μM and 20 μM BBR for 6, 12 and 24 h. To further address the role of NAMPT in BBR mediated effects, C2C12 cells were treated with 5 μM BBR or 5 μM BBR plus 10 nM FK866 for 12 h.
2.5. SIRT1 gene silencing in C2C12 cells
SIRT1 shRNA and control shGFP lentivirus were produced by co-transfection of 293T cells with plasmids encoding Vsv-g, Δ8.9, pLKO-shSIRT1 (mouse) or pLKO-shGFP using FuGENE HD in accordance with the manufacturer’s protocol. The Oligo ID for the SIRT1 mouse hairpin used was TRCN0000039296 (Open Biosystems). Media was changed 24 h post-transfection. Virus was harvested after 48 h, filtered, and used to infect C2C12 cells in the presence of 5 μg/mL polybrene via spin infection (2500 rpm, 30 min). Selection of resistant colonies was initiated 24 h later using 2 μg/mL puromycin.
2.6. Mitochondrial function
Skeletal muscle mitochondria were isolated as described previously [36] with minor modifications. Oxygen consumption of isolated mitochondria was polarographically determined with a Clark oxygen electrode as described [37]. State 3 respiration was induced with the addition of 200 nmol of ADP and FCCP-induced respiration was induced by the addition of 1 μM of FCCP. Succinate Dehydrogenase and Cytochrome c Oxidase activities were polarographically measured as previously described [38–40]. ATPase activity was determined spectrophotometrically as described [40]. Mitochondrial membrane potential in C2C12 cells was measured with a fluorescent probe, Tetramethylrhodamine methyl ester (TMRM) (Sigma) as described before [41] and the ATP content was measured with a commercial kit according to the manufacturer’s instructions (Roche). Endogenous mitochondrial ATP was extracted using an alkaline extraction procedure and was measured using a commercially available kit according to the manufacture’s instruction (Sigma).
2.7. Gene expression and mtDNA analysis
RNA from skeletal muscle tissue and C2C12 cells were extracted with RNeasy mini kit (QIAGEN) according to the instructions and quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific). cDNA was synthesized with an iSCRIPT cDNA synthesis kit (BioRad) using 200 ng of RNA. Quantitative RT-PCR reactions were performed using 1 μM of primers and LightCycler® 480 SYBR Green Master (Roche) on a LightCycler® 480 detection system (Roche). Calculations were performed by a comparative method (2−ΔΔCT) using actin as an internal control. For mtDNA analysis, total DNA was extracted with a DNeasy blood and tissue kit (QIAGEN). mtDNA was amplified using primers specific for the mitochondrial cytochrome c oxidase subunit 2 (COX2) gene and normalized to genomic DNA by amplification of the ribosomal protein s18 (rps18) nuclear gene. Primers were designed using the IDT software (IDT) and the primer sequences can be found in the Supplemental Information.
2.8. Citrate synthase activity
Citrate synthase activity was measured in both skeletal muscle tissue and C2C12 cell lysates spectrophotometrically at 412 nm at 30 °C, as previous described [42]. Tissue or cell protein extracts (20 μg) were added to buffer containing 10 mM Tris pH 8, 200 μM Acetyl CoA and 500 μM 5,5-dithio-bis-(2-nitrobenzoic) acid. The reaction was initiated by the addition of 1 mM oxaloacetate.
2.9. Mitochondrial mass
Mitochondrial mass was evaluated using the fluorescent probe N-nonyl acridine orange (NAO). Briefly, C2C12 cells were incubated in DMEM containing 10 nM of NAO for 30 min at 37 °C in the dark. The cells were then trypsinized and resuspended in DMEM without NAO. The NAO fluorescence intensity was determined by flow cytometry on the FACSCalibur (BD Biosciences) using the 488 nm laser.
2.10. Immunoblot
Protein extracts from tissue or C2C12 cells were obtained by lysis in ice-cold lysis buffer (150 mM NaCl, 10 mM Tris HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.5% NP-40) supplemented with a cocktail of protease and phosphatase inhibitors (Roche). Protein content was determined by the bicinchoninic acid protein assay (Pierce), and 50 μg of protein was run on SDS-PAGE under reducing conditions. The separated proteins transferred to a polyvinylidene difluoride membrane (Perkin-Elmer). Proteins of interest were revealed with specific antibodies: anti-phospho-AMPKα (Thr 172), anti- AMPKα, anti-phospho-ACC (Ser79) (Cell Signaling Technology), anti-SIRT1 (Sigma-Aldrich), anti-PGC1alpha (H300) (Santa Cruz Biotechnology), anti-NRF-1 (Abcam), anti-TFAM (Aviva Biosciences), anti-COXI, anti-COXIV (Mitosciences) and anti-β-Actin (Sigma-Aldrich) overnight at 4 °C. The immunostaining was detected using horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin for 1 h at room temperature. After the visualization of phospho-ACC or phospho-AMPKα, membranes were stripped with Stripping buffer (100 mM Mercaptoethanol, 2% SDS and 62.5 mM Tris–HCl buffer at pH 6.7) at 50 °C for 15 min and further probed for the visualization of total ACC or AMPKα. Bands were revealed using Amersham ECL detection system (GE Healthcare) and quantitated by densitometry using Image J.
2.11. NAD+/NADH measurement
NAD+/NADH ratio from C2C12 cells was quantified with a commercially available kit (BioVision) according to the manufacturer’s instructions.
2.12. Statistical analysis
Data were analyzed by a two-tailed unpaired Student’s t-test. All data are reported as mean±SEM. Statistical analysis was performed using Excel software.
3. Results
3.1. BBR reverts high fat diet-induced obesity
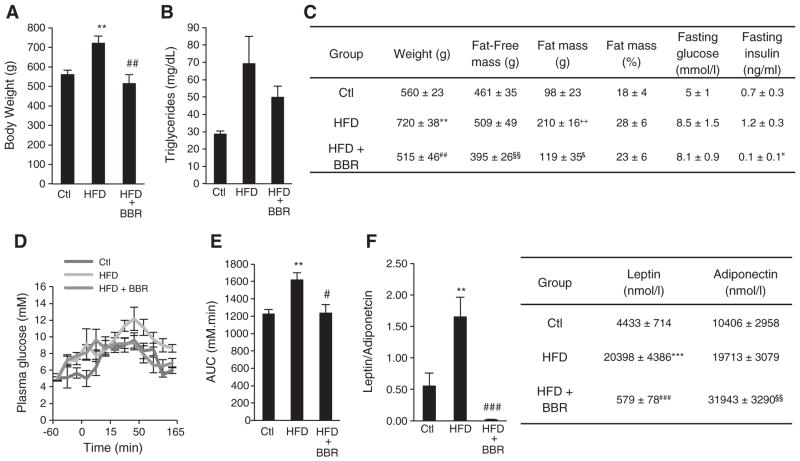
Previous work has demonstrated that BBR protects from High fat diet (HFD)-induced obesity and its deleterious effects. Also, BBR has been shown to prevent the natural onset of obesity in several animal models [25,26,28,30]. In our present work, we decided to investigate the metabolic effects of BBR on skeletal muscle in a model of diet-induced obesity. We administered BBR (100 mg/kg/day) in the drinking water for 4 weeks to rats previously fed with a HFD for 12 weeks. Body weight measurements at the end of the experimental treatment validated the HFD feeding as a model for obesity, since the HFD rats showed a significant increase in body weight when compared to control rats (on average 160 g more) (Fig. 1A). Moreover, we observed that both fat and lean masses were contributing to this increase (Fig. 1C). This was also correlated with an increase in the triglyceride content in skeletal muscle of HFD fed rats (Fig. 1B). BBR significantly reduced this increase in body weight to values similar to control rats (Fig. 1A) due to reversion of the HFD inducing increase in both fat and lean mass induced by HFD feeding (Fig. 1C), since weight loss is not purely from adipose mass loss but also includes lean mass loss. BBR treatment also decreased the triglyceride content in the skeletal muscle of these rats (Fig. 1B).
Fig. 1.
Berberine protects from obesity and obesity-induced impairment in glucose homeostasis in Sprague–Dawley Rats. (A) Body Weight of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=5 experiments **p<0.01 versus Ctl, ## p<0.01 versus HFD). (B) Triglycerides content expressed in mg/dL in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=4 experiments p≥0.05). (C) Comparative whole body characteristics of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=5 experiments **p<0.01 versus Ctl, ## p<0.01 versus HFD, §§ p<0.01 versus HFD, ++ p<0.01 versus Ctl, & p<0.05 versus HFD, p<0.05 versus HFD). (D and E) Plasma glucose concentrations at the indicated time points after glucose challenge were measured (D) and values for areas under the insulin curve were calculated (E) in rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=5 experiments **p<0.01 versus Ctl, # p<0.05 versus HFD). (F) Leptin and Adiponectin content in the plasma of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR). (n=4 experiments **p<0.01 ***p<0.001 versus Ctl, ### p<0.001 versus HFD &&& p<0.001 versus HFD). All data represent mean±SEM.
3.2. BBR normalizes hormonal levels and glucose homeostasis
Hyperglycemia and hyperinsulinemia are common symptoms associated with development of the Metabolic Syndrome [4]. BBR supplementation reduced HFD-induced fasted plasma insulin levels (Fig. 1C), indicating that BBR is able to decrease HFD-induced hyperinsulinemia. However, despite abolishing the HFD-induced hyperinsulinemia BBR supplementation did not effectively change fasting plasma glucose levels (Fig. 1C). To further study the effects of BBR in glucose homeostasis and insulin sensitivity, an oral glucose tolerance test was performed. Concomitant with an increase in body weight, HFD feeding decreased the ability to clear blood glucose, as demonstrated by glucose excursions (Fig. 1D) and AUC (Fig. 1E). Despite the weak effect on fasting glucose plasma levels, BBR supplementation was able to significantly improve glucose tolerance and hyperinsulinemia over HFD fed rats to values comparable to the control group (Fig. 1C–E). Together this data revel that BBR supplementation has a positive impact on glucose and insulin homeostasis upon HFD feeding.
Leptin and adiponectin levels were also assessed since alterations in the levels of these two adipokines have been extensively correlated with obesity and obesity-related disorders [4]. Indeed, adiponectin and leptin are the two main adipokines thought to contribute to systemic abnormalities in both lipid and glucose homeostasis [43]. HFD fed rats presented a marked increase in leptin levels (Fig. 1F), which was reverted by BBR supplementation (Fig. 1F). Moreover, BBR supplementation also induces a dramatic increase in the plasmatic levels of adiponectin (Fig. 1F), resulting in mitigation of the dramatic increase in the leptin/adiponectin ratio induced by HFD.
3.3. Mitochondrial function is rescued by BBR
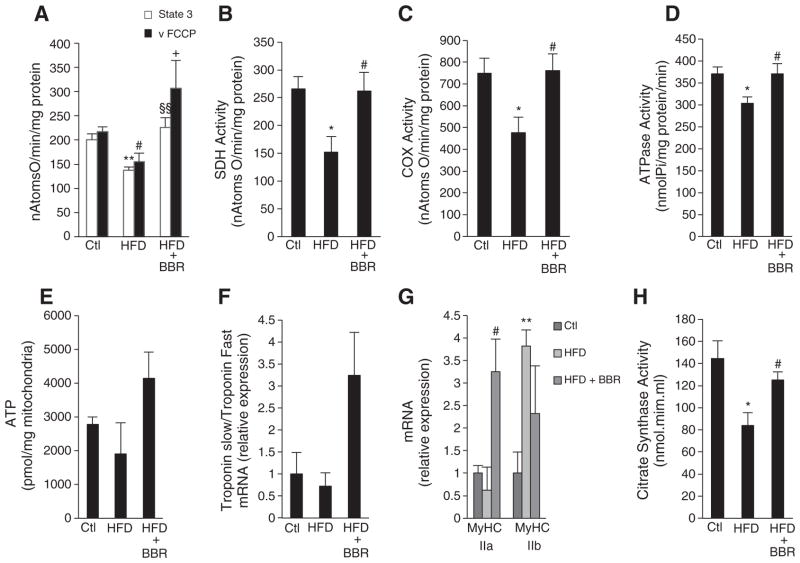
Mitochondria play an important role in the development of insulin resistance and hyperglycemia [8,44]. Boosting mitochondrial activity provides, at least in part, a potential mechanism by which several therapeutic agents act [19–21]. In order to address whether the beneficial effects of BBR are related to its ability to alter mitochondrial function, we evaluated the function of isolated mitochondria. As reported before, HFD feeding induced mitochondrial dysfunction in skeletal muscle (Fig. 2A–E). This was demonstrated by decreased oxygen consumption (State 3), and decreased maximal oxygen consumption in the presence of FCCP (vFCCP), which is dependent only on the oxidative efficiency of the electron transport chain (Fig. 2A). These defects in mitochondrial respiration were rescued by treatment with BBR. In accordance with decreased mitochondrial function, mitochondrial ATP content was also decreased by HFD feeding and this was rescued by BBR (Fig. 2E). The changes in mitochondrial function induced by HFD feeding, seem to be the result of impairments in the mitochondrial ATPase (Fig. 2D) and electron transport chain (ETC) complexes like Succinate dehydrogenase (SDH) (Fig. 2B) and Cytochrome c Oxidase (COX) (Fig. 2C). BBR supplementation was able to revert mitochondrial dysfunction induced by HFD by restoring ATPase and ETC activities (Fig. 2B–D). Although BBR has previously been shown to negatively impact hepatic mitochondrial function in in vitro assays or in cells exposed to concentrations higher than the one used in our study [27,30,45,46], we also observed an improvement in the function of hepatic mitochondria isolated from animals treated with BBR (data not shown). This suggests a dose-dependent, indirect but positive action of BBR on mitochondria.
Fig. 2.
Berberine Protects from obesity-induced skeletal muscle mitochondrial dysfunction in Sprague–Dawley Rats. (A) State 3 respiration and FCCP-induced respiration of isolated skeletal muscle mitochondria from rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) measured polarographically and expressed in nAtomsO/min/mg protein (n=5 experiments **p<0.01 versus Ctl, # p<0.05 versus Ctl, §§ p<0.01 versus HFD, + p<0.05 versus HFD). (B and C) Succinate Dehydrogenase (B) and Cytochrome c Oxidase (C) activity in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) measured polarographically and expressed in nAtomsO/min/mg protein (n=4 experiments *p<0.05 versus Ctl, ## p<0.01 versus HFD). (D) ATPase activity measured spectrophotometrically in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) expressed in nmolPi/mgprotein/min (n=4 experiments *p<0.05 versus Ctl, # p<0.05 versus HFD). (E) ATP content in isolated mitochondria from skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=5 experiments p≥0.05). (F) Amount of oxidative fibers analyzed by the ratio of Troponin 1 slow and Troponin 1 fast mRNA measured by means of quantitative RT-PCR in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+ BRR) (n=4 experiments p≥0.05). (G) MyHCIIa and MyHCIIb mRNA analyzed by means of quantitative RT-PCR in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) (n=4 experiments ** p<0.01 versus Ctl, # p<0.05 versus HFD). (H) Citrate Synthase activity measured spectrophotometrically in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR) expressed in nmol.mim.ml (n=4 experiments *p<0.05 versus Ctl, # p<0.05 versus HFD). All data represent mean±SEM.
HFD-induced mitochondrial dysfunction was also correlated with decreased oxidative type 1 fibers (Fig. 2F) and a switch towards the glycolytic type II fibers (Fig. 2G) while BBR supplementation increased oxidative fiber type in skeletal muscle (Fig. 2F and G) and slightly decreased glycolytic type IIb fibers (Fig. 2G). Citrate synthase activity in HFD fed rats was also decreased, and this effect was again rescued by BBR supplementation (Fig. 2H). These observations led us to speculate that BBR supplementation may be rescuing mitochondrial function from HFD damage by increasing mitochondrial biogenesis.
3.4. BBR rescues mitochondrial function in a SIRT1-dependent manner
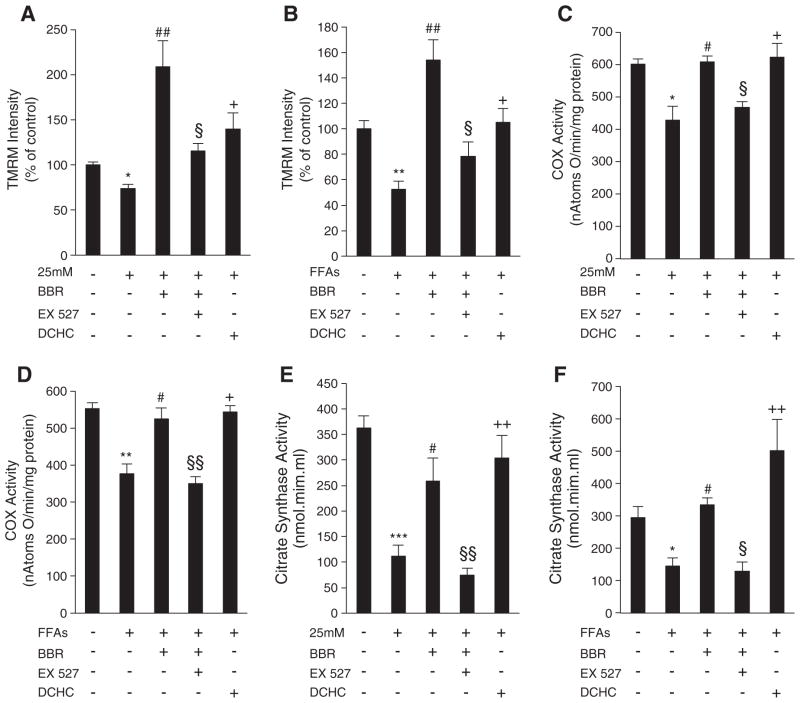
The observations from the in vivo studies were extended to cell culture models by investigating potential mechanisms for BBR’s beneficial effects on mitochondrial function under conditions of excess of nutrients. SIRT1 has been highlighted as a potential therapeutic target for numerous mitochondrial pathologies [19–21]. Its beneficial effects are associated with an induction of genes involved in oxidative phosphorylation and mitochondrial biogenesis, thereby increasing mitochondrial ability to oxidize substrates. Since BBR has been shown to activate AMPK, an upstream regulator of SIRT1 and mitochondrial biogenesis [31], we tested if SIRT1 is involved in the protective mechanisms of BBR in mitochondrial function. Initially, C2C12 myotubes were exposed to high glucose and high fatty acids (FFAs) for 96 h. These exposures induced mitochondrial dysfunction, as demonstrated by a decrease in mitochondrial membrane potential and COX activity (Fig. 3A–D). Interestingly, supplementation with BBR for 96 h prevented this loss of mitochondrial function (Fig. 3A–D). Since it has been recently shown that 10 μM BBR induces an increase in mitochondrial-derived superoxide that leads to AMPK activation in endothelial cells [45], we evaluated ROS generation in cells treated with BBR 5 μM for 96 h. No statistically significant changes in ROS production were observed with BBR supplementation (Data not shown).
Fig. 3.
SIRT1 is involved in the rescue of mitochondrial dysfunction induced by hyperglycemia and fatty acids in C2C12 myotubes by Berberine. (A and B) Mitochondrial membrane potential measured with TMRM fluorescent probe and normalized to mg of protein in cells after 96 h of treatment with hyperglycemia (25 mM) (A) or fatty acids (FFAs) (B) and exposed to 5 μM BBR, 10 μM DCHC and 5 μM BBR plus 1 μM EX- 527. Data was analyzed as percentage of untreated cells taken as 100% (n=5 experiments *p<0.05 **p<0.01 versus Ctl, ## p<0.01 versus 25 mM or FFAs, § p<0.05 versus 25 mM+BBR or FFAs+BBR, + p<0.05 versus 25 mM or FFAs). (C and D) Cytochrome c Oxidase activity measured polarographically in cells after 96 h of treatment with hyperglycemia (25 mM) (C) or fatty acids (FFAs) (D) and exposed to 5 μM BBR, 10 μM DCHC and 5 μM BBR plus 1 μM EX- 527. Data is expressed in nAtomsO/min/mg protein. (n=4 experiments *p<0.05 **p<0.01 versus Ctl, # p<0.05 versus 25 mM or FFAs, § p<0.05 §§ p<0.01 versus 25 mM+BBR or FFAs+BBR, + p<0.05 versus 25 mM or FFAs). (E and F) Citrate Synthase activity measured spectrophotometrically in cells after 96 h of treatment with hyperglycemia (25 mM) (E) or fatty acids (FFAs) (F) and exposed to 5 μM BBR, 10 μM DCHC and 5 μM BBR plus 1 μM EX- 527. Data is expressed in nAtomsO/min/mg protein. (n=4 experiments ***p<0.001 *p<0.05 versus Ctl, # p<0.05 versus 25 mM or FFAs, §§ p<0.01 § p<0.05 versus 25 mM+BBR or FFAs+BBR, + p<0.05 versus 25 mM or FFAs). All data represent mean±SEM.
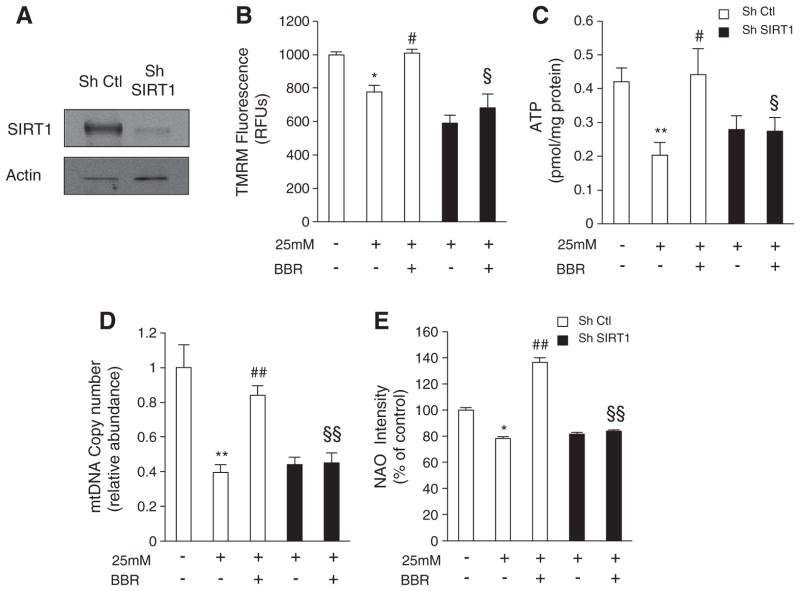
Similar to BBR, DCHC, a SIRT1 activator (Fig. 3A–D), also prevented the loss of mitochondrial function caused by high glucose or FFAs. Moreover, incubation of C2C12 cells with both BBR and EX-527 (a known inhibitor of SIRT1), completely blocked BBR-mediated prevention of mitochondrial dysfunction induced by both high glucose and FFAs (Fig. 3A–D). This indicates that the beneficial effects of BBR on mitochondrial function are at least partly mediated through SIRT1. To confirm this, C2C12 SIRT1 knockdown cells were generated (Fig. 4A). Importantly, these cells were unable to differentiate into myotubes, and consequently they were used in the myoblast form. Mitochondrial membrane potential and ATP content were decreased in both control and SIRT1 knockdown cells exposed to high glucose (Fig. 4B and C). Again, BBR protected against mitochondrial dysfunction in the control cells. However, in the SIRT1 knockdown cells BBR was unable to protect from hyperglycemia-induced mitochondrial damage (Fig. 4B and C).
Fig. 4.
Berberine rescues mitochondrial dysfunction and mitochondrial biogenesis induced by hyperglycemia in a SIRT1-dependent manner in C2C12 myoblasts. (A) Representative Immunoblot for SIRT1 and Actin in C2C12 cells infected with SIRT1 Sh RNA (Sh SIRT1) or non targeting Sh RNA (Sh Ctl). (B and C) Mitochondrial membrane potential measured with TMRM fluorescent probe (B) and ATP content (C) measured in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (Sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. (n=5 experiments *p<0.05 **p<0.01 versus Ctl, # p<0.05 versus 25 mM, § p<0.05 versus 25 mM+BBR). (D) Mitochondrial DNA amount analyzed by means of quantitative PCR in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. Relative units are expressed in comparison to untreated cells taken as 1.0. (n=5 experiments **p<0.01 versus Ctl, ## p<0.01 versus 25 mM, §§ p<0.01 versus 25 mM+BBR). (E) Mitochondrial mass measured by quantification of NAO intensity in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. Data is expressed as percentage of untreated cells taken as 100% (n=5 experiments *p<0.05 versus Ctl, ## p<0.01 versus 25 mM, §§ p<0.01 versus 25 mM+BBR). All data represent mean±SEM.
3.5. Mitochondrial biogenesis is increased by BBR in a SIRT1-dependent-manner
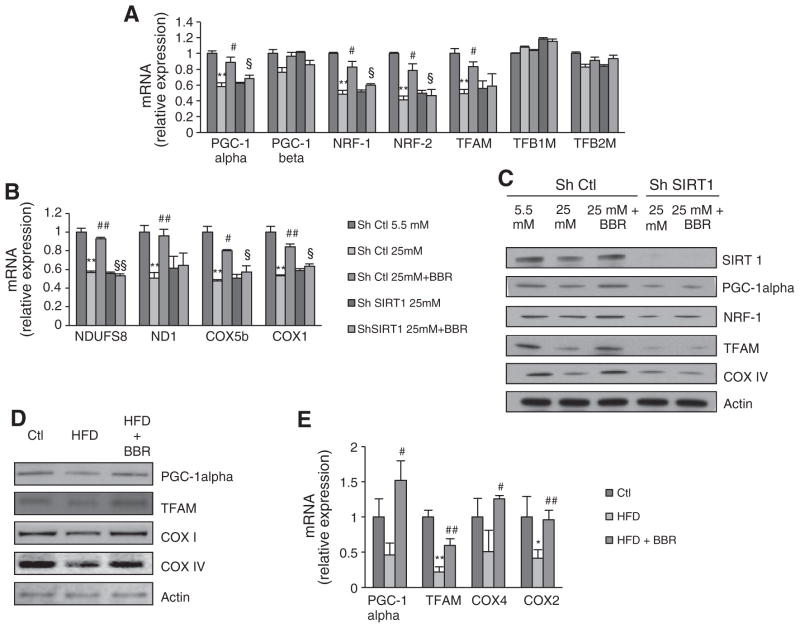
Hyperglycemia has been reported to induce mitochondrial dysfunction by decreasing mitochondrial biogenesis [8,47]. Moreover, recent studies report that lipid overload in skeletal muscle may promote mitochondrial dysfunction by supplying an excessive amount of reducing equivalents to the ETC-complexes, resulting in their inhibition [35,48]. Therefore, increasing mitochondrial biogenesis may constitute an important approach in preventing FFA-induced mitochondrial dysfunction. The increase in citrate synthase activity in the in vivo study suggested that BBR treatment increased mitochondrial biogenesis (Fig. 2H). Furthermore, citrate synthase activity in C2C12 myotubes was decreased by both hyperglycemia and FFAs while both BBR and DCHC prevented this decrease (Fig. 3E and F). Interestingly, the beneficial effects of BBR were again blocked by treatment with EX-527 (Fig. 3E and F). To address if BBR indeed increases mitochondrial biogenesis in a SIRT1-dependent manner, we examined several parameters of mitochondrial biogenesis in SIRT1 knockdown and control cells. BBR prevented the decrease in mitochondrial DNA content (Fig. 4D) and mitochondrial mass (Fig. 4E) induced by hyperglycemia in control cells. However, BBR did not affect mitochondrial DNA content (Fig. 4D) nor mitochondrial mass (Fig. 4E) in SIRT1 knockdown cells. In control cells, BBR prevented hyperglycemia-induced decrease in the expression of several genes that regulate mitochondrial biogenesis, such as PGC-1alpha, NRF-1, NRF-2 and TFAM (Fig. 5A), as well as nuclear-encoded (NDUFS8 and COX5b) and mitochondrial-encoded mitochondrial genes (ND1 and COX1) (Fig. 5B). However, in the SIRT1 knockdown cells, BBR did not show any effect on the aforementioned genes. These results were also confirmed at the protein level (Fig. 5C). To assess the effects of BBR on mitochondrial biogenesis in vivo, the expression and protein level of mitochondrial biogenesis regulators such as PGC-1alpha and TFAM and mitochondrial genes COX1, COX2, and COX IV were analyzed. HFD feeding decreased the expression of these genes, and in all cases, this was rescued by BBR treatment (Fig. 5D and E).
Fig. 5.
Berberine regulates mitochondrial biogenesis in a SIRT1-dependent manner in C2C12 myoblasts and also in skeletal muscle of Sprague–Dawley Rats. (A) PGC-1alpha, PGC-1beta, NRF-1, NRF-2, TFAM, TFB1M and TFB2M mRNA analyzed by means of quantitative RT-PCR in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. Relative expression values of the untreated cells were taken as 1.0. (n=4 experiments **p<0.01 versus Ctl, # p<0.05 versus 25 mM, § p<0.05 versus 25 mM+BBR). (B) NDUFS8, ND1, COX5b, COX1 mRNA analyzed by means of quantitative RT-PCR in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. Relative expression values of the untreated cells were taken as 1.0. (n=4 experiments **p<0.01 versus Ctl, # p<0.05 versus 25 mM, § p<0.05 versus 25 mM+BBR). (C) Representative Immunoblot for SIRT1, PGC-1alpha, NRF-1, TFAM, COXIV and Actin in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and 5 μM BBR. (D) Representative immunoblot for PGC-1alpha, TFAM, COXI, COXIV and Actin in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR). (E) PGC-1alpha, TFAM, COX4, COX2 mRNA analyzed by means of RT-PCR in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR). (n=4 experiments *p<0.05 **p<0.01 versus Ctl, # p<0.05 ## p<0.01 versus HFD). All data represent mean±SEM.
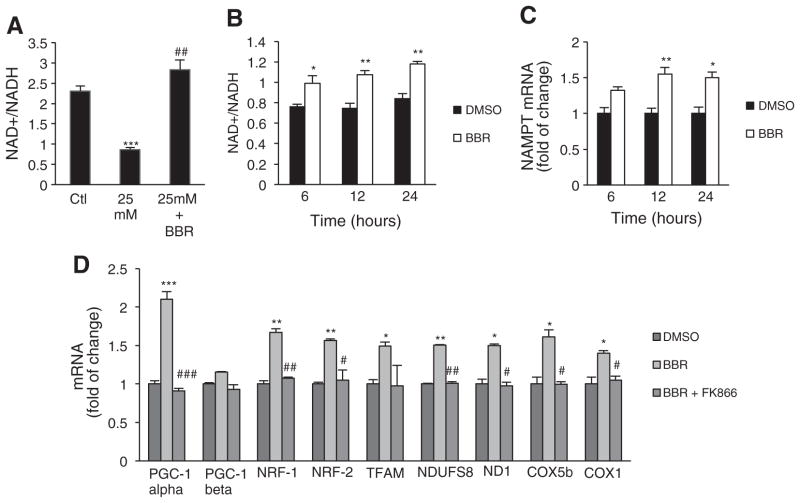
3.6. BBR increases the NAD+/NADH ratio
After observing that the effects of BBR on mitochondrial function and biogenesis were dependent on SIRT1, we sought to elucidate the mechanism by which BBR activates SIRT1. BBR did not directly activate SIRT1 as evaluated with an in vitro activity assay (data not shown), indicating that it likely does not function in the same manner as the commonly used Sirtuin Activating Compounds (STACs) [49]. Given that SIRT1 is known to be regulated by NAD+ and the NAD+/NADH ratio, we measured their abundance in cells treated with BBR. Interestingly, BBR prevented the decrease in the NAD+/NADH ratio caused by hyperglycemia (Fig. 6A) in C2C12 myotubes. To explain the increase in NAD+/NADH induced by BBR, we evaluated the expression of nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting factor in NAD+ biosynthesis. A decrease in NAMPT expression and consequent depletion of cellular NAD+ has been shown as a mechanism linking high glucose and FFAs with SIRT1 downregulation [50]. Therefore, we tested whether BBR modulates NAMPT expression in C2C12 cells. Gene expression of NAMPT markedly increased after 6 h of BBR exposure (Fig. 6C), which was paralleled by an increase in the NAD+/NADH ratio (Fig. 6B). Moreover, FK866, an inhibitor of NAMPT, blocked the induction of genes that regulate mitochondrial biogenesis and also in nuclear-encoded and mitochondrial-encoded mitochondrial genes by BBR (Fig. 6D).
Fig. 6.
Berberine increases NAD+/NADH ratio through induction of NAMPT expression in C2C12 cells. (A) NAD+/NADH ratio measured spectophotometricaly in C2C12 myotubes treated for 96 h with hyperglycemia and exposed to 5 μM BBR (n=4 experiments ***p<0.001 Ctl, ## p<0.01 versus 25 mM). (B) NAD+/NADH ratio measured spectophotometricaly in C2C12 myoblasts treated for 6 h, 12 h and 24 h with 5 μM BBR (n=4 experiments *p<0.05 **p<0.01 versus Ctl). (C) NAMPT mRNA analyzed by means of quantitative RT-PCR in C2C12 cells after 6 h, 12 h and 24 h of treatment with 5 μM BBR. Relative expression values of the untreated cells were taken as 1.0. (n=4 experiments *p<0.05 **p<0.01 versus Ctl). (D) PGC-1alpha, PGC1-beta, NRF-1, NRF-2, TFAM, NDUFS8, ND1, COX5b, COX1 mRNA analyzed by means of quantitative RT-PCR in C2C12 cells after 12 h of treatment with 5 μM BBR or 5 μM BBR+10 nM FK866. Relative expression values of the untreated cells were taken as 1.0. (n=4 experiments *p<0.05 **p<0.01 ***p<0.001 versus Ctl, # p<0.05 ## p<0.01 ### p<0.001 versus BBR). All data represent mean±SEM.
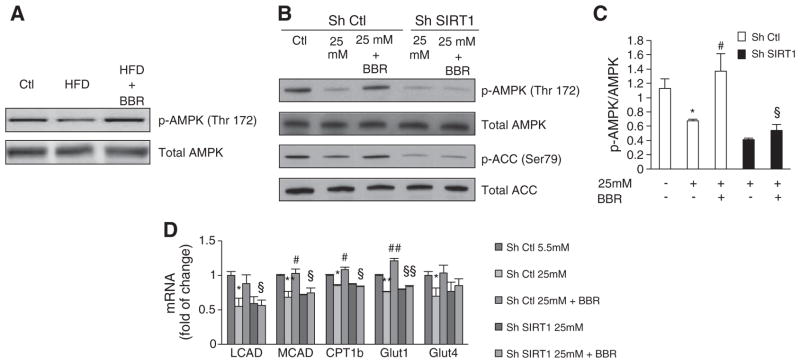
3.7. SIRT1 is involved in AMPK phosphorylation induced by BBR
BBR is known to activate AMPK [25,29] but the precise mechanism by which BBR activates AMPK however is not well established. SIRT1 is known to regulate AMPK through deacetylation and activation of LKB1 [32]. Thus, we aimed to address in our models if BBR activates AMPK, and if this activation is SIRT1-dependent. As described previously, BBR induces AMPK activation in vivo (Fig. 7A). In C2C12 control cells, BBR rescued the decrease in AMPK phosphorylation (Thr172) induced by hyperglycemia (Fig. 7B and C), as well as the decrease in ACC phosphorylation (Ser79) (a direct target of AMPK activity) (Fig. 7B). However, these effects were not observed in SIRT1 knockdown cells (Fig. 7B and C). Moreover, genes involved in fatty acid oxidation (LCAD, MCAD, CPT1b) that are targets of AMPK, as well as glucose uptake genes (GLUT1 and GLUT4), were also upregulated by BBR in a SIRT1-dependent manner (Fig. 7D).
Fig. 7.
Berberine activates AMPK in skeletal muscle of Sprague–Dawley Rats and in C2C12 myoblasts in a SIRT1-dependent way. (A) Representative Immunoblot for total AMPK, p-AMPK (Thr172), total ACC and p-ACC (Ser79) in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and to 5 μM BBR. (B) Quantification of AMPK activation by Berberine in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and to 5 μM BBR. (n=4 experiments *p<0.05 versus Ctl, # p<0.05 versus 25 mM, § p<0.05 versus 25 mM+BBR. (C) AMPK activity accessed by immunoblot in skeletal muscle of rats fed with Chow diet (Ctl), High Fat Diet (HFD) and High Fat Diet supplemented with BBR (HFD+BRR). A representative immunoblot is shown for total AMPK and p-AMPK(Thr172). (D) LCAD, MCAD and CPT1b mRNA analyzed by means of quantitative RT-PCR in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 96 h of treatment with hyperglycemia (25 mM) and to 5 μM BBR. Relative expression values of the untreated cells were taken as 1.0. (n=4 experiments *p<0.05 **p<0.01 versus Ctl, # p<0.05 versus 25 mM, § p<0.05 versus 25 mM+BBR). All data represent mean±SEM.
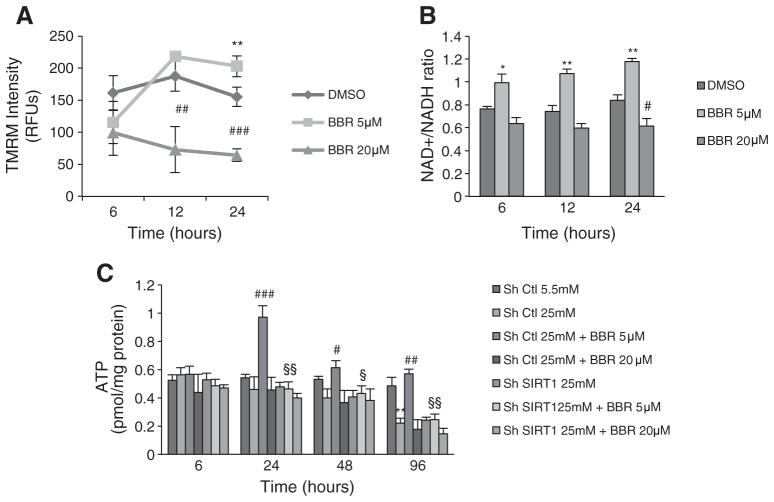
Since a dose and time-dependent mechanism for AMPK activation by BBR, dependent on superoxide-generation has been shown [45], we compared the effects of 5 and 20 μM BBR on C2C12 cells, at 6, 12 and 24 h. While at 5 μM, BBR increased mitochondrial membrane potential, 20 μM BBR decreased this parameter at all time points (Fig. 8A). This was associated with a decrease in ATP content elicited by 20 μM BBR, that was not affected by SIRT1 knockdown (Fig. 8C). This effect is consistent with published data showing inhibition of mitochondrial oxygen consumption by this range of BBR concentrations. Opposingly, 5 μM BBR increased NAD+/NADH ratio as early as 6 h incubation (Fig. 8B), which led to an increase in ATP content only after 12 h of incubation with BBR (Fig. 8C). This data further supports a dose-dependent, indirect but positive action of BBR on mitochondria that is primarily mediated by SIRT1 activation.
Fig. 8.
Different concentrations of Berberine have different effects in mitochondrial function in C2C12. (A) Mitochondrial membrane potential measured with TMRM fluorescent probe (B) and NAD+/NADH ratio measured spectophotometricaly in C2C12 cells after 6 h, 12 h and 24 h of treatment with 5 μM and 20 μM BBR. (n=4 experiments *p<0.05 **p<0.01 versus DMSO, # p<0.05 ## p<0.01 ### p<0.001 versus DMSO). (C) ATP content measured in C2C12 cells infected with SIRT1 shRNA (Sh SIRT1) or nontargeting shRNA (sh Ctl) after 6 h, 24 h, 48 h and 96 h of treatment with hyperglycemia (25 mM), 5 μM and 20 μM BBR. (n=4 experiments **p<0.01 versus Ctl, # p<0.05 ## p<0.01 ### p<0.001 versus 25 mM, § p<0.05 §§ p<0.01 versus 25 mM+BBR). All data represent mean±SEM.
4. Discussion
In the present study we have demonstrated that oral supplementation of BBR (100 mg/kg/day) rescues key features of the metabolic syndrome, including insulin resistance and hyperleptinemia. This is in agreement with several other studies that have demonstrated a role for BBR in the protection against HFD-induced insulin resistance and diabetes [25,30,46]. Notably, BBR not only acts in the insulin resistant tissues, but has also been shown to protect pancreatic beta cells function in high caloric and streptozotocin-induce diabetic models [51–53]. Several studies have shown that BBR needs to be administrated in high doses (380–560 mg/kg/day) in order to have a beneficial effect [25,27]. However, our data and a recent study [46] show that BBR can protect from HFD feeding at a much lower dose than previously described (100 mg/kg/day).
Here, we show for the first time, that the beneficial effects of BBR are accompanied by an increase in mitochondrial function and biogenesis in skeletal muscle. Our results also confirm these findings in a cell-based model. It is important to note that short term treatments with BBR were previously reported to inhibit ETC complex I [27], decrease ATP content in hepatocytes [29] and cause mitochondrial fragmentation, depolarization, and oxidative stress in K1735-M2 cells when used at concentrations 2–5 times higher than the concentrations used in this study [54]. Moreover, when BBR is incubated with isolated liver mitochondria, mitochondrial respiration is inhibited and mitochondrial permeability transition is induced [55]. Therefore, while our findings demonstrate improved mitochondrial function by BBR, they do not rule out the possibility of toxic effects of BBR on mitochondria under different conditions or at higher concentrations.
PGC-1alpha and some of its targets, such as NRF-1, NRF-2 and TFAM, are known to be the main regulators of the mitochondrial biogenesis pathway [56] and ultimately regulate mitochondrial function and fiber-type switch in skeletal muscle [57]. Our results show that BBR rescues skeletal muscle from mitochondrial dysfunction caused by HFD feeding, as well as from hyperglycemia and fatty acid exposure. Our data strongly indicate that this effect is due to enhanced mitochondrial biogenesis through the PGC-1alpha signaling pathway. Furthermore, our results show that the beneficial effects of BBR on mitochondrial biogenesis and function are dependent on the presence of SIRT1, pointing to an essential role for SIRT1 in the molecular pathway mediating the effects of BBR.
A recent study by Xia and colleagues showed that in hepatocytes, short term treatment with BBR results in ATP depletion and that results in AMPK activation [30]. Despite the apparent contradiction, we hypothesize that short term treatments with higher doses of BBR induce mitochondrial toxicity and ATP depletion, while a long term, lower dose results in a SIRT1-dependent AMPK activation and increased mitochondrial biogenesis. This culminates in the prevention and reversion of mitochondrial dysfunction induced by HFD, hyperglycemia and fatty acid exposure. The ability of BBR to activate AMPK has been extensively studied [25,29,58]. Nevertheless, the exact mechanism by which this occurs remains elusive. It was previously reported that BBR inhibits ETC complex I, thereby increasing the AMP/ATP ratio and thus activating AMPK [27]. Furthermore it was recently showed that mitochondrial-derived superoxide and peroxynitrite are required for BBR-induced AMPK activation in endothelial cells [47].
In this work, we demonstrate another mechanism by which BBR is able to counteract the negative effects of excess nutrients on metabolic homeostasis. In this case, AMPK activation is not a primary target but probably a consequence of SIRT1 activation by BBR. This model explains why BBR is able to improve mitochondrial and insulin sensitivity in animals previously fed a high fat diet, and therefore exhibiting mitochondrial damage with impaired ATP formation, prior to BBR treatment. Therefore, under the conditions of our study inhibition of ATP synthesis by BBR probably is not the main trigger for AMPK activation and improvement of mitochondrial function. This is supported by assays with C2C12 cells exposed to 5 or 20 μM BBR for 6, 12 or 24 h. Indeed, while 20 μM BBR decreases mitochondrial membrane potential and ATP content, 5 μM BBR has the opposite effect being dependent on the formation of NAD+ associated with increased NAMPT expression and SIRT1 activation. These observations provide evidence that SIRT1 plays a pivotal role in the cellular effects of BBR but does not rule out the involvement of AMPK. Since SIRT1 regulates LKB1 through deacetylation [32], a necessary step for AMPK activation, SIRT1 activation by BBR may lead to a secondary activation of AMPK. SIRT1 silencing blocks AMPK (Thr172) and ACC (Ser67) phosphorylation. Our results show that gene silencing of SIRT1 in skeletal muscle cells prevented BBR-induced AMPK activation as well as ACC (Ser67) phosphorylation. Further investigation will be needed to sort out the interplay between LKB1, AMPK, and SIRT1 in mediating the effects of BBR. However, recent work has shown that the hypoglycemic action of BBR can be attributed to its acute activation of the transport activity of GLUT1, an effect independent of AMPK activation [59]. Thus, the beneficial effects of BBR on metabolic abnormalities may involve mechanisms that are yet to be elucidated.
5. Conclusions
In summary, we provided new evidence that low concentrations of BBR greatly improve mitochondrial function in both animal and cell-based models of insulin resistance and diabetes. We report for the first time that these beneficial effects involve the stimulation of mitochondrial biogenesis via a SIRT1-mediated mechanism, thereby protecting against the deleterious effects of HFD feeding, hyperglycemia and fatty acids. We propose that BBR may be a natural therapeutic agent useful in the treatment of type 2 diabetes, obesity and the metabolic syndrome.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Portuguese Foundation for Science and Technology (PTDC/SAU-OSM/72443/2006). A.P.G, F.V.D, J.S.T and A.T.V are recipients of individual fellowships from the Portuguese Foundation for Science and Technology (SFRH/BD/44674/2008, SFRH/BD/38372/2007, SFRH/BD/38467/2007 and SFRH/BD/44796/2008 respectively). B.P.H. was supported by an NSERC PGS-D fellowship. D.A.S is an Ellison Medical Foundation senior scholar and is supported by The Paul F. Glenn Foundation for medical research and a grant from NIH/NIA (ROI AG 028730).
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10. 1016/j.bbadis.2011.10.008.
Footnotes
Conflict of interest declaration
D.A.S. is a consultant for Sirtris, a GSK company developing sirtuin-based medicines. No other potential conflicts of interest were reported.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Hegarty BD, Furler SM, Ye J, Cooney G, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–383. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- 4.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 5.Krebs M, Roden M. Nutrient-induced resistance in human skeletal muscle. Curr Med Chem. 2004;11:901–908. doi: 10.2174/0929867043455620. [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR. Role of adiponectin in human skeletal muscle biogenesis. Cell Metab. 2006;4:75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal mucle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 10.Mootha VK, Lindfren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamaro P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 11.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92:1467–1473. doi: 10.1210/jc.2006-2210. [DOI] [PubMed] [Google Scholar]
- 13.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset HJ. Mitochondrial dysfunction results from oxidative stress in skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y, Hamaguchi S, Sobirin MA, Ono T, Suga T, Kuroda S, Tanaka S, Terasaki F, Okita K, Tsutsui H. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;297:H1069–H1077. doi: 10.1152/ajpheart.00267.2009. [DOI] [PubMed] [Google Scholar]
- 15.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kin SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haigis MC, Sinclair DA. Mammalian sirtuins: biological inights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodgers JT, Lerin C, Haas W, Gygi SP, Speigelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 19.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliot P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kin JBL. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 23.Bova S, Padrini R, Goldman WF, Berman DM, Cargnelli G. On the mechanism of vasodilating action of berberine: possible role of inositiol lipid signaling system. J Pharmacol Exp Ther. 1992;261:318–323. [PubMed] [Google Scholar]
- 24.Choi MS, Yuk DY, Oh JH, Jung HY, Han SB, Moon DC, Hon JT. Berberine inhibits human neuroblastoma cell growth through induction of p53-dependent apoptosis. Anticancer Res. 2008;28:3777–3784. [PubMed] [Google Scholar]
- 25.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 26.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: and additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–1288. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 28.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, Oh GT, Park HS, Lee KU, Lane MD, Kim JB. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–E819. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JA, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Xia X, Yan J, Shen J, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One. 2011;6:e16556. doi: 10.1371/journal.pone.0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan F, Cacicedo JM, Riderman N, Ido Y. SIRT1 modulation of the acetylation satus, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti L, Massillon D, Barzilai N, Vuguin P, Chen W, Hawkins M, Wu J, Wang J. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272:27758–27763. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 34.Jones JG, Fagulha A, Barosa C, Bastos M, Barros L, Baptista C, Caldeira MM, Carvalheiro M. Noninvasive analysis of hepatic glycogen kinetics before and after breakfast with deuterated water and acetaminophen. Diabetes. 2006;55:2294–2300. doi: 10.2337/db06-0304. [DOI] [PubMed] [Google Scholar]
- 35.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JRB, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver muscle and culture fibroblasts. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 37.Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Bile acids effect liver mitochondrial bioenergetics: possible relevance for cholestasis therapy. Toxicol Sci. 2000;57:177–185. doi: 10.1093/toxsci/57.1.177. [DOI] [PubMed] [Google Scholar]
- 38.Brautigan DL, Ferguson-Miller S, Margoliash E. Mitochondrial cytochrome c: preparation and activity of native and chemically modified cytochromes c. Methods Enzymol. 1978;53:128–133. doi: 10.1016/s0076-6879(78)53021-8. [DOI] [PubMed] [Google Scholar]
- 39.Singer TP. Determination of the activity of succinate, NADH, choline and glycerophosphate dehydrogenases. Biochim Biophys Acta. 1994;1363:100–124. [Google Scholar]
- 40.Varela AT, Gomes AP, Simões AM, Teodoro JS, Duarte FV, Rolo AP, Palmeira CM. Indirubin-3′-oxime impairs mitochondrial oxidative phosphorylation and prevents mitochondrial permeability transition induction. Toxicol Appl Pharmacol. 2008;233:179–185. doi: 10.1016/j.taap.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Rolo AP, Palmeira CM, Wallace KB. Mitochondrially mediated synergistic cell killing by bile acids. Biochim Biophys Acta. 2003;1637:127–132. doi: 10.1016/s0925-4439(02)00224-7. [DOI] [PubMed] [Google Scholar]
- 42.Srere PA, Brazil H, Gonen L. The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand. 1963;17:S129–S134. [Google Scholar]
- 43.Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144:166–171. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- 44.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2000;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 45.Han Y, Wang Q, Song P, Zhu Y, Zou MH. Redox regulation of AMP-activated protein kinase. PLoS One. 2010;5:e15420. doi: 10.1371/journal.pone.0015420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen C, Zhang Y, Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397:543–547. doi: 10.1016/j.bbrc.2010.05.153. [DOI] [PubMed] [Google Scholar]
- 47.Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol. 2007;225:214–220. doi: 10.1016/j.taap.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 49.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Dish JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2011;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang ZQ, Lu FE, Leng SH, Fang XS, Chen G, Wang ZS, Dong LP, Yan ZQ. Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity. World J Gastroenterol. 2008;14:6004–6011. doi: 10.3748/wjg.14.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu SS, Yu YL, Zhu HJ, Liu XD, Liu L, Liu YW, Wang P, Xie L, Wang GJ. Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol. 2009;200:159–165. doi: 10.1677/JOE-08-0419. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Zhou S, Tang J, Zhang K, Guang L, Haung Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin-and high carbohydrate/high-fat diet-induced diabetic rats. Euro J Pharmacol. 2009;606:262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 54.Pereira GC, Branco AF, Matos JA, Pereira SL, Parke D, Perkins EL, Serafim TL, Sardão VA, Santos MS, Moreno AJ, Holy J, Oliveira PJ. Mitochondrially targeted effects of berberine [Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] on K1735-M2 mouse melanoma cells: comparison with direct effects on isolated mitochondrial fractions. J Pharmacol Exp Ther. 2007;323:636–649. doi: 10.1124/jpet.107.128017. [DOI] [PubMed] [Google Scholar]
- 55.Pereira CV, Machado NG, Oliveira PJ. Mechanisms of berberine (natural yello 18)-induced mitochondrial dysfunction: interaction with the adenine nucleotide translocator. Toxicol Sci. 2008;105:408–417. doi: 10.1093/toxsci/kfn131. [DOI] [PubMed] [Google Scholar]
- 56.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 57.Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1alpha regulates HIF2alpha-dependent switch in skeletal muscle fiber type. Proc Natl Acad Sci U S A. 2010;107:21866–21871. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMPK-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010;110:697–705. doi: 10.1002/jcb.22580. [DOI] [PubMed] [Google Scholar]
- 59.Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187–1192. doi: 10.1016/j.biochi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.