Abstract
The 22q13.3 deletion syndrome, also known as Phelan-McDermid syndrome, is a contiguous gene disorder resulting from deletion of the distal long arm of chromosome 22. In addition to normal growth and a constellation of minor dysmorphic features, this syndrome is characterized by neurological deficits which include global developmental delay, moderate to severe intellectual impairment, absent or severely delayed speech, and neonatal hypotonia. In addition, more than 50% of patients show autism or autistic-like behavior, and therefore it can be classified as a syndromic form of autism spectrum disorders (ASD). The differential diagnosis includes Angelman syndrome, velocardiofacial syndrome, fragile X syndrome, and FG syndrome. Over 600 cases of 22q13.3 deletion syndrome have been documented. Most are terminal deletions of ∼100 kb to >9 Mb, resulting from simple deletions, ring chromosomes, and unbalanced translocations. Almost all of these deletions include the gene SHANK3 which encodes a scaffold protein in the postsynaptic densities of excitatory synapses, connecting membrane-bound receptors to the actin cytoskeleton. Two mouse knockout models and cell culture experiments show that SHANK3 is involved in the structure and function of synapses and support the hypothesis that the majority of 22q13.3 deletion syndrome neurological defects are due to haploinsufficiency of SHANK3, although other genes in the region may also play a role in the syndrome. The molecular connection to ASD suggests that potential future treatments may involve modulation of metabotropic glutamate receptors.
Key Words: 22q13.3 deletion syndrome, Autism spectrum disorders, SHANK3
Introduction
The 22q13.3 deletion syndrome (Phelan-McDermid syndrome) typically results from the loss of the distal long arm of chromosome 22. The loss of material may result from a terminal or interstitial deletion of chromosome 22, an unbalanced translocation that may be inherited or de novo, or from other structural rearrangements involving chromosome 22. The abnormalities may be cryptic and virtually always involve haploinsufficiency for the SHANK3 gene. Major features of the syndrome include neonatal hypotonia, moderate to severe intellectual impairment, severe or absent expressive language delay, and normal growth. Common facial characteristics include dolicocephaly, flat midface, wide brow, wide nasal bridge, deep-set eyes, full cheeks, puffy eyelids, long eyelashes, and bulbous nose. Large fleshy hands, dysplastic toenails, sacral dimple, and large poorly formed ears are frequently observed. Behavior is autistic-like with impaired communication, reduced social interaction, poor eye contact, anxiety, and self-stimulatory conduct. This report summarizes many aspects of the clinical and genetic facets of the 22q13.3 deletion syndrome.
History of 22q13.3 Deletion Syndrome
The first case of pure monosomy for the distal long arm of chromosome 22 was reported by Watt et al. [1985] in a 14-year-old male with profound intellectual disability, absent speech, minor dysmorphic features, and normal tone. Meiotic recombination of a maternal pericentric inversion resulted in the loss of 22q12 to the 22q terminus. Herman et al. [1988] reported a terminal deletion of chromosome 22q13.31 in a 16-month-old male with features of Goldenhar complex. Phelan et al. [1988] described hypotonia associated with a de novo deletion of 22q13.3 in a newborn male who subsequently demonstrated global developmental delay, diffuse hypotonia, normal growth, and minor dysmorphic features. Biochemical studies revealed a deficiency of arylsulfatase A (ARSA) associated with deletion of one chromosome 22 and a pseudodeficiency allele on the homologous 22. Molecular studies refined the breakpoint to 22q13.31 and demonstrated that the deletion was on the paternal chromosome 22 [Phelan et al., 1992].
Intellectual impairment, hypotonia, normal to accelerated growth, absent to severely delayed speech, and minor dysmorphic features emerged as common characteristics of individuals with deletion of 22q13.3 [Watt et al., 1985; Kirshenbaum et al., 1988; Romain et al., 1990; Zwaigenbaum et al., 1990; Narahara et al., 1992]. Nesslinger et al. [1994] first suggested that this was the recognizable phenotype of deletion 22q13.3, and they narrowed the critical region of overlap from a proximal breakpoint below D22S97 to a region distal to ARSA, a distance of less than 25.5 cM.
The following year, Flint et al. [1995] reported telomere studies in a group of 99 individuals with varying degrees of idiopathic intellectual disability. They identified 3 individuals with subtelomeric deletions, 2 of which involved chromosome 22. One of these individuals had a de novo cryptic unbalanced translocation between the telomere of 9q and the telomere of 22q. The other individual had a 130-kb deletion of 22q13.3 involving only the most distal locus tested, D22S163. His phenotype was less severe than previously reported cases, with mild intellectual impairment and expressive speech delay. This report was seminal in emphasizing the importance of studying the ends of the chromosomes for cryptic changes and in defining deletion of 22q13 as a significant cause of intellectual impairment.
Cryptic deletions of 22q13.3 resulting from malsegregation of maternal translocations were reported by Smith et al. [1996] and Doheny et al. [1997]. Doheny et al. [1997] also reported a de novo cryptic deletion. Yong et al. [1997] reported the first case of mosaicism for deletion 22q13.2 in a 5-year-old female with global developmental delay, failure to thrive, seizures, dysmorphic features, and abnormal skin pigmentation. Riegel et al. [2000] reported the prenatal detection of a mosaic deletion 22q13 in a fetus with a cystic ‘tumor’ of the neck.
Slavotinek et al. [1997] reported mother-to-son transmission of deletion 22q13.3. The mother had a direct insertion of a segment of the long arm of chromosome 7 (7q21.2–q22.1) into chromosome 22 at 22q13.3 resulting in a submicroscopic deletion of chromosome 22. She had a history of learning problems and delayed speech. Her son, who inherited the abnormal chromosome 22, had global developmental delay, severely delayed speech, hypotonia, normal growth, and dysmorphic features. The son was also trisomic for a segment of chromosome 7.
Two cases of deletion 22q13 reported by Precht et al. [1998] were fortuitously detected by fluorescence in situ hybridization (FISH). The first individual had been referred to rule out Angelman syndrome. Her blood was inadvertently used as a control sample for a DiGeorge/velocardiofacial (VCF) syndrome study, and she was found to be deleted for the distal probe D22S39. The second individual was referred to rule out VCF and was deleted for the distal probe D22S75. Both deletions occurred on the paternal chromosome 22. Schroder et al. [1998] reported 3 unrelated individuals with bilateral hearing loss, possibly contributing to speech delay. Two of the 3 patients had renal abnormalities. Parental origin studies, conducted in 2 of the individuals, revealed that the deletion occurred on the paternal chromosome 22.
Three individuals with deletion 22q13.3, normal to advanced growth, developmental delay, and pervasive developmental disorders were reported by Prasad et al. [2000]. Several subsequent reports have linked autism spectrum disorders with deletion 22q13.3 [Goizet et al., 2000; Manning et al., 2004; Cusmano-Ozog et al., 2007].
In 2001, Phelan et al. [2001] compared the features of 37 individuals with deletion 22q13 to features of 24 cases previously described in the literature. The most frequently observed features in their 37 cases were global developmental delay (100%), absent/severely delayed speech (100%), hypotonia (97%), and normal to accelerated growth (95%). These were also the most common features observed in the 24 cases from the literature. Of interest, 17 of 18 individuals evaluated by the Child Autism Rating Scale scored in the autistic range (12 in the moderate to severe range and 5 in the mild range). Difficulty in diagnosing the deletion by chromosome analysis and/or FISH was demonstrated by the fact that 32% of individuals had previous chromosome studies that failed to detect the deletion. These included 3 deletions that were not detected by prenatal chromosome studies.
In 2001, Bonaglia et al. [2001] described mild intellectual impairment, severe expressive language delay, hypotonia, joint laxity, and minor facial dysmorphism in a 4.5-year-old male with a de novo translocation between 12q24.1 and 22q13. Although the translocation was balanced with no apparent loss of genetic material, the breakpoint on chromosome 22 was within the SHANK3 locus, suggesting that disruption of SHANK3 may be responsible for at least some of the phenotypic features of 22q13.3 deletion syndrome.
In 2003, 2 groups identified SHANK3 as the most likely candidate gene for the neurological impairments (speech delay and intellectual disability) in individuals with 22q13.3 deletion syndrome. Luciani et al. [2003] studied 33 individuals, including 17 with ring chromosome 22, with deletion size ranging from 160 kb to 9 Mb and refined the critical region to include SHANK3, ACR, and RABL2. They found 74% of the deletions were on the paternal chromosome 22. Wilson et al. [2003] reported a larger series of 56 individuals with deletion size ranging from 130 kb to over 9 Mb. Similarly, they concluded that SHANK3, ACR, and RABL2 were in the critical region. The paternal chromosome 22 was deleted in 70% of cases. The authors recommended screening for SHANK3 mutations in individuals with idiopathic intellectual disability and suggested that other members of the SHANK3 family of proteins or proteins interacting with SHANK3 in the postsynaptic density may be candidate genes for neurological impairments.
The high percentage of deletions of paternal origin is consistent with findings in many terminal deletion syndromes including Wolf-Hirschhorn syndrome (deletion 4p) [Zollino et al., 2008], cri-du-chat syndrome (deletion 5p) [Mainardi et al., 2001], 9p deletion syndrome [Micale et al., 1995], 18q deletion syndrome [Cody et al., 1997], and others [Thomas et al., 2006]. This phenomenon has generally been attributed to greater susceptibility of male germ cells to chromosome breakage related to the increased number of cell divisions when compared to female germ cells.
Bisgaard et al. [2009] described a male with deletion of 22q13.2 resulting from a de novo t(13;22) and a pathogenic mutation of the arylsulfatase A (ARSA) gene on the non-deleted chromosome 22. ARSA deficiency due to deletion of one copy and mutation of the other resulted in the autosomal recessive disease metachromatic leukodystrophy. A second child with deletion of 22q13 and a pseudeficiency allele for ARSA did not have the disease.
Uncommon clinical complications in individuals with 22q13.3 deletion syndrome have been reported; it is uncertain if these are coincidental or rare associations for which individuals should be carefully monitored. One case of central diabetes insipidus was diagnosed in a 2-day-old female with deletion 22q13.31 [Barakat et al., 2004]. The diabetes resolved by 27 months of age. Sathyamoorthi et al. [2009] described an atypical teratoid/rhabdoid tumor in a 2-year-old female with a 7.2-Mb deletion of 22q13.2–q13.33. The child died at 26 months of age. Study of frozen tissue from the tumor demonstrated loss of the deleted chromosome and an acquired mutation of the INI1 gene, a tumor suppressor gene located on 22q11.2. The authors report a second case of atypical teratoid/rhabdoid tumor which was not included in their case report due to lack of sufficient clinical and molecular testing.
Two cases of fulminant autoimmune hepatitis have been described. The first case was a 7-year-old female with a 1.5-Mb deletion of 22q13 who required a liver transplant to treat her disease [Tufano et al., 2009]. The second case was a 3-year-old female with a 5.7-Mb deletion of 22q13 who developed hyperacute autoimmune hepatitis triggered by a viral infection [Bartsch et al., 2010]. Following a liver transplant, marked improvement in social interaction, sequential planning, and imitation of complex movements was observed. The authors suggested that the SHANK3 gene product may play a role in autoimmunological response. The deleted region also includes the oncogene PIM3 which has previously been associated with liver disease in rats [Liu et al., 2010]. PIM3 encodes a serine/threonine protein kinase that is reported to be aberrantly expressed in human and mouse hepatitis but not normal liver. One role of PIM3 in rats seems to be the protection against fulminant hepatic liver failure. Haploinsufficiency for PIM3 may prevent or prolong recovery from hepatic injury [Bartsch et al., 2010]. At ∼775 kb proximal to SHANK3, PIM3 would be deleted in most 22q13.3 deletion patients.
Currently, over 600 cases of 22q13.3 deletion syndrome are known world-wide. Historically, this condition has been under-diagnosed due to the failure to recognize the clinical and behavioral phenotypes and the difficulty in detecting the deletion by available laboratory methods. Recent microarray techniques are capable of identifying microdeletions of 22q13.3 that would have been undetectable in the past. The wide range of deletion sizes from <100 kb to >9 Mb is accompanied by a broad spectrum of phenotypic variability. The association between autistic spectrum disorders (ASD) and deletion 22q13.3 warrants that 22q13.3 deletion syndrome be recognized as a syndromic form of autism.
Clinical Features
The 22q13.3 deletion syndrome is characterized by neonatal hypotonia, normal growth, absent or delayed speech, moderate to profound developmental delay, and minor dysmorphic features [Cusmano-Ozog et al., 2007; Dhar et al., 2010; Phelan and Betancur, 2011]. Neonatal hypotonia is the first presenting symptom and can contribute to poor feeding, speech difficulty, reduced reflexes, and delayed motor milestones. Hypotonia may persist in children and adults, leading to a lethargic or drowsy appearance, delayed or unstable gait, and muscle weakness. The poor muscle control and neurological impairment may cause individuals with deletion 22q13.3 to be misdiagnosed with cerebral palsy. Extensive data on the clinical features of deletion of 22q13 have been collected since 1998 through parent questionnaires and physical examinations conducted in conjunction with Phelan-McDermid Syndrome Support Group family meetings which are held biennially with the support of the Greenwood Genetic Center, South Carolina. Much of the information in this section is reflective of that data.
Infants with 22q13.3 deletion syndrome may babble at an appropriate age, and toddlers may possess a limited vocabulary until 3 or 4 years. At this age many children seem to lose the ability to speak, although through aggressive therapy and communication training they may regain and increase their vocabulary. Nonetheless, speech will remain impaired throughout life [Phelan et al., 2010].
In contrast to other autosomal chromosome abnormalities that are typically associated with growth deficiency, most individuals with 22q13.3 deletion syndrome are in the normal range for growth. Only about 10% of affected children are small for their age, and some show growth beyond the 95th percentile. Varying degrees of developmental delay are present. Motor milestones, such as sitting up, rolling over, crawling, and walking, generally occur at a later age than usual. The average age at rolling and sitting is 18 month. Walking is characterized by an unsteady gait and occurs at an average of 27 months, although some individuals remain non-ambulatory [Prasad et al., 2000]. Mild to profound intellectual impairment is typical.
The craniofacial features of 22q13.3 deletion syndrome are relatively subtle (fig. 1). The head tends to be long or dolichocephalic. Facial features include a wide brow, deep-set eyes, puffy eyelids, bulbous nose, and long thick eyelashes. The midface may be flat with a wide nasal bridge and puffy cheeks. The chin is often pointed and may become prominent with age. Ears are typically large and may be prominent or poorly formed. Less frequently observed features are strabismus, ptosis, epicanthal folds, high arched palate, and long philtrum [Cusmano-Ozog et al., 2007; Dhar et al., 2010; Phelan et al., 2010]. The subtle facial features and variability in facial manifestations, most likely associated with deletion size, makes it difficult to diagnose this syndrome based solely on the facial phenotype.
Fig. 1.
These patients with 22q13.3 deletion syndrome demonstrate the wide differences in facial phenotype that may make clinical diagnosis difficult. Many children will have facial hypotonia, mild periorbital fullness, long eyelashes, full cheeks, and smoothing of the philtrum.
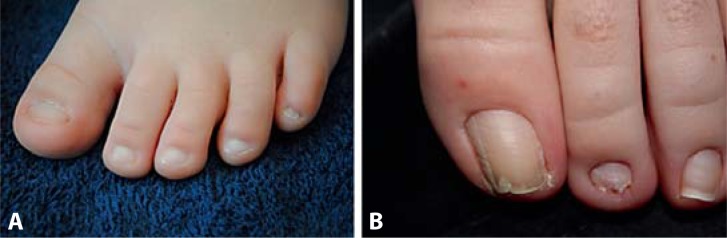
Other relatively common features are large fleshy hands, dysplastic toenails (fig. 2), lymphedema, and decreased perception of pain. Less common features are arachnoid cysts (15%), cortical visual impairment, hypothyroidism, and hearing loss. Subarachnoid cysts associated with deletion of 22q13 are not distinct from those observed in typical individuals and should be managed by conventional methods. The cysts may be present at birth or develop postnatally and should be monitored periodically for change in size. Symptoms including headache, vomiting, seizures, hearing and visual impairment, loss of balance, and incoordination may lead parents or clinicians to suspect the presence of arachnoid cysts. The association of cortical visual impairment with deletion 22q13 is not clear, although the presence of neurological impairment, abnormal brain development, and seizures may be predisposing factors.
Fig. 2.
Dysplastic toenails. A Small and poorly formed toenails of a 3-year-old child. B Jagged and thick toenails of an 11-year-old girl. Toenails were small and thin during early childhood, then became thicker with a tendency to become ingrown.
Seizures are present in about 25% of individuals. Cardiac abnormalities, including tricuspid valve regurgitation, atrial septal defect, patent ductus arteriosus, and total anomalous pulmonary return, occur in >25% of individuals. Renal abnormalities (>25%) include absent kidney, structural abnormalities of the kidney, hydronephrosis, and kidney reflux. These abnormalities can be detected by renal ultrasound before the clinical symptoms are manifested. Frequent vomiting can lead to weight loss and irritation of the esophagus. Cyclic vomiting is reported in 25% of individuals. The vomiting episodes tend to recur every few months and may be accompanied by headaches, lethargy, and dehydration [Phelan et al., 2010]. Two recently described cases have presented with sudden and severe autoimmune liver failure requiring transplant [Tufano et al., 2009; Bartsch et al., 2010]. Bartsch et al. [2010] suggested that liver function tests should be part of the management of patients with 22q13.3 deletion syndrome.
Behavior is characterized by impaired communication and impaired social interactions. Affected individuals may be diagnosed with autism or are described as having ‘autistic-like’ behavior even before the chromosome deletion is detected [Manning et al., 2004]. Young children may avoid eye contact, become anxious in social situations, exhibit tactile defensiveness, and display self-stimulatory behavior such as hand flapping or rocking. About 70% of individuals demonstrate persistent chewing and mouthing of non-food items. Others show teeth grinding or tongue thrusting.
A summary of features is given in table 1. The phenotypic features are shown in figures 1 and 2.
Table 1.
Features associated with 22q13.3 deletion syndrome [Cusmano-Orog et al., 2007; Dhar et al., 2010; Phelan et al., 2010]
| >75% cases | >50% cases | >25% cases | <25% cases |
|---|---|---|---|
|
|
|
|
Differential Diagnosis
The differential diagnosis for the 22q13.3 deletion syndrome (OMIM #606232) includes the following:
Autism Spectrum Disorders
The behavioral phenotype of impaired communication, impaired socialization, and repetitive or self-stimulatory movements is common to ASDs and to 22q13.3 deletion syndrome. The subtle dysmorphic features associated with deletion of 22q13.3 may be overlooked, causing an individual to be diagnosed with non-syndromic, or simple, autism [Manning et al., 2004; Durand et al., 2007].
Cerebral Palsy
Cerebral palsy is a term used to describe neurological impairment that is typically present at birth. Hypotonia, feeding difficulties, poor coordination, and subtle dysmorphic features may lead to a diagnosis of cerebral palsy in an individual with 22q13.3 deletion syndrome [Phelan, 2007].
Angelman Syndrome (OMIM #105830)
Features that are shared with the 22q13.3 deletion syndrome include hypotonia, global developmental delay, absent speech, poor coordination, and minor dysmorphic features [Phelan, 2007].
Velocardiofacial Syndrome (OMIM #192430)
The 22q13.3 deletion syndrome has been fortuitous diagnosed in individuals referred for FISH analysis for velocardiofacial syndrome [Precht et al., 1998]. The neurologic problems in this disease are not as severe as in 22q13.3 deletion syndrome, although hypotonia, speech delay, epicanthal fold, and developmental delay are common to both disorders.
Fragile X Syndrome (OMIM #300624)
Males with fragile X syndrome may have autistic-like behavior and speech delay in addition to developmental delay, similar to individuals with deletion of 22q13.3. Physical features of older males with fragile X syndrome, including tall stature, long face, and large ears, are similar to features seen in some males with 22q13.3 deletion syndrome [Phelan, 2007].
FG Syndrome (OMIM #305450)
Shared features include hypotonia, intellectual impairment, delayed speech, autistic-like behavior, and gastroesophageal reflux. There are many distinct features of FG syndrome that are not seen in 22q13.3 deletion syndrome including intestinal/anal atresia, chronic constipation, short stature, and vertebral malformations [Phelan, 2007].
As microarray CGH becomes a standard of care for testing individuals with intellectual impairment with or without dysmorphic features, additional cases of 22q13.3 deletion syndrome and other chromosome deletion and duplication disorders will be identified.
Natural History
Pregnancy is typically uneventful with no characteristic ultrasound findings. In most instances, birth weight, length, and head circumference are normal. Neonatal hypotonia may result in poor suck and difficulty feeding. Infants will coo and babble at the appropriate ages and acquire a few words, although regression in speech may occur at 2–3 years. Major milestones will be delayed. Toilet training is particularly difficult. With intensive therapies children may regain speech, although they will always have mild to severe impairment of expressive speech. Receptive language skills are more advanced than expressive skills. Communication can be improved through assistive technologies, touch screens, message boards, card exchange, and sign language.
Seizures typically develop around the time of puberty, although younger children can be affected. Lymphedema may become problematic in teens and young adults. Other medical problems occurring in over 25% of individuals include gastroesophageal reflux, renal abnormalities, cardiac defects, and cyclic vomiting. Arachnoid cysts may be present, warranting baseline brain imaging studies and repeat studies as indicated by behavioral changes. Both precocious puberty and delayed puberty have been reported.
Children may be hyperactive or aggressive with tongue thrusting and teeth grinding. They may have trouble sleeping. Most children exhibit incessant chewing on non-food items, possibly as a self-stimulatory behavior. Autism and autistic-like behavior are common. Reduced perspiration with the tendency to overheat necessitates that individuals avoid direct sunlight and stay hydrated. The reduced perception of pain in the non-verbal child requires the caregiver to be particularly vigilant to detect any changes in behavior that may indicate pain or injury. The risk for hypertension and other age-related cardiac change is uncertain due to the relatively low number of affected adults.
No individuals with 22q13.3 deletion syndrome have been known to reproduce, with the exception of the complex case described in Slavotinek et al. [1997]. There would be a significant risk of producing a similarly affected child. It is unlikely that adults could live independently, although group homes are a viable option for many teens and adults. Because so few adults have been identified, the life expectancy is uncertain, although several studies have suggested that individuals with intellectual disability have shorter life expectancies than the general population. The lack of behaviors that promote wellbeing, such as an appropriate diet and adequate physical activity, contribute to the shortened life span [Sutherland et al., 2002]. Co-morbid conditions such as seizures, cerebral palsy, and asthma further reduce life expectancy [Katz, 2003].
Recommendations for Management
Hypotonia
Persistent hypotonia can contribute to speech difficulty, reduced reflexes, and delayed motor milestones. A neurological evaluation is warranted for neonatal hypotonia. Therapies directed at increasing muscle tone such as physical therapy and occupational therapy should be implemented.
Feeding Problems
Feeding problems often are related to hypotonia. An infant may have difficulty sucking or swallowing and should be monitored for weight loss. Persistent feeding problems should be evaluated by a feeding specialist, occupational therapist, or speech pathologist. Strategies to increase muscle tone may alleviate the feeding difficulties.
Gastroesophageal Reflux
Smaller feeds, holding the baby upright for about 30 minutes after feeding, thickening the milk or cereal, and raising the head of the crib may be effective. Reflux can often be controlled by medication and usually resolves by 12 months of age. If the infant loses weight or reflux continues after 1 year of age, the child should be evaluated by a feeding specialist or gastroenterologist. Persistent cases may require surgery.
Cyclic Vomiting
Persistent vomiting may require hospitalization and intravenous fluids to prevent dehydration. The decreased perception of pain and impaired communication in individuals with 22q13.3 deletion syndrome makes it extremely difficult to determine if migraine headaches, anxiety, fatigue, or other causes of discomfort trigger the cyclic vomiting. Brain imaging studies are recommended to determine if the cyclic vomiting is related to the presence of an arachnoid cyst.
Delayed Milestones
Varying degrees of developmental delay are present. Motor milestones should be monitored and early intervention programs should be designed to meet the child's developmental needs. Physical therapy, occupational therapy, exercise and adaptive sports help improve the muscle tone.
Behavior
The individual should be evaluated by a developmental specialist to assess for ASD. Behavior modification programs are indicated if aggression or other undesirable behavior is present. The individual typically requires frequent interactions and positive reinforcement to affect a change in behavior.
Speech and Language
Expressive and receptive language skills should be evaluated by a speech pathologist. Neuropsychological testing may be beneficial to determine if delayed speech results from delay in processing auditory stimuli. Speech therapy, occupational therapy, and physical therapy should be directed toward improving the child's ability to communicate. Assistive technologies, such as a touch screen computer, are often beneficial in enhancing the child's ability to communicate.
Sleep Disturbance
Although children and adults frequently have sleep disturbances, sleep apnea is uncommon. Difficulty falling to sleep and staying asleep is a fairly common problem in individuals with developmental disabilities and/or neurological dysfunction. Establishing a schedule of defined wake-up and bedtimes, avoiding active play prior to bedtime, and soothing music or massage may enhance sleep. Some parents suggest that treatment with melatonin helps their children sleep through the night. Primary care physicians should be consulted regarding the appropriate dose of melatonin [Wirojanan et al., 2009]. Individuals with a severe sleep disorder can be referred for a sleep study in an attempt to determine the cause of the sleep disturbance.
Hypothyroidism
Behavioral changes including lethargy, decreased activity, cognitive regression, and loss of coordination have been associated with hypothyroidism in some individuals with 22q13.3 deletion syndrome. A thyroid blood panel should be obtained on individuals who exhibit these symptoms. Conventional methods of treating hypothyroidism should be implemented. Treatment includes introduction of synthetic T4 replacement with periodic monitoring of T4 and TSH levels [Phelan et al., 2010].
Lymphedema
The legs are more often affected than the arms, and the condition may worsen with age. Massage and elevation of the affected limb may lessen the swelling, while compression stockings or bandages may help improve circulation. Devices such as compression boots with a pneumatic pump to push fluid away from the distal extremity are available for severe cases. Painful lymphedema should be evaluated by a vascular surgeon. In one case severe lymphedema led to ascites and pleural effusions requiring repeated drainage of ascitic fluid beginning at about 12 years of age and repeated drainage of pleural effusions by about 14 years until surgical intervention [McGaughran et al., 2010].
Hearing
Hearing is typically normal although affected individuals often have a delayed response to verbal or auditory cues. Difficulty distinguishing words from background noises may contribute to this delay. If a hearing impairment is suspected, testing should be performed by a specialist who is experienced in testing individuals with developmental delay. Hearing deficits should be managed by standard methods.
Eyes
Vision is normal in most cases, although about one third have strabismus and about 6% have cortical visual impairment. Both of these conditions can cause problems with depth perception. When a child has a vision exam, the individual performing the testing should be experienced in working with children who have developmental delay. Vision problems should be treated in the standard manner.
Seizures
Grand mal seizures, focal seizures, and absence seizures have been reported and may indicate an increased risk of developing epilepsy. Although no specific electroencephalogram findings have been associated with 22q13.3 deletion syndrome, seizures should be evaluated by an electroencephalogram. Standard anticonvulsant medication should be used to treat recurrent seizures.
Arachnoid Cysts
Symptoms of intracranial pressure, such as incessant crying bouts, irritability, severe headaches, cyclic vomiting, and seizures, may lead to the suspicion of an arachnoid cyst. Baseline brain imaging studies, such as magnetic resonance imaging and computed tomography scan, are recommended for children with 22q13.3 deletion syndrome. Even if early studies show no evidence of a cyst, repeat studies prompted by the onset of symptoms may demonstrate that a cyst is present. Surgical placement of a shunt to relieve intracranial pressure may be required in some cases. Other abnormalities such as delayed myelination, frontal lobe hypoplasia, agenesis of the corpus callosum, and ventriculomegaly have been revealed by brain imaging.
Renal
It is recommended that individuals have a baseline renal ultrasound as soon as reasonably possible after deletion of 22q13.3 is diagnosed. Problems including absent kidney, structural abnormalities of the kidney, hydronephrosis, and kidney reflux have been identified by ultrasound before symptoms have been manifested. Standard methods of treatment are indicated. One child with a unilateral multicystic kidney detected at prenatal ultrasound and Wilms’ tumor in the contralateral, non-cystic kidney diagnosed at 22 months of age has been described [Kirkpatrick and El-Khechen, 2011]. A regimen of chemotherapy, tumor resection, and radiation was used to treat the tumor.
Medication
There is no evidence of an increased risk associated with anesthesia or specific medications. As in typical children who are on medication for seizures, hyperactivity, or other issues, certain medications will be more effective in some children than others. The primary care physician should monitor maintenance medications for adverse interactions.
Genetics of 22q13.3 Deletion Syndrome
The 22q13.3 deletion syndrome results from a de novo deletion of chromosome 22 in 80–85% of individuals, and approximately 70% of the deletions are paternal in origin [Luciani et al., 2003; Wilson et al., 2003]. The syndrome also results from an unbalanced chromosome rearrangement involving chromosome 22 in 15–20% of cases, of which approximately 50% are inherited from a balanced carrier parent. The rearrangement is equally likely to be inherited from the mother or the father. In cases of familial rearrangement, prenatal testing may be warranted for at risk pregnancies.
Four studies have compared a total of 111 patients with simple deletions, ring chromosomes, and unbalanced translocations [Luciani et al., 2003; Wilson et al., 2003; Koolen et al., 2005; Dhar et al., 2010]. These terminal deletions range from ∼185 kb (previously reported as 100 kb, see below) to >9 Mb [Wilson et al., 2003]. None of the studies found a correlation between the severity of the clinical features and the size of the deletion, with the exception that in 56 patients Wilson et al. [2003] observed a correlation of size to some but not all measures of developmental assessment. However, the general lack of correlation of the phenotype to the deletion size has led to a focus on the gene SHANK3, 1 of 3 genes lost in all terminal deletions.
SHANK3
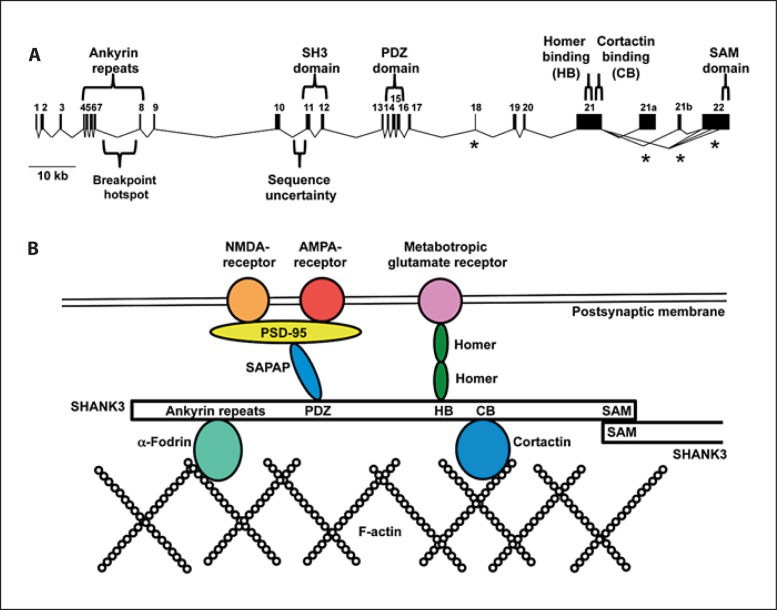
Of the more than 90 genes that may be deleted in 22q13.3 deletion syndrome, the majority of the neurological features are thought to be caused by haploinsufficiency of SHANK3 (SH3 and multiple ankyrin repeat domains 3), previously known as ProSAP2 (proline-rich synapse-associated protein 2) (fig. 3). SHANK3 was first implicated as a major contributor to 22q13.3 deletion syndrome in a child with a de novo reciprocal balanced translocation t(12;22)(q24.1;q13.3) that interrupted the gene in exon 21 [Bonaglia et al., 2001]. This child had features typical of 22q13.3 deletion syndrome, including severe speech delay, mild intellectual impairment, mild hypotonia, and typical dysmorphic features. Small terminal deletions give further support for the importance of SHANK3 in 22q13.3 deletion syndrome. Three of the smallest independent deletions all break within the D22S167 minisatellite repeat, implying a deletion hotspot [Bonaglia et al., 2006]. They interrupt SHANK3 between exons 7 and 8 (fig. 3a), resulting in a deletion originally estimated at 100 kb but now estimated to be 185 kb (Hg19 assembly). One patient showed absence of expressive speech and severe intellectual impairment with autistic traits and dysmorphic features. The other 2 cases were described previously and showed a milder phenotype: Anderlid et al. [2002] described a woman with mild intellectual impairment, delayed expressive speech, autistic symptoms, and facial dysmorphia, while the other patient showed mild intellectual impairment and delayed expressive speech [Flint et al., 1995]. A smaller 142-kb deletion starting in intron 8 was detected in family 1 of Durand et al. [2007] and was associated with autism, absent language, and moderate intellectual impairment.
Fig. 3.
SHANK3 structure and function. A Genomic structure of SHANK3. Alternatively spliced exons are marked with an asterisk. The locations of protein motifs are shown above the gene. The breakpoint hotspot consists of a complex microsatellite (D22S163) and is the location of at least 3 independent deletion breakpoints. ‘Sequence uncertainty’ refers to a small stretch of sequence not included in the Hg19 reference sequence, resulting in the incorrect annotation of an additional exon. B A representation of some of the PSD proteins that bind to SHANK3 which acts as a PSD scaffold. SHANK3 binds to various membrane-bound receptors through Homer, SAPAP, and PSD-95 proteins. SHANK3 connects to the actin cytoskeleton through proteins such as α-Fodrin and Cortactin. Two or more SHANK3 proteins can multimerize through the SAM domains [based on Kreienkamp, 2008].
SHANK3 acts as a scaffolding protein in the postsynaptic density (PSD) of excitatory glutamatergic synapses [Naisbitt et al., 1999; Böckers et al., 2002]. The PSD is a large complex of proteins under the postsynaptic membrane at the end of dendritic spines [reviewed in Kreienkamp, 2008]. Through the PSD the cell surface receptors are connected to the actin cytoskeleton. Components of the PSD are interconnected through the SHANK family of proteins (1, 2, and 3) protein binding domains which include (fig. 3b): ankyrin repeats that bind to α-fodrin and sharpin; a PDZ domain that binds through SAPAP to PSD95 and the NMDA and AMPA receptors; a domain that binds to Homer, which binds to metabotropic glutamate receptors; a domain that binds to Cortactin, which binds F-actin; and a SAM domain through which SHANK proteins can multimerize [reviewed in Kreienkamp, 2008]. SHANK proteins show differential, although partially overlapping, expression patterns in the postnatal developing brain and adult brain [Böckers et al., 2004], and the physiological relationship of these 3 proteins to one another is not clear. SHANK3 is involved in both forming the PDS by recruiting proteins and being part of the signaling system between the receptors and the actin cytoskeleton [Sala et al., 2001; Roussignol et al., 2005]. The levels of SHANK3 must be tightly controlled. A change in SHANK3 expression in cultured mouse neurons leads to abnormalities of spine density and function [Roussignol et al., 2005]. RNA interference-mediated knockdown of SHANK3 resulted in decreased dendritic spine density in hippocampal neurons, while transgenic overexpression induced the growth of spines in aspiny cerebellar neurons and led to increased synaptic contacts and increased spine size.
Proteins at the synapses of excitatory neurons have been linked to ASD [reviewed in Bourgeron, 2009]. Rare mutations in patients with ASD have been found in both neuroligins (NLGN3 and 4) [Jamain et al., 2003; Laumonnier et al., 2004] and in neurexin 1 (NRXN1) [Kim et al., 2008]. Neurexins in the presynaptic membrane bind to neuroligins in the postsynaptic membrane. Together these proteins are involved in the formation of synapses and the balance between excitatory and inhibitory synapses. Neuroligins bind to SHANK3 in the PSD, and thus it is not surprising that mutations associated with ASDs have also been found in SHANK3. Durand et al. [2007] screened 324 cases with ASD and found 3 families with mutations in SHANK3. One was a small de novo deletion starting in intron 8 in an individual with moderate intellectual impairment and no speech. The second family had 2 brothers with a G insertion in exon 21, creating a frameshift and loss of the SAM domain in exon 22. Neither parent carried the mutation, and it was hypothesized that the mother was a germline mosaic. Both brothers showed severe speech delay and intellectual impairment. The third family had 2 affected children as a result of a paternal translocation t(14;22)(p11.2;q13.33). The daughter with severe language delay had an 800-kb terminal 22q deletion. However, her brother had the reciprocal translocation, resulting in 3 copies of SHANK3 and 24 other genes. Although he showed precocious speech, he also showed impaired social communication and was diagnosed with Asperger syndrome, a mild ASD disorder. In addition, this study identified 7 families with non-synonymous SNPs in children with ASD and their normal parents, but not in controls. Two of these SHANK3 variants when overexpressed in cell culture showed abnormal synaptic clustering, suggesting that these SNPs may result in elevated susceptibility rather than being causative. Moessner et al. [2007] screened 400 patients with ASD and found 3 families with SHANK3 deletions that include the entire gene. The deletion in family ASD2 was again the unbalanced result of a paternal translocation t(14;22)(q32.33;q13.31). One child with ASD had a 3.2-Mb 22q13.3 deletion, while her sister had the reciprocal duplication and a diagnosis of attention-deficit/hyperactivity disorder (ADHD) and mild cognitive impairment. Thus, both the loss and gain of one copy of SHANK3 can be associated with neurological impairment. Two possibly causative SHANK3 mutations were also found in a screen of 427 ASD patients [Gauthier et al., 2009]. However, no SHANK3 copy number variants or SNP associations were seen in 330 multiplex ASD families in another study [Sykes et al., 2009], nor was a SHANK3 SNP association seen in screening 305 Chinese Han trios [Qin et al., 2009], although neither of these studies sequenced the gene. A screen of 396 patients with ASD and 184 patients with intellectual impairment revealed 2 individuals with deletions in SHANK2 associated with both ASD and intellectual impairment. Also identified were other SHANK2 mutations including a nonsense mutation, 6 missense mutations, and a 6-bp duplication in affected individuals but not in 659 controls, indicating that both SHANK2 and 3 are associated with ASD [Berkel et al., 2010]. However, SHANK mutations, like NLGN3/4 and NRXN1 mutations, are not a common cause of autism. Mutations in each gene have been estimated to be responsible for only 1% or less of ASD cases. Nevertheless, these cases do support the hypothesis that haploinsufficiency for SHANK3 is responsible for the ASD symptoms seen in many patients with 22q13.3 deletion syndrome. Interestingly, of 185 schizophrenia cases screened, 3 brothers with schizophrenia and mild to moderate intellectual impairment but not dysmorphic features were found to have a nonsense mutation (R1117X) in exon 21 of SHANK3, suggesting a connection between autism and schizophrenia [Gauthier et al., 2010].
Screening for mutations and deletions in SHANK3 can be problematic, since it is a complex gene with multiple alternative transcripts and regions that are extremely CG-rich [Wilson et al., 2003]. Furthermore, there is still confusion over its genomic structure (fig. 3a). Numerous papers have reported 24 exons covering ∼56.7 kb [Wilson et al., 2003; Durand et al., 2007; Moessner et al., 2007; Sykes et al., 2009]; however, SHANK3 is currently annotated with 23 exons in the Hg19 assembly (high coverage assembly build 19 from the Genome Reference Consortium, February 2009). Wilson et al. [2003] determined that the consensus sequence contains a small deletion in a very CG-rich region just proximal to and including part of exon 11, which results in missing codons from the 5′ end of that exon, as determined by comparing the human genomic sequence to rat cDNA AF133301. Sequencing of a human genomic clone isolated with part of the rat cDNA resulted in base pairs being added to the exon 11 sequence. A further addition of 4 amino acids in exon 11 to better match the rat protein sequence lead to a prediction of human exon 11 (Accession ABA00478), the full protein sequence (Accession BAJ09793, 1,731 amino acids), and mRNA sequence (Accession AB569469). These sequences closely match both the rat and mouse SHANK3 genes. However, the current Hg19 annotation has an incorrect prediction of the 5′ end of this exon as well as a short additional preceding ‘exon 11’ thus shifting the following exon numbering by one. This additional predicted exon does not match the rat and mouse cDNAs. This discrepancy was noted by Kolevzon et al. [2011] who described a patient with ASD and speech delay that had a SHANK3 variant in Hg19 ‘exon 11’. The variant was a 1-bp insertion that would create a frameshift in the middle of the gene, yet the same variant was present in the healthy mother and in ∼1% of normal controls (4/382). Since the exon size is not a multiple of 3 (49 bp), the exon could not simply be alternatively spliced out without affecting the reading frame. The observation of this variant supports the original annotations and indicates that the Hg19 ‘exon 11’ is not part of the transcript. The original exon 11 region is extremely CG-rich, and cDNAs containing the entire exon do not exist. It has been reported that both exon 1 and 11 cannot be amplified reliably [Moessner et al., 2007], which adds to the difficulty of screening for mutations. A further complication that has not been explored in detail is the discovery of 2 truncated isoforms of SHANK3 [Maunakea et al., 2010], which may or may not be deleted/mutated in all cases of 22q13.3 deletion syndrome.
The numbering of the last 3 exons varies. Exon 21 [as numbered in Bonaglia et al., 2001; Wilson et al., 2003; Durand et al., 2007; Moessner et al., 2007; Sykes et al., 2009] is a large, CG-rich exon containing Homer and Cortactin-binding domains. Following this exon, there have been 3 alternatively spliced exons described, usually referred to as exons 21a, 21b, and 22. Exons 21a and 21b are absent from the Hg19 annotation. Most transcripts appear to skip these 2 exons [Durand et al., 2007] and splice exon 21 directly to 3 alternative 5′ beginnings of the terminal exon 22 (referred to as exon 24 in Wilson et al. [2003]). The exon numbering system given in Durand et al. [2007] is used in this review (fig. 3a).
Further evidence supporting the importance of SHANK3 in 22q13.3 deletion syndrome and ASDs comes from 2 independent reports of Shank3 knockout mice. Bozdagi et al. [2010] produced a mouse line with a deletion of exons 4–9. Both hetero- and homozygotes are viable and fertile with grossly normal brains. Homozygotes have not been studied in detail, but heterozygotes showed synaptic abnormalities that lead to behavioral defects. Hippocampal cells showed reduced basal neurotransmission, abnormal long-term potentiation, fewer synapses, and decreased spine expansion. Males showed reduced social interactions with females in estrus. Peca et al. [2011] deleted the ankyrin-binding repeats to eliminate the main Shank3 isoform and, in a second line, deleted the PDZ domain which additionally eliminated or reduced the remaining 2 isoforms. Homozygotes showed structural and physiological abnormalities of the basal ganglia and cortico-striatum. Homozygotes displayed compulsive/repetitive behavior including self-injurious grooming, anxiety-related behavior, and abnormal social interactions. These mouse lines provide possible models to test pharmacological treatments for 22q13.3 deletion syndrome [Bozdagi et al., 2010]. Interestingly, mice lacking SHANK1 also show abnormalities of dendritic spines and PSDs, as well as a complex behavioral phenotype. Shank1 knockouts show increased anxiety, abnormal fear memory, and enhanced spatial learning associated impaired long-term memory [Hung et al., 2008]. The authors speculate that these symptoms are reminiscent of those of ASDs.
Other Genes in 22q13.3 Deletion Syndrome
The smallest 22q13.3 terminal deletions also include genes ACR (acrosin) and RABL2B (RAB, member of RAS oncogene family-like 2B), the last 2 known genes before the telomeric sequences. ACR codes for a serine protease in the sperm acrosome [Klemm et al., 1991]. The active enzyme causes a localized breakdown of the oocyte zona pellucida, allowing penetration of the sperm. It is unlikely that this gene contributes to the syndrome. RABL2B is a member of the RAS GTPase superfamily and has a near identical paralogue at 2q13 [Wong et al., 1999]. It has recently been shown that these paralogues do not express equally, and that RABL2b on chromosome 22 is preferentially expressed in all tissues tested, particularly in brain and placenta [Kramer et al., 2010]. Thus, it is possible that haploinsufficiency for RABL2b in 22q13.3 deletions may play a role in the syndrome phenotypes. However, a major role for RABL2b is unlikely since 2 patients with interstitial deletions of SHANK3, which leave RABL2b intact, have been associated with features typical of the 22q13.3 deletion syndrome. An interstitial deletion with a distal breakpoint within SHANK3 was seen in a patient with developmental delay, delayed expressive speech, and dysmorphic features [Delahaye et al., 2009]. A patient with a 17.6-kb t(22;X)-associated interstitial deletion involving the last 2 exons of SHANK3 and part of ACR had moderate intellectual impairment, delayed and simple expressive speech, hypotonia, abnormal social interactions, stereotypic hand movements, ritualistic behavior, and dysmorphic features [Misceo et al., 2011].
Another gene deleted in most 22q13.3 deletion syndrome cases is IB2 (islet brain 2), also known as MAPK8IP2 (mitogen-activated protein kinase 8-interacting protein 2), which is located ∼70 kb proximal to SHANK3. In mice this gene is expressed in the brain, and the protein is enriched in PSDs [Giza et al., 2010]. Mice deficient for IB2 show normal PSD structure but have reduced cerebellar AMPA and enhanced NMDA receptor-associated transmission. Behavioral defects include motor deficits and decreased social interaction and exploratory behavior, reminiscent of the autism-like features of mice with other mutated PSD genes. These defects were all characterized in the homozygous IB2 mutant, and it remains to be seen whether IB2 shows haploinsufficiency, as has been shown for a Shank3 mutation in mice [Bozdagi et al., 2010]. However, the location of the IB2 protein in the PSD and the phenotype of the IB2-deficient mice suggest that IB2 may play a role in the neurological features of most patients with 22q13.3 deletion syndrome.
Interestingly, 2 children with some features of 22q13.3 deletion syndrome were reported with overlapping interstitial deletions that do not include the SHANK3 or IB2 regions, but are contained within the largest 22q13.3 deletion syndrome terminal deletions [Wilson et al., 2008]. Both children showed intellectual impairment, severe speech delay, developmental delay, hypotonia, macrocephaly, abnormal MRI scans, and dysmorphic features. This suggests that there are additional haploinsufficient genes in the 22q13.3 region that contribute to cognitive and speech development and are involved in the phenotype of at least the larger 22q13.3 deletions. However, a parent of one of the children also had the interstitial deletion with only mild speech problems and normal cognition, implying that haploinsufficiency of these genes may be more variable in their effects.
Research into Potential Therapeutic Approaches
Intranasal Insulin
Treatment of the neurologic symptoms of 22q13.3 deletion syndrome is hindered by the inability of potentially beneficial therapeutic agents to cross the blood-brain barrier. Intranasal administration is an approach that circumvents this problem and avoids systemic complications [Born et al., 2002]. Intranasal insulin has been shown to improve declarative memory in individuals with Alzheimer's disease and in normal individuals [Benedict et al., 2007]. These results have led to speculation that intranasal delivery of therapeutic agents may improve the neurologic deficits associated with 22q13.3 deletion syndrome.
In 2009, Schmidt et al. [2009] conducted a clinical trial in which intranasal insulin was administered to 6 children with 22q13.3 deletion syndrome. Five of the 6 children showed marked short-term (first 6 weeks) improvements in gross- and fine-motor skills and cognitive function. One child was removed from the study due to adverse reactions. Long-term (12 months) affects were measured in 4 children and also suggested improved motor skills and cognitive function. The mechanism by which intranasal insulin improved the skills in these children was not clear. Earlier studies by Bockmann et al. [2002] demonstrated that an insulin receptor kinase substrate, IRSp53, appeared to interact with SHANK3 to improve the PSD. After insulin receptor activation, IRSp53 may play a role in the morphological reorganization of spines and synapses. When SHANK3 is deficient, as in 22q13.3 deletion syndrome, intranasal insulin may improve the process by enhancing the expression of a local dendritic scaffolding protein PSD95 which would partially counteract the decreased SHANK3, resulting in improved cognitive ability [Schmidt et al., 2009]
In this study there were no adverse effects on height, weight, or head circumference and no treatment effects on blood levels of glucose, HBA1c, cortisol, and insulin antibodies after 12 months [Schmidt et al., 2009]. However, the long-range effect of this treatment on the health of these children is unknown. The individuals in the study were of various ages with different levels of cognitive impairment and were unmatched for the specific deletion type. Longitudinal studies with larger samples sizes and more stringently controlled conditions are needed before recommendations regarding treatment with intranasal insulin can be recommended.
Risperidone
Pasini et al. [2010] demonstrated a dose-dependent effect of oral risperidone in an 18-year-old female with severe intellectual impairment, intense psychomotor agitation, and aggressive behavior associated with 22q13.3 deletion syndrome. Although standard doses of risperidone resulted in worsened behavior, a lower dose gave a favorable response. Following treatment the patient experienced rapid improvement in mood and behavior, which the authors attributed to a dose-dependent effect of risperidone on glutamate receptors.
Treatments Targeted for Other Forms of Autism
Additional therapeutic strategies for autism spectrum disorders and other neurological and behavioral disorders are under investigation. The success of targeted therapy for fragile X syndrome in humans [Bear et al., 2004; D'Hulst and Kooy, 2007] and for Rett syndrome in mouse models [Tropea et al., 2009] demonstrate that treatment of autistic disorders is feasible. Induced pluripotent stem cells are a potential source of replacement therapy in neurodegenerative conditions such as Alzheimer's disease, Parkinson disease, amyotrophic lateral sclerosis, and spinal cord injuries [Chen and Xiao, 2011]. Extensive research is required to determine if the use of induced pluripotent stem cells is a viable option for these and other neurological disorders. Although animal models are being developed to mimic the human condition, very few have reached the clinical trial stage. As more therapies for autism and similar disorders are shown to be safe and effective, their application to 22q13.3 deletion syndrome remains to be determined.
Conclusions
22q13.3 deletion syndrome manifests as a wide range of clinical and behavioral characteristics. The phenotype continues to evolve as an increasing number of cases are diagnosed as a result of advanced laboratory technologies. Elucidation of the behavioral phenotype is of particular interest as recent research has demonstrated that haploinsufficiency of SHANK3 is also responsible for about 1% of ASDs. Over 50% of individuals with 22q13.3 deletion syndrome are reported to have autistic features. This is certain to be an underestimate since all individuals with 22q13.3 deletion syndrome have not been evaluated for ASDs and all individuals with ASDs have not been tested for deletion of 22q13. Regardless of the true incidence, evidence supports a 22q13.3 deletion syndromic autism disorder. Further testing of individuals with ASDs with microarray CGH and sequencing studies will give a more accurate estimate of the incidence of ASDs associated with 22q13.3 deletion syndrome.
Acknowledgement
We wish to thank Dr. Curtis Rogers of the Greenwood Genetics Center for clinical photographs.
References
- Anderlid BM, Schoumans J, Anneren G, Tapia-Paez I, Dumanski J, et al. FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet. 2002;110:439–443. doi: 10.1007/s00439-002-0713-7. [DOI] [PubMed] [Google Scholar]
- Barakat AJ, Pearl PL, Acosta MT, Runkle BP. 22q13 deletion syndrome with central diabetes insipidus: a previously unreported association. Clin Dysmorphol. 2004;13:191–194. doi: 10.1097/01.mcd.0000134479.65125.08. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Schneider E, Damatova N, Weis R, Tufano M, et al. Fulminant hepatic failure requiring liver transplantation in 22q13.3 deletion syndrome. Am J Med Genet A. 2010;152A:2099–2102. doi: 10.1002/ajmg.a.33542. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86:136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Bisgaard AM, Kirchhoff M, Nielsen JE, Kibaek M, Lund A, et al. Chromosomal deletion unmasking a recessive disease: 22q13 deletion syndrome and metachromatic leukodystrophy. Clin Genet. 2009;75:175–179. doi: 10.1111/j.1399-0004.2008.01113.x. [DOI] [PubMed] [Google Scholar]
- Böckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins – a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- Böckers TM, Segger-Junius M, Iglauer P, Bockmann J, Gundelfinger ED, et al. Differential expression and dendritic transcript localization of Shank family members: identification of a dendritic targeting element in the 3′ untranslated region of Shank1 mRNA. Mol Cell Neurosci. 2004;26:182–190. doi: 10.1016/j.mcn.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Bockmann J, Kreutz MR, Gundelfinger ED, Bockers TM. ProSAP/Shank postsynaptic density proteins interact with insulin receptor tyrosine kinase substrate IRSp53. J Neurochem. 2002;83:1013–1017. doi: 10.1046/j.1471-4159.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, et al. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001;69:261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Mani E, Aceti G, Anderlid BM, et al. Identification of a recurrent breakpoint within the SHANK3 gene in the 22q13.3 deletion syndrome. J Med Genet. 2006;43:822–828. doi: 10.1136/jmg.2005.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Xiao SF. Induced pluripotent stem cells and neurodegenerative diseases. Neurosci Bull. 2011;27:107–114. doi: 10.1007/s12264-011-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody JD, Pierce JF, Brkanac Z, Plaetke R, Ghidoni PD, et al. Preferential loss of the paternal alleles in the 18q-syndrome. Am J Med Genet. 1997;69:280–286. doi: 10.1002/(sici)1096-8628(19970331)69:3<280::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cusmano-Ozog K, Manning MA, Hoyme HE. 22q13.3 deletion syndrome: a recognizable malformation syndrome associated with marked speech and language delay. Am J Med Genet C Semin Med Genet. 2007;145C:393–398. doi: 10.1002/ajmg.c.30155. [DOI] [PubMed] [Google Scholar]
- Delahaye A, Toutain A, Aboura A, Dupont C, Tabet AC, et al. Chromosome 22q13.3 deletion syndrome with a de novo interstitial 22q13.3 cryptic deletion disrupting SHANK3. Eur J Med Genet. 2009;52:328–332. doi: 10.1016/j.ejmg.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Dhar SU, del Gaudio D, German JR, Peters SU, Ou Z, et al. 22q13.3 deletion syndrome: clinical and molecular analysis using array CGH. Am J Med Genet A. 2010;152A:573–581. doi: 10.1002/ajmg.a.33253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Doheny KF, McDermid HE, Harum K, Thomas GH, Raymond GV. Cryptic terminal rearrangement of chromosome 22q13.32 detected by FISH in two unrelated patients. J Med Genet. 1997;34:640–644. doi: 10.1136/jmg.34.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Wilkie AO, Buckle VJ, Winter RM, Holland AJ, McDermid HE. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat Genet. 1995;9:132–140. doi: 10.1038/ng0295-132. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, Piton A, Lafreniere RG, Laurent S, et al. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Champagne N, Lafreniere RG, Xiong L, Spiegelman D, et al. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci USA. 2010;107:7863–7868. doi: 10.1073/pnas.0906232107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza J, Urbanski MJ, Prestori F, Bandyopadhyay B, Yam A, et al. Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2. J Neurosci. 2010;30:14805–14816. doi: 10.1523/JNEUROSCI.1161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goizet C, Excoffier E, Taine L, Taupiac E, El Moneim AA, et al. Case with autistic syndrome and chromosome 22q13.3 deletion detected by FISH. Am J Med Genet. 2000;96:839–844. [PubMed] [Google Scholar]
- Herman GE, Greenberg F, Ledbetter DH. Multiple congenital anomaly/mental retardation (MCA/MR) syndrome with Goldenhar complex due to a terminal del(22q) Am J Med Genet. 1988;29:909–915. doi: 10.1002/ajmg.1320290423. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RT. Life expectancy for children with cerebral palsy and mental retardation: implications for life care planning. NeuroRehabilitation. 2003;18:261–270. [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick BE, El-Khechen D. A unique presentation of 22q13 deletion syndrome: multicystic kidney, orofacial clefting, and Wilms' tumor. Clin Dysmorphol. 2011;20:53–54. doi: 10.1097/MCD.0b013e32833effb1. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum G, Chmura M, Rhone DP. Long arm deletion of chromosome 22. J Med Genet. 1988;25:780. doi: 10.1136/jmg.25.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm U, Muller-Esterl W, Engel W. Acrosin, the peculiar sperm-specific serine protease. Hum Genet. 1991;87:635–641. doi: 10.1007/BF00201716. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Cai G, Soorya L, Takahashi N, Grodberg D, et al. Analysis of a purported SHANK3 mutation in a boy with autism: clinical impact of rare variant research in neurodevelopmental disabilities. Brain Res. 2011;1380:98–105. doi: 10.1016/j.brainres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Koolen DA, Reardon W, Rosser EM, Lacombe D, Hurst JA, et al. Molecular characterisation of patients with subtelomeric 22q abnormalities using chromosome specific array-based comparative genomic hybridisation. Eur J Hum Genet. 2005;13:1019–1024. doi: 10.1038/sj.ejhg.5201456. [DOI] [PubMed] [Google Scholar]
- Kramer M, Backhaus O, Rosenstiel P, Horn D, Klopocki E, et al. Analysis of relative gene dosage and expression differences of the paralogs RABL2A and RABL2B by pyrosequencing. Gene. 2010;455:1–7. doi: 10.1016/j.gene.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ. Scaffolding proteins at the postsynaptic density: Shank as the architectural framework. Handb Exp Pharmacol. 2008;186:365–380. doi: 10.1007/978-3-540-72843-6_15. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LM, Zhang JX, Wang XP, Guo HX, Deng H, Luo J. Pim-3 protects against hepatic failure in D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest. 2010;40:127–138. doi: 10.1111/j.1365-2362.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- Luciani JJ, de Mas P, Depetris D, Mignon-Ravix C, Bottani A, et al. Telomeric 22q13 deletions resulting from rings, simple deletions, and translocations: cytogenetic, molecular, and clinical analyses of 32 new observations. J Med Genet. 2003;40:690–696. doi: 10.1136/jmg.40.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi PC, Perfumo C, Cali A, Coucourde G, Pastore G, et al. Clinical and molecular characterisation of 80 patients with 5p deletion: genotype-phenotype correlation. J Med Genet. 2001;38:151–158. doi: 10.1136/jmg.38.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, et al. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004;114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D'Souza C, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughran J, Hadwen T, Clark R. Progressive edema leading to pleural effusions in a female with a ring chromosome 22 leading to a 22q13 deletion. Clin Dysmorphol. 2010;19:28–29. doi: 10.1097/MCD.0b013e3283301f58. [DOI] [PubMed] [Google Scholar]
- Micale MA, Haren JM, Conroy JM, Crowe CA, Schwartz S. Parental origin of de novo chromosome 9 deletions in del(9p) syndrome. Am J Med Genet. 1995;57:79–81. doi: 10.1002/ajmg.1320570118. [DOI] [PubMed] [Google Scholar]
- Misceo D, Rodningen OK, Baroy T, Sorte H, Mellembakken JR, et al. A translocation between Xq21.33 and 22q13.33 causes an intragenic SHANK3 deletion in a woman with Phelan-McDermid syndrome and hypergonadotropic hypogonadism. Am J Med Genet A. 2011;155:403–408. doi: 10.1002/ajmg.a.33798. [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Narahara K, Takahashi Y, Murakami M, Tsuji K, Yokoyama Y, et al. Terminal 22q deletion associated with a partial deficiency of arylsulphatase A. J Med Genet. 1992;29:432–433. doi: 10.1136/jmg.29.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesslinger NJ, Gorski JL, Kurczynski TW, Shapira SK, Siegel-Bartelt J, et al. Clinical, cytogenetic, and molecular characterization of seven patients with deletions of chromosome 22q13.3. Am J Hum Genet. 1994;54:464–472. [PMC free article] [PubMed] [Google Scholar]
- Pasini A, D'Agati E, Casarelli L, Curatolo P. Dose-dependent effect of risperidone treatment in a case of 22q13.3 deletion syndrome. Brain Dev. 2010;32:425–427. doi: 10.1016/j.braindev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K: 22q13.3 deletion syndrome; in Pagon RA, Bird TD, Dolan CR, Stephens K (eds): GeneTests: GeneReviews [Internet], (University of Washington, Seattle, WA 2007). Available at http://www.ncbi.nlm.nih.gov/books/NBK1198/
- Phelan K, Betancur C. Clinical utility gene card for: deletion 22q13 syndrome. Eur J Hum Genet. 2011:19. doi: 10.1038/ejhg.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan MC, Rogers RC, Stevenson RE. A de novo terminal deletion of 22q. Am J Hum Genet. 1988;43:A118. [Google Scholar]
- Phelan MC, Thomas GR, Saul RA, Rogers RC, Taylor HA, et al. Cytogenetic, biochemical, and molecular analyses of a 22q13 deletion. Am J Med Genet. 1992;43:872–876. doi: 10.1002/ajmg.1320430524. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, et al. 22q13 deletion syndrome. Am J Med Genet. 2001;101:91–99. doi: 10.1002/1096-8628(20010615)101:2<91::aid-ajmg1340>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Stapleton GA, Rogers RC. The Management of Genetic Syndromes. Hoboken: Wiley-Liss, Inc.; 2010. [Google Scholar]
- Prasad C, Prasad AN, Chodirker BN, Lee C, Dawson AK, et al. Genetic evaluation of pervasive developmental disorders: the terminal 22q13 deletion syndrome may represent a recognizable phenotype. Clin Genet. 2000;57:103–109. doi: 10.1034/j.1399-0004.2000.570203.x. [DOI] [PubMed] [Google Scholar]
- Precht KS, Lese CM, Spiro RP, Huttenlocher PR, Johnston KM, et al. Two 22q telomere deletions serendipitously detected by FISH. J Med Genet. 1998;35:939–942. doi: 10.1136/jmg.35.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Jia M, Wang L, Lu T, Ruan Y, et al. Association study of SHANK3 gene polymorphisms with autism in Chinese Han population. BMC Med Genet. 2009;10:61. doi: 10.1186/1471-2350-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel M, Baumer A, Wisser J, Acherman J, Schinzel A. Prenatal diagnosis of mosaicism for a del(22)(q13) Prenat Diagn. 2000;20:76–79. doi: 10.1002/(sici)1097-0223(200001)20:1<76::aid-pd752>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Romain DR, Goldsmith J, Cairney H, Columbano-Green LM, Smythe RH, Parfitt RG. Partial monosomy for chromosome 22 in a patient with del(22)(pter–q13.1::q13.33–qter) J Med Genet. 1990;27:588–589. doi: 10.1136/jmg.27.9.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussignol G, Ango F, Romorini S, Tu JC, Sala C, et al. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J Neurosci. 2005;25:3560–3570. doi: 10.1523/JNEUROSCI.4354-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthi S, Morales J, Bermudez J, McBride L, Luquette M, et al. Array analysis and molecular studies of INI1 in an infant with deletion 22q13 (Phelan-McDermid syndrome) and atypical teratoid/rhabdoid tumor. Am J Med Genet A. 2009;149A:1067–1069. doi: 10.1002/ajmg.a.32775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Kern W, Giese R, Hallschmid M, Enders A. Intranasal insulin to improve developmental delay in children with 22q13 deletion syndrome: an exploratory clinical trial. J Med Genet. 2009;46:217–222. doi: 10.1136/jmg.2008.062141. [DOI] [PubMed] [Google Scholar]
- Schroder K, Schuffenhauer S, Seidel H, Bartsch O, Blin N, et al. Deletion mapping by FISH with BACs in patients with partial monosomy 22q13. Hum Genet. 1998;102:557–561. doi: 10.1007/s004390050739. [DOI] [PubMed] [Google Scholar]
- Slavotinek A, Maher E, Gregory P, Rowlandson P, Huson SM. The phenotypic effects of chromosome rearrangement involving bands 7q21.3 and 22q13.3. J Med Genet. 1997;34:857–861. doi: 10.1136/jmg.34.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DP, Floyd M, Say B. Detection of a familial cryptic translocation by fluorescent in situ hybridisation. J Med Genet. 1996;33:84. doi: 10.1136/jmg.33.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G, Couch MA, Iacono T. Health issues for adults with developmental disability. Res Dev Disabil. 2002;23:422–445. doi: 10.1016/s0891-4222(02)00143-9. [DOI] [PubMed] [Google Scholar]
- Sykes NH, Toma C, Wilson N, Volpi EV, Sousa I, et al. Copy number variation and association analysis of SHANK3 as a candidate gene for autism in the IMGSAC collection. Eur J Hum Genet. 2009;17:1347–1353. doi: 10.1038/ejhg.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NS, Durkie M, Potts G, Sandford R, Van Zyl B, et al. Parental and chromosomal origins of microdeletion and duplication syndromes involving 7q11.23, 15q11–q13 and 22q11. Eur J Hum Genet. 2006;14:831–837. doi: 10.1038/sj.ejhg.5201617. [DOI] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, et al. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M, Della Corte C, Cirillo F, Spagnuolo MI, Candusso M, et al. Fulminant autoimmune hepatitis in a girl with 22q13 deletion syndrome: a previously unreported association. Eur J Pediatr. 2009;168:225–227. doi: 10.1007/s00431-008-0732-z. [DOI] [PubMed] [Google Scholar]
- Watt JL, Olson IA, Johnston AW, Ross HS, Couzin DA, Stephen GS. A familial pericentric inversion of chromosome 22 with a recombinant subject illustrating a ‘pure' partial monosomy syndrome. J Med Genet. 1985;22:283–287. doi: 10.1136/jmg.22.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wong ACC, Shaw SR, Tse WY, Stapleton GA, et al. Molecular characterization of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/ PROSAP2 in the major neurological symptoms. J Med Genet. 2003;40:575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Crolla JA, Walker D, Artifoni L, Dallapiccola B, et al. Interstitial 22q13 deletions: genes other than SHANK3 have major effects on cognitive and language development. Eur J Hum Genet. 2008;16:1301–1310. doi: 10.1038/ejhg.2008.107. [DOI] [PubMed] [Google Scholar]
- Wirojanan J, Jacquemont S, Diaz R, Bacalman S, Anders TF, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5:145–150. [PMC free article] [PubMed] [Google Scholar]
- Wong AC, Shkolny D, Dorman A, Willingham D, Roe BA, McDermid HE. Two novel human RAB genes with near identical sequence each map to a telomere-associated region: the subtelomeric region of 22q13.3 and the ancestral telomere band 2q13. Genomics. 1999;59:326–334. doi: 10.1006/geno.1999.5889. [DOI] [PubMed] [Google Scholar]
- Yong YP, Knight LA, Yong MH, Lam S, Ho LY. Partial monosomy for chromosome 22 in a girl with mental retardation. Singapore Med J. 1997;38:85–86. [PubMed] [Google Scholar]
- Zollino M, Murdolo M, Marangi G, Pecile V, Galasso C, et al. On the nosology and pathogenesis of Wolf-Hirschhorn syndrome: genotype-phenotype correlation analysis of 80 patients and literature review. Am J Med Genet C Semin Med Genet. 2008;148C:257–269. doi: 10.1002/ajmg.c.30190. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Siegel-Bartelt J, Teshima I, Ho C. Two patients with 22q13.3 deletions have similar facies and developmental patterns. Am J Hum Genet. 1990;47:A45. [Google Scholar]