Abstract
While heterozygous variants in CNTNAP2 and NRXN1 are reported as susceptibility factors for neuropsychiatric disorders, homozygous or compound heterozygous defects in either gene were reported as causative for severe neurodevelopmental disorders. This review provides an overview of the clinical aspects in patients with recessive defects in CNTNAP2 and NRXN1.
Key Words: CNTNAP2, Epilepsy, Intellectual disability, Mental retardation, NRXN1
History of the Syndrome
During recent years, heterozygous copy-number and missense variants in CNTNAP2 and NRXN1 have repeatedly been reported as susceptibility factors for a wide spectrum of neuropsychiatric disorders such as developmental language delay and autism spectrum disorders, epilepsy and schizophrenia [Verkerk et al., 2003; Feng et al., 2006; Autism Genome Project Consortium, 2007; Belloso et al., 2007; Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; Friedman et al., 2008; Kim et al., 2008; Kirov et al., 2008; Marshall et al., 2008; Vrijenhoek et al., 2008; Zahir et al., 2008; Bucan et al., 2009; Rujescu et al., 2009; Awadalla et al., 2010; Bradley et al., 2010; Ching et al., 2010; Magri et al., 2010; Mefford et al., 2010; Wiśniowiecka-Kowalnik et al., 2010].
A homozygous stop mutation in CNTNAP2 in 10 Old Order Amish children was reported to cause a distinct disorder, cortical dysplasia-focal epilepsy syndrome (MIM 610042), characterized by cortical dysplasia and early onset, intractable focal epilepsy leading to language regression, and behavioral and mental deterioration [Strauss et al., 2006; Jackman et al., 2009]. Recently, homozygous or compound heterozygous defects in CNTNAP2 or NRXN1 were reported to cause a severe intellectual disability disorder resembling Pitt-Hopkins syndrome (MIM 610954) [Zweier et al., 2009]. These 4 patients had an initially negative TCF4-testing and showed severe intellectual disability and additional variable symptoms such as epilepsy, breathing anomalies and stereotypies.
In total, 13 patients with homozygous or compound heterozygous defects in CNTNAP2 and 1 patient with a compound heterozygous defect in NRXN1 are reported to date.
Clinical Features (table 1)
Table 1.
Clinical findings in patients with recessive CNTNAP2 or NRXN1 defects
| CNTNAP2 (n = 13) | NRXN1 (n = 1) | |
|---|---|---|
| Age at clinical assessment | 2–20 years | 18 years |
| Normal body height | 11/13 | 1/1 |
| Head circumference | 1 <P3, others P18-P99, mean >P75 | P50-P75 |
| Severe intellectual disability | 13/13 | 1/1 |
| Age of walking | normal-30 months | 2 years |
| Speech | none or single words | none |
| Developmental regression | 11/13 | 1/1 |
| Seizures with age of onset | 13/13, 4–30 months | 0/1 |
| MRI anomalies | CD 3/10, CH 1/10, PL 1/10 | 0/1 |
| Behavioral anomalies | 9/13 | 1/1 |
| Decreased deep tendon reflexes | 9/10 | 1/1 |
| Breathing anomalies | 3/3 | 1/1 |
P = Centile; CD = cortical dysplasia; CH = cerebellar hypoplasia; PL = periventricular leukomalacia. Data for CNTNAP2: Jackman et al., 2009; Strauss et al., 2006; Zweier et al., 2009. Data for NRXN1: Zweier et al., 2009.
Facial Gestalt
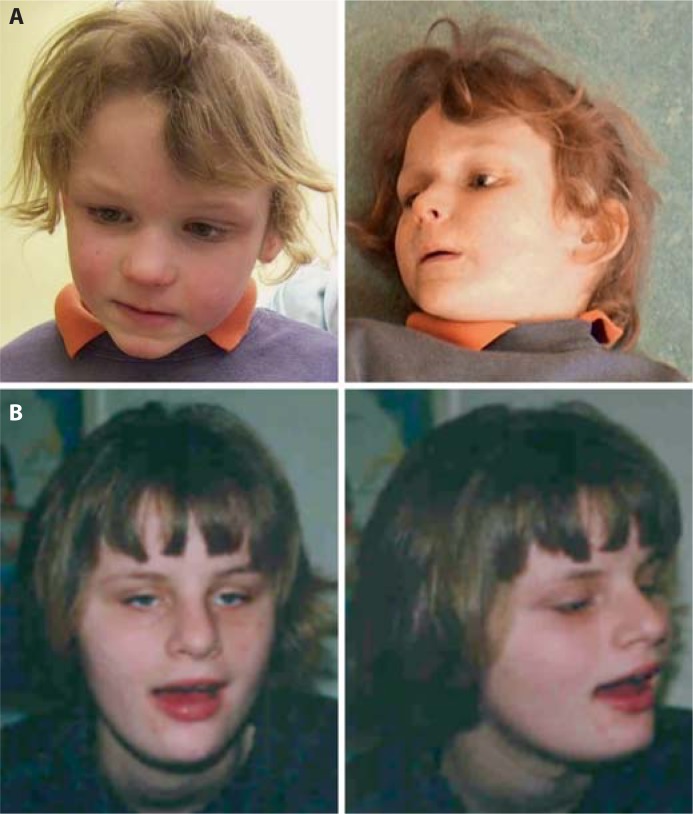
In contrast to patients with Pitt-Hopkins syndrome, the facial phenotype in patients with recessive CNTNAP2 and NRXN1 defects is rather unsuspicious. Two patients with a homozygous deletion within CNTNAP2 [Zweier et al., 2009] had previously been published as possible Pitt-Hopkins syndrome patients due to a wide mouth and thick lips [Orrico et al., 2001], but as indicated by Peippo et al. [2006] and supported by the present knowledge and perspective, they do not have the distinct Pitt-Hopkins syndrome facial phenotype. The remaining patients do not show specific facial dysmorphisms [Zweier et al., 2009] (fig. 1), neither are any reported for the Amish patients [Strauss et al., 2006; Jackman et al., 2009].
Fig. 1.
Facial appearance of patients with compound heterozygous defects in CNTNAP2 (A) or NRXN1 (B). Note a wide mouth in the patient with NRXN1 defects but otherwise unsuspicious facial gestalts in both patients. Reprinted from Zweier et al. [2009], with permission from Elsevier.
Intellectual Disability
Intellectual disability is reported to be severe in all patients. Developmental testing of 3 patients with cortical dysplasia-focal epilepsy syndrome at the ages of 32, 40 and 73 months revealed global mental ages of 21, 17 and 13 months, respectively [Strauss et al., 2006]. Particularly speech impairment is severe with either no or very limited speech development or regression of speech abilities. Both receptive and expressive language was reported to be maintained at the 1-year level in a 7-year-old patient [Jackman et al., 2009]. In comparison, motor delay is rather mild with a normal or mildly delayed walking age. Developmental regression was not noted in 2 patients [Orrico et al., 2001], but was reported in the other patients with CNTNAP2 defects, starting at the same age as onset of epilepsy between 4 and 30 months [Strauss et al., 2006; Zweier et al., 2009]. Later on, the degree of mental impairment seems to be stable. The early onset mandatory epilepsy with concurrent developmental regression in patients with CNTNAP2 defects might be a discriminating aspect regarding other disorders with regression phenotypes such as Rett syndrome. Furthermore, the discrepancy between severe speech impairment and rather mild motor delay seems to be quite specific for recessive CNTNAP2 defects. Similarly, in the patient with the NRXN1 defect, only mild motor delay with a walking age of 2 years but no speech development was reported [Zweier et al., 2009].
Seizures
The single patient with biallelic NRXN1 defects does not have epilepsy [Zweier et al., 2009]. Due to the limited number of patients, no conclusion about frequency of seizures in NRXN1 related intellectual disability can be made.
All patients with recessive defects in CNTNAP2 do show epilepsy with an early onset between 4 and 30 months of age [Strauss et al., 2006; Jackman et al., 2009; Zweier et al., 2009]. Concurrent with the onset of epilepsy, language regression and deterioration of social behavior occur in most of the patients [Strauss et al., 2006]. Regarding available data reported by Strauss et al. [2006], the average peak seizure frequency (number of events per week) is 50 with complex partial, simple partial, secondarily generalized, and status epilepticus types of seizures.
Growth Parameters
As far as data is available, birth measurements are normal. Apart from 2 siblings with short stature [Orrico et al., 2001; Zweier et al., 2009], later body measurements are reported to be normal as well. Interestingly, many patients show a tendency to rather large head circumferences in relation to body height [Strauss et al., 2006]. This might be a suitable discriminating factor in differential diagnosis to other severe intellectual disability disorders where microcephaly is common.
Behavioral Observations
Behavioral anomalies with autistic traits or pervasive developmental delay, stereotypic movements and attention deficit-hyperactivity disorder are common [Strauss et al., 2006; Jackman et al., 2009; Zweier et al., 2009].
Magnetic Resonance Imaging
MRI examination of the brain showed focal malformations in 3 patients. Two of them had unilateral dysplasia of the anterior temporal lobe, and 1 had a malformation of the left striatum [Strauss et al., 2006]. In 2 other patients, periventricular leukomalacia [Jackman et al., 2009] and cerebellar hypoplasia were observed, respectively [Orrico et al., 2001; Zweier et al., 2009].
Other Findings
Breathing anomalies [Zweier et al., 2009] and decreased deep tendon reflexes [Strauss et al., 2006; Jackman et al., 2009] were reported in some patients; hepatomegaly was observed in 1 girl [Jackman et al., 2009].
Natural History
CNTNAP2
Early infant development appears to be normal or with only mild motor delay and is followed by onset of seizures, language regression or no speech development, with social and behavioral disturbances, and moderate to severe intellectual disability by late childhood [Orrico et al., 2001; Strauss et al., 2006; Jackman et al., 2009; Zweier et al., 2009].
NRXN1
The development of the patient was reported to be normal for the first year before severe intellectual disability with a walking age of 2 years and no speech development was noted [Zweier et al., 2009].
Recommendations for Management
Not much information about treatment is available. In 1 patient, zonisamide was used for treatment of seizures [Jackman et al., 2009]. Electrocorticography-guided epilepsy surgery for disabling complex partial seizures in 3 patients resulted in a temporarily seizure-free period of 6–15 months but with recurrence after this time [Strauss et al., 2006].
Genetics
Information about the Genes and Protein Function
CNTNAP2 is one of the largest genes in the human genome spanning 2.3 Mb on chromosome 7q35–36.1 and consisting of 24 coding exons (NM_014141). NRXN1 also belongs to the largest known genes in humans with spanning 1.1 Mb on chromosome 2p16.3. The classical neurexin genes in mammals have 2 promoters, generating longer α- and shorter β- neurexins, and are subject to additional extensive alternative splicing, generating a large number of variants [Missler and Südhof, 1998]. The representative NRXN1 isoform α 1 (NM_004801) consists of 21 coding exons.
CNTNAP2 encodes for CASPR2, a protein distantly related to the neurexins and regulating neuron-glia contact in vertebrates and glia-glia contact in insects [Bellen et al., 1998]. Vertebrate Caspr2 has been shown to colocalize with Shaker-like K+ channels in the juxtaparanodal areas of Ranvier nodes in myelinated axons of both the CNS and PNS [Arroyo et al., 2001; Poliak et al., 2003]. Furthermore, it seems to play a role in human cortical histogenesis as signs of neuronal migration anomalies were observed in brain samples of patients with a homozygous CNTNAP2 mutation [Strauss et al., 2006].
The presynaptic neurexins like NRXN1 and their postsynaptic binding partners, the neuroligins, are crucial synapse molecules [Li et al., 2007], with α-neurexins playing a role in normal neurotransmitter release and the function of synaptic calcium channels [Missler et al., 2003].
Recently, findings in Drosophila indicated that not only NrxI (NRXN1), but also NrxIV (CNTNAP2) might be involved in synaptic organization and that both proteins might be linked by a common target, presynaptic protein bruchpilot [Zweier et al., 2009].
Mode of Inheritance
The mutations and deletions in the published patients were inherited in an autosomal recessive manner with homozygous or compound heterozygous defects in the patients and heterozygosity for 1 of the defects in each parent [Strauss et al., 2006; Jackman et al., 2009; Zweier et al., 2009]. The carrier parents were reported to be healthy; therefore, penetrance of clinical symptoms associated with heterozygous defects in either gene might be lower than possibly appreciated from previous reports on neuropsychiatric disorders [Verkerk et al., 2003; Feng et al., 2006; Autism Genome Project Consortium, 2007; Belloso et al., 2007; Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; Friedman et al., 2008; Kim et al., 2008; Kirov et al., 2008; Marshall et al., 2008; Vrijenhoek et al., 2008; Zahir et al., 2008; Bucan et al., 2009; Rujescu et al., 2009; Awadalla et al., 2010; Bradley et al., 2010; Ching et al., 2010; Magri et al., 2010; Mefford et al., 2010; Wiśniowiecka-Kowalnik et al., 2010]. However, an increased risk for variable neuropsychiatric disorders has to be considered in carrier individuals.
Frequency of Certain Mutations/Copy Number Variations in Certain Patient Cohorts
The number of patients carrying recessive defects in CNTNAP2 or NRXN1 is too small to give frequencies of certain mutations/copy number variations. In the Amish population, 1 specific stop mutation c.3709delG in the C-terminal region of CNTNAP2 is reported [Strauss et al., 2006]. The other published patients either harbor a homozygous deletion of exons 2–9 or a compound heterozygous intragenic deletion of exons 5–8 and a splice site mutation in exon 10 of CNTNAP2 [Zweier et al., 2009]. For NRXN1, to date only a deletion of exons 1–4 in compound heterozygosity with a stop mutation in exon 15 is known.
Genotype-Phenotype Correlation
Some phenotypic differences such as lack of speech development versus regression or the presence/absence of cortical dysplasia and episodes of hyperbreathing between the Amish patients with the C-terminal stop mutation and the other patients with rather N-terminal defects in CNTNAP2 can be noted [Zweier et al., 2009]. However, it remains currently elusive if these observations might indicate a real genotype-phenotype correlation or if they are due to clinical bias as the phenotype in the Amish patients was classified as an epilepsy syndrome with developmental deterioration, while the other patients were initially classified as having primary intellectual disability with epilepsy.
References
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS. Internodal specializations of myelinated axons in the central nervous system. Cell Tissue Res. 2001;305:53–66. doi: 10.1007/s004410100403. [DOI] [PubMed] [Google Scholar]
- Autism Genome Project Consortium. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadalla P, Gauthier J, Myers RA, Casals F, Hamdan FF, et al. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am J Hum Genet. 2010;87:316–324. doi: 10.1016/j.ajhg.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin – novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Belloso JM, Bache I, Guitart M, Caballin MR, Halgren C, et al. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur J Hum Genet. 2007;15:711–713. doi: 10.1038/sj.ejhg.5201824. [DOI] [PubMed] [Google Scholar]
- Bradley WE, Raelson JV, Dubois DY, Godin E, Fournier H, et al. Hotspots of large rare deletions in the human genome. PLoS One. 2010;5:e9401. doi: 10.1371/journal.pone.0009401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, et al. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:937–947. doi: 10.1002/ajmg.b.31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Schroer R, Yan J, Song W, Yang C, et al. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neurosci Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- Jackman C, Horn ND, Molleston JP, Sokol DK. Gene associated with seizures, autism, and hepatomegaly in an Amish girl. Pediatr Neurol. 2009;40:310–313. doi: 10.1016/j.pediatrneurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Gumus D, Chen W, Norton N, Georgieva L, et al. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Sacchetti E, Traversa M, Valsecchi P, Gardella R, et al. New copy number variations in schizophrenia. PLoS One. 2010;5:e13422. doi: 10.1371/journal.pone.0013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Südhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Orrico A, Galli L, Zappella M, Lam CW, Bonifacio S, et al. Possible case of Pitt-Hopkins syndrome in sibs. Am J Med Genet. 2001;103:157–159. doi: 10.1002/ajmg.1523. [DOI] [PubMed] [Google Scholar]
- Peippo MM, Simola KO, Valanne LK, Larsen AT, Kähkönen M, et al. Pitt-Hopkins syndrome in two patients and further definition of the phenotype. Clin Dysmorphol. 2006;15:47–54. doi: 10.1097/01.mcd.0000184973.14775.32. [DOI] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, et al. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/s0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Vrijenhoek T, Buizer-Voskamp JE, van der Stelt I, Strengman E. Genetic Risk and Outcome in Psychosis (GROUP) Consortium, et al: Recurrent CNVs disrupt three candidate genes in schizophrenia patients. Am J Hum Genet. 2008;83:504–510. doi: 10.1016/j.ajhg.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniowiecka-Kowalnik B, Nesteruk M, Peters SU, Xia Z, Cooper ML, et al. Intragenic rearrangements in NRXN1 in three families with autism spectrum disorder, developmental delay, and speech delay. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:983–993. doi: 10.1002/ajmg.b.31064. [DOI] [PubMed] [Google Scholar]
- Zahir FR, Baross A, Delaney AD, Eydoux P, Fernandes ND, et al. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1alpha. J Med Genet. 2008;45:239–243. doi: 10.1136/jmg.2007.054437. [DOI] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, et al. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]