Abstract
Purpose.
Mechanisms by which visual impairment (VI) increases mortality risk are poorly understood. We estimated the direct and indirect effects of self-rated VI on risk of mortality through mental well-being and preventive care practice mechanisms.
Methods.
Using complete data from 12,987 adult participants of the 2000 Medical Expenditure Panel Survey with mortality linkage through 2006, we undertook structural equation modeling using two latent variables representing mental well-being and poor preventive care to examine multiple effect pathways of self-rated VI on all-cause mortality. Generalized linear structural equation modeling was used to simultaneously estimate pathways including the latent variables and Cox regression model, with adjustment for controls and the complex sample survey design.
Results.
VI increased the risk of mortality directly after adjusting for mental well-being and other covariates (hazard ratio [HR] = 1.25 [95% confidence interval: 1.01, 1.55]). Poor preventive care practices were unrelated to VI and to mortality. Mental well-being decreased mortality risk (HR = 0.68 [0.64, 0.74], P < 0.001). VI adversely affected mental well-being (β = −0.54 [−0.65, −0.43]; P < 0.001). VI also increased mortality risk indirectly through mental well-being (HR = 1.23 [1.16, 1.30]). The total effect of VI on mortality including its influence through mental well-being was HR 1.53 [1.24, 1.90]. Similar but slightly stronger patterns of association were found when examining cardiovascular disease-related mortality, but not cancer-related mortality.
Conclusions.
VI increases the risk of mortality directly and indirectly through its adverse impact on mental well-being. Prevention of disabling ocular conditions remains a public health priority along with more aggressive diagnosis and treatment of depression and other mental health conditions in those living with VI.
Analysis using structural equation modeling of data from 12,987 adults of the 2000 Medical Expenditure Panel Survey with mortality linkage through 2006 showed visual impairment increases mortality risk directly and indirectly through mental well-being but not through poor preventive care practices.
Introduction
Anumber of studies have shown a relationship between visual impairment (VI) and increased risk of mortality.1–12 Visual impairment is associated with psychosocial conditions including social isolation,13,14 cognitive impairment,15 increased dependency on others,16,17 poor self-rated health,1,14 and depression.18 Poor vision in older adults has a greater impact on overall mental health than stroke and heart attack.19
Several studies have attempted to clarify the mechanisms and pathways explaining the association between VI and increased mortality. Christ and associates20 found self-reported VI affected mortality through general health and disability mechanisms among participants of the National Health Interview Survey. Karpa and colleagues21 reported disability in walking as a major indirect pathway between VI and increased mortality in persons age 49 years and older in the Blue Mountain Eye Study. Freeman and coauthors22 found insufficient evidence to conclude that depressive symptoms mediate the relationship between VI and increased mortality risks in participants age 65 years and older in the Salisbury Eye Evaluation Project.
The influence of a broader morbidity indicator, such as mental well-being, on the relationship between VI and increased mortality has not been examined. Lower mental well-being is associated with increased risk of mortality.23 Mojon-Azzi and colleagues24 analyzed mental well-being indicators in persons aged 50 years and older using data from the 2004 Survey of Health, Ageing, and Retirement in Europe (SHARE) and found self-reported low vision was associated with lower psychological well-being. Although related to depression, overall psychological well-being is a broader indicator of self-rated or perceived psychosocial mental health.
Another unexamined mechanism that potentially contributes to the relationship between VI and mortality is poor compliance with preventive health care guidelines such as routine physical examinations. Numerous investigators have found factors such as sociodemographic status and levels of psychological distress influence preventive care behaviors.25–28 Preventive care practices are likely to lower the risk of mortality, but to our knowledge, the association between general preventive care practices and risk of mortality has not been examined adequately. Also, to date it is unknown if preventive health behaviors vary as a function of VI status and ocular conditions.
Constructs such as mental well-being and preventive care behavior can be modeled effectively using structural equation modeling (SEM), which permits the estimation of constructs free of random measurement error.29 This protects against biased associations owing to correlated measurement error in observed measures. With few exceptions, the application of SEM to examine the association between VI and mortality has been infrequently applied in the literature.20,21 In addition to estimating mental well-being and preventive practices without random measurement error, our SEM model allows simultaneous estimation of mediation pathways such as between VI and mental well-being, preventive care practice, and risk of mortality.
The purpose of the study is to use SEM to examine the relationship between vision and mental well-being, preventive care, and mortality by estimating the direct and indirect effects of self-rated VI on mortality through mental well-being and preventive care.
Methods
Study Population and Design
This study included participants age 18 years and older from the 2000 Medical Expenditure Panel Survey (MEPS). The MEPS is a nationally representative subsample of the National Health Interview Survey (NHIS), a continuous multipurpose and multistage area probability survey of the US civilian noninstitutionalized population living at addressed dwellings.30 MEPS participants age 18 years and older were linked with the National Death Index (NDI) through December 31, 2006, via its linkage with NHIS.31 The present analysis included 12,987 participants with complete data.
Assessment of VI
Participants were asked a set of VI questions. Their answers were summarized into a five-category VI status variable: 1, No difficulty seeing; 2, Some difficulty seeing, can read newsprint; 3, Some difficulty seeing, cannot read newsprint, can recognize familiar people; 4, Some difficulty seeing, cannot read newsprint, cannot recognize familiar people but is not blind; 5, Blind. Initially, participants falling into categories 2 through 4 were combined into one single category designated as “Some VI,”30 resulting in a three-category–ordered VI measure: no VI, some VI, and Blind. Because of the small sample size of the “Blind” group, with only 48 participants and 0.4% of study population, category 5 “Blind” was pooled together with “Some VI” to form the group “Any VI,” resulting in a two-category dichotomized VI measure: No VI versus Any VI. The model that used the two-category VI variable fit the data better than the model using the three-category VI variable, based on Akaike Information Criteria and Bayesian Information Criteria testing.32 Therefore, VI was treated as a two-category variable in all the final models.
Latent Variables
Latent variables are unobserved variables measured as a function of multiple observed indicators.29 The multiple observed indicators are optimally combined into a composite representing the latent variable, and random measurement error is estimated separately from the latent variable, thereby improving the measurement quality of data. Two such variables were used for the current study: mental well-being and preventive care.
Mental well-being was analyzed as a latent variable with five indicators. Each indicator was derived from one of the following questions: 1, “How much of the time during the past 4 weeks have you felt calm and peaceful?” 2, “How much of the time during the past 4 weeks have you felt downhearted and blue?” 3, “How much of the time during the past 4 weeks did you have a lot of energy?” 4, “Problems with anxiety/depression: no problem, some problem, major problem?” 5, “Perceived mental health status: poor, fair, good, very good, excellent?” Questions 1 to 3 were from the mental component measure of the SF-12,33 and possible answers were as follows: none of the time, a little of the time, some of the time, good bit of time, most of time, all of the time. Question 4 was from the mental status domain of the EuroQol 5-D.34 Question 5 was a self-rated mental health measure. Data were recoded such that higher values indicated better mental well-being. These indicators measured different aspects of mental well-being and combined well as a latent variable based on model fit statistics reported below.
Preventive care was analyzed as a latent variable with three indicators: 1, “About how long since last blood cholesterol check by a doctor or health professional?” 2, “How long since last routine check-up by doctor or other health professional for assessing overall health?” 3, “How long since last flu shot?” Possible answers to these three questions were as follows: within past year, within past 2 years, within past 3 years, within past 5 years, more than 5 years, or never. Each question was selected from the preventive care questions of MEPS 2000.30 These preventive care measurements were chosen because they are applicable to all adults 18 years and older, and are not sex specific. These three questions combined well, with a higher value indicating worse preventive care status.
The variance of latent variables was fixed at one, so that the distribution was standardized as a normal distribution with mean of zero and standard deviation (SD) of 1. The effect estimates for relationships with latent variables were presented in terms of SD units, which may be interpreted as effects sizes comparable with Cohen's D.35 Most consider 0.20 and lower as a small effect, approximately 0.50 as a moderate effect, and 0.80 or higher as a large effect size.35
Covariates
Several social demographic and health behavior variables that affect mortality were included in the model as controls: age in years (range 18–90), sex (male reference), a three-category racial identity variable (white [reference], black, other races), smoking status at time of interview (nonsmoker [reference]), health insurance status (private insurance [reference], public only insurance, no insurance), and education in years (range 0–17). To protect privacy, MEPS coded all participants age 90 years or older as age 90 years; there were 62 (0.48%) participants who fell into this category.
Cause of Death
Cause of death information was obtained from National Death Index through linkage with NHIS. The underlying cause of death was determined by Underlying Cause of Death 113 Groups (ucod_113), which follows the ninth revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-9).36 Our study examined all-cause mortality, cardiovascular-related mortality (ucod_113 code 053–075), and cancer-related mortality (ucod_113 code 019–043).
Analysis
Descriptive and model-based analyses were completed with adjustments for sample weights and design effects using SAS 9.2 (SAS Institute Inc., Cary, NC)37 and Mplus 6 statistical packages38 (Muthen & Muthen, Los Angeles, CA), respectively.
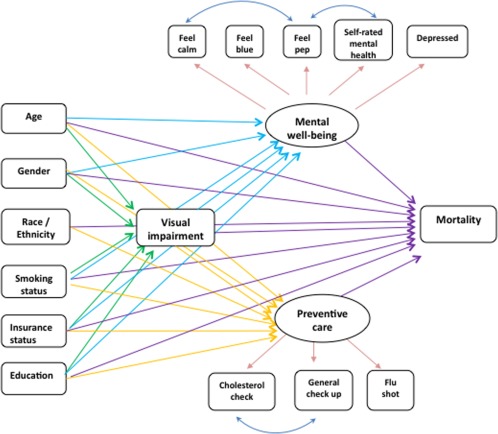
The latent variables were evaluated first as a submodel and subsequently used in the full SEM model, which served as a starting point for our analysis (Fig. 1). The confirmatory factor analysis of the two latent variables, mental well-being and preventive care, fit the data well. The model fit indices were as following: comparative fit index (CFI) = 0.98, root mean square error of approximation (RMSEA) = 0.045, and standard root mean square residual (SRMR) = 0.031. A good model fit indicated the variances and covariances implied by the proposed model replicated well the variance and covariance among the observed variables. The P value of the chi-square model fit test was less than 0.001, likely owing to the large sample size of approximately 13,000.
Figure 1.
Initial path diagram of visual impairment and mortality structural equation model.
Next, all relationships in Figure 1, with the exception of the effect on mortality, were evaluated. Three regression models were fit simultaneously: (1) a logistic regression model of VI on the six demographic variables, (2) a multiple linear regression model of the mental well-being latent variable on six demographic variables and VI status, and (3) a multiple linear regression model of the preventive care latent variable on the same variables as in 2. To improve model fit, paths from race to VI and race to mental well-being were removed because of statistical nonsignificance. The fit indices were CFI 0.918, RMSEA 0.038, and SRMR 0.031, indicating a moderate to good model fit.
Next, the mortality component using Cox Proportional Hazard Regression was added to the model. In this model, VI was hypothesized to directly affect mortality, as well as affect mortality indirectly through mental well-being and preventive care latent variables. All model equations were estimated simultaneously using a pseudo-maximum likelihood estimator38 with robust standard errors.39 Regression coefficients from the Cox Proportional Hazard Regression were exponentiated to obtain the hazard ratios (HRs), whereas coefficients from the logistic models were similarly exponentiated to obtain odds ratios. All features of the MEPS complex survey design were accounted for in model estimation.
Indirect effects were calculated by multiplying the two parameters involved in the mediation relationship. For example, the effect of VI on mental well-being was multiplied by the effect of mental well-being on mortality. The new parameter was exponentiated to obtain the hazard ratio for the indirect effect. Total hazard ratio effects were calculated by taking the exponentiation of the summed direct and indirect coefficients. Standard errors for both indirect and total effects were obtained using the delta method.40
Results
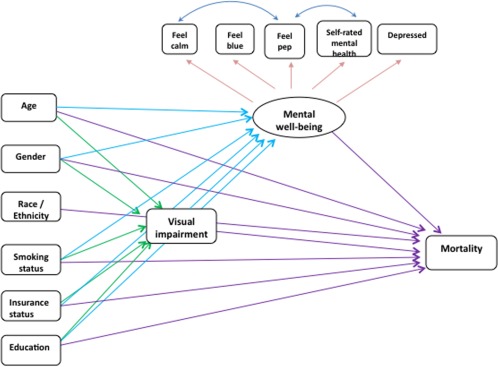
We analyzed the model depicted in Figure 1 and found the preventive care latent variable was not significant in predicting all-cause and cause-specific mortality, nor was it significantly associated with VI. To provide the most parsimonious model, the preventive care practice latent variable was excluded in the final model, as shown in Figure 2.
Figure 2.
Final path diagram of visual impairment and mortality structural equation model.
Table 1 shows the demographic characteristics of the study population. The study sample of 12,987 adults represented 155 million adults in the noninstitutionalized US population. Mortality linkage with the National Death Index with follow-up to December 31, 2006, identified 881 observed deaths in the study sample, of which 325 were cardiovascular disease (CVD)-related deaths and 218 were cancer-related deaths. The average age of this population was 47.0 years. The population included 46.0% men, 83.4% White, 13.6% African American, 3% Other races. The average education was 12.4 years. At the time of interview, 22.2% of participants were smokers, 71.2% had private health insurance, 15.1% had public only insurance, and 13.7% had no health insurance. With respect to VI, 94.3% had “No VI,” and 5.7% had VI.
Table 1.
Demographic Characteristics of Study Sample, Medical Expenditure Panel Survey (MEPS) 2000
|
Characteristic |
|
| Average age, y | 47.0 |
| Sex, No. (%) | |
| Male | 5968 (46.0) |
| Female | 7019 (54.0) |
| Race, No. (%) | |
| American Indian, Aleut, or Eskimo | 102 (0.8) |
| Asian or Pacific Islander | 288 (2.2) |
| Black | 1769 (13.6) |
| White | 10,828 (83.4) |
| Average years of education | 12.4 |
| Smoker at time of survey, No. (%) | |
| Yes | 2882 (22.2) |
| No | 10,105 (77.8) |
| Health insurance, No. (%) | |
| Any private insurance | 9284 (71.2) |
| Public only insurance | 1965 (15.1) |
| Uninsured | 1774 (13.7) |
| Visual impairment status, No. (%) | |
| No impairment | 12,248 (94.3) |
| Any impairment | 735 (5.7) |
| Survival status at 12/31/2006, No. (%) | |
| Alive | 12,106 (93.2) |
| Deceased | 881 (6.8) |
| Cardiovascular disease-related deaths | 321 (2.5) |
| Cancer-related deaths | 281 (1.7) |
| Total | 12,987 (100) |
Tables 2 through 5 present the estimates from the final model with mortality outcome depicted in Figure 2. Table 2 displays odds ratio estimates from the logistic regression of VI on demographic variables where VI was a two-category variable of “No VI” versus “Any VI.” Table 3 shows the multiple linear regression results for the latent variable mental well-being. VI was associated with decreased mental well-being. A change of VI status from no VI to any VI, was associated with 0.54 SD decrease in mental well-being status (β = −0.54, P < 0.001). Those with public only insurance and uninsured were 0.58 SD and 0.18 SD, respectively lower in mental well-being status compared with those with private insurance (both P < 0.001). Women (β = −0.23, P < 0.001) and smokers (β = −0.28, P < 0.001) had worse mental well-being compared with men and nonsmokers. With each additional year of education there was a 0.03-SD unit increase in mental well-being status (P < 0.001).
Table 2.
Logistic Regression of Visual Impairment on Demographic Variables
|
Variables |
Odds Ratio |
95% Confidence Interval |
PValue |
| Sex | |||
| Male | 1.00 | ||
| Female | 1.42 | [1,23, 1.64] | < 0.001 |
| Age, y | 1.03 | [1.03, 1.04] | < 0.001 |
| Health insurance | |||
| Private insurance | 1.00 | ||
| Public only insurance | 1.53 | [1.16, 2.02] | 0.002 |
| Uninsured | 1.83 | [1.38, 2.43] | < 0.001 |
| Smoking status | |||
| Nonsmoker | 1.00 | ||
| Current smoker | 1.32 | [1.07, 1.62] | 0.01 |
| Education, y | 0.97 | [0.95, 1.00] | 0.044 |
Table 3.
Multiple Regression of Latent Variable: Mental Well-being* on Demographic Variables and Visual Impairment
|
Variables |
Coefficients |
95% Confidence Interval |
PValue |
| Visual impairment | −0.54 | [−0.65, −0.43] | < 0.001 |
| Female | −0.23 | [−0.27, −0.19] | < 0.001 |
| Age, y | −0.002 | [0.004, 0.00] | 0.088 |
| Current smoker | −0.28 | [−0.34, −0.21] | < 0.001 |
| Public only insurance | −0.58 | [−0.68, −0.48] | < 0.001 |
| Uninsured | −0.18 | [−0.27, −0.09] | < 0.001 |
| Education | 0.03 | [0.02, 0.04] | < 0.001 |
Coefficients are partially standardized with predictors in original units and latent variable in standard deviation units.
Table 4 provides the Cox proportional hazard model regression results of the effect of all model variables on all-cause mortality. Visual impairment was significant in predicting mortality (HR = 1.25; 95% confidence interval: [1.01, 1.55], P = 0.039). The mental well-being latent variable also had a significant effect on the mortality hazard. A 1 SD increase in the mental well-being latent variable decreased the hazard of death by a factor of 0.68 (P < 0.001). As expected, age predicted mortality; a 1-year increase in age increased the hazard of death by a factor of 1.09 (P < 0.001). Females had decreased mortality hazard compared with males (HR = 0.59, P < 0.001). Blacks compared with Whites had increased mortality hazard (HR = 1.45, P < 0.001), as did smokers relative to nonsmokers (HR = 1.61, P < 0.001).
Table 4.
Cox Proportional Hazard Model: Direct Effect on All-Cause Mortality*
|
Variables |
Hazard Ratio of Mortality |
95% Confidence Interval |
PValue |
| Mental well-being | 0.68 | [0.64, 0.74] | < 0.001 |
| Visual impairment | 1.25 | [1.01, 1.55] | 0.039 |
| Female | 0.59 | [0.48, 0.74] | < 0.001 |
| Age, y | 1.09 | [1.08, 1.09] | < 0.001 |
| Black | 1.45 | [1.18, 1.83] | 0.001 |
| Other | 0.48 | [0.22, 1.05] | 0.068 |
| Public only insurance | 1.17 | [0.98, 1.39] | 0.081 |
| Uninsured | 1.43 | [0.99, 2.06] | 0.059 |
| Current smoker | 1.61 | [1.36, 1.93] | < 0.001 |
| Education | 0.98 | [0.96, 1.00] | 0.123 |
Coefficients are partially standardized with predictors in original units and latent variable in standard deviation units.
Because of its association with both VI and all-cause mortality, mental well-being served as a mediator between the relationship of VI and mortality. The path from VI to mental well-being, the presumed mediator, was significant (β = −0.54, P < 0.001). Second, the path from mental well-being to mortality, the outcome, was also significant (HR = 0.68, P < 0.001). The direct effect of VI on mortality remained significant after including mental well-being in the regression (HR = 1.25, P = 0.039). Therefore, the mediation effect of mental well-being on VI and all-cause mortality was a partial mediation effect.41 The indirect effect of VI on all-cause mortality through mental well-being was HR = 1.23 [1.16, 1.30] (Table 6). The total effect of VI on all-cause mortality, which combines the direct effect and the indirect effect through mental well-being was 1.53 [1.24, 1.90].
Table 6.
Total and Indirect Effect of Visual Impairment on Mortality through Mental Well-being
|
All-cause Mortality |
Cardiovascular- related Mortality |
Cancer- related Mortality |
|
| Indirect effect | 1.23* [1.16, 1.30] | 1.22* [1.13, 1.32] | 1.16* [1.05, 1.27] |
| Direct effect | 1.25* [1.01, 1.55] | 1.47* [1.08, 2.01] | 1.11 [0.70, 1.78] |
| Total effect | 1.53* [1.24, 1.90] | 1.80* [1.33, 2.44] | 1.29 [0.83, 2.00] |
P values < 0.05.
Table 5 presents the HRs of cause-specific mortality. VI was significant in predicting CVD-related mortality (HR = 1.47 [1.08, 2.01], P = 0.015). The mental well-being latent variable was also significant in predicting CVD-related mortality (HR = 0.69 [0.61, 0.77], P < 0.001). Because VI was also significantly associated with the mental well-being latent variable (β = −0.54, P < 0.001), VI affected CVD-related mortality not only directly, but also indirectly through the effect on mental well-being. The total effect of VI on CVD-related mortality was HR = 1.80 [1.33, 2.44] (Table 6). This pattern of association was similar to the relationship between VI and all-cause mortality, except that it was slightly stronger.
Table 5.
Hazard Ratio from Cox Proportional Hazard Model: Direct Effect on Cause-specific Mortality*
|
Variables |
Cardiovascular- related Mortality |
Cancer- related Mortality |
| Mental well-being | 0.69† [0.61, 0.77] | 0.76† [0.65, 0.89] |
| Visual impairment | 1.47† [1.08, 2.01] | 1.11 [0.70, 1.78] |
| Female | 0.60† [0.45, 0.80] | 0.64† [0.44, 0.92] |
| Age, y | 1.11† [1.10, 1.12] | 1.08† [1.07, 1.09] |
| Black | 1.56† [1.09, 2.24] | 1.55† [1.04, 2.32] |
| Other | 0.08† [0.01, 0.59] | 0.60 [0.19, 1.91] |
| Public only insurance | 1.20 [0.90, 1.60] | 1.23 [0.86, 1.77] |
| Uninsured | 1.58 [0.80, 3.10] | 0.97 [0.48, 1.94] |
| Current smoker | 1.67† [1.20, 2.33] | 2.03† [1.53, 2.71] |
| Education | 1.01 [0.98, 1.05] | 0.98 [0.95, 1.02] |
Coefficients are partially standardized with latent variables in standard deviation units.
P values < 0.05.
As shown in Table 5, VI was not significant in directly predicting cancer-related mortality (HR = 1.11 [0.70, 1.78], P = 0.65). Even though the indirect effect of VI on cancer mortality through mental well-being was significant (1.16 [1.05, 1.27]), the total effect of VI on cancer-related mortality was not significant (HR = 1.29 [0.83, 2.00]) (Table 6).
Discussion
Our results indicate mental well-being is a significant mechanism by which VI affects the risk of mortality. Although VI increased the risk of mortality directly after adjusting for mental well-being and other covariates, mental well-being serves as an important indirect pathway from VI to increased mortality risk. In contrast, our results also indicate VI does not have a significant effect on preventive care practice and preventive care practice is not a significant predictor of mortality.
Our study confirms that persons with VI have reduced mental well-being and that mental well-being is a strong predictor of mortality. Using 11 variables to capture emotional well-being from the population-based 2004 SHARE, Monjon-Azzi and associates found a significant relationship between lower vision and well-being.24 In a systematic review of 29 quantitative studies between 2001 and 2008, Nyman and coworkers42 noted working-age adults with VI were significantly more likely to report lower levels of mental health, social functioning, and quality of life. With respect to well-being and mortality, Chida and Steptoe23 performed meta-analysis of 35 studies in initial healthy populations and 35 studies of disease populations and documented positive psychological well-being has a favorable effect on survival in both healthy and diseased populations.
Using latent variables in SEM, we measured the association between VI and reduced mental well-being status. A change of VI status from no impairment to any impairment was associated with more than a half SD decrease in mental well-being. In comparison with other sociodemographic variables, VI had the strongest effect in lowering mental well-being. The effect of VI on mental well-being was stronger than sex, 1-year increase in age, 1-year increase in education, smoking status, or having health insurance.
Similar patterns of association were found when we repeated our analyses using CVD-related mortality as the outcome. VI not only directly increased the risk of CVD-related mortality, it also indirectly increased the risk of mortality through its effect on mental well-being. Furthermore, these associations were slightly stronger in comparisons to results for overall survival; however, the direct and total effect of VI on cancer-related mortality was not significant. These CVD and cancer mortality findings were consistent with previous research. For example, Lee and associates9 found VI increased the risk of CVD-related mortality but not cancer-related mortality in a large population-based NHIS study. In the Beaver Dam Eye Study, Knutson and colleagues12 concluded increased diabetic retinopathy severity was associated with heart disease mortality, and increased nuclear sclerosis severity was associated with stroke mortality; however, there was no significant association between ocular conditions including VI and cancer mortality.
Although the total effects of VI on cancer mortality risk was nonsignificant, there was a significant but relatively small indirect effect on cancer mortality through VI's effect on mental well-being (HR = 1.16 [1.05, 1.27], P < 0.05). Lower mental well-being is associated with increased risk of cancer mortality, although most studies focused on all-cause mortality and did not report cancer-specific findings.23 A recent Danish cohort study reported that a global quality-of-life question predicted the development of cancer over a 13-year follow-up period (poor versus very good: adjusted HR = 1.90 [1.1, 3.4]).43
One potential mechanism by which VI may indirectly affect cancer mortality is through the influence of low mental well-being on cancer screening behaviors. Higher levels of depressive symptomatology were found to be associated with reduced rates of breast cancer screening behaviors in women.44–47 On the other hand, similar associations were not found between cervical cancer screening and depressive symptoms,45 nor was a direct association found for colorectal cancer in women and men.48
When the analysis was performed by restricting to those participants with “No VI” and “Some VI” by excluding the blind participants, the direct effect of VI on all-cause mortality had the same directionality and was weakened; HR decreased from HR = 1.25 [1.01, 1.55], P < 0.05, to HR = 1.14 [0.91, 1.43], P = 0.25. The indirect and total effect of VI on mortality through mental well-being remained significant; the indirect effect remained essentially unchanged from HR = 1.23 [1.16, 1.30], P < 0.05, to 1.22 [1.15, 1.29], P < 0.05, and the total effect decreased from HR = 1.53 [1.24, 1.90], P < 0.05, to HR = 1.39 [1.11, 1.74], P < 0.05. When the analysis was performed by restricting to those participants with “No VI” and “Some VI” for the CVD mortality analysis, a similar pattern of changes was obtained. These findings indicate a consistent relationship between VI and mortality through mental well-being.
In each of our overall and cause-specific mortality analyses, we found at least some evidence of an indirect effect of VI through its influence on mental well-being. These findings reinforce the notion that the adverse effects of VI on psychological health indicators is a phenomenon that is partially “hidden” from view and should therefore receive greater attention by both clinicians and the research community at large. For example, strategies for the enhancement of well-being and overall quality of life among those living with VI should be further developed. In addition, clinicians treating the visually impaired should be aware that rapid depression screening tools are available to identify subpopulations that may benefit from appropriate follow-up care from mental health professionals.49–51
Our results also indicate VI does not have a significant effect on preventive care practice and preventive care practice is not a significant predictor of mortality. Persons with VI may have more barriers in seeking preventive care.52 Conversely, persons with VI may be more likely to come into contact with the health care system, either because of medical comorbidities or because of ongoing ocular care.53,54 To further study the relationship among preventive care, VI, and mortality, we repeated the analysis replacing preventive care latent variable with just a single preventive care practice indicator: flu shot status. We selected this indicator because the results from a previous report suggested it could lower mortality risk.55 In our multivariable model, however, the direct pathway from flu shot to mortality did not reach statistical significance (HR = 0.97 [0.93, 1.01], P = 0.15). Therefore, our findings suggest the effect of VI on mortality is not via the pathway of affecting preventive care, although this conclusion is somewhat tempered by the rather limited number of preventive care indicators available in the MEPS dataset.
Although there is no established methodology of assessing mental well-being, the five quantitative mental well-being indicators used in this study are consistent with those of Monjon-Azzi and associates24 and measure overall psychological well-being. Rather than studying each indicator individually, we used a latent variable in the SEM setting to create a more comprehensive composite measure of mental well-being. The advantages of using latent variables within SEM to model mental well-being allow the estimation of mental well-being free of random measurement error. To our knowledge, our group is the first to use such a latent variable to assess the effect of mental well-being on the relationship between VI and mortality. Moreover, SEM allows the assessment of multiple pathways simultaneously.
Limitations of our study include the fact that all variable information was based on participant self-report and was therefore subject to misclassification. For example, self-reported questions like those for VI could be context-specific or influenced by culture, and therefore may be subject to differing interpretations across the diverse sample of adult participants of the NHIS. To our knowledge, the specific VI questions in the MEPS have not been validated, although validations of similar questions have been reported in the literature. For example, concordance of Snellen distance acuity results with the question “Are you able to recognize a face from a distance of 4 meters” was reported to be 79% in one clinic-based study.56 The MEPS distance acuity question was very similar in its structure: “(With glasses or contacts) can you see well enough to recognize familiar people if they are 2 or 3 feet away?” In the Rand Health Insurance Study, the sensitivity and specificity for the question, “Without glasses, can you recognize a friend across the street” were 90% and 78%, respectively, when using 20/100 or worse distance visual acuity in the better seeing eye as the criterion.57 The Rand question, “Without glasses can you read ordinary newsprint?” had a sensitivity of 87% and a specificity of 91% when using 20/100 near visual acuity as the criterion. This question was similar to the one used in the MEPS: “(With glasses or contacts), can you see well enough to read ordinary newspaper print, even if you cannot read.” Sensitivities for both RAND items were lower when cut points were reduced to include mild levels of near and distance impairment (e.g., for 20/40 or worse: distance sensitivity 58%, near sensitivity 59%). The use of visual acuity as a “gold-standard” criterion to evaluate self-reported visual impairment is subject to its own limitations, however, given that other visual system components can affect visual functioning. For example, contrast sensitivity, stereoacuity, and visual acuity are independently associated with self-reported near and far VIs in community-residing populations.58
Information such as history of ocular diseases such as cataract and retinopathy and the causes of visual impairment were not available in MEPS. Furthermore, as indicated previously, the number of assessed preventive care practices was somewhat limited in the MEPS. The preventive care indicators used may not be sensitive enough to reflect the health behavior effect on mortality, and we may have failed to capture with our latent variable other preventive care practices that may be related to both VI and mortality. For example, preventive services targeting the reduction in CVD (e.g., aspirin prophylaxis, pharmaceutical treatment of hypertension, and hyperlipidemia) have been shown to reduce all-cause mortality, although these reductions are somewhat modest (relative risk range, 0.84–0.93).55
In summary, our study shows that VI not only directly increases the risk of mortality but also indirectly increases mortality risk through its adverse impact on mental well-being. Mental well-being represents an important pathway through which VI affects the risk of mortality. Ignoring this indirect effect may lead to an underestimation of the impact of VI on mortality. The persistent increased mortality risk among the visually impaired documented across epidemiologic studies is unlikely to be a result of the impact of VI on preventive care practices. Our study underscores the importance of improving mental well-being of those with VI, which will improve the quality of life and potentially reduce the risk of mortality.
Footnotes
Supported by Grant R21 EY019096 from the National Eye Institute.
Disclosure: D.D. Zheng, None; S.L. Christ, None; B.L. Lam, None; K.L. Arheart, None; A. Galor, None; D.J. Lee, None
References
- 1. Wang JJ, Mitchell P, Simpson JM, Cumming RG, Smith W. Visual impairment, age-related cataract, and mortality. Arch Ophthalmol. 2001;119:1186–1190 [DOI] [PubMed] [Google Scholar]
- 2. Thompson JR, Gibson JM, Jagger C. The association between visual impairment and mortality in elderly people. Age Ageing. 1989;18:83–88 [DOI] [PubMed] [Google Scholar]
- 3. McCarty CA, Nanjan MB, Taylor HR. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egge K, Zahl PH. Survival of glaucoma patients. Acta Ophthalmol Scand. 1999;77:397–401 [DOI] [PubMed] [Google Scholar]
- 5. Podgor MJ, Cassel GH, Kannel WB. Lens changes and survival in a population-based study. N Engl J Med. 1985;313:1438–1444 [DOI] [PubMed] [Google Scholar]
- 6. Hu FB, Hankinson SE, Stampfer MJ, et al. Prospective study of cataract extraction and risk of coronary heart disease in women. Am J Epidemiol. 2001;153:875–881 [DOI] [PubMed] [Google Scholar]
- 7. Meddings DR, Hertzman C, Barer ML, et al. Socioeconomic status, mortality, and the development of cataract at a young age. Soc Sci Med. 1998;46:1451–1457 [DOI] [PubMed] [Google Scholar]
- 8. West SK, Munoz B, Istre J, et al. Mixed lens opacities and subsequent mortality. Arch Ophthalmol. 2000;118:393–397 [DOI] [PubMed] [Google Scholar]
- 9. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002;120:1544–1550 [DOI] [PubMed] [Google Scholar]
- 10. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Glaucoma and survival: the National Health Interview Survey 1986–1994. Ophthalmology. 2003;110:1476–1483 [DOI] [PubMed] [Google Scholar]
- 11. Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual impairment and unintentional injury mortality: the National Health Interview Survey 1986–1994. Am J Ophthalmol. 2003;136:1152–1154 [DOI] [PubMed] [Google Scholar]
- 12. Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–249 [DOI] [PubMed] [Google Scholar]
- 13. Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993;41:401–407 [DOI] [PubMed] [Google Scholar]
- 14. Wallhagen MI, Strawbridge WJ, Shema SJ, Kurata J, Kaplan GA. Comparative impact of hearing and vision impairment on subsequent functioning. J Am Geriatr Soc. 2001;49:1086–1092 [DOI] [PubMed] [Google Scholar]
- 15. Wahl HW, Tesch-Romer H, Rott C. Vision and cognitive functioning in old age. : Silverstone B, Lang MA, Rosenthal MA, Faye EE. The Lighthouse Handbook on Vision Impairment and Vision Rehabilitation. New York: Oxford University Press; 2000:431–439 [Google Scholar]
- 16. Salive ME, Guralnik J, Glynn RJ, Christen W, Wallace RB, Ostfeld AM. Association of visual impairment with mobility and physical function. J Am Geriatr Soc. 1994;42:287–292 [DOI] [PubMed] [Google Scholar]
- 17. Jette AM, Branch LG. Impairment and disability in the aged. J Chronic Dis. 1985;38:59–65 [DOI] [PubMed] [Google Scholar]
- 18. Tournier M, Moride Y, Ducruet T, Moshyk A, Rochon S. Depression and mortality in the visually-impaired, community-dwelling, elderly population of Quebec. Acta Ophthalmol. 2008;86:196–201 [DOI] [PubMed] [Google Scholar]
- 19. Chia EM, Wang JJ, Rochtchina E, Smith W, Cumming RR, Mitchell P. Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2004;45:71–76 [DOI] [PubMed] [Google Scholar]
- 20. Christ SL, Lee DJ, Lam B, Zheng D, Arheart K. Assessment of the effect of visual impairment on mortality through multiple health pathways using structural equation modeling. Invest Ophthalmol Vis Sci. 2008;49:3318–3323 [DOI] [PubMed] [Google Scholar]
- 21. Karpa MJ, Mitchell P, Beath K, Rochtchina E, Cumming RG, Wang JJ. Direct and indirect effects of visual impairment on mortality risk in older persons. Arch Ophthalmol. 2009;127:1347–1353 [DOI] [PubMed] [Google Scholar]
- 22. Freeman EE, Egleston BL, West SK, Bandeen-Roche K, Rubin G. Visual acuity change and mortality in older adults. Invest Ophthalmol Vis Sci. 2005;46:4040–4045 [DOI] [PubMed] [Google Scholar]
- 23. Chida Y, Steptoe A. Positive psychological well-being and mortality: a quantitative review of prospective observational studies. Psychosom Med. 2008;70:741–756 [DOI] [PubMed] [Google Scholar]
- 24. Mojon-Azzi SM, Sousa-Poza A, Mojon DS. Impact of low vision on well-being in 10 European countries. Ophthalmologica. 2008;222:205–212 [DOI] [PubMed] [Google Scholar]
- 25. Corbie-Smith G, Flagg EW, Doyle JP, O'Brien MA. Influence of usual source of care on differences by race/ethnicity in receipt of preventive services. J Gen Intern Med. 2002;17:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felix-Aaron K, Moy E, Kang M, Patel M, Chesley FD, Clancy C. Variation in quality of men's health care by race/ethnicity and social class. Med Care. 2005;43:I72–181 [DOI] [PubMed] [Google Scholar]
- 27. Thorpe JM, Kalinowski CT, Patterson ME, Sleath BL. Psychological distress as a barrier to preventive care in community-dwelling elderly in the United States. Med Care. 2006;44:187–191 [DOI] [PubMed] [Google Scholar]
- 28. Shi L, Stevens GD. Vulnerability and the receipt of recommended preventive services: the influence of multiple risk factors. Med Care. 2005;43:193–198 [DOI] [PubMed] [Google Scholar]
- 29. Bollen K. Structural Equations with Latent Variables. New York, NY: Wiley; 1989. [Google Scholar]
- 30. Agency for Healthcare Research and Quality. MEPS HC-050: 2000 Full Year Consolidated Data File. Available at: http://www.meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h50/h50doc.shtml. Accessed September 28, 2011. [DOI] [PubMed]
- 31. NCHS. National Health Interview Survey Linked Mortality File. Available at: http://www.cdc.gov/nchs/r&d/nchs_datalinkage/nhis_data_linkage_activities.htm. Accessed February 15, 2005.
- 32. Raftery AE. Bayesian model selection in structural equation models. : Bollen KA, Long JS, eds Testing Structural Equation Models. Newbury Park, CA: Sage Publications; 1993:163–180 [Google Scholar]
- 33. Ware J, Jr, , Kosinski M, Keller SDA. 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233 [DOI] [PubMed] [Google Scholar]
- 34. Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72 [DOI] [PubMed] [Google Scholar]
- 35. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 36. NCHS. The National Health Interview Survey 1986–2004 Linked Mortality Files Public-use File Layout. Available at: http://www.cdc.gov/nchs/data/datalinkage/nhis_file_layout_public_2010.pdf. 2010. Accessed February 5, 2012.
- 37. Skinner CJ. Domain means, regression and multivariate analysis. : Skinner CJ, Holt D, Smith TMF. Analysis of Complex Surveys. New York, NY: Wiley; 1989:59–87 [Google Scholar]
- 38. Muthén LK, Muthén BO. Mplus User's Guide. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- 39. Binder D. On the variance of asymptotically normal estimators from complex surveys. International Statistical Review. 1983;51:279–292 [Google Scholar]
- 40. Davison AC. Statistical Models. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 41. Baron MKD. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 42. Nyman SR, Gosney MA, Victor CR. Psychosocial impact of visual impairment in working-age adults. Br J Ophthalmol. 2010;94:1427–1431 [DOI] [PubMed] [Google Scholar]
- 43. Flensborg-Madsen T, Johansen C, Gronbaek M, Mortensen EL. A prospective association between quality of life and risk for cancer. Eur J Cancer. 2011;47:2446–2452 [DOI] [PubMed] [Google Scholar]
- 44. Husaini BA, Sherkat DE, Bragg R, et al. Predictors of breast cancer screening in a panel study of African American women. Women Health. 2001;34:35–51 [DOI] [PubMed] [Google Scholar]
- 45. Aggarwal A, Freund K, Sato A, et al. Are depressive symptoms associated with cancer screening and cancer stage at diagnosis among postmenopausal women? The Women's Health Initiative observational cohort. J Womens Health (Larchmt). 2008;17:1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Price MA, Butow PN, Charles M, et al. Predictors of breast cancer screening behavior in women with a strong family history of the disease. Breast Cancer Res Treat. 2010;124:509–519 [DOI] [PubMed] [Google Scholar]
- 47. Ludman EJ, Ichikawa LE, Simon GE, et al. Breast and cervical cancer screening specific effects of depression and obesity. Am J Prev Med. 2010;38:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miles A, Rainbow S, von Wagner C. Cancer fatalism and poor self-rated health mediate the association between socioeconomic status and uptake of colorectal cancer screening in England. Cancer Epidemiol Biomarkers Prev. 2011;20:2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rees G, Fenwick EK, Keeffe JE, Mellor D, Lamoureux EL. Detection of depression in patients with low vision. Optom Vis Sci. 2009;86:1328–1336 [DOI] [PubMed] [Google Scholar]
- 50. O'Connor EA, Whitlock EP, Beil TL, Gaynes BN. Screening for depression in adult patients in primary care settings: a systematic evidence review. Ann Intern Med. 2009;151:793–803 [DOI] [PubMed] [Google Scholar]
- 51. Mitchell AJ. Clinical utility of screening for clinical depression and bipolar disorder. Curr Opin Psychiatry. 2012;25:24–31 [DOI] [PubMed] [Google Scholar]
- 52. Spencer C, Frick K, Gower EW, Kempen JH, Wolff JL. Disparities in access to medical care for individuals with vision impairment. Ophthalmic Epidemiol. 2009;16:281–288 [PubMed] [Google Scholar]
- 53. Lee DJ, Lam BL, Arora S, et al. Reported eye care utilization and health insurance status among US adults. Arch Ophthalmol. 2009;127:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14 [DOI] [PubMed] [Google Scholar]
- 55. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–609 [DOI] [PubMed] [Google Scholar]
- 56. Eekhof JA, De Bock GH, Schaapveld K, Springer MP. Screening for hearing and visual loss among elderly with questionnaires and tests: which method is the most convincing for action? Scand J Prim Health Care. 2000;18:203–207 [DOI] [PubMed] [Google Scholar]
- 57. Rubinstein R, Lohr K, Brook R, Goldberb G. Conceptualization and Measurement of Physiologic Health for Adults. Volume 12: Vision Impairments. Rand Corporation; 1982. [Google Scholar]
- 58. Valbuena M, Bandeen-Roche K, Rubin GS, Munoz B, West SK. Self-reported assessment of visual function in a population-based study: the SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 999; 40:280–288 [PubMed] [Google Scholar]