Abstract
The pentatricopeptide repeat (PPR) gene family represents one of the largest gene families in higher plants. Accumulating data suggest that PPR proteins play a central and broad role in modulating the expression of organellar genes in plants. Here we report a rice (Oryza sativa) mutant named young seedling albino (ysa) derived from the rice thermo/photoperiod-sensitive genic male-sterile line Pei'ai64S, which is a leading male-sterile line for commercial two-line hybrid rice production. The ysa mutant develops albino leaves before the three-leaf stage, but the mutant gradually turns green and recovers to normal green at the six-leaf stage. Further investigation showed that the change in leaf color in ysa mutant is associated with changes in chlorophyll content and chloroplast development. Map-based cloning revealed that YSA encodes a PPR protein with 16 tandem PPR motifs. YSA is highly expressed in young leaves and stems, and its expression level is regulated by light. We showed that the ysa mutation has no apparent negative effects on several important agronomic traits, such as fertility, stigma extrusion rate, selfed seed-setting rate, hybrid seed-setting rate, and yield heterosis under normal growth conditions. We further demonstrated that ysa can be used as an early marker for efficient identification and elimination of false hybrids in commercial hybrid rice production, resulting in yield increases by up to approximately 537 kg ha−1.
The completion of the genome sequence of several higher plants species, including Arabidopsis (Arabidopsis thaliana; Arabidopsis Genome Initiative, 2000), rice (Oryza sativa; Goff et al., 2002; Yu et al., 2002), maize (Zea mays; Schnable et al., 2009), sorghum (Sorghum bicolor; Paterson et al., 2009), castor bean (Ricinus communis; Chan et al., 2010), soybean (Glycine max; Schmutz et al., 2010), potato (Solanum tuberosum; Xu et al., 2011), salt cress (Thellungiella halophila; Dassanayake et al., 2011), and most recently Medicago truncatula (Young et al., 2011), has opened an unprecedented opportunity for functional genomics studies in higher plants. Meanwhile, it poses a significant challenge to the plant science community to elucidate the functions of all plant genes. One of the largest and perhaps the most mysterious gene families uncovered by bioinformatic analyses is the pentatricopeptide repeat (PPR) protein family, which is characterized by the tandem array of a PPR motif, a highly degenerate unit consisting of 35 canonical amino acid. The PPR motif is predicted to resemble the structure of the tetratricopeptide repeat motif that consists of a protein sequence constituting two antiparallel α-helices. This motif is found in a few animal and fungal proteins but the family has expanded greatly in higher plants, with 466 members in Arabidopsis and 480 in rice (Small and Peeters, 2000; Lurin et al., 2004). The vast majority of these proteins are predicted to localize to chloroplasts or mitochondria, but a small portion is predicted as untargeted and they likely localize outside the organelles of the plant cells (Lurin et al., 2004; Ding et al., 2006).
Functional studies of PPR proteins in higher plants remain very sparse. Accumulating data point to an involvement in posttranscriptional processes in organelles. Fisk et al. (1999) first reported a maize PPR gene, (for CHLOROPLAST RNA PROCESSING1), which was implicated by genetic analysis in processing and translation of plastid pet transcripts. Similar effects on plastid transcripts were subsequently observed in other mutants from Arabidopsis (Hashimoto et al., 2003; Meierhoff et al., 2003; Yamazaki et al., 2004; Chateigner-Boutin et al., 2008, 2011; Chi et al., 2008; Okuda et al., 2009, 2010; Yu et al., 2009; Johnson et al., 2010), rice (Kazama and Toriyama, 2003; Komori et al., 2004; Gothandam et al., 2005), and maize (Williams and Barkan, 2003; Schmitz-Linneweber et al., 2006; Pfalz et al., 2009; Prikryl et al., 2011). Additional evidence for a role of PPR proteins in regulating organelle gene expression has also come from positional cloning of several cytoplasmic male sterility (CMS) restorer genes from petunia (Petunia hybrida; Bentolila et al., 2002) and radish (Raphanus sativus; Brown et al., 2003; Desloire et al., 2003; Koizuka et al., 2003). Genetic and biochemical data, and structural modeling of PPR tracts based on established tetratricopeptide repeat proteins together suggest that PPR proteins typically bind directly to specific organellar RNA sequences through a surface created by the stacked helical repeating units. However, still very little is known about the functions, substrates, and regulatory mechanisms for the vast majority of PPR proteins.
Food security is a global concern. The development and utilization of hybrid rice has made a great contribution to ensuring food sufficiency in China, and the annual planting area of hybrid rice has been maintained at about 16 million ha since the early 1990s. In 2010, hybrid rice accounted for about 58% of China’s 196 million tons of rice production. The average yield of hybrids is 7.1 to 7.2 tons per ha compared with 5.8 to 5.9 tons per ha for inbred varieties (Normile, 2008). Recently, hybrid rice has been adopted in other countries. So far, more than 40 countries have participated in the introduction, research, and promotion of hybrid rice worldwide. In 2008, the cultivation area of hybrid rice has reached 1.3 million ha in India, 0.65 million ha in Vietnam, and 0.34 million ha in Philippines. Compared with the traditional CMS-based three-line hybrid rice system, the development of two-line hybrid rice system based on thermo/photoperiod-sensitive genic male-sterile (T/PGMS) lines has raised the yield potential of rice by another 10% (Sing and Limani, 1990; Yuan, 2004). However, a major constraint in utilizing two-line hybrid rice, especially for indica rice, is the unpredictable influence of prevailing temperature and photoperiod conditions on the fertility of the T/PGMS line during the male-sterility-inductive period (Yuan, 1997). Normally, the T/PGMS lines are male sterile under high-temperature and long-day conditions, but they become fertile under lower-temperature and short-day conditions—these conditions are used for the propagation and maintenance of T/PGMS lines. This means that a significant fall in temperature during the male-sterility-inductive period could cause the male-sterile lines to become partially fertile, resulting in contamination of the hybrid seeds by T/PGMS selfed seeds and, as a consequence, a decrease in hybrid yield (Bi et al., 1990).
Great efforts have been made to minimize the environmental effect in hybrid rice production. However, previous attempts using leaf-color mutants as screening markers to monitor seed purity in two-line hybrid rice production have achieved limited success (Wu et al., 2002). To search for markers better suited for use in early removal of false hybrids, we sought to identify markers that allow early detection of false hybrids resulted from selfed T/PGMS seeds. In this study, we isolated a rice mutant named young seedling albino (ysa), which develops albino leaves before the three-leaf stage. Map-based cloning revealed that YSA encodes a PPR protein. We demonstrated that the ysa mutation could be used as an early marker for efficient identification and elimination of false hybrids in commercial hybrid rice production, resulting in a grain yield increase of up to approximately 537 kg ha−1.
RESULTS
Phenotypic Analysis of the ysa Mutant
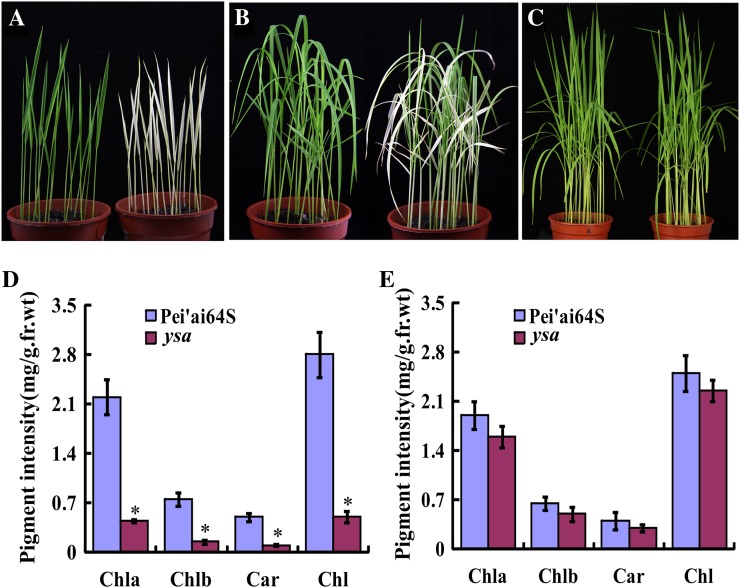
Leaves of ysa mutant appeared albinic albino before the three-leaf stage (Fig. 1A), but gradually turned green from the four-leaf stage onward (Fig. 1B) and appeared normal green like the leaves of parental Pei'ai64S plants after the six-leaf stage (Fig. 1C). The contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid in ysa plants before the three-leaf stage were drastically lower than those in the parental Pei'ai64S plants (Fig. 1D). After regreening, the levels of these pigments in the ysa mutant increased and became similar to Pei'ai64S plants (Fig. 1E). These results suggest that the albino phenotype of the young ysa seedlings is caused by a reduction in total chlorophyll content, rather than reduction of a particular pigment.
Figure 1.
Phenotypic analysis of the ysa mutant plants. A to C, Phenotypes of Pei'ai64S (left) and ysa mutant (right) seedlings at 1 (A), 2 (B), and 3 (C) weeks after sowing. D, The pigment contents in leaves of 1-week-old ysa mutants are much lower than that in Pei'ai64S. E, The pigment contents in leaves of 6-week-old ysa mutants are similar to that of Pei'ai64S plants. Chla, Chlorophyll a; Chlb, chlorophyll b; Chl, total chlorophyll; Car, carotenoid. Bars represent sds of three measurements. Student’s t test was performed on the raw data; asterisk indicates statistical significance at P < 0.01.
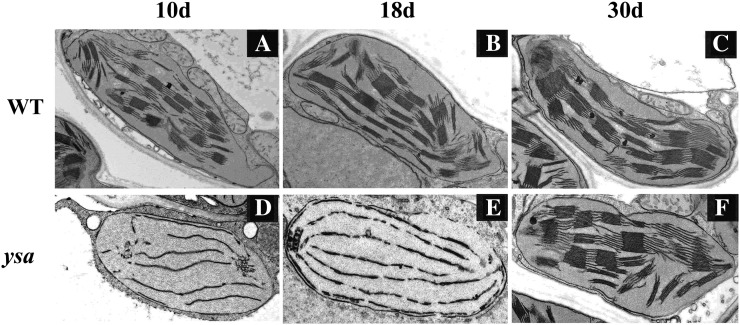
We next examined whether the lack of photosynthetic pigments in the ysa mutant were accompanied by ultrastructural changes in the chloroplasts. We compared the ultrastructure of chloroplasts in the ysa mutant and Pei'ai64S at three developmental stages using transmission electron microscopy. Granal stacks in Pei'ai64S plants were dense and well structured at all three developmental stages (Fig. 2, A–C). The granal stacks in ysa mutant were less dense and lacked well-structured thylakoid membranes before the three-leaf stage (Fig. 2D). Subsequently, the thylakoid membranes slightly increased (Fig. 2E), and the granal stacks in ysa mutant were similar to those of wild-type plants after the six-leaf stage (Fig. 2F). These observations revealed that the ysa mutation primarily affects chloroplast development in the first few leaves.
Figure 2.
Transmission electron microscopic images of chloroplasts of wild-type Pei'ai64S and ysa mutant plants at 10 d (A and D), 18 d (B and E), and 30 d (C and F) after sowing. Chloroplasts of Pei'ai64S have abundant, well-ordered stacks membranes after sowing for 10, 18, or 30 d, but ysa have normal stacked membranes only after 30 d.
Cloning of the YSA Gene
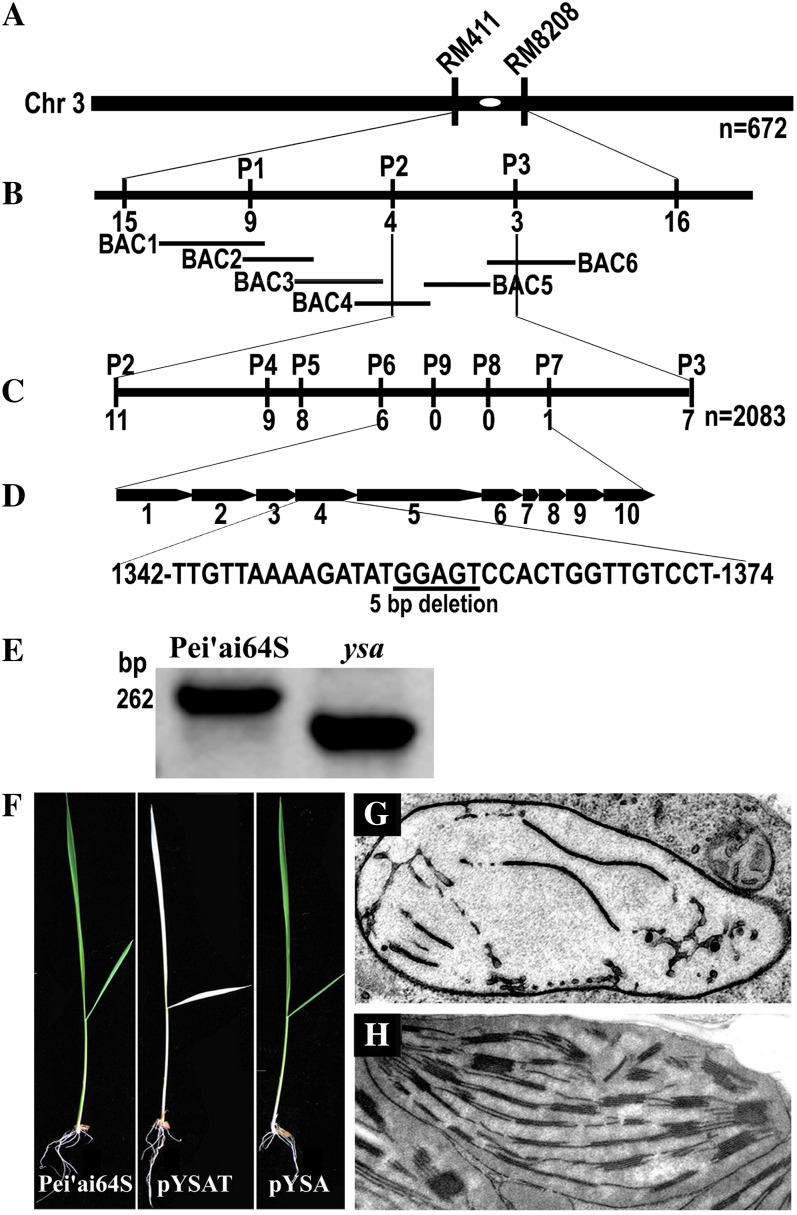
To understand the molecular basis of the phenotype, we used map-based cloning to isolate the YSA gene. Genetic analyses from reciprocal crosses between the ysa mutant and the two indica rice cultivars 93-11 and Taiziyuzhu showed that the albino phenotype in the ysa mutant is recessive and controlled by a single gene (Supplemental Table S1). Molecular analysis of an F2 population from the cross Taiziyuzhu × ysa placed the YSA locus between the markers RM411 and RM8208 on chromosome 3 (Fig. 3A). Five simple sequence repeat markers, four derived cleaved amplified polymorphic sequence (dCAPS) markers, and one insertion-deletion polymorphism (InDel) marker were developed between RM411 and RM8208 (Supplemental Table S2). The YSA locus was further narrowed down to a 45-kb region, which includes 10 putative open reading frames (ORFs; Fig. 3, B–D). We sequenced all ORFs and found a 5-bp deletion in Os03g40020, causing a premature stop codon (Fig. 3D). A newly developed InDel marker P9 can be used to distinguish ysa mutant and wild-type Pei'ai64S by the size of the amplified genomic DNA fragment (Fig. 3E). Molecular complementation experiment showed that all of the 25 independent transgenic lines transformed with vector pYSA containing the wild-type Os03g40020 gene completely rescued the ysa mutant phenotype as judged by the color of the first few leaves and their thylakoid structure, whereas 20 independent lines transformed with vector pYSAT containing the shifted ORF all failed to rescue the ysa mutant (Fig. 3, F and G). These results suggested that the 5-bp deletion in Os03g40020 is responsible for the albino phenotype of ysa mutant.
Figure 3.
Cloning of the YSA gene. A, The YSA locus was initially mapped to the centromeric region between markers RM411 and RM8208 on chromosome 3 (Chr. 3). B, Mapping of the YSA locus with markers P1 to P3 developed based on bacterial artificial chromosome clone sequence (BAC1, OSJNBa0010D22; BAC2, OSJNBb0085A04; BAC3, OSJNBa0027H16; BAC4, OSJNBb0056O10; BAC5, OSJNBb0042N11; BAC6, OSJNBa0087M10). C, Fine mapping of the YSA locus with markers P4 to P9. The YSA locus was narrowed down to a genomic DNA region of 45 kb between dCAPS markers P6 and P7. The number of recombinants identified from 2,083 recessive individuals was shown for each marker. D, Diagram of the predicted ORFs (highlighted with arrows) and the mutation site. A 5-bp deletion (underlined) in ORF4 results in a premature stop codon. E, The difference between ysa mutant and Pei'ai64S is shown by the size of amplified genomic DNA by a newly developed InDel marker P9. F, Complementation of the ysa mutant. The wild-type Pei'ai64S plants (left) and the ysa mutants transformed with pYSA vector (right) shows normal green leaves, whereas the mutant transformed with pYSAT vector has albino leaves. G, Chloroplasts of plants transformed with pYSAT have few membrane stacks. H, Chloroplasts of plants transformed with pYSA have abundant and well-ordered stacks.
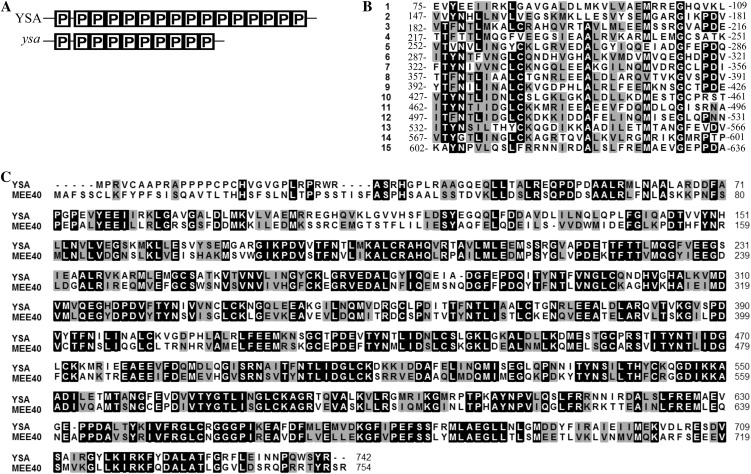
The predicted ORF of YSA encodes a 742-amino acid polypeptide with a calculated molecular mass of 82.5 kD. Database searches using Pfam (Finn et al., 2006) revealed that YSA encodes a member of the P subfamily of PPR-containing proteins. The gene product contains a tandem repeat of 15 PPR motifs with varying degrees of conservation (Fig. 4A). The second to 15th repeats are contiguous, and the sequence is not well conserved (Fig. 4B). The PPR motif is a degenerate 35-amino acid repeat often arranged in tandem arrays of two to 27 repeats per polypeptide. Thus YSA is a new member of the superfamily of PPR proteins.
Figure 4.
Sequence analysis of YSA. A, The YSA protein has 15 PPR motifs, whereas the ysa mutant protein has only 10 PPR motifs (P). B, Comparison of the PPR motifs of YSA. Amino acids fully or semiconserved are shaded black and gray, respectively. C, Amino acid sequence alignment of YSA and MEE40. Amino acids fully or semiconserved are shaded black and gray, respectively.
BLAST searches of the available genome sequences revealed that in the rice genome, there is another gene (Os11g03850) encoding a PPR protein that shares high sequence homology to YSA (33.4% amino acid identity). Close homologs of YSA were also identified in sorghum, maize, Arabidopsis, Populus trichocarpa, M. truncatula, and soybean (Supplemental Fig. S1); however, the functions of these genes are largely unknown. Notably, YSA and the Arabidopsis protein MEE40 (encoded by At3G53700) possess a similar domain structure and they share the highest sequence homology across their entire lengths (51% amino acid identity; Fig. 4C). Interestingly, MEE40 protein was previously reported to be essential for embryogenesis (Pagnussat et al., 2005).
Expression Analysis of YSA
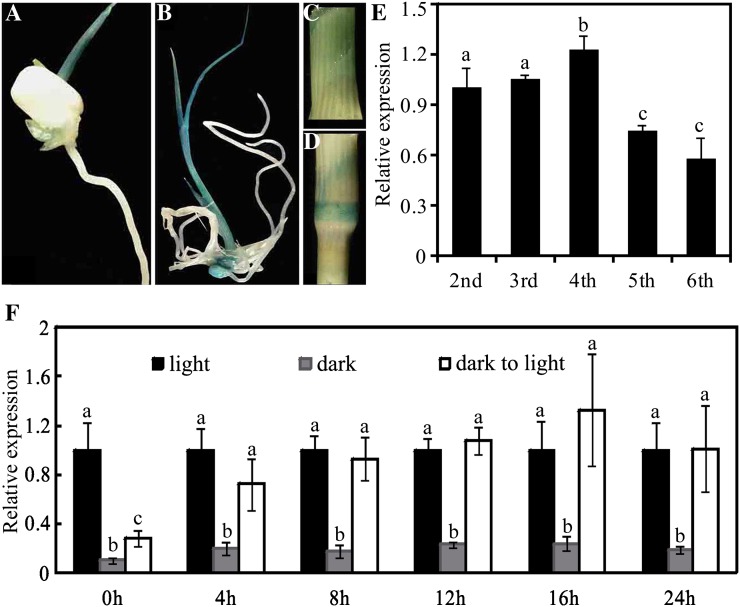
To examine the tissue-specific expression pattern of YSA, we generated transgenic plants expressing a GUS reporter gene driven by the YSA promoter (pYSA::GUS). Histochemical assay revealed that YSA is highly expressed in young leaves and stems, but not in the roots (Fig. 5, A–D). Quantitative real-time reverse transcription (RT)-PCR analysis revealed that the expression of YSA peaked in the fourth leaf (Fig. 5E). Thus, the expression pattern of YSA is consistent with the seedling-stage-specific albino phenotype of ysa mutant and further supports the notion that YSA plays an important role in chloroplast development in the first few leaves of rice seedlings, but plays more minor roles in later stages.
Figure 5.
Expression analysis of YSA. A to D, GUS staining shows that YSA is highly expressed in young leaves and stem, but not in the roots and mature stems. E, Transcript levels of YSA in leaves of different developmental stages. The YSA RNA level in the Pei'ai64S plants of two-leaf stage was set to 1.0, and the relative YSA RNA levels in other developmental stages were calculated accordingly. F, YSA expression in 10-d-old etiolated Pei'ai64S plants after different times of illumination. After growing in darkness for 10 d, the etiolated rice seedlings were illuminated for 4, 8, 12, 16, or 24 h. The YSA RNA level in seedlings growing under illumination was set to 1.0, and the relative YSA RNA level in seedlings growing under dark or dark-to-light condition were calculated accordingly. Seedlings growing under continuous light or dark were chosen as the control. Error bars (sds) are based on three independent experiments. Bars with different letters indicate significant differences at P < 0.05 level based on the one-way ANOVA assay.
Light is one of the most important environmental signals that trigger the differentiation of nonphotosynthetic proplastids into fully functional photosynthetic chloroplasts. To examine the effects of light on the expression of YSA, we examined the accumulation of YSA transcripts during light-induced greening of Pei'ai64S seedlings. As shown in Figure 5F, YSA transcript level was high under light, but much lower in the dark in 10-d-old etiolated seedlings. Furthermore, the expression level of YSA was highly induced after illumination for 4 h and reached maximal levels after 16 h of illumination (Fig. 5F). These observations suggest that light plays a major role in regulating YSA expression.
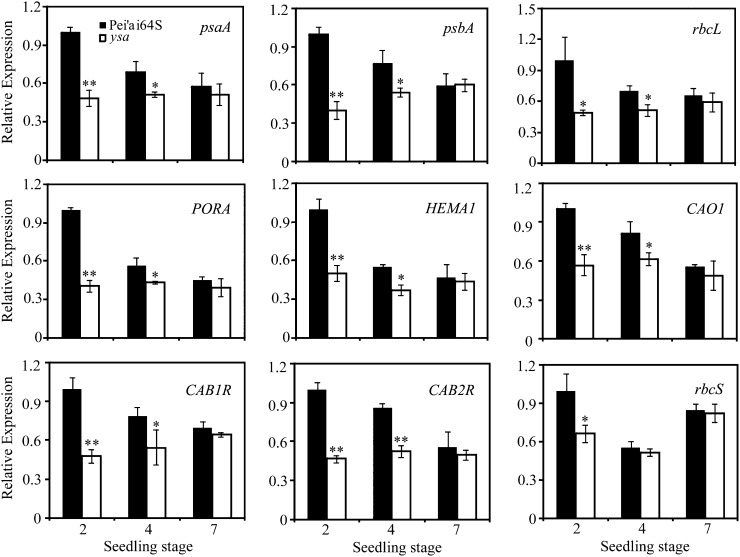
We next examined the transcription levels of other genes associated with chlorophyll biosynthesis, chloroplast development, or photosynthesis in the ysa mutant. Nine genes were selected, including those encoding a glutamyl-tRNA reductase (HEMA1), CHLOROPHYLLIDE A OXYGENASE1 (CAO1), NADPH: PCHLIDE OXIDOREDUCTASE, light-harvesting Chl a/b-binding protein of PSII (CAB1R and CAB2R; Matsuoka, 1990), two reaction center polypeptides (psaA and psbA), Rubisco large subunit, and Rubisco small subunit (Kyozuka et al., 1993). Quantitative real-time PCR analysis showed that the transcripts of both nuclear-encoded genes (such as CAB1R, CAB1R, HEMA1, CAO1, and Rubisco small subunit) and plastid-encoded genes (such as psaA, psbA, and Rubisco large subunit) were all severely suppressed at the two-leaf stage in the ysa mutant (Fig. 6), but the suppression became alleviated in the ysa mutant at the four-leaf stage, and was fully restored to wild-type levels at the seven-leaf stage. These results indicated that YSA plays an important role in regulating gene expression associated with chlorophyll biosynthesis, chloroplast development, and photosynthesis in the first few leaves.
Figure 6.
Expression analysis of genes associated with chlorophyll biosynthesis, photosynthesis, or chloroplast development by real-time PCR. The relative expression level of each gene was normalized using Actin1 as an internal control. The expression level of each gene at the two-leaf stage in Pei'ai64S was set as 1.0 and other samples were calculated accordingly. Bars represent sds of three independent experiments. Asterisks indicate statistically significant differences compared with Pei'ai64S (Student’s t test: *P < 0.05; **P < 0.01).
Subcellular Localization of YSA Protein
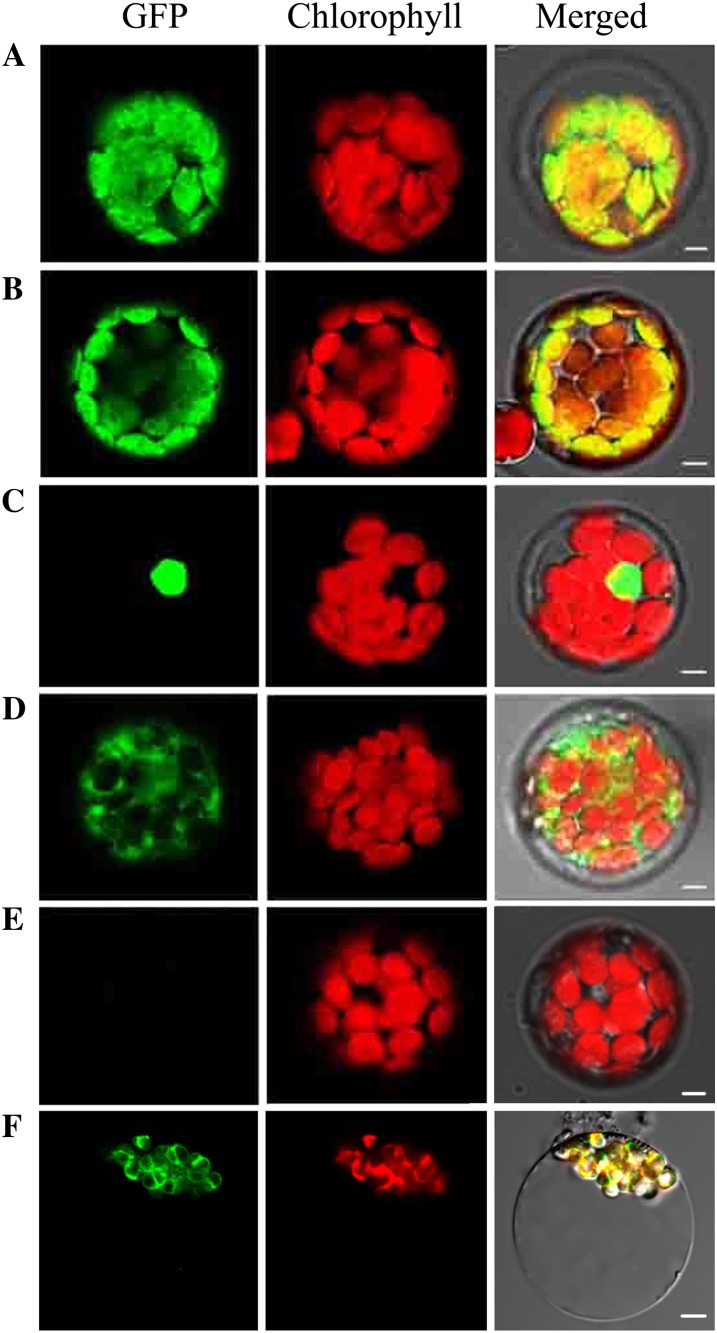
Most PPR proteins are predicted to be targeted to either mitochondria or chloroplasts (Small and Peeters, 2000). The YSA protein is predicted to localize to chloroplasts by ChloroP (Emanuelsson et al., 1999) and TargetP (Emanuelsson et al., 2000). To investigate the actual cellular localization of YSA, we constructed a chimeric gene expressing a fusion protein consisting of the 200 N-terminal amino acids of YSA and GFP under the control of the 35S promoter. The plasmid containing the chimeric gene was transformed into Arabidopsis protoplasts, and confocal microscopy was used to observe the fluorescent signals 16 h after transformation. The green fluorescent signals of YSA-GFP fusion proteins colocalized with the autofluorescent signals of chlorophylls in the chloroplasts, consistent with the results obtained for GFP fused to the transit peptide of the small subunit of Arabidopsis ribulose bisphosphate carboxylase (Fig. 7, A and B). When GFP fused to the nuclear localization signal of the fibrillarin protein, GFP signals located specifically in the nucleus of Arabidopsis protoplasts (Fig. 7C). In addition, the protoplasts transformed with the empty GFP vector without a specific targeting sequence had green fluorescent signals in both the cytoplasm and the nucleus (Fig. 7, D and E). To further confirm the subcellular localization of YSA protein, we transformed the plasmid containing the YSA-GFP fusion constructs into rice protoplasts. Confocal microscopy observations revealed that GFP-YSA was exclusively detected in the chloroplasts (Fig. 7F). These findings suggest that YSA protein is localized to the chloroplast.
Figure 7.
Subcellular localization of YSA protein. Fluorescence signals were visualized using confocal laser-scanning microscopy. Green fluorescence shows GFP, red fluorescence indicates chloroplast autofluorescence, and yellow fluorescence indicates images with the two types of fluorescence merged. A, GFP signals of the YSA-GFP fusion protein. B, GFP signals from the transit peptide of ribulose bisphosphate carboxylase small subunit (control). C, GFP signals from the nuclear localization signal of fibrillarin (control). D, Empty GFP vector without a specific targeting sequence. E, Untransformed chloroplasts. F, Subcellular localization of YSA protein in rice protoplasts. Bars = 5 μm. A to E are Arabidopsis protoplasts and F is rice protoplasts.
Utilization of ysa as an Early Marker to Remove Off-Type Seedlings during T/PGMS Line Propagation and Hybrid Seed Production
To use the albino phenotype of ysa mutant as a leaf-color marker in hybrid rice production, we first examined the potential effect of the ysa mutation on several important agronomic traits of T/PGMS lines. There were not any discernible differences between the parental Pei'ai64S plants and the ysa plants in heading date, plant height, tiller number, panicle length, floret number, stigma extrusion rates, selfed seed-setting rate, hybrid seed-setting rate, 1,000-grain weight, and yield per plant (Supplemental Table S3). In addition, we examined the potential effect of the ysa mutation on male sterility, using the standard methods of T/PGMS authentication used by the China National Rice Research Institute. The results showed that Pei'ai64S and the ysa mutant had similar seed-setting rates when grown under different temperature and photoperiod conditions (Supplemental Table S4). These data indicated that the ysa mutation did not affect the agronomic traits of the parental Pei'ai64S T/PGMS line.
Yield heterosis is the key trait for the male-sterile parent in hybrid rice production and F1 hybrid yield is the main measurement for yield heterosis in the field. Using the same restorer line 93-11, we found that all F1 hybrid plants derived from Pei'ai64S or ysa displayed normal green leaves and similar grain yields and other agronomic traits, indicating that the ysa mutation did not have any negative effects on yield heterosis (Supplemental Table S3). We next tested the ysa mutation as a marker for removing off-type seedlings in T/PGMS line propagation. Results showed that only the true male-sterile plants derived from self-pollination develop albino leaves, whereas off-type seedlings from cross-pollination are green (Fig. 8A). We randomly sampled 5,000 seeds from self-pollinated ysa T/PGMS line grown under conditions necessary for full fertility, and sowed them in different fields in 2006 and 2007. Off-type seeds arising from biological mixtures (cross-pollination) or mechanical mixtures were identified at the seedling stage. This sampling showed that the seeds produced from selfed ysa were approximately 99.8% pure each year, and the seed purity reached 100% after removal of the false-positive green seedlings (Table I). It is worth noting that although the false-positive seedlings were only approximately 0.12% of the total seedlings in the trials, the improvement in the self-pollinated ysa T/PGMS seeds is significant in commercial seed production. With an average planting density of 300,000 plants ha−1, and at an average productivity of 500 seeds plant−1, there will be approximately 168,000 off-type T/PGMS seeds produced per ha in commercial seed production.
Figure 8.
Application of the ysa marker in T/PGMS propagation and two-line hybrid seed production. A, The ysa marker identifies the green off-type seedlings during propagation of T/PGMS seeds. B, ysa identifies albino off-type seedlings during two-line hybrid seed production.
Table I. Utilization of the ysa marker to remove off-type plants in T/PGMS line propagation and F1 hybrid rice production.
The number of off-type seeds were estimated according to the formula: 300,000 plants/ha × 500 seeds/plant × percentage of off-type seedlings (6/5,000 and 3/5,000 for 2006 and 2007, respectively). The estimated yield increase was calculated based on the formula: 300,000 plants/ha × percentage of false hybrids (183/5,000 or 121/5,000 for 2006 and 2007, respectively) × yield per plant (average yields of ysa × 93-11 F1 hybrid were 48.6 and 48.3 g for 2006 and 2007, respectively). NA, Data not available.
| Parameters Investigated | ysa T/PGMS Line | F1 (ysa × 93-11) | ||
|---|---|---|---|---|
| Year |
2006 |
2007 |
2006 |
2007 |
| Seed number | 5,000 | 5,000 | 5,000 | 5,000 |
| Green seedlings | 6 | 3 | 4817 | 4,879 |
| No. of off-type plants | 6 | 3 | 183 | 121 |
| Original seed purity (%) | 99.88 | 99.94 | 96.34 | 95.78 |
| Seed purity after removal of the false-positive seedlings (%) | 100 | 100 | 100 | 100 |
| Increase in seed purity after early removal of off-type seedlings utilizing the ysa marker (%) | 0.12 | 0.06 | 3.66 | 2.42 |
| Estimated off-type seeds produced per ha | 168,000 | 84,000 | NA | NA |
| Estimated yield increase (kg/ha) | NA | NA | 537 | 367 |
On the other hand, for hybrid seed production, it is the albino seedlings that are off-type seeds (false hybrid) and derived from self-pollination of the ysa mutant, and it is the green seedlings that are true F1 hybrids derived from cross-pollination with the restorer (Fig. 8B). We also randomly sampled 5,000 F1 seeds generated from crosses involving ysa with the 93-11 restorer line and sowed them in a field. We found 183 and 121 albino seedlings in the 2006 and 2007 trial, respectively (Table I). Although these off-type seedlings were only approximately 3.66% of the total samples, their removal increased the hybrid seeds purity to 100%. According to these data, we estimated that the use of the ysa mutation increased hybrid seed yield by up to 537 kg/ha and 367 kg/ha in 2006 and 2007, respectively (Table I).
To further test and expand the utility of the ysa marker, we introduced the ysa mutation into Guangzhan63S, which is one of the most popular commercial T/PGMS lines currently used for hybrid seed production in China. Analysis of agronomic traits and the heterosis confirmed that ysa worked well as an efficient marker for T/PGMS propagation and hybrid seed production (Supplemental Table S3). Recently, a new hybrid rice variety with good grain quality and high yield potential, harboring the ysa marker, was released, and tested in provincial trials. It is estimated that approximately 10,000 ha of hybrid rice developed by utilizing the ysa marker are now being grown in the middle and lower regions of Yangtze River, and that the acreage of hybrid rice based on this ysa system will reach approximately 0.2 million ha by 2012 or shortly thereafter.
DISCUSSION
Here, we showed that a rice PPR protein, YSA, is required for chloroplast development in early seedling leaves, and disruption of its function causes a seedling-stage-specific albino phenotype, but the plant recovers and develops normal green leaves from the four-leaf stage onward. We showed that the ysa mutation could be used as an early marker for efficient identification and elimination of false hybrids in commercial hybrid rice production.
YSA Encodes a PPR Protein Essential for Chloroplast Biogenesis in Early Leaves
The PPR protein family is one of the largest families in plants. Although PPR proteins are found in other eukaryotes, their large number (466 members in Arabidopsis and 480 in rice) is probably required for plants to meet the specific needs of organellar gene expression. Most PPR proteins are predicted to localize to plastids or mitochondria. It is generally believed that the repeats of PPR proteins form a superhelical structure to bind a specific ligand, probably a single-stranded RNA molecule, and modulate its expression (Lurin et al., 2004).
Functional analyses on this group of proteins in plants have just begun. PPR proteins have been implicated in many crucial functions including chloroplast biogenesis, embryogenesis fertility restoration in CMS plants, and plant development (Saha et al., 2007). An emerging theme of PPR protein function is regulation of various aspects of gene expression in plastids, including transcription, splicing, RNA cleavage, RNA editing, translation, and RNA stabilization (Schmitz-Linneweber and Small, 2008). For example, the Arabidopsis dg1 (for delayed greening1) and ys1 mutants (for yellow seedling1) display a seedling-stage-specific albino and yellow seedling phenotype, respectively. Both DG1 and YS1 encode chloroplast-targeted PPR proteins and YS1 has been shown to be required for editing of rpoB transcripts (Chi et al., 2008; Zhou et al., 2009). The null mutation of the maize PPR2 gene leads to albino seedlings, but does not affect seed development or gametogenesis (Williams and Barkan, 2003). Maize PPR4 (ZmPPR4) is a chloroplast-targeted protein harboring both a PPR tract and an RNA recognition motif and it facilitates transsplicing of chloroplast rps12 premRNA (Schmitz-Linneweber et al., 2006). ZmPPR10 bound to its native binding site in the chloroplast atpI-atpH intergenic region stabilizes RNA and activates translation (Pfalz et al., 2009; Prikryl et al., 2011).
So far, only one PPR protein affecting chloroplast development in rice has been reported. OsPPR1 is a chloroplast-targeted PPR protein with 11 PPR motifs, and is required for chloroplast biogenesis (Gothandam et al., 2005). This study reports the second PPR protein, YSA, to be required for chloroplast biogenesis in rice. This finding adds further support to a central role of PPR proteins in regulating chloroplast development in a staple crop plant. However, there are several important differences between OsPPR1 and YSA proteins and their respective mutant phenotypes. First, although both OsPPR1 and YSA belong to the PPR gene family and share similar secondary structure, they do not share significant primary sequence homology (amino acid identity is only 15.64%). Phylogenetic analysis revealed that the two proteins are grouped in different clades (Supplemental Fig. S1), suggesting that they might have been evolved from distinct immediate ancestors. Second, the albino phenotype of the ysa mutant is seedling stage specific and the mutant is viable, whereas the OsPPR1 antisense transgenic plants are lethal and their pigmentation is not reversible. Third, no internal membranes or clear prolamellar body were detected, with only proplastid-like structures being detected in the chloroplast of OsPPR1 antisense transgenic plants (Gothandam et al., 2005), whereas chloroplasts with reduced thylakoid membrane were detected in the ysa mutant leaves. Further, the expression levels of a number of chloroplast development-associated genes were significantly down-regulated in the two-leaf-stage ysa mutant as compared with Pei'ai64S, but the differences became less at the four-leaf stage, and disappeared at the seven-leaf stage (Fig. 6). However, it was reported that the expression level of psaA and psbA rarely decreased in the OsPPR1 antisense plants (Gothandam et al., 2005). Taken together, these results suggest that YSA and OsPPR1 may play distinct roles in regulating chloroplast development. Future studies will be required to further dissect the functional relationship between YSA and OsPPR1 and the molecular mechanisms linking YSA to chloroplast development.
Why the ysa mutant displays a seedling-stage-specific albino phenotype is an intriguing question. A possible explanation is that other related genes may compensate for the absence of YSA during later developmental stages. It is also possible that YSA is not required for later developmental stages of chloroplast development, as the expression level of YSA decreased as their development proceeded (Fig. 5E). This phenotype, in essence, is very similar to a previously described virescent (v) phenotype, which is defined as presenting young leaves with reduced chlorophyll but can accumulate almost normal amounts of chlorophyll as they mature (Archer and Bonnett, 1987). Due to the unique features of their chloroplast biogenesis and potential value in agriculture, v mutants have been studied for more than half a century in various plant species, including maize, rice, cotton (Gossypium hirsutum), tobacco (Nicotiana tabacum), peanut (Arachis hypogaea), and Arabidopsis (Zhou et al., 2009). To date, only a few genes responsible for v phenotype have been isolated and their molecular mechanisms remain largely unknown. For example, the rice yellow-green leaf1 (ygl1) mutant showed yellow-green leaves in young plants with decreased chlorophyll synthesis and delayed chloroplast development. The ygl1 mutant carries a missense mutation (Pro-198 to Ser) in the rice chlorophyll synthase gene (Wu et al., 2007). Such mutants became green with near-normal chlorophyll accumulation at the mature stage. Why the ygl1 mutation, like ysa, dramatically affects chlorophyll biosynthesis in the early developmental stages but appears to be dispensable in the later stages is also not clear. The rice v2 mutant develops chlorotic leaves at the restrictive temperature (20°C), but develops nearly normal green leaves at the permissive temperature (30°C; Iba et al., 1991). The v2 mutation affects activation of the chloroplast translation machinery and/or expression of the plastid-to-nucleus signaling pathway during the P4 stage of leaf development (Sugimoto et al., 2004). The V2 gene was cloned and shown to encode a novel type of guanylate kinase (Sugimoto et al., 2007). Clearly, chloroplast development and pigmentation is under complex genetic control and elucidating the molecular mechanisms regulating chloroplast and leaf development in higher plants remains a major task for future studies.
On the other hand, despite the overwhelming accumulated evidence supporting the notion that the majority of PPR proteins are involved in organellar RNA metabolism, studies of their biochemical function have remained quite limited. Attempts to identify the in vivo RNA ligands have only been made for a few PPR proteins (Schmitz-Linneweber and Small, 2008; Okuda et al., 2009, 2010; Pfalz et al., 2009; Yu et al., 2009; Johnson et al., 2010; Chateigner-Boutin et al., 2011; Prikryl et al., 2011). Many of the postulated activities of PPR proteins still lack direct biochemical proof and thus leave open the possibility that their roles are more indirect than suspected and may require additional, unidentified factors. Further studies will be aimed at identifying the RNA ligands and/or interacting proteins that YSA binds to and determining the biochemical activity of YSA.
Advantages of Utilizing ysa as a Seedling-Stage-Specific Marker for Enhancing Seed Purity during Hybrid Seed Production
The discovery of T/PGMS lines opened up a new avenue for the development of two-line hybrid rice systems (Sing and Limani, 1990). However, a major constraint in utilizing two-line hybrid rice, especially for indica rice, is the unpredictable influence of prevailing temperature and photoperiod conditions on the fertility of the T/PGMS line during the male-sterility-inductive period (Liao et al., 2001). Previous efforts using leaf-color mutations as screening markers in two-line hybrid rice production achieved only limited success (Dong et al., 1995; Cao et al., 1999; Wu et al., 2002; Song and Song, 2007). These mutations affect pigmentation throughout the life cycle of the rice plant, resulting in lower photosynthetic rates and decreased grain yields in both T/PGMS lines and hybrids developed from them. Additionally, the pale-green and light-yellow leaves are not easily distinguishable when grown in the field, making the mutations inefficient as the screening tools.
Compared with other methods currently used to remove false hybrids in the two-line hybrid rice production system, the ysa marker offers two major advantages. In ysa T/PGMS line propagation and hybrid seed production, off-type contaminants arising from biological mixtures or mechanical mixtures can be easily identified visually and removed prior to transplanting (which is still a largely manual process in China and many other Asian countries), ensuring pure line in the paddy field. Assuming that 1 ha T/PGMS seedlings in a nursery will be transplanted to 20 ha paddy field, and that 30 man hours are needed to eliminate all the off-type contaminants per ha, at least 600 man hours of labor are needed for each 20 ha of paddy rice in traditional cultivation. Utilizing the ysa marker, only 15 man hours are needed to eliminate the false positives, equivalent to a 40-fold increase in efficiency. In addition, identification and removal of the green seedlings does not require any special training. Thus, the ysa marker can be the basis of a simple, rapid, and economic seed purity determination method requiring only 1 week to achieve results. In comparison, other practices require examination of the hybrid plants at the heading and flowering stages, which is costly, time consuming, and not reliable.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The rice (Oryza sativa) albino leaf mutant ysa was identified from a M2 population of the indica cultivar Pei'ai64S irradiated with Cobalt-60 γ rays at a total dose of 300 Gy and a dosage rate of 5 Gy/min. M2 seeds were sown on a seedling bed under standard field conditions. Mutants displaying an albino phenotype at the two-leaf or three-leaf stage were labeled and transplanted to the paddy rice field at the five-leaf or six-leaf stages. Mature M3 seeds were then harvested from each plant developed as plant lines uniformly showing the trait. One mutant expressing young seedling albino termed ysa was observed in subsequent M4 to M7 generations. Homozygous M8 ysa mutants were selected and used for in-depth analysis. Rice plants were cultivated in experimental fields in Beijing (39°54′N, summer season, temperate climate), Zhejiang (30°15′N, summer season, temperate climate), and Hainan (18°16′N, winter season, subtropical climate) under local growing conditions.
Cloning of YSA
For genetic analysis, F2 populations were generated from crosses between the ysa mutant and three wild-type varieties, Pei'ai64S, 93-11, and Taiziyuzhu. The population from the cross between ysa and Taiziyuzhu, which includes 7,760 normal green individuals and 2,240 mutant plants, was used for fine mapping of the YSA locus. Genomic DNA was extracted from F2 plants and analyzed for cosegregation using available simple sequence repeat markers (McCouch et al., 2002). New markers were developed based on the entire genomic sequences of the Nipponbare variety (Goff et al., 2002) and the indica variety 93-11 (Yu, et al., 2002). dCAPS markers were designed using the Web server program dCAPS Finder 2.0 (http://helix.wustl.edu/dcaps/dcaps.html). Full-length cDNAs for candidate genes were amplified using the GeneRacer kit following the manufacturer’s instructions (Invitrogen, L1500-02).
Complementation of the ysa Mutant
Plasmids for transgenic plants were constructed using standard molecular techniques. To create the complementation construct pYSA, the genomic fragment encoding YSA was amplified by RT-PCR from Pei'ai64S with the primers 5′-cgcaagcttctagagggacgacgacatcgccggcggg-3′ and 5′-ctggatcctcggtaactccattgagggttg-3′. To create the pYSAT construct, the frame shifted coding region of ysa was amplified by RT-PCR with the primers 5′-cgcaagcttctagagggacgacgacatcgccggcggg-3′ and 5′-ctggatccttatatgtaattgtacttcgaggacaacc-3′. The resulting fragments were inserted into the binary vector pCUbi1390 in which the YSA gene was under the control of the constitutive maize (Zea mays) ubiquitin promoter. The constructs were introduced into ysa mutant by Agrobacterium tumefaciens-mediated transformation as described previously (Jeon et al., 2000).
Chlorophyll and Carotenoid Content Measurement
Chlorophyll and carotenoid contents were determined according to the method of Wu et al. (2007). Leaves (approximately 0.2 g fresh weight) were cut and homogenized in 5 mL of 9:1 acetone:0.1 m NH4OH, and centrifuged at 3,000g for 20 min. The supernatants were combined and washed successively with an equal volume of hexane for three times and then the pigment content was measured with a DU 800 UV/Vis spectrophotometer (Beckman Coulter).
Transmission Electron Microscopy
Samples of wild-type and ysa mutant leaves were prepared for transmission electron microscopy by cutting into small pieces, fixed in 2.5% glutaraldehyde in a phosphate buffer at 4°C for 4 h, rinsed and incubated overnight in 1% OsO4 at 4°C, dehydrated through an ethanol series, and infiltrated with a graded series of epoxy resin, and then embedded in Epon 812 resin. Thin sections were obtained using a diamond knife and a Reichert OM2 ultramicrotome, stained in 2% uranyl acetate, and 10 mm lead citrate, pH 12, and then viewed with a JEOL 100 CX electron microscope.
Subcellular Localization of GFP Proteins
To investigate the subcellular localization of YSA, a gene fragment encoding the N-terminal region (amino acids 1–200) of the YSA protein was amplified by PCR and ligated into the PA7-GFP vector (kindly provided by Dr. Hongquan Yang), in frame with GFP, resulting in pA7-YSA-GFP. The primer pair used for YSA was: 5′-ggaccatggacatgccccgcgtttgcgccgcccctcg-3′ and 5′-catcactagtttgctcccctccacaagaacattgag-3′. The vectors for the nuclear localization signal of the fibrillarin protein and the transit peptide of the small subunit of ribulose bisphosphate carboxylase of Arabidopsis (Arabidopsis thaliana) were kindly provided by Dr. W. Chi (Chi et al., 2008). The constructs were introduced into Arabidopsis protoplasts according to the protocols described previously (Yoo et al., 2007). The transfected protoplast suspensions were incubated overnight on 12-well plates in the dark.
To investigate the subcellular localization of YSA in rice protoplasts, the pA7-YSA-GFP vector was transformed into rice protoplasts according to the protocols described previously (Chen et al., 2006). GFP fluorescence in the transformed protoplasts was imaged using a confocal laser-scanning microscope (LSM 510; Carl Zeiss).
Histochemical GUS Assay
To create the YSA::GUS reporter gene construct, a 1,855-bp genomic sequence upstream of the YSA transcription start site was amplified from Pei'ai64S and inserted into the binary vector pCAMBIA1304. The primers used were: 5′-cttcgctgcagcatgttcccaaccggaacag-3′ and 5′-gttcagatctgctcagctacctgaaaatcc-3′. Transgenic rice plants were produced by the Agrobacterium-mediated cocultivation method as described previously (Jeon et al., 2000).
Histochemical assays in transgenic lines were performed as described (Ai et al., 2009). Seedlings or adult plants were incubated at 37°C in 100 mm sodium phosphate pH 7.5, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, 10 mm EDTA, and 0.1% Triton X-100 containing 1 mm 5-bromo-4-chloro-β-d-gluclonide. After incubation for 10 h, the samples were vacuum infiltrated. Samples were subsequently transferred to 70% ethanol to remove the chlorophyll. The stained tissues were photographed using an OLYMPUS MVX10 stereomicroscope, with a color CCD camera.
RNA Preparation and RT-PCR Analysis
Total rice RNA was extracted with a RNA prep pure kit (Tiangen) according to the manufacturer’s instructions. The first-strand cDNA was synthesized from 2 μg of total RNA using the QuantiTect reverse transcription kit (Qiagen). Primer pairs were designed using Primer Express (Applied Biosystems) and listed in Supplemental Table S5. Real-time PCR analysis was conducted using ABI7300HT fast real-time PCR system with the SYBR Premix Ex Taq (TaKaRa; RR041A). The program was as follows: initial polymerase activation for 10 s at 95°C followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. For each sample, real-time PCR was performed with three technical replicates on three biological replicates. Efficiency-corrected ΔCT values were calculated, and ΔΔCT was used to quantify relative differences in transcript accumulation. Representative PCR products for all sample types and gene targets were sequenced to confirm target specificity.
Sequence data of YSA can be found in the GenBank library under the accession number JN167987.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phylogenetic tree of OsYSA and other PPR proteins in plants.
Supplemental Table S1. Segregation of wild-type and mutant plants in the F2 populations derived from four different crosses.
Supplemental Table S2. The PCR-based molecular markers developed for fine mapping of ysa.
Supplemental Table S3. Major agronomic traits and yield heterosis ability of ysa mutant and its F1 hybrids.
Supplemental Table S4. Comparison of the male-sterility trait between ysa mutant and Pei'ai64S grown in the phytotrons (PAR 384 μmol m−2 s−1).
Supplemental Table S5. Primers used in quantitative real-time RT-PCR.
Supplementary Material
Acknowledgments
We thank Dr. Hongquan Yang (Shanghai Jiaotong University, China) for the gift of plasmid PA7-GFP, Dr. LiXin Zhang (Institute of Botany, Chinese Academy of Sciences, Beijing) for providing the GFP control vectors, and Dr. Chentao Lin (University of California, Los Angeles) for critical comments on the manuscript.
References
- Ai PH, Sun SB, Zhao JN, Fan XR, Xin WJ, Guo Q, Yu L, Shen QR, Wu P, Miller AJ, et al. (2009) Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Archer EK, Bonnett HT. (1987) Characterization of a virescent chloroplast mutant of tobacco. Plant Physiol 83: 920–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S, Alfonso AA, Hanson MR. (2002) A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc Natl Acad Sci USA 99: 10887–10892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi C, Li Z, Wan J. (1990) Effect of the lower temperature in mid-summer on the fertility stability of HPGMR. Chin J Rice Sci 4: 181–184 [Google Scholar]
- Brown GG, Formanová N, Jin H, Wargachuk R, Dendy C, Patil P, Laforest M, Zhang J, Cheung WY, Landry BS. (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35: 262–272 [DOI] [PubMed] [Google Scholar]
- Cao L, Qian Q, Zhu X, Zheng D, Min S, Xiong Z. (1999) Breeding of a photoperiod-sensitive genic male sterile indica rice Zhongzi S with a purple leaf marker and the heterosis of its hybrid rice produced with it. Acta Agron Sin 25: 44–49 [Google Scholar]
- Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, Melake-Berhan A, Jones KM, Redman J, Chen G, et al. (2010) Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol 28: 951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateigner-Boutin AL, des Francs-Small CC, Delannoy E, Kahlau S, Tanz SK, de Longevialle AF, Fujii S, Small I. (2011) OTP70 is a pentatricopeptide repeat protein of the E subgroup involved in splicing of the plastid transcript rpoC1. Plant J 65: 532–542 [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin AL, Ramos-Vega M, Guevara-García A, Andrés C, de la Luz Gutiérrez-Nava M, Cantero A, Delannoy E, Jiménez LF, Lurin C, Small I, et al. (2008) CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J 56: 590–602 [DOI] [PubMed] [Google Scholar]
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL. (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol Plant Pathol 7: 417–427 [DOI] [PubMed] [Google Scholar]
- Chi W, Ma J, Zhang D, Guo J, Chen F, Lu C, Zhang L. (2008) The pentratricopeptide repeat protein DELAYED GREENING1 is involved in the regulation of early chloroplast development and chloroplast gene expression in Arabidopsis. Plant Physiol 147: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassanayake M, Oh DH, Haas JS, Hernandez A, Hong H, Ali S, Yun DJ, Bressan RA, Zhu JK, Bohnert HJ, et al. (2011) The genome of the extremophile crucifer Thellungiella parvula. Nat Genet 43: 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, et al. (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4: 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Liu NY, Tang ZS, Liu J, Yang WC. (2006) Arabidopsis GLUTAMINE-RICH PROTEIN23 is essential for early embryogenesis and encodes a novel nuclear PPR motif protein that interacts with RNA polymerase II subunit III. Plant Cell 18: 815–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong FG, Zhu XD, Xiong ZM, Cheng SH, Sun ZX, Min SK. (1995) Breeding of a photo-thermoperiod sensitive genie male sterile indica rice with a pale-green-leaf marker. Chinese Rice Sci 9: 65–70 [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, von Heijne G. (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Mistry J, Schuster-Böckler B, Griffiths-Jones S, Hollich V, Lassmann T, Moxon S, Marshall M, Khanna A, Durbin R, et al. (2006) Pfam: clans, web tools and services. Nucleic Acids Res (Database issue) 34: D247–D251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk DG, Walker MB, Barkan A. (1999) Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J 18: 2621–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100 [DOI] [PubMed] [Google Scholar]
- Gothandam KM, Kim ES, Cho H, Chung YY. (2005) OsPPR1, a pentatricopeptide repeat protein of rice is essential for the chloroplast biogenesis. Plant Mol Biol 58: 421–433 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T. (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36: 541–549 [DOI] [PubMed] [Google Scholar]
- Iba K, Takamiya K, Toh Y, Satoh H, Nishimur M. (1991) Formation of functionally active chloroplasts is determined at a limited stage of leaf development in virescent mutants of rice. Dev Genet 12: 342–348 [Google Scholar]
- Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, Lee J, Kim C, Jang S, Yang K, Nam J, et al. (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22: 561–570 [DOI] [PubMed] [Google Scholar]
- Johnson X, Wostrikoff K, Finazzi G, Kuras R, Schwarz C, Bujaldon S, Nickelsen J, Stern DB, Wollman FA, Vallon O. (2010) MRL1, a conserved pentatricopeptide repeat protein, is required for stabilization of rbcL mRNA in Chlamydomonas and Arabidopsis. Plant Cell 22: 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama T, Toriyama K. (2003) A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett 544: 99–102 [DOI] [PubMed] [Google Scholar]
- Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Kawasaki S, Imamura J. (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34: 407–415 [DOI] [PubMed] [Google Scholar]
- Komori T, Ohta S, Murai N, Takakura Y, Kuraya Y, Suzuki S, Hiei Y, Imaseki H, Nitta N. (2004) Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J 37: 315–325 [DOI] [PubMed] [Google Scholar]
- Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K. (1993) Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion genes in transgenic rice. Plant Physiol 102: 991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Yuan L, Yang Y. (2001) Sterility purification of the photoperiod-thermo sensitive genic male rice line Pei'ai64S. Chin J Rice Sci 15: 1–6 [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, et al. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M. (1990) Classification and characterization of cDNA that encodes the light-harvesting chlorophyll a/b binding protein of photosystem II from rice. Plant Cell Physiol 31: 519–526 [Google Scholar]
- McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, et al. (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res 9: 199–207 [DOI] [PubMed] [Google Scholar]
- Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G. (2003) HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 15: 1480–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. (2008) Agricultural research: reinventing rice to feed the world. Science 321: 330–333 [DOI] [PubMed] [Google Scholar]
- Okuda K, Chateigner-Boutin AL, Nakamura T, Delannoy E, Sugita M, Myouga F, Motohashi R, Shinozaki K, Small I, Shikanai T. (2009) Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21: 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Hammani K, Tanz SK, Peng L, Fukao Y, Myouga F, Motohashi R, Shinozaki K, Small I, Shikanai T. (2010) The pentatricopeptide repeat protein OTP82 is required for RNA editing of plastid ndhB and ndhG transcripts. Plant J 61: 339–349 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Yu HJ, Ngo QA, Rajani S, Mayalagu S, Johnson CS, Capron A, Xie LF, Ye D, Sundaresan V. (2005) Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132: 603–614 [DOI] [PubMed] [Google Scholar]
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Pfalz J, Bayraktar OA, Prikryl J, Barkan A. (2009) Site-specific binding of a PPR protein defines and stabilizes 5′ and 3′ mRNA termini in chloroplasts. EMBO J 28: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl J, Rojas M, Schuster G, Barkan A. (2011) Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein. Proc Natl Acad Sci USA 108: 415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Prasad AM, Srinivasan R. (2007) Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol Biochem 45: 521–534 [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Small I. (2008) Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends Plant Sci 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A. (2006) A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 18: 2650–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183 [DOI] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Sing R, Limani SS. (1990) Recent progress in the technology and development of hybrid rice in Asia. Int Rice Comm Newsl 39: 133 [Google Scholar]
- Small ID, Peeters N. (2000) The PPR motif—a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Song K, Song Z. (2007) Discovery and preliminary research of the yellowish leaf mutant Annongbiao 810S in rice. Hybrid Rice 22: 71–73 [Google Scholar]
- Sugimoto H, Kusumi K, Noguchi K, Yano M, Yoshimura A, Iba K. (2007) The rice nuclear gene, VIRESCENT 2, is essential for chloroplast development and encodes a novel type of guanylate kinase targeted to plastids and mitochondria. Plant J 52: 512–527 [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Kusumi K, Tozawa Y, Yazaki J, Kishimoto N, Kikuchi S, Iba K. (2004) The virescent-2 mutation inhibits translation of plastid transcripts for the plastid genetic system at an early stage of chloroplast differentiation. Plant Cell Physiol 45: 985–996 [DOI] [PubMed] [Google Scholar]
- Williams PM, Barkan A. (2003) A chloroplast-localized PPR protein required for plastid ribosome accumulation. Plant J 36: 675–686 [DOI] [PubMed] [Google Scholar]
- Wu D, Shu Q, Xia Y. (2002) In vitro mutagenesis induced novel thermo/photoperiod-sensitive genic male sterile indica rice with green-revertible xanthan leaf color marker. Euphytica 123: 195–202 [Google Scholar]
- Wu Z, Zhang X, He B, Diao L, Sheng S, Wang J, Guo X, Su N, Wang L, Jiang L, et al. (2007) A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Pan S, Cheng S, Zhang B, Mu D, Ni P, Zhang G, Yang S, Li R, Wang J, et al. (2011) Genome sequence and analysis of the tuber crop potato. Nature 475: 189–195 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Tasaka M, Shikanai T. (2004) PPR motifs of the nucleus-encoded factor, PGR3, function in the selective and distinct steps of chloroplast gene expression in Arabidopsis. Plant J 38: 152–163 [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Young ND, Debellé F, Oldroyd GE, Geurts R, Cannon SB, Udvardi MK, Benedito VA, Mayer KF, Gouzy J, Schoof H, et al. (2011) The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 480: 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296: 79–92 [DOI] [PubMed] [Google Scholar]
- Yu QB, Jiang Y, Chong K, Yang ZN. (2009) AtECB2, a pentatricopeptide repeat protein, is required for chloroplast transcript accD RNA editing and early chloroplast biogenesis in Arabidopsis thaliana. Plant J 59: 1011–1023 [DOI] [PubMed] [Google Scholar]
- Yuan L. (1997) Current status and developing prospects in two-line hybrid rice research in China. China Res Agric Modern 18: 1–3 [Google Scholar]
- Yuan L. (2004) Hybrid rice technology for food security in the world. International Conference on Sustainable Rice Systems. FAO, Rome, Italy [Google Scholar]
- Zhou W, Cheng Y, Yap A, Chateigner-Boutin AL, Delannoy E, Hammani K, Small I, Huang J. (2009) The Arabidopsis gene YS1 encoding a DYW protein is required for editing of rpoB transcripts and the rapid development of chloroplasts during early growth. Plant J 58: 82–96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.