Background: Clearance of insoluble debris and apoptotic cells occurs via phagocytosis of non-opsonized particles, i.e. innate phagocytosis.
Results: A group of serum glycoproteins was found to inhibit phagocytosis of non-opsonized beads and apoptotic cells by monocyte/macrophages.
Conclusion: Certain metal-interacting glycoproteins are able to inhibit the scavenger activity.
Significance: This is the first time to show serum glycoproteins associated with Alzheimer disease inhibit innate phagocytosis.
Keywords: Amyloid Precursor Protein, Glycoprotein, p2x7, Peptide Interactions, Phagocytosis, Serum, Cerebrospinal Fluid, Ceruloplasmin, Serum Amyloid P-component, Tetraethylenepentamine
Abstract
Rapid phagocytosis of non-opsonized particles including apoptotic cells is an important process that involves direct recognition of the target by multiple scavenger receptors including P2X7 on the phagocyte surface. Using a real-time phagocytosis assay, we studied the effect of serum proteins on this phagocytic process. Inclusion of 1–5% serum completely abolished phagocytosis of non-opsonized YG beads by human monocytes. Inhibition was reversed by pretreatment of serum with 1–10 mm tetraethylenepentamine, a copper/zinc chelator. Inhibitory proteins from the serum were determined as negatively charged glycoproteins (pI < 6) with molecular masses between 100 and 300 kDa. A glycoprotein-rich inhibitory fraction of serum not only abolished YG bead uptake but also inhibited phagocytosis of apoptotic lymphocytes or neuronal cells by human monocyte-derived macrophages. Three copper- and/or zinc-containing serum glycoproteins, ceruloplasmin, serum amyloid P-component, and amyloid precursor protein, were identified, and the purified proteins were shown to inhibit the phagocytosis of beads by monocytes as well as phagocytosis of apoptotic neuronal cells by macrophages. Human adult cerebrospinal fluid, which contains very little glycoprotein, had no inhibitory effect on phagocytosis of either beads or apoptotic cells. These data suggest for the first time that metal-interacting glycoproteins present within serum are able to inhibit the scavenger activity of mononuclear phagocytes toward insoluble debris and apoptotic cells.
Introduction
Human monocytes suspended in saline media without serum have long been known to rapidly phagocytose a range of foreign particles, although this process has been less studied than phagocytosis in the presence of serum opsonins (1). This innate phagocytic function requires recognition of the foreign particle by one or more scavenger receptors on the phagocyte surface and is not confined to particulate matter but extends to the “silent” and rapid removal of apoptotic or aged cells in the absence of inflammation (2). The search for scavenger receptors responsible for the recognition and clearance of apoptotic cells has focused on molecules that recognize and bind phosphatidylserine on its surface of apoptotic cells (3). At least three transmembrane receptors have been predominantly identified as phosphatidylserine receptors and shown to be important for the binding and engulfment of apoptotic cells by macrophages. These include T-cell immunoglobulin and mucin-domain containing molecules (Tim-1 and Tim-4) (a family of Type-1 single transmembrane proteins) (4), brain-specific angiogenesis inhibitor (BAI-1) (an adhesion-type G-protein-coupled receptor family) (5), and stabilin-2 (a large multifunctional scavenger receptor) (6). We have recently shown another pathway for the removal of apoptotic cells involving P2X7 receptors on the phagocyte surface (7–9). The highest expression of P2X7 receptors is on professional phagocytes of monocyte/macrophage lineage including microglia (10), but P2X7 also has ubiquitous distribution, and other cell types (e.g. epithelial and both mature and progenitor neuronal cells) are able to recognize and engulf apoptotic cells at slower rates (11–13). Apart from certain complement components (14), physiological regulators in the process of non-inflammatory removal of apoptotic cells have yet to be identified. In this study we show that human serum is a potent inhibitor of non-opsonized particle phagocytosis. Through serum fractionation, we identify ceruloplasmin (CP), serum amyloid P-component (SAP), and amyloid precursor protein (APP) as prominent glycoproteins each of which inhibits bead phagocytosis including that mediated by P2X7 receptors. Concentrations of these glycoproteins are minimal in CSF, and accordingly human CSF had no effect on engulfment of beads or apoptotic cells by monocytes or macrophages. Without the presence of these inhibitors in CSF, the scavenger activity of receptors such as P2X7 would be unimpeded in the removal of apoptotic cells and insoluble debris from the central nervous system.
EXPERIMENTAL PROCEDURES
Materials
ATP, cytochalasin D (CytD), tetraethylenepentamine pentahydrochloride (TEPA), EDTA, CuSO4, and CP2 were purchased from Sigma. Ammonium sulfate was from Amresco (Solon, OH). The recombinant human interferon-γ and Mini-complete Protease Inhibitor Tablets were from Roche Applied Science. Alexa 488-conjugated Escherichia coli and Saccharomyces aureus were from Invitrogen. Fluoresbrite yellow green carboxylate microspheres (1-μm YG beads) were from Polysciences (Warrington, PA). Recombinant inter-α-trypsin inhibitor heavy chain (H1) (ITIH1) and histidine-rich glycoprotein were purchased from Abnova (Taipei, Taiwan). The purified human complements, including C3, C4, C4a, C4b, C8, factor B, and factor H, were purchased from Complement Technology, Inc. (Tyler, Texas). The mouse anti-human CP (clone 3B11) and mouse anti-SAP monoclonal antibodies (clone 6E6) were from Abcam (Cambridge, UK). The Capto Q, Q-Sepharose, Capto S, protein A, protein G, concanavalin A (Con A), Butyl-Fast Flow (FF), phenyl-Sepharose, heparin and Superdex 200 columns, and resins were from GE Healthcare.
Sources of Cells
Human peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation over Ficoll-Hypaque, washed once in RPMI 1640 medium, and resuspended in HEPES-buffered NaCl medium (140 mm NaCl, 5 mm NaOH, 5 mm KCl, 10 mm HEPES (pH 7.5), plus 5 mm glucose, 0.1% BSA, and 0.1 mm CaCl2). The human monocytic cell line THP-1 was cultured in RPMI 1640 medium containing 10% fetal calf serum and 5 μg/ml gentamycin. The THP-1 were stimulated into a macrophage-like cell by incubating with 100 nm phorbol 12-myristate 13-acetate at a 0.5 × 106/ml count for 24 h according to the previously described method (15). The study was approved by Human Research Ethics Committee of Sydney West Area Health Service (06/058) and Eastern Health of Melbourne (E05/1011). Informed consent was provided according to the Declaration of Helsinki.
Phagocytosis of YG Beads in Vitro
PBMC (4 × 106/ml) or THP-1 (2 × 106/ml) cells were resuspended in 1.0 ml of NaCl medium with 0.1 mm CaCl2. All samples were stirred, and temperature was controlled at 37 °C using a Time Zero module (Cytek Development Inc.). 5 μl of YG beads were added, and cells were analyzed at ∼1500 events/s on a FACSCalibur flow cytometer (BD Bioscience) that gated the cells by forward and side scatter as well as by cell-type-specific antibodies. The linear mean channel of fluorescence intensity for each gated subpopulation over successive 10-s intervals was analyzed by WinMDI software (by Joseph Trotter, The Scripps Research Institute, La Jolla, CA) and plotted against time. The area under YG bead uptake curve in the first 6.5 min was calculated as the phagocytosis index using a function within Excel (Microsoft).
Isolation of Serum Proteins That Inhibit Phagocytosis of Non-opsonized Particles
Human serum or heparin anti-coagulated plasma from normal subjects was precipitated with ammonium sulfate (25∼40% saturation) to deplete immunoglobulin, and the supernatant was further precipitated by 40∼60% ammonium sulfate saturation. The latter pellet was dissolved in water and dialyzed over 20 mm Tris buffer (pH 8.0) before the dialysate was loaded onto a Capto Q column (150 ml) and eluted with a NaCl gradient from 0 to 1.0 m to remove cationic charged proteins (pI > 7). Proteins eluting at higher NaCl fractions inhibited bead uptake by monocytes, and these were pooled and run through a HiTrap Protein A column (5 ml) and a HiTrap Protein G column (5 ml) to remove remaining immunoglobulin. Immunoglobulin-free samples were then loaded onto a Con A-Sepharose 4B column (100 ml), and glycoproteins were separated on a gradient of 20 mm Tris-HCl containing 0.5 m NaCl (pH 7.0) to 0.1 m borate buffer (pH 6.5). The glycoprotein-rich fractions that inhibited bead uptake were dialyzed overnight against 10 mm phosphate buffer (pH 7.0) before running through a HiTrap Heparin column (5 ml) and eluted with a NaCl gradient from 0 to 1.0 m. Eluted fractions were pooled, added to 1.4 m ammonium sulfate (pH 7.0), and run through a HiScreen butyl-Sepharose 4 Fast Flow column (4.7 ml), and samples were collected in 50 mm sodium phosphate buffer containing an ammonium sulfate gradient from 1.4 to 0 m. Fractions of interest were pooled, dialyzed against PBS, and concentrated as the serum extract. The minimum concentration of the serum extract at which bead uptake by monocytes was completely blocked was used to study its effect on phagocytosis of apoptotic cells as well as live and heat-killed bacteria. The serum extract (1.0 ml) was then loaded onto a Superdex 200 column (120 ml, 1.6 mm diameter) and eluted at 0.1 ml/min in PBS. The inhibitory effect of each fraction (0.5 ml) on YG beads uptake was measured. Most of the chromatography work was performed on an AKTA Primer Plus instrument. The whole procedure was repeated three times with reproducible results.
Western Blotting Analysis
Proteins from chromatographic fractions (30 μl each) were separated on 4–20% PAGE (Bis-Tris, Invitrogen) and transferred to nitrocellulose membranes. Primary antibodies used were mouse monoclonal antibodies (mAb) against CP (1:1000), SAP (1:1000, clone 6E6), and the N-terminal APP (1:2000, clone 22C11, in house). Proteins were visualized with ECL (Amersham Biosciences) or SuperSignal West Pico (Pierce) using a LAS-3000 Imaging suite and analyzed using Multi Gauge (Fuji).
Purification of Serum CP
Purification of CP was performed according to a method described previously (16). Briefly, 300 ml of Sepharose 2B was sequentially treated with 25 ml epichlorohydrine in 5 n NaOH and 140 ml of chloroethylamine in 10 n NaOH at 70 °C for 2 h each. Human serum (200 ml) was diluted 1:5 with 20 mm 6-amino-n-hexanoic acid (pH 7.4), filtered through a 0.22-μm membrane, and loaded to the derived Sepharose 2B column. After extensive washing with phosphate buffer (pH 7.4) at increasing concentrations, CP was eluted with 300 mm phosphate buffer (pH 7.4). The eluate was dialyzed against Hepes-buffered saline and concentrated.
Purification of SAP
Purification of SAP was performed with minor modifications to a previously described method (17). Briefly, human heparin-anticoagulated plasma (500 ml) was fractionated with saturated ammonium sulfate, and the 40∼65% fraction was collected and dialyzed against 50 mm Tris (pH 7.0). The solution was precipitated twice at 4 °C overnight after the addition of 100 mm CaCl2 each time. The filtered (0.22 μm) supernatant was loaded onto a Sepharose 2B column (200 ml) equilibrated with the loading buffer (0.1 m Tris, 0.15 m NaCl, 0.1 m CaCl2 (pH 7.8)). The SAP was eluted with elution buffer (0.1 m Tris, 0.15 m NaCl, 4 mm EDTA (pH 7.8)). The eluate was dialyzed against Hepes-buffered saline and concentrated.
Production and Purification of Recombinant APP
The recombinant fragments of the human soluble APP isoforms APP695α, APP751α, and APP770α were expressed in the methylotrophic yeast Pichia pastoris strain GS115 and purified as previously described (18) using anion exchange on a Q-Sepharose column (1.6 × 25 cm column) followed by hydrophobic exchange with phenyl-Sepharose (0.5 × 5 cm column) using an AKTA FPLC (GE Healthcare). The protein was dialyzed against Hepes-buffered saline and concentrated at 4.0 mg/ml containing 10 μm CuCl2. The deglycosylated APP695α was obtained using the Glycofree Chemical Deglycosylation kit (Prozyme, Hayward, CA) according to the manufacturer's instruction.
Phagocytosis of Bacteria in Vitro
PBMC (2.0 × 107/ml in 150 μl) were pretreated with various conditions and then incubated with 20 μg of Alexa 488-conjugated heat-killed S. aureus or E. coli (Invitrogen, 2 mg/ml) for 20 or 40 min, respectively, followed by fixation with an equal volume of 4% paraformaldehyde at 4 °C. Equal volume of 20 mm filtered sodium acetate buffer containing 1% trypan blue (pH 4.5) was added 30 s before the samples were analyzed by a BD Biosciences FACSCalibur flow cytometry in settings gated for a monocyte population.
Phagocytosis of Live S. aureus in Vitro
S. aureus (live clinical standard) was cultured in LB broth until the A690 reached 0.9. The culture was continued for another 2 h after the addition of 40 μm carboxyfluorescein diacetate succinimidyl ester (CFSE). The bacteria were washed 3 times with PBS in a 1-h period and resuspended to ∼2 mg protein/ml (equal to heat killed Alexa 488-conjugated S. aureus). Human PBMC (2 × 107/ml in 160 μl of sodium medium) were incubated with CFSE-labeled live S. aureus for 60 min followed by the addition of 100 μl of 0.25% trypsin in PBS with 1 mm EDTA for a further 2-min incubation. Cells were vigorously stirred and fixed with equal volume of 4% paraformaldehyde at 4 °C.
Phagocytosis of Apoptotic Lymphocytes in Vitro
Human mononuclear cells were isolated from 400 ml of peripheral blood by centrifugation over Ficoll. The monocytes were further separated from lymphocytes by plastic adhesion and cultured in RPMI 1640 medium plus 10% FCS and 100 units/ml interferon-γ for 5 days followed by labeling with 10 μm CMTMR for 2 h. Cells were washed once and resuspended in complete medium with interferon-γ overnight. Macrophages were then washed twice with Na+ medium and treated with various reagents or fixed with 2% paraformaldehyde. The lymphocytes were kept in RPMI 1640 medium plus 10% FCS or autologous serum before labeling with 5 μm CFSE for 2 h. Cells were washed once and incubated in complete medium with 1.0 μm staurosporine overnight. Viable lymphocytes were then separated by centrifugation over Ficoll and washed twice with Na+ medium. The proportion of apoptotic cells was assayed using annexin V and 7-AAD dual staining (data not shown). Apoptotic autologous lymphocytes (60∼85% in total cell population) suspension was added into pretreated adherent macrophages with a target cell/phagocyte ratio of 5:1. The cell mixture was incubated at 37 °C for 3 h with gentle shaking, and cells were collected by trypsin/EDTA digestion. Samples were vortexed thoroughly and fixed at 4 °C before analysis by flow cytometry.
Peptide Binding Assay
The peptide binding assay was performed as previously described (9). Short biotin-labeled peptides identical to the P2X7extracellular domain sequence were used to bind to the surface of particles, including peptide#5 (EQR LCP EYP TRR TL), #7 (CSS DRG CKK GWM DPQ), #8 (GRC VVH EGN QKT C), and #22 (KRT LIK VFG IRF DIL) for apoptotic cells, peptides #22 and #23 (KFD IIQ LVV YIG STL) for live E. coli and S. aureus, and peptides #6 (PTR RTL CSS DRG C) and #22 for 3-μm beads. YG beads (3 μm size, 5 μl), live S. aureus or E. coli (10 μl, A690 = 0.9), and staurosporine (0.5 μm)-pretreated human lymphocytes (5 × 106/ml, 200 μl) were incubated with mixed peptides (5 μg/ml each) in the presence or absence of 10% human serum, 0.5 mg/ml glycoprotein extract, 200 μg/ml purified CP, 200 μg/ml APP695, or 100 μg/ml SAP for 15 min followed by two washes. Beads and bacteria were incubated with 100 μl of HRP-labeled streptavidin (1:2000) for 15 min. After two washes, 500 μl of SuperSignal West Pico was added, and the chemiluminescence was measured by a Glo 20/20 luminometer (Promega). Apoptotic cells were incubated with FITC-conjugated streptavidin, allophycocyanin (APC)-conjugated annexin V, and 7-AAD (5 μg/ml) for 30 min. Cells were then analyzed by flow cytometry on gated annexin V+:7-AAD−/+ population.
Mass Spectrometry
The serum proteins in selected fractions after size exclusion chromatography (Superdex 200) were separated by 10% SDS-PAGE in non-reduced conditions. The gel was stained overnight with Colloidal Coomassie as described previously (19). The protein bands of interest were cut, destained, and tryptic-digested before analysis by matrix-assisted laser desorption ionization (MALDI) mass spectroscopy. For one-dimensional-electrospray ionization, the resulting digested peptides were separated by nano-LC using a CapLC system (Agilent 1100 Series, Agilent Technologies). The LC eluent was subjected to positive ion nanoflow electrospray analysis on an Applied Biosystems QSTAR XL mass spectrometer. The peak list of the peptides was submitted to the data base search program, Mascot (Matrix Science Ltd, London UK), against Homo sapiens in the SwissProt data base.
RESULTS
Serum Inhibits Phagocytosis of Non-opsonized Beads
Standard phagocytic assays are generally of several hours duration during which serum can opsonize particles and promote phagocytosis mediated by Fc and complement receptors (20). We have developed a time-resolved flow cytometry method based on previous publications (21, 22) to examine the effect of serum on phagocytosis over a short time course (<10 min). Fluorescent YG 1.0-μm beads were added to monocyte preparations, and the uptake of beads by the gated CD14+ monocytes was followed over 6–7 min at 37 °C with stirring (8). Engulfment of beads by monocytes was observed at a linear rate, whereas cell surface adhesion of beads to monocytes did not significantly contribute to the value of bead uptake as the addition of CytD, an inhibitor of phagocytosis, reduced uptake by >90% (Fig. 1a). This flow cytometric method gave the surprising result that serum was a potent inhibitor of phagocytosis of non-opsonized beads by human monocytes and was more potent than the classic phagocytosis inhibitor CytD (Fig. 1a). Autologous human serum (1–5%) or 56 °C heat-inactivated fetal bovine serum (2–5%) almost completely inhibited the phagocytosis of fluorescent latex beads by fresh peripheral blood monocytes from >40 healthy subjects. 5% serum from other mammalian species, including sheep, rabbit, rat, and mouse showed a similar inhibitory effect (data not shown).
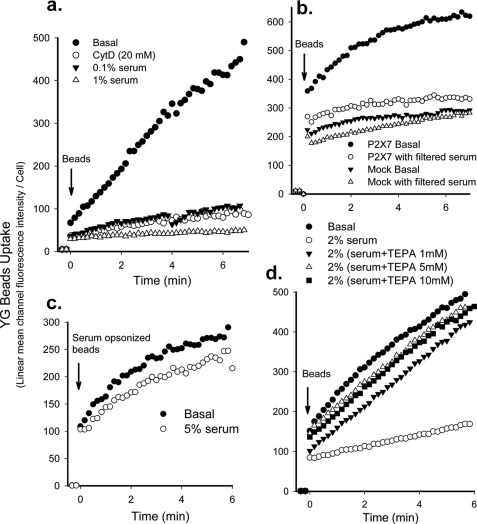
FIGURE 1.
Serum inhibits phagocytosis of non-opsonized beads by monocytes. Human monocytes (2 × 106/ml) were labeled with APC-conjugated CD14 and incubated with 20 μm CytD for 20 min or with serum for 1 min before the addition of 1-μm YG beads. The fluorescence intensity of CD14+ monocytes was analyzed by time-resolved flow cytometry. a, a typical YG beads uptake curve shows complete inhibition of bead phagocytosis by freshly prepared human monocytes by 1% serum. The curve is representative of >10 human serum samples. b, shown is phagocytosis of YG beads by HEK-293 cells transfected with DsRed-tagged P2X7 constructs or DsRed-monomer-N1 vector (Mock) in the presence or absence of 2% serum pre-filtered through a 300-kDa ultrafiltration tube. Cells were gated on DsRed for analysis. c, uptake of YG beads opsonized by preincubation with 5% serum for 3 days at 4 °C is shown. 5% serum failed to inhibit uptake of serum-opsonized beads. d, serum pretreated with 1–10 mm TEPA showed little or no inhibition of YG bead uptake.
Transfection of HEK-293 cells with wild type P2X7 conferred phagocytosis ability on this epithelial cell line as described in our previous study (8). However, phagocytosis of non-opsonized YG beads by these P2X7-transfected cells was almost completely inhibited by 2% whole serum or serum prefiltered through 300-kDa membrane (Fig. 1b). In contrast, serum had no inhibitory effect on phagocytosis by monocytes of beads precoated with IgG or serum (Fig. 1c). Pretreatment of serum with 1–10 mm TEPA (a high affinity copper and zinc chelator) for 1–3 days at 4 °C completely neutralized the inhibitory effect of serum (Fig. 1d). EDTA also neutralized the inhibitory effect of serum, although to a lesser extent than TEPA (data not shown). These data show that serum inhibits scavenger-mediated uptake of beads (including that mediated by P2X7) and suggest that divalent cations may be involved in this inhibitory effect.
Characterization of Serum Anti-phagocytic Proteins
The inhibitory effect of serum was unaltered by storage at 4 °C or heating serum at 56 °C for 30 min. To characterize the inhibitory component, serum was filtered through ultrafiltration tubes, allowing passage of proteins of size 3, 10, 30, 50, 100, and 300 kDa. Only the filtrate from the 300-kDa filter inhibited phagocytosis of YG beads by monocytes or P2X7-transfected HEK-293 cells (Fig. 1b). Therefore, the inhibitory effect was unlikely to be due to free divalent cations in serum; rather, it was due to proteins with molecular masses around or >100 kDa and <300 kDa. To examine the inhibitory effect of large serum proteins (>300 kDa), a serum lipoprotein fraction was prepared. Serum density was adjusted to 1.019, 1.063, 1.125, and 1.210 g/liter with NaBr, and sera were centrifuged at 38,000 rpm with a T70 rotor for 24 h. The upper lipoprotein-rich fractions at all four densities had little or no inhibitory effect on phagocytosis of beads. The lower lipoprotein-poor fractions showed significant inhibitory effects, suggesting that the inhibitory protein was not a lipoprotein (data not shown). We found that the inhibitory fraction of serum was able to bind the anion exchange column Capto Q but not the cation exchange column Capto S at pH 7.0, indicating that the pI of serum inhibitory protein was below 6.0.
Broad Inhibitory Effect of Serum Anti-phagocytic Proteins
We fractionated serum by sequential ammonium sulfate precipitation and subsequent chromatography with Capto Q anion exchange, removal of immunoglobulin (protein A and protein G affinity column), Con A affinity binding, heparin affinity binding, and finally butyl hydrophobic polishing (supplemental Fig. S1). Glycoprotein-rich fractions after the butyl column completely inhibited phagocytosis of latex beads and apoptotic human lymphocytes by autologous macrophages as well as S. aureus (live and heat-killed) and E. coli (heat-killed) by human monocytes (Fig. 2). Inhibition by glycoprotein fractions was comparable with CytD (Fig. 2). Preincubation of monocytes or macrophages with extracellular ATP (0.5 mm) inhibited phagocytosis of beads or bacteria, which we have previously reported as a feature of P2X7-mediated phagocytosis (8, 9).
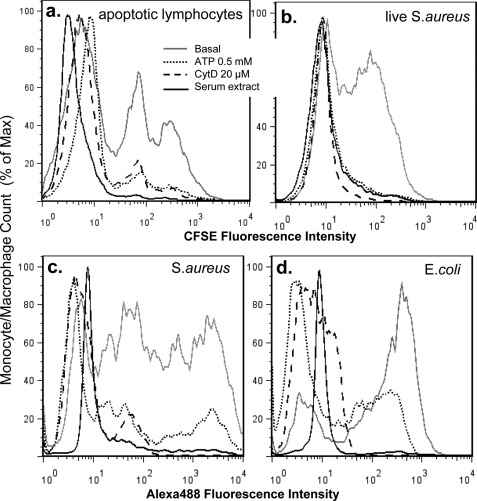
FIGURE 2.
The anti-phagocytic effect of extracted serum glycoprotein-rich fraction. Human monocyte-derived macrophages (a) or fresh monocytes (b, c, and d) were pretreated with 0.5 mm ATP for 15 min, 20 μm CytD for 30 min, or ∼250 μg/ml glycoprotein-rich serum fraction for 1 min (a concentration at which the phagocytosis of non-opsonized YG beads by monocytes was completely inhibited). a, phagocytosis of CFSE-labeled apoptotic lymphocytes by autologous monocyte-derived macrophages is shown. b, phagocytosis of CFSE-labeled live S. aureus by human monocytes is shown. c and d, phagocytosis of heat-killed Alexa 488-conjugated S. aureus (c) or E. coli. (d) by human monocytes is shown.
Identification of CP and SAP as Serum Anti-phagocytic Proteins by Mass Spectrometry
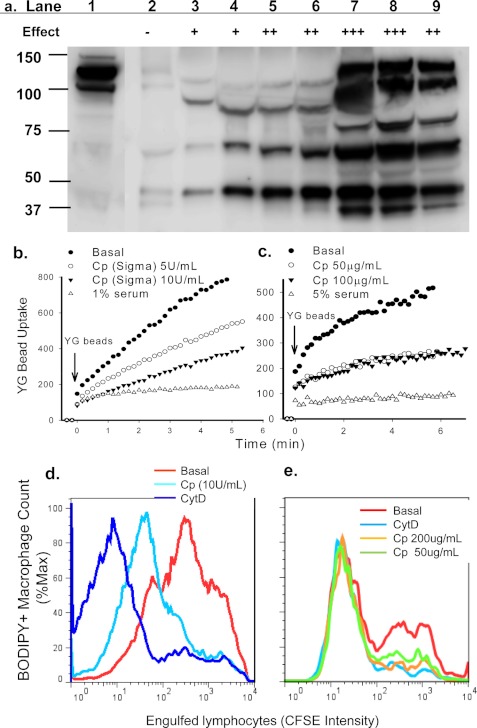
The inhibitory glycoprotein-rich fractions extracted from serum were further fractionated by size exclusion chromatography (Superdex 200). Proteins in each fraction were separated by SDS-PAGE, and eluted bands with maximum anti-phagocytic activity were analyzed by mass spectrometry (supplemental Fig. S2). CP was among the proteins identified with a high score of matching peptides (Table 1). The presence of CP in the inhibitory gel filtered glycoprotein fractions was confirmed by Western blotting assay (Fig. 3a). Full-length CP is a glycoprotein with a molecular mass of 151 kDa and pI of 4.4; however, smaller sized fragments were invariably present. Even with extensive degradation after multiple column runs, inhibition of phagocytosis was maintained in fractions containing the lower molecular mass forms of CP (120, 110, 75, and 60 kDa). Both commercial CP (Sigma) and CP purified from serum by a one-step method (16) inhibited YG bead uptake by monocytes (Fig. 3, b and c) as well as phagocytosis of apoptotic lymphocytes by autologous macrophages (Fig. 3, d and e). Concentrations of CP around 5–10 units/ml (∼100–200 μg/ml) and 50–100 μg/ml gave maximal effects on phagocytosis of beads and apoptotic cells, respectively. CP is the major copper carrier in serum, and its inhibitory effect on phagocytosis was copper-dependent as CP reconstituted in a buffer containing 5 mm EDTA (from Abcam) failed to show any inhibitory effect (data not shown).
TABLE 1.
Possible serum anti-phagocytic proteins identified by mass spectrometry
Only proteins identified at least in two out of three experiments are included.
| Protein | ID | Mr | Best score | Number of matching peptides |
|---|---|---|---|---|
| Complement factor B | CFAB_HUMAN | 85,479 | 3501 | 80 |
| Inter-α-trypsin inhibitor heavy chain H1 | ITIH1_HUMAN | 101,326 | 2645 | 51 |
| Complement component C8 α-chain | CO8A_HUMAN | 65,121 | 1805 | 37 |
| Inter-α-trypsin inhibitor heavy chain H2 | ITIH2_HUMAN | 106,370 | 1549 | 31 |
| Ceruloplasmin | CERU_HUMAN | 122,128 | 1404 | 30 |
| Histidine-rich glycoprotein | HRG_HUMAN | 59,541 | 1341 | 34 |
| Complement component C8 γ-chain | CO8G_HUMAN | 22,264 | 1270 | 22 |
| Complement component C4a | CO4A_HUMAN | 192,650 | 910 | 28 |
| Complement component C3 | CO3_HUMAN | 187,030 | 879 | 24 |
| Serum amyloid P-component | SAMP_HUMAN | 25,371 | 273 | 7 |
FIGURE 3.
CP inhibits phagocytosis of beads and apoptotic cells. a, immunoreactive CP protein in column fractions was detected by Western blotting. Lane 1, starting material; lanes 2∼9, fractions (30 μl each) eluted from Superdex 200 gel filtration. The relative inhibitory effect of each fraction on YG bead uptake by monocytes is indicated (−, no inhibition; + to +++, increasing strength of inhibition). b and c, human PBMC labeled with APC-conjugated anti-CD14 mAb were resuspended in sodium medium with 0.1 mm Ca2+. CP or serum was added 1 min before the addition of YG beads. The fluorescence intensity of CD14+ monocytes containing engulfed beads were analyzed by time-resolved flow cytometry. d and e, flow cytometry histograms show phagocytosis of CFSE-labeled apoptotic lymphocytes by autologous monocyte-derived macrophages labeled with CMTMR or BODIPY. Mixed cells were incubated with CP or CytD (20 μm) for 3 h before cells were collected and fixed for flow cytometry assay. Inhibitors used were commercially obtained CP (∼50 unit/mg) (b and d), CP purified from human serum (c and e), or human serum (b and c).
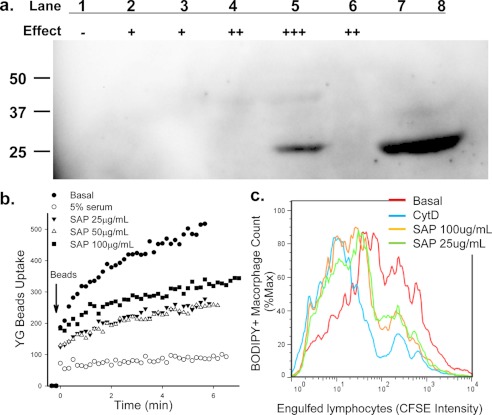
Mass spectrometry also gave a good peptide matching score for SAP (Table 1), although this score was lower than for ceruloplasmin. Western blotting confirmed the presence of SAP in the inhibitory size exclusion chromatography fractions (Fig. 4a). Moreover, purified SAP inhibited both phagocytosis of YG beads by monocytes (Fig. 4b) and phagocytosis of apoptotic lymphocytes by autologous macrophages (Fig. 4c). Inhibition by SAP was only found to be partial compared with 5% serum, even at a concentration of 100 μg/ml.
FIGURE 4.
SAP inhibits phagocytosis of beads and apoptotic cells. a, SAP protein in column fractions was detected by Western blotting. Lane 1–6, fractions were eluted from Superdex 200 gel filtration. The relative inhibitory effect of each fraction on YG bead uptake by monocytes is indicated; lanes 7 and 8 are 5 and 10 μl of purified SAP (1.0 mg/ml), respectively. b, human PBMC labeled with APC-conjugated anti-CD14 mAb were resuspended in sodium medium with 0.1 mm Ca2+. Purified SAP was added 1 min before the addition of YG beads. The uptake of YG beads by CD14+ monocytes was analyzed by time-resolved flow cytometry. c, flow cytometry histograms show phagocytosis of CFSE-labeled apoptotic lymphocytes by autologous monocyte-derived macrophages labeled with CMTMR or BODIPY. Mixed cells were incubated with SAP or CytD (20 μm) for 3 h before cells were collected and fixed for flow cytometry assay.
Upon examination of other proteins in Table 1 with high matching scores, anti-phagocytic activity was unable to be detected with purified complement C3, C4, C4a, C4b, C8, and Factor B (100 μg/ml) or with recombinant ITIH1 and histidine-rich glycoprotein (50 μg/ml) as measured with YG bead uptake by monocytes (data not shown).
Identification of APP as Another Serum Anti-phagocytic Protein
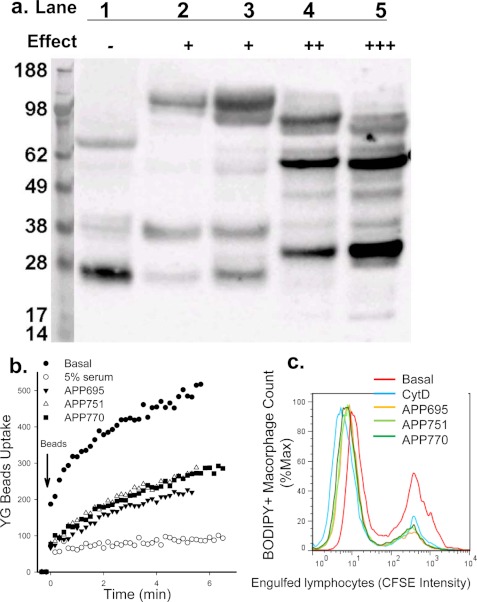
We noted the purification steps for the inhibitory glycoproteins in serum were similar to methods previously described for isolation of APP from tissue and biological fluids. Although mass spectrometry failed to identify peptides matching APP, we studied whether APP could inhibit phagocytosis. We confirmed the presence of APP in size exclusion chromatography fractions high in phagocytic inhibition by Western blotting using an antibody against the N terminus of APP (clone 22C11) (Fig. 5a). Multiple immunoreactive bands of APP were detected on gels, suggesting APP underwent extensive degradation after column chromatography runs potentially through proteolytic degradation as reported previously (23). The inhibitory effect of APP was observed with all three predominant soluble isoforms of APP (sAPP695α, sAPP751α, and sAPP770α) at concentrations of 100–200 μg/ml, as assayed by phagocytosis of beads by monocytes (Fig. 5b) and phagocytosis of apoptotic lymphocytes by autologous macrophages (Fig. 5c). The similar inhibitory effect of all three soluble isoforms of APP suggested the inhibitory region of the ectodomain of full-length APP is within a conserved domain.
FIGURE 5.
APP inhibits phagocytosis of beads and apoptotic cells. a, shown is APP protein expression detected by Western blotting with anti-APP mAb (clone 22C11). Lanes 1∼5, fractions eluted from Superdex 200 gel filtration. The relative inhibitory effect of each fraction on YG bead uptake by monocytes is indicated. b, human PBMC labeled with APC conjugated anti-CD14 mAb were resuspended in sodium medium with 0.1 mm Ca2+. Recombinant APP695α, APP751α, or APP770α (100 μg/ml each) was added 1 min before the addition of YG beads. The uptake of YG beads by CD14+ monocytes were analyzed by time-resolved flow cytometry. c, flow cytometry histograms show phagocytosis of CFSE-labeled apoptotic lymphocytes by autologous monocyte-derived macrophages labeled with CMTMR or BODIPY. Mixed cells were incubated with isoforms of APP (200 μg/ml) or CytD (20 μm) for 3 h before cells were collected and fixed for flow cytometry assay.
Serum Anti-phagocytic Proteins Interfere with Particle Recognition by P2X7
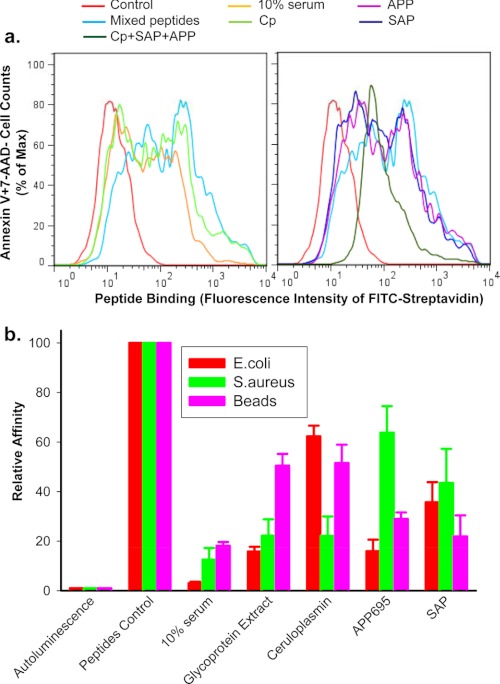
Our previous studies have shown that the P2X7 receptor expressed on the phagocyte surface directly recognizes beads, bacteria, and apoptotic cells via critical peptide sequences in its extracellular loop that recognize these targets (8, 9). We then examined whether the anti-phagocytic proteins found in serum exerted their inhibitory effect by blocking the interaction between target particle and P2X7. Short peptides, with P2X7-mimicking sequences known to bind to beads, bacteria, and apoptotic cells were incubated with these targets in the presence or absence of 10% serum, the serum glycoprotein-rich fraction, CP, APP, or SAP. Serum and all three purified glycoproteins were found to reduce the binding affinity of peptides to the surface of apoptotic cells (Fig. 6a) as well as to the surface of beads, E. coli, and S. aureus (Fig. 6b).
FIGURE 6.
Serum glycoproteins inhibit peptide binding to phagocytic target particles. Short biotin-labeled peptides, identical to the P2X7 extracellular domain sequence, show binding to the surface of apoptotic lymphocytes (a) or live E. coli, S. aureus, and 3-μm latex beads (b). Particles were incubated with mixed peptides (5 μg/ml each) in the presence or absence of 10% human serum, 0.5 mg/ml glycoprotein extract, 200 μg/ml purified CP, 200 μg/ml APP695, 100 μg/ml SAP for 15 min, or all three glycoproteins together. a, shown is a flow cytometry histogram of peptides binding. Apoptotic cells were incubated with FITC-conjugated streptavidin, APC-conjugated annexin V, and 7-AAD for 30 min. Cells were then analyzed by flow cytometry on gated Annexin V+7-AAD−/+ population. b, shown is peptide binding as measured by chemiluminescence assay. Beads and bacteria were incubation with 100 μl of HRP-labeled streptavidin (1:2000) for 15 min. Data were normalized to binding of peptide control in the absence of serum or glycoproteins and are presented as the mean ± S.D. (n = 3).
Possible Additive Effect of CP, APP, and SAP
Compared with CP, APP, or SAP alone, a combination of CP (100 μg/ml), SAP (50 μg/ml), and sAPP695α (100 μg/ml) showed enhanced inhibition on bead uptake by monocytes as well as on phagocytosis of apoptotic lymphocytes by autologous macrophages (supplemental Fig. S3). In addition, a combination of all three glycoproteins showed a more potent inhibitory effect on peptide binding (Fig. 6a).
Deglycosylated sAPP695α Retained Inhibitory Effect
Because CP, SAP, and APP are all glycoproteins, we investigated whether the linked glycan was essential for the inhibitory effect exerted by these three proteins. Soluble APP695α (100 μg/ml), deglycosylated with anhydrous trifluoromethanesulfonic acid, showed a similar inhibitory effect on phagocytosis of beads and apoptotic cells as compared with previous results with recombinant sAPP695α (supplemental Fig. 4).
Cerebrospinal Fluid Does Not Inhibit Phagocytosis of Beads or Apoptotic Neuronal Cells
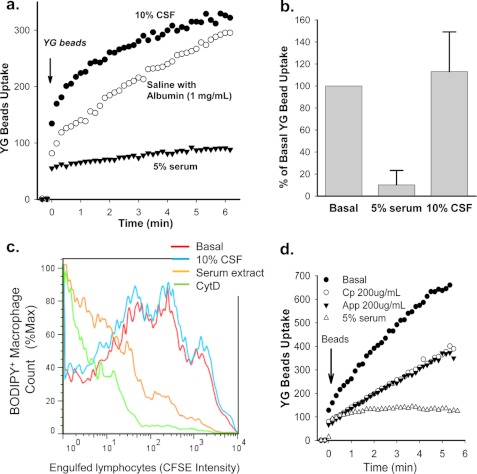
The major protein in CSF is albumin, and little or no glycoprotein is present (24). Inclusion of 10% CSF collected from subjects (n = 15) with non-neurological disease failed to inhibit phagocytosis of non-opsonized beads and in most cases slightly increased phagocytosis of these YG beads (Fig. 7, a and b). Similarly, inclusion of 10% CSF had no effect on the phagocytosis of apoptotic neuronal cells by human monocyte-derived macrophages (Fig. 7c). This result contrasts with the marked inhibition of phagocytosis of apoptotic neuronal cells by the serum glycoprotein-rich fraction (Fig. 7c). It is worth noting that 5% serum as well as purified CP and recombinant APP695α retained their potent inhibitory effect on bead uptake in a buffered medium containing 50% CSF (Fig. 7d), suggesting that these serum glycoproteins would exert a similar inhibitory effect on phagocytosis of non-opsonized particles within the central nervous system.
FIGURE 7.
The effect of CSF on phagocytosis of YG beads and apoptotic neuronal cells by human monocyte/macrophages. a, a typical YG beads uptake curve shows phagocytosis of beads by human monocytes is inhibited by 5% serum but not by 10% CSF. b, composite results for YG bead uptake at 5 min from 15 CSF samples and >40 human serum samples (*, p < 0.001). c, shown is phagocytosis of apoptotic SH-SY5Y neuroblastoma cells by human monocyte-derived macrophages. SH-SY5Y cells were induced to apoptosis by 0.2 μm staurosporine for 30 min and labeled with 10 μm CFSE. Before the addition of SH-SY5Y cells, BODIPY 630/650-SE (5 μm)-labeled human macrophages were incubated with 10% CSF or 10% serum glycoprotein-rich fraction or 20 μm CytD. Mixed cells were incubated for 3 h before collection and fixation. Results are representative of eight experiments with different CSF samples. d, phagocytosis of YG beads by human monocytes is shown. Cells were resuspended in sodium medium containing 50% mixed human CSF with or without 200 μg/ml CP, 200 μg/ml APP695α, or 5% serum before the addition of YG beads.
DISCUSSION
It is well known that serum promotes phagocytosis by providing various opsonins, e.g. immunoglobulin and complement. However, the effect of serum proteins on phagocytosis of non-opsonized particles, i.e. innate phagocytosis, has been less studied. In this study we show that as little as 1–5% serum from healthy subjects completely abolishes phagocytosis of non-opsonized particles and apoptotic cells by mononuclear phagocytes. The inhibitory effect is due to a group of negatively charged copper/zinc-binding glycoproteins, including CP, APP, and SAP. Moreover, we found that the inhibitory effect of serum can be reversed by chelating divalent metals including copper and zinc. In normal conditions the levels of these glycoproteins are much lower in CSF than in serum, which is consistent with our data showing that phagocytosis of non-opsonized particles and apoptotic cells by mononuclear phagocytes is unimpeded in the presence of CSF (Fig. 7). During chronic inflammation or infection, the serum levels of both SAP and ceruloplasmin increase, but it is not known whether this affects CSF levels of these glycoproteins.
Chromatographic and MS analyses of this glycoprotein-rich fraction of serum showed CP, APP, and SAP were all inhibitory to innate phagocytosis at concentrations similar to those present in plasma. CP is the major copper-carrying protein in blood and utilizes these multiple coppers for its broad oxidase activity on ferrous iron (25), nitric oxide (26), and amines (27). APP in its full-length form is an integral membrane protein with neuroprotective properties that interacts with copper as well as zinc. Similar to CP, APP has recently been found to have both ferroxidase (28) and amine oxidase activity (29); however, it is not known if these enzymatic activities are modified by the presence of P2X7. APP is transcribed into a number of isoforms, with APP695 being the predominant form expressed in neuronal tissue, whereas isoforms APP751 and APP770 (containing the Kunitz protease inhibitor domain, respectively, without and with the Ox-2 domain) are widely expressed in non-neuronal cells (30). All isoforms are further modified through proteolytic cleavage, yielding major products including soluble APPα and APPβ present in most biological fluids including serum as well as the Aβ peptides that make up the major components of amyloid deposition in cardiovascular and brain tissue. Recent evidence has indicated that neuronal P2X7 receptors trigger α-secretase-dependent cleavage of APP to yield the neuroprotective soluble APPα product (23, 31). Significantly, ITIH1 and -H2 contain a similar Kunitz protease inhibitor domain to the longer isoforms of APP (32). Although the recombinant ITI-H1 failed to inhibit the phagocytosis of non-opsonized beads by monocytes as shown with APP751 and APP770, we are currently investigating if modifications to the Kunitz protease inhibitor domain present in both glycoproteins are of relevance for non-opsonized phagocytosis. Our data show that SAP is a third glycoprotein that inhibits the phagocytosis of non-opsonized beads and apoptotic cells. Serum amyloid P component exists as a 150-kDa pentamer in serum and is thought to inhibit proteolytic cleavage and, hence, impede protein scavenging mechanisms. SAP has been shown to be an important contributor to amyloidogenic pathogenesis and is a major contributor to Aβ containing amyloid deposits. As with CP and APP, SAP is known to bind copper as well as other divalent metals such as calcium, cadmium, and zinc (33).
Currently, the full mechanism by which these serum glycoproteins inhibit phagocytosis is unknown. None of the three identified inhibitory glycoproteins abolished phagocytosis of beads to the level observed with 5% serum, suggesting that the serum effect may either be additive from a group of glycoproteins as inferred in this study, or other inhibitory proteins yet to be identified are present. Whatever the identity of this unknown protein(s), it is likely that inhibition is dependent on bound metal ions as preincubation of serum with the copper/zinc selective chelator TEPA abolished inhibition by serum.
The potent inhibitory effect of the serum glycoprotein-rich fraction on innate phagocytosis suggested that glycosylation may be a contributing factor to the inhibition. Therefore, it was important to determine whether these proteins retained their inhibitory effect after deglycosylation. To date, enzymatic digestion has been unable to reliably remove O-linked glycan. Therefore, we used a chemical method to remove both N- and O-linked glycoconjugates on CP, APP, and SAP. Unfortunately, soluble deglycosylated CP or SAP was unable to be obtained using this technique, and thus further investigation was carried out with APP. Although this remains to be elucidated in all proteins, deglycosylated sAPP695α continued to have an inhibitory effect, suggesting that the glycosylation state is not of importance to the inhibition of innate phagocytosis by these glycoproteins.
Serum concentrations of CP, APP, and SAP are well characterized, but the concentrations of these glycoproteins in CSF are less than 1% of values in serum. For example, average concentration of CP in serum is 150–600 μg/ml compared with values in CSF of 1.57 μg/ml (34). Concentrations of SAP, APPα, and APPβ in CSF are likewise extremely low with average values of 4.5 (35), 298, and 932 ng/ml (36), respectively. Based on our data in Figs. 3, 4, and 5, glycoproteins at these concentrations are unlikely to inhibit phagocytosis of beads or apoptotic cells. Direct measurement of phagocytosis in the presence of 50% CSF (Fig. 7d) confirmed that phagocytosis was unimpeded by CSF and that purified CP and APP, which are largely excluded from CSF, remained inhibitory to phagocytosis in the CSF medium. Interestingly, all three inhibitory glycoproteins identified in this study have also been implicated in neurodegeneration, particularly Alzheimer disease (37, 38). Our identification of CP, SAP, and APP as inhibitory proteins for phagocytosis of apoptotic cells mediated by P2X7 or other scavenger receptors may provide new insights into the pathogenesis of neurodegenerative disease.
Our previous data have established P2X7 as one of the scavenger receptors involved in the recognition and removal of particles and apoptotic cells in the absence of extracellular ATP and serum (7–9). Fig. 1b shows that a glycoprotein-rich filtrate of serum inhibited the phagocytosis of beads by P2X7-transfected HEK-293 cells, whereas Fig. 7 shows glycoproteins inhibited phagocytosis of neuronal cells by human macrophages, a cell type in which P2X7 is greatly up-regulated on its surface. Binding assays of peptides, which mimic P2X7 extracellular sequence, support this data and suggest that serum glycoproteins interfere with the interaction between P2X7 and the target particle. Our previous study has identified an extremely positively charged (pI = 11.5) region of the P2X7 extracellular domain located at amino acid residues 306–320 (same sequence as peptide #22 used in all peptide binding assay) that recognizes beads, bacteria, and apoptotic cells (9). It is possible that negatively charged CP, APP, and SAP bind to this 306–320 region of P2X7 via weak electrostatic force. However, we consider the binding affinity is low, as stringent washing easily reversed this inhibition. The P2X7 receptor is in a tight complex with nonmuscle myosin heavy chain IIA, and it is this complex, involving the cytoskeleton, that regulates the engulfment of a broad range of non-opsonized targets such as latex beads, bacteria, and apoptotic cells of both neuronal and non-neuronal origin (7–9). Nevertheless it is likely that multiple scavenger receptors mediate phagocytosis of non-opsonized particles and apoptotic cells, and not only P2X7 but other scavenger receptors such as Tim-4, BAI-1, stabilin-1, scavenger receptor (SR) type A receptors (e.g. CD204), or SR type B receptors (e.g. CD36) may be sensitive to inhibition by serum glycoproteins (39, 40). Indeed, SAP is already known to interact with type B scavenger receptor CD36 (41).
Phagocytosis is a critical physiological process for tissue remodeling that removes apoptotic or aged cells in a regulated manner in the absence of inflammation (3). Specific recognition of the apoptotic cell by the phagocyte occurs without regard to the species or tissue origin of the target or the nature of the apoptotic stimulus and can occur without the involvement of soluble factors (42). Our present data show that as little as 1–5% serum from many animal species and healthy humans completely inhibits this innate phagocytosis of apoptotic cells and that three glycoproteins purified from human serum, CP, SAP, and APP, contribute in a major way to this inhibitory effect of serum. Each of these three glycoproteins interacts with metal ions (copper and/or zinc) that also appear essential for their inhibitory role. We have previously shown that P2X7 expressed on the phagocyte surface is directly involved in the recognition and binding to apoptotic targets most likely by thiol-disulfide bond rearrangement (9). Our present data suggest that scavenger receptors including P2X7 on mononuclear phagocytes of the play an important role in the non-inflammatory removal of apoptotic neuronal cells and insoluble debris. Furthermore, disruption of the blood brain barrier may allow entry of serum glycoproteins into CSF and inhibit this scavenger function with deleterious neurological effects.
Supplementary Material
Acknowledgments
We thank Dr. Steve Fuller for organizing some blood donors and Kristen K. Skarratt, Chun Sun, and Linh Lam for technical assistance. Part of the mass spectrometry work was undertaken at Australian Proteome Analysis Facility, the infrastructure provided by the Australian Government through the National Collaborative Research Infrastructure Strategy.
This work was supported by the Cure Cancer Australia Foundation, the Leukemia Foundation of Australia, and the National Health and Medical Research Council, Australia.

This article contains supplemental Figs. 1–4.
- CP
- ceruloplasmin
- SAP
- serum amyloid P-component
- APP
- amyloid precursor protein
- CSF
- cerebrospinal fluid
- TEPA
- tetraethylenepentamine
- CytD
- cytochalasin D
- PBMC
- peripheral blood mononuclear cells
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- IRIH
- inter-α-trypsin inhibitor heavy chain
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- 7-AAD
- 7-aminoactinomycin D
- Con A
- concanavalin A
- SR
- Scavenger Receptor
- APC
- allophycocyanin.
REFERENCES
- 1. Cline M. J., Lehrer R. I. (1968) Phagocytosis by human monocytes. Blood 32, 423–435 [PubMed] [Google Scholar]
- 2. Ravichandran K. S. (2003) “Recruitment signals” from apoptotic cells. Invitation to a quiet meal. Cell 113, 817–820 [DOI] [PubMed] [Google Scholar]
- 3. Ravichandran K. S., Lorenz U. (2007) Engulfment of apoptotic cells. Signals for a good meal. Nat. Rev. Immunol. 7, 964–974 [DOI] [PubMed] [Google Scholar]
- 4. Wong K., Valdez P. A., Tan C., Yeh S., Hongo J. A., Ouyang W. (2010) Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc. Natl. Acad. Sci. U.S.A. 107, 8712–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park D., Tosello-Trampont A. C., Elliott M. R., Lu M., Haney L. B., Ma Z., Klibanov A. L., Mandell J. W., Ravichandran K. S. (2007) BAI-1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 [DOI] [PubMed] [Google Scholar]
- 6. Park S. Y., Jung M. Y., Kim H. J., Lee S. J., Kim S. Y., Lee B. H., Kwon T. H., Park R. W., Kim I. S. (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 [DOI] [PubMed] [Google Scholar]
- 7. Gu B. J., Rathsam C., Stokes L., McGeachie A. B., Wiley J. S. (2009) Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex. This dissociation regulates P2X(7) pore formation. Am. J. Physiol. Cell Physiol. 297, C430–D439 [DOI] [PubMed] [Google Scholar]
- 8. Gu B. J., Saunders B. M., Jursik C., Wiley J. S. (2010) The P2X7-nonmuscle myosin membrane complex regulates phagocytosis of nonopsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood 115, 1621–1631 [DOI] [PubMed] [Google Scholar]
- 9. Gu B. J., Saunders B. M., Petrou S., Wiley J. S. (2011) P2X(7) is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J. Immunol. 187, 2365–2375 [DOI] [PubMed] [Google Scholar]
- 10. Wiley J. S., Sluyter R., Gu B. J., Stokes L., Fuller S. J. (2011) The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332 [DOI] [PubMed] [Google Scholar]
- 11. Lu Z., Elliott M. R., Chen Y., Walsh J. T., Klibanov A. L., Ravichandran K. S., Kipnis J. (2011) Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat. Cell Biol. 13, 1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sexton D. W., Al-Rabia M., Blaylock M. G., Walsh G. M. (2004) Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin. Exp. Allergy 34, 1514–1524 [DOI] [PubMed] [Google Scholar]
- 13. Bowen S., Ateh D. D., Deinhardt K., Bird M. M., Price K. M., Baker C. S., Robson J. C., Swash M., Shamsuddin W., Kawar S., El-Tawil T., Roos J., Hoyle A., Nickols C. D., Knowles C. H., Pullen A. H., Luthert P. J., Weller R. O., Hafezparast M., Franklin R. J., Revesz T., King R. H., Berninghausen O., Fisher E. M., Schiavo G., Martin J. E. (2007) The phagocytic capacity of neurones. Eur. J. Neurosci. 25, 2947–2955 [DOI] [PubMed] [Google Scholar]
- 14. Trouw L. A., Blom A. M., Gasque P. (2008) Role of complement and complement regulators in the removal of apoptotic cells. Mol. Immunol. 45, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 15. Humphreys B. D., Dubyak G. R. (1998) Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J. Leukoc. Biol. 64, 265–273 [DOI] [PubMed] [Google Scholar]
- 16. Calabrese L., Carbonaro M., Musci G. (1988) Chicken ceruloplasmin. Evidence in support of a trinuclear cluster involving type 2 and 3 copper centers. J. Biol. Chem. 263, 6480–6483 [PubMed] [Google Scholar]
- 17. Urbanyi Z., Medzihradszky D. (1992) Rapid method to isolate serum amyloid P component from human plasma. Characterization of the isolated protein. J. Chromatogr. B. Biomed. Appl. 578, 130–133 [DOI] [PubMed] [Google Scholar]
- 18. Henry A., Masters C. L., Beyreuther K., Cappai R. (1997) Expression of human amyloid precursor protein ectodomains in Pichia pastoris. Analysis of culture conditions, purification, and characterization. Protein Expr. Purif. 10, 283–291 [DOI] [PubMed] [Google Scholar]
- 19. Brymora A., Valova V. A., Robinson P. J. (2004) Protein-protein interactions identified by pull-down Experiments and mass spectrometry. Curr. Protoc. Cell Biol. Chapter 17. Unit 17.5 [DOI] [PubMed] [Google Scholar]
- 20. Olds J. W., Reed W. P., Eberle B., Kisch A. L. (1974) Corticosteroids, serum, and phagocytosis. In vitro and in vivo studies. Infect. Immun. 9, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schroeder F., Kinden D. A. (1983) Measurement of phagocytosis using fluorescent latex beads. J. Biochem. Biophys. Methods 8, 15–27 [DOI] [PubMed] [Google Scholar]
- 22. Steinkamp J. A., Wilson J. S., Saunders G. C., Stewart C. C. (1982) Phagocytosis. Flow cytometric quantitation with fluorescent microspheres. Science 215, 64–66 [DOI] [PubMed] [Google Scholar]
- 23. Delarasse C., Auger R., Gonnord P., Fontaine B., Kanellopoulos J. M. (2011) The purinergic receptor P2X7 triggers α-secretase-dependent processing of the amyloid precursor protein. J. Biol. Chem. 286, 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maurer M. H. (2010) Proteomics of brain extracellular fluid (ECF) and cerebrospinal fluid (CSF). Mass Spectrom. Rev. 29, 17–28 [DOI] [PubMed] [Google Scholar]
- 25. Osaki S. (1966) Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin). J. Biol. Chem. 241, 5053–5059 [PubMed] [Google Scholar]
- 26. Shiva S., Wang X., Ringwood L. A., Xu X., Yuditskaya S., Annavajjhala V., Miyajima H., Hogg N., Harris Z. L., Gladwin M. T. (2006) Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2, 486–493 [DOI] [PubMed] [Google Scholar]
- 27. Schosinsky K. H., Lehmann H. P., Beeler M. F. (1974) Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin. Chem. 20, 1556–1563 [PubMed] [Google Scholar]
- 28. Duce J. A., Tsatsanis A., Cater M. A., James S. A., Robb E., Wikhe K., Leong S. L., Perez K., Johanssen T., Greenough M. A., Cho H. H., Galatis D., Moir R. D., Masters C. L., McLean C., Tanzi R. E., Cappai R., Barnham K. J., Ciccotosto G. D., Rogers J. T., Bush A. I. (2010) Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer disease. Cell 142, 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duce J. A., Ayton S., Miller A. A., Tsatsanis A., Lam L. Q., Leone L., Corbin J. E., Butzkueven H., Kilpatrick T. J., Rogers J. T., Barnham K. J., Finkelstein D. I., Bush A. I. (2012) Amine oxidase activity of β-amyloid precursor protein modulates systemic and local catecholamine levels. Mol. Psychiatry, doi: 10.1038/mp.2011.168 [DOI] [PubMed] [Google Scholar]
- 30. Tanaka S., Shiojiri S., Takahashi Y., Kitaguchi N., Ito H., Kameyama M., Kimura J., Nakamura S., Ueda K. (1989) Tissue-specific expression of three types of β-protein precursor mRNA. Enhancement of protease inhibitor-harboring types in Alzheimer disease brain. Biochem. Biophys. Res. Commun. 165, 1406–1414 [DOI] [PubMed] [Google Scholar]
- 31. Caillé I., Allinquant B., Dupont E., Bouillot C., Langer A., Müller U., Prochiantz A. (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131, 2173–2181 [DOI] [PubMed] [Google Scholar]
- 32. Reisinger P., Hochstrasser K., Albrecht G. J., Lempart K., Salier J. P. (1985) Human inter-α-trypsin inhibitor. Localization of the Kunitz-type domains in the N-terminal part of the molecule and their release by a trypsin-like proteinase. Biol. Chem. Hoppe. Seyler 366, 479–483 [DOI] [PubMed] [Google Scholar]
- 33. Potempa L. A., Kubak B. M., Gewurz H. (1985) Effect of divalent metal ions and pH upon the binding reactivity of human serum amyloid P component, a C-reactive protein homologue, for zymosan. Preferential reactivity in the presence of copper and acidic pH. J. Biol. Chem. 260, 12142–12147 [PubMed] [Google Scholar]
- 34. Loeffler D. A., DeMaggio A. J., Juneau P. L., Brickman C. M., Mashour G. A., Finkelman J. H., Pomara N., LeWitt P. A. (1994) Ceruloplasmin is increased in cerebrospinal fluid in Alzheimer disease but not Parkinson disease. Alzheimer Dis. Assoc. Disord. 8, 190–197 [DOI] [PubMed] [Google Scholar]
- 35. Kimura M., Asada T., Uno M., Machida N., Kasuya K., Taniguchi Y., Fujita T., Nishiyama E., Iwamoto N., Arai H. (1999) Assessment of cerebrospinal fluid levels of serum amyloid P component in patients with Alzheimer disease. Neurosci. Lett. 273, 137–139 [DOI] [PubMed] [Google Scholar]
- 36. Perneczky R., Tsolakidou A., Arnold A., Diehl-Schmid J., Grimmer T., Förstl H., Kurz A., Alexopoulos P. (2011) CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology 77, 35–38 [DOI] [PubMed] [Google Scholar]
- 37. Capo C. R., Arciello M., Squitti R., Cassetta E., Rossini P. M., Calabrese L., Rossi L. (2008) Features of ceruloplasmin in the cerebrospinal fluid of Alzheimer disease patients. Biometals 21, 367–372 [DOI] [PubMed] [Google Scholar]
- 38. Squitti R., Zito G. (2009) Anti-copper therapies in Alzheimer disease. New concepts. Recent Pat. CNS Drug Discov. 4, 209–219 [DOI] [PubMed] [Google Scholar]
- 39. Komohara Y., Terasaki Y., Kaikita K., Suzuki H., Kodama T., Takeya M. (2005) Clearance of apoptotic cells is not impaired in mouse embryos deficient in class A scavenger receptor types I and II (CD204). Dev. Dyn. 232, 67–74 [DOI] [PubMed] [Google Scholar]
- 40. Greenberg M. E., Sun M., Zhang R., Febbraio M., Silverstein R., Hazen S. L. (2006) Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stewart C. R., Tseng A. A., Mok Y. F., Staples M. K., Schiesser C. H., Lawrence L. J., Varghese J. N., Moore K. J., Howlett G. J. (2005) Oxidation of low-density lipoproteins induces amyloid-like structures that are recognized by macrophages. Biochemistry 44, 9108–9116 [DOI] [PubMed] [Google Scholar]
- 42. Cvetanovic M., Mitchell J. E., Patel V., Avner B. S., Su Y., van der Saag P. T., Witte P. L., Fiore S., Levine J. S., Ucker D. S. (2006) Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J. Biol. Chem. 281, 20055–20067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.