Background: The neural cell adhesion molecule L1 is important in the developing and adult nervous system.
Results: L1 stimulation leads to sumoylation and proteolytic processing of L1 and translocation of a sumoylated transmembrane fragment to the nucleus.
Conclusion: Sumoylation and nuclear localization of the L1 fragment are required for L1-dependent functions.
Significance: Unraveling the molecular mechanisms underlying L1-activated cellular responses helps understanding L1-linked disorders.
Keywords: Cell Adhesion, Cell Signaling, Development, Neurite Outgrowth, Nuclear Translocation, Protease, Protein Sorting, Sumoylation, Proteolytic Processing
Abstract
The functions of the cell adhesion molecule L1 in the developing and adult nervous system are triggered by homophilic and heterophilic interactions that stimulate signal transductions that activate cellular responses. Here, we show that stimulation of signaling by function-triggering L1 antibodies or L1-Fc leads to serine protease-dependent cleavage of full-length L1 at the plasma membrane and generation of a sumoylated transmembrane 70-kDa fragment comprising the intracellular and transmembrane domains and part of the extracellular domain. The 70-kDa transmembrane fragment is transported from the plasma membrane to a late endosomal compartment, released from endosomal membranes into the cytoplasm, and transferred from there into the nucleus by a pathway that depends on importin and chromatin-modifying protein 1. Mutation of the sumoylation site at Lys1172 or of the nuclear localization signal at Lys1147 abolished L1-stimulated generation or nuclear import of the 70-kDa fragment, respectively. Nuclear import of the 70-kDa fragment may activate cellular responses in parallel or in association with phosphorylation-dependent signaling pathways. Alterations in the levels of the 70-kDa fragment during development and in the adult after spinal cord injury or in a mouse model of Alzheimer disease suggest that this fragment is functionally implicated in development, regeneration, neurodegeneration, tumorigenesis, and possibly synaptic plasticity in the mature nervous system.
Introduction
The neural cell adhesion molecule L1 not only plays crucial roles during development of the nervous system, such as neuronal migration and survival, axon outgrowth and fasciculation, and myelination, but it is also involved in functions of the adult brain, such as synaptic plasticity, learning, and memory as well as regeneration after central and peripheral nervous system trauma (1–9). Dynamic events such as cell migration, neuritogenesis, and synaptic plasticity depend on regulated proteolysis of transmembrane adhesion molecules to disconnect contacts between cells or between cells and the extracellular matrix and to trigger signal transduction and gene expression (10, 11). The functions of L1 also depend on regulated proteolytic cleavage as first described for neurite outgrowth. The 200-kDa full-length molecule L1, which consists of extracellular immunoglobulin- and fibronectin type III (FNIII)3-like domains, a transmembrane domain, and a cytoplasmic tail (12), is proteolytically processed at different sites by different enzymes. Cleavage by trypsin, plasmin, or proprotein convertase PC5a generates a soluble extracellular 140-kDa fragment and an 80-kDa transmembrane fragment (13–16). The membrane-proximal cleavage of the full-length molecule or the 80-kDa fragment by metalloproteases, such as ADAM10 and ADAM17, or by the serine protease neuropsin yields soluble extracellular 180- and 50-kDa fragments as well as a 32-kDa membrane-bound fragment (16–20). The 32-kDa transmembrane fragment is further processed by γ-secretase, generating a soluble intracellular 28-kDa fragment that enters the nucleus and leads to nuclear signaling and gene expression (20).
Here, we show that stimulation of L1 signaling leads to serine protease-dependent cleavage of full-length L1, generating a soluble extracellular 135-kDa fragment and a transmembrane 70-kDa fragment comprising the intracellular and transmembrane domains and part of the extracellular domain. We provide evidence that the 135- and 70-kDa fragments are different from the previously described 140- and 80-kDa fragments. Nuclear import of the 70-kDa fragment depends on sumoylation and a nuclear localization signal and results from trafficking via endosomes and the cytoplasm. The generation of the 70-kDa fragment is regulated during development, after spinal cord injury, and in a mouse model of Alzheimer disease, suggesting its importance in various cellular responses.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6 mice bred and maintained at the Universitätsklinikum Hamburg-Eppendorf were used for all experiments. Generation and breeding of the L1-deficient mice were described (7). Additionally, 7-month-old transgenic APPPS1-21 (C57BL/6J-TgN; Thy1-APPKM670/671NL; Thy1-PS1L166P) mice were obtained from a breeding colony at University of Tübingen, Germany. These mice co-express mutated human amyloid precursor protein (Swedish double mutation) and a mutated presenilin-1 under a neuron-specific murine Thy-1 promoter element on a C57BL/6J background (21). Animals were housed at 25 °C on a 12-h light/12-h dark cycle with ad libitum access to food and water. All animal experiments were approved by the local authorities of the State of Hamburg (animal permit numbers ORG 535 and G09/098) and conform to the guidelines set by the European Union.
Reagents and Antibodies
Polyclonal antibodies to mouse L1 that react with the extracellular domain and rat monoclonal antibodies 557 and 555 against distinct epitopes at the N terminus of the third FNIII domain or between the second and third FNIII domains, respectively, have been described (22). Monoclonal L1 antibody 172-R against the intracellular domain of L1 was obtained from HISS Diagnostics. All secondary antibodies were obtained from Dianova. Antibodies against importin-α, importin-β, histone H1, and heterochromatin-associated protein 1-γ (HP1γ) were purchased from Sigma-Aldrich, Abcam, MBL International, Millipore, and Cell Signaling Technology, respectively. Antibodies against protein-disulfide isomerase, actin, apoptosis-linked gene-2-interacting protein X (Alix), tumor susceptibility gene 101 (Tsg101), vacuolar protein sorting-associated protein 4 (Vps4), and chromatin-modifying protein 1 (CHMP1) were obtained from Santa Cruz Biotechnology. Pan-ubiquitin and pan-small ubiquitin-like modifier (SUMO) antibodies were obtained from Santa Cruz Biotechnology or Abgent. Mouse L1-Fc was prepared as described (16). Aprotinin was purchased from Sigma-Aldrich. Primers were from Metabion. Vectors encoding GFP-SUMO-1, GFP-SUMO-2, and GFP-SUMO-3 were kindly provided by Hans Will (Heinrich-Pette-Institut and Leibniz Institute for Experimental Virology, Hamburg, Germany). OptiPrep was from Sigma-Aldrich.
Site-directed Mutagenesis of L1
To disrupt the nuclear localization site Lys1147 (exchange of KRSK to RRSK), the sumoylation site Lys1172 (exchange of MKDE to MRDE), or concomitantly the nuclear localization signal and the sumoylation site Lys1235 (exchange of GKKE to GRKE) the primer pairs up1 (5′-CTC ATC CTC TGC TTC ATC AGA CGC AGC AAG GGT GGC AAA TAC-3′) and down1 (5′-A TTT GCC ACC CTT GCT GCG TCT GAT GAA GCA GAG GAT GAG CA-3′), up2 (5′-TA GAT TCC GAG GCC CGG CCC ATG AGA GAC GAG ACC TTC GGC GA-3′) and down2 (5′-T GTA CTC GCC GAA GGT CTC GTC TCT CAT GGG CCG GGC CTC GGA AT-3′), or up3 (5′-T TTC ATC GGC CAG TAC AGT GGC AGG AAA GAG AAG GAG GCA GCA-3′) and down3 (5′-T GCC TCC TGC TGC CTC CTT CTC TTT CCT GCC ACT GTA CTG GCC GA-3′) (bold letters indicate the exchanges), respectively, were used in GENEART® Site-Directed Mutagenesis System (Invitrogen).
Transfection of HEK Cells
HEK293TN (BioCat) cells were plated in 6-well plates (Nunc) at a density of 2 × 105 cells/well; maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with glutamine, 4.5 mg/ml glucose, 10% fetal calf serum, and 100 μg/ml penicillin/streptomycin for 24 h; and then transfected using 6 μl of TurboFect (Fermentas) and 4 μg of vector DNA in 200 μl of serum-free DMEM according to the manufacturer's instructions.
Cultures and Treatments of Cerebellar Neurons and SH-SY5Y Cells
Cerebellar neurons were cultured as described (23). SH-SY5Y (ATCC number CRL-2266TM) cells were cultured in 6-well plates (Nunc) for 24 h in high glucose (4.5 g/liter) DMEM supplemented with 10% fetal calf serum, 1 mm sodium pyruvate (PAA Laboratories), 2 mm l-glutamine (Invitrogen), and 100 units/ml penicillin and streptomycin (Invitrogen). Cells were maintained at 37 °C, 5% CO2, and 90% humidity.
SH-SY5Y cells, freshly dissociated cerebellar neurons, or transfected HEK293TN cells were seeded into 6-well plates (Nunc) at a density of 190,000 cells/well, maintained for 24 h, and serum-deprived for 5 h. Cells were then treated with rabbit polyclonal L1 antibody or rabbit non-immune control serum (corresponding to 5 μg of IgG/ml; Jackson ImmunoResearch Laboratories), with monoclonal L1 antibody 557 or rat non-immune control IgG (50 μg/ml; Jackson ImmunoResearch Laboratories), or with L1-Fc or Fc (10 μg/ml) in the absence or presence of 1 μm aprotinin for 1 h at 37 °C.
Cell Surface Biotinylation of SH-SY5Y Cells
At 70–80% confluence, SH-SY5Y cells were incubated in 20 15-cm dishes with serum-free medium for 8–12 h. Cells were washed three times with PBS-2+ (phosphate-buffered saline, pH 7.3 (PBS), 0.5 mm CaCl2, 2 mm MgCl2) and incubated for 30 min at room temperature with 0.5 mg/ml sulfo-NHS-LS-biotin (Pierce) in PBS-2+ followed by washing the cells twice with 100 mm glycine at room temperature. Cells were washed with PBS-2+ and treated with L1 or control antibodies at 37 °C for 1 h. After removal of the culture medium, the cells were washed twice with PBS, resuspended in hypotonic buffer (100 mm HEPES, pH 7.8, 20 mm KCl, 2 mm EGTA) containing one protease inhibitor mixture tablet (Roche Diagnostics)/50 ml (added just before the experiment), and harvested using a rubber policeman. Cells were centrifuged for 5 min at 600 × g and 4 °C. The packed cell volume of the pellet was measured, 2 volumes of isotonic buffer (50 mm HEPES, pH 7.8, 0.25 m sucrose, 20 mm KCl, protease inhibitor mixture) were added to the pellet, and the pellet was homogenized using a Dounce homogenizer and passed through a 27-gauge needle several times at 4 °C.
Isolation of Subcellular Fractions
Brains of 2-day-old mice or SH-SY5Y cells were homogenized in homogenization buffer (0.32 m sucrose, 10 mm Tris-HCl, pH 7.4). The brain homogenate was incubated for 1 h at 37 °C to increase the amount of L1 fragments. After centrifugation at 1,000 × g for 10 min at 4 °C, the 1,000 × g pellet containing nuclei was saved as the nuclear fraction, and the resulting 1,000 × g postnuclear supernatant was centrifuged at 17,000 × g for 20 min at 4 °C. All subsequent steps were carried out at 4 °C. The 17,000 × g supernatant was further centrifuged at 100,000 × g for 45 min. The 100,000 × g pellet was taken as the microsomal fraction, and the supernatant containing soluble proteins was taken as the cytoplasmic fraction.
For the isolation of the plasma membrane fraction, the 17,000 × g pellet was washed once with homogenization buffer and subjected to hypotonic shock by resuspending the pellet in 9 volumes of ice-cold H2O containing one protease inhibitor mixture tablet/50 ml. The resuspended fraction was adjusted to 5 mm Tris-HCl by adding 1 m Tris-HCl (pH 7.5), stirred for 30 min, and centrifuged at 25,000 × g for 20 min. The pellet was homogenized in homogenization buffer using a Dounce homogenizer and by passing through a 27-gauge needle and loaded onto a discontinuous sucrose gradient, which consisted of 0.8, 1.0, and 1.2 m sucrose. After centrifugation at 150,000 × g for 2 h, the material at the interphase between 1.0 and 1.2 m sucrose, which contains plasma membranes, was collected and diluted with 2 volumes of homogenization buffer.
For the isolation of endosomes, the 100,000 × g microsomal pellet was resuspended in 2 m sucrose and applied to a gradient containing 0.25, 0.8, 1.15, and 1.3 m sucrose. After centrifugation at 100,000 × g for 2 h, the fraction at the interphase between 0.8 and 1.15 m sucrose, which contains endosomes, was collected.
For the isolation of nuclei, the 1,000 × g nuclear pellet was homogenized in homogenization buffer; applied to a gradient of 35, 30, and 25% OptiPrep; and centrifuged at 10,000 × g for 20 min. Nuclei were collected from the 30/35% interphase, diluted with 2 volumes of homogenization buffer, and centrifuged at 1,000 × g for 20 min. The pellet was again resuspended in homogenization buffer and centrifuged at 1,000 × g for 10 min. The nuclear pellet was then resuspended in 200 μl of extraction buffer (10 mm HEPES, 10 mm KCl, 2 mm MgCl2, 500 mm NaCl, 25% glycerol, pH 7.5, protease inhibitor mixture) and incubated on ice for 30 min. After centrifugation at 10,000 × g for 5 min, the supernatant was collected, and the pellet was resuspended in SDS sample buffer (see below) and passed through a 27-gauge needle. The supernatant was designated the “nuclear protein extract,” and the pellet was designated “insoluble nuclear protein.”
For the isolation of exosomes, cell culture supernatants were collected and centrifuged for 10 min at 1,000 × g and for 15 min at 17,000 × g to remove cellular debris. Exosomes were collected by centrifuging the resulting cell-free supernatant at 100,000 × g for 1 h.
For the isolation of the endoplasmic reticulum (ER) fraction, an ER isolation kit (Sigma) was used following the manufacturer's instructions. Briefly, cells were centrifuged at 1,000 × g, resuspended in hypotonic buffer containing the protease inhibitor mixture, incubated for 20 min on ice, and centrifuged at 600 × g for 5 min. The pellet was suspended in 2 volumes of isotonic buffer containing the protease inhibitor mixture and homogenized with a Dounce homogenizer and by passing through a 27-gauge needle. The samples were centrifuged at 1,000 × g for 10 min, and the resulting supernatants were centrifuged at 12,000 × g for 15 min followed by centrifugation of the resulting supernatants at 100,000 × g for 1 h. The pellets were homogenized in homogenization buffer, adjusted to 20% OptiPrep, layered between 30 and 15% OptiPrep, and centrifuged at 150,000 × g for 3 h. The material from the 15/20% interphase containing smooth ER (SER) and the material from the 20/30% interphase containing rough ER (RER) was collected. The Qproteome Nuclear Protein kit (Qiagen) and Subcellular Protein Fractionation kit (ThermoScientific) were used for subcellular fractionation according to the manufacturers' instructions.
Western Blot Analysis, Streptavidin Pulldown, and Immunoprecipitation
Western blot analysis has been described in detail (24). For isolation of biotinylated proteins after cell surface biotinylation, streptavidin-conjugated magnetic beads (Pierce) were incubated with cell lysates or cellular subfractions overnight at 4 °C. After washing the beads, biotinylated proteins bound to the streptavidin beads were eluted by boiling the beads in SDS sample buffer (60 mm Tris-HCl, pH 6.8, 2% SDS, 1% β-mercaptoethanol, 10% glycerol, 0.02% bromphenol blue) for 5 min at 95 °C. For immunoprecipitation, samples were resuspended in radioimmune precipitation assay buffer (25 mm Tris-HCl, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, pH 7.6) and subjected to preclearing and immunoprecipitation using Protein A/G-Agarose Plus (Santa Cruz Biotechnology). Beads were then washed with PBS and boiled in SDS sample buffer for 5 min at 95 °C.
Retrotranslocation and Nuclear Import Assay
The endosomal or ER fractions were resuspended in homogenization buffer and incubated with the cytoplasmic fraction or the buffer used for the isolation of the fractions in translocation buffer (10 mm HEPES, 40 mm magnesium acetate, pH 7.2, 1 mm DTT, 0.1 mm PMSF) in the presence or absence of 3 μg of antibodies for 60 min at 4 °C. The samples were then centrifuged at 100,000 × g for 20 min at 4 °C. The pellets and supernatants were collected, and streptavidin-coupled magnetic beads (Invitrogen) were used to isolate the biotinylated proteins.
For the analysis of nuclear import, nuclei isolated from SH-SY5Y cells or from mouse brain were incubated in the absence or presence of 3 μg of antibodies in nuclear translocation buffer (25 mm HEPES, pH 7.4, 12.5 mm KCl, 2.5 mm MgCl2, 1.25 mm CaCl2, 0.1 mm ATP), with the buffer used for the isolation of nuclei, or with the cytoplasmic fraction isolated from SH-SY5Y cells after cell surface biotinylation and stimulation of L1 functions or from mouse brain homogenates after incubation at 37 °C for 1 h. Streptavidin-coupled magnetic beads were used to isolate biotinylated proteins.
Neurite Outgrowth and Immunocytochemistry of Cerebellar Neurons and Image Acquisition
Cerebellar neurons were seeded on substrate-coated poly-l-lysine and maintained in serum-free medium as described (24). Antibody 557 or control antibody (50 μg/ml) and aprotinin (0.042 trypsin inhibitory unit) were added 2 h after the cell seeding. Neurite outgrowth was analyzed by measuring the total length of neurites in the Kontron microscope equipped with the IBAS imaging system (Carl Zeiss). Differences between the groups were statistically evaluated using Student's t test followed by one-way analysis of variance.
For immunostaining, cultured neurons were washed with ice-cold PBS, fixed in 4% formaldehyde in PBS for 15 min at room temperature, washed, permeabilized with 1% Triton X-100 in PBS for 5 min, and blocked in 1% BSA in PBS for 30 min at 4 °C. Primary antibodies in blocking solution were incubated overnight at 4 °C, and Cy2- or Cy3-conjugated secondary antibodies were incubated for 1 h in 1% BSA in PBS at room temperature under exclusion of light. To counterstain nuclei, DRAQ5TM (Biostatus Ltd.) was diluted 1:1,000 in PBS and applied for 15 min at 4 °C. Coverslips were embedded in Fluoromount G (SouthernBiotech). Images of neurons were acquired using an Axiophot 2 microscope (Carl Zeiss) equipped with a digital camera (AxioCam HRc), AxioVision software (version 3.1), and a Plan-Neofluar 40× objective (numerical aperture, 0.75) or using a confocal laser-scanning microscope (LSM510, Carl Zeiss), LSM510 software (version 3), and an oil Plan-Neofluar 40× objective (numerical aperture, 1.3) at 3× digital zoom. Contrast and brightness of the images were further adjusted in Adobe Photoshop CS2. For quantification of the fluorescence intensity of nuclear areas, one image per cell at the level of the largest nuclear area from stacks of images 1 μm apart was used to measure fluorescence intensity in the area using ImageJ software. Area fluorescence intensity of nuclei of 10 cells per group was determined, and statistical comparisons between the groups were performed using Student's t test.
Spinal Cord Injury
Lower thoracic compression spinal cord injury was performed on 3-month-old C57BL/6 female mice as described (25, 26). Briefly, mice were anesthetized by intraperitoneal injection of ketamine and xylazine (100 mg of Ketanest (Pfizer) and 5 mg of Rompun (Bayer)/kg of body weight). Laminectomy was performed at the T7–T9 level with mouse laminectomy forceps (Fine Science Tools). An electromagnetic compression device (27) was used to elicit injury by compressing the delaminated spinal cord for 1 s by a time-controlled 12-V (maximum voltage) current flow through the device. After surgery, mice were allowed to recover at 35 °C and were provided water and food ad libitum. Seven days after spinal cord injury mice were sacrificed, and 5-mm-long segments from individual spinal cords were dissected from the part of the spinal cord 5 mm rostral to the injury site. Samples were mechanically dissociated in radioimmune precipitation assay buffer using a Dounce homogenizer and processed for Western blot analysis as described above.
RESULTS
70-kDa Transmembrane L1 Fragment with Extracellular Portion Is Generated and Translocated to Nucleus upon L1 Stimulation
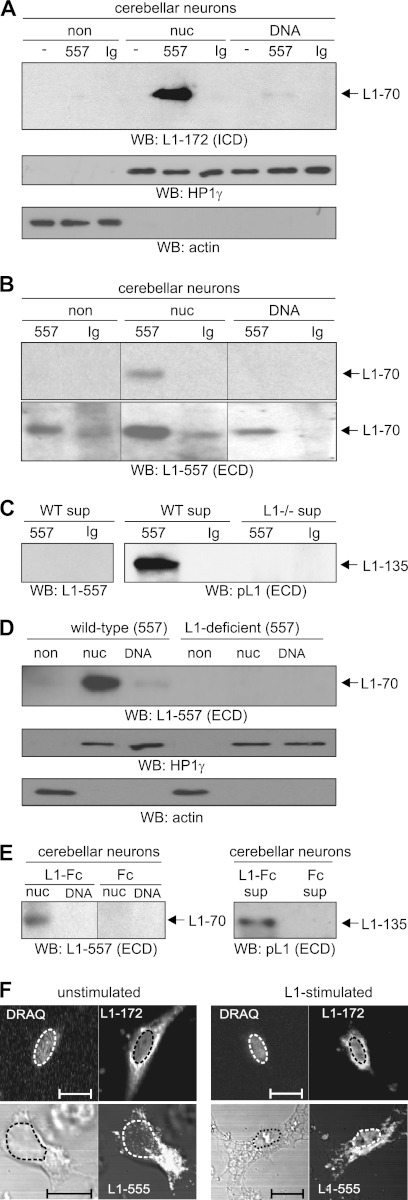
We started the present study by asking whether triggering the neurite outgrowth-promoting functions of L1 at the cell surface would influence the generation and nuclear import of its proteolytic fragments. Therefore, we applied the monoclonal L1 antibody 557, which triggers L1-dependent cellular responses, such as neurite outgrowth (22), to cultured cerebellar neurons and analyzed non-nuclear and nuclear fractions for the presence of L1 fragments. Western blot analysis with the L1 antibody 172-R directed against an L1-specific epitope in the intracellular domain showed an L1 fragment with an apparent molecular mass of ∼70 kDa mainly in the fraction containing soluble nuclear proteins after application of L1 antibody 557 (Fig. 1A). This fragment was not detectable in fractions from mock-treated cells or cells treated with a non-immune control antibody (Fig. 1A). Because the calculated molecular mass of the intracellular domain of L1 is ∼12 kDa, we assumed that the nuclear 70-kDa fragment comprises not only the intracellular domain but also the transmembrane domain and part of the extracellular domain of L1. To test this assumption, the antibody 557, which recognizes an epitope at the N terminus of the third FNIII domain, was used for Western blot analysis of the non-nuclear and nuclear fractions and showed the 70-kDa fragment mainly in the soluble nuclear protein fraction (Fig. 1B), indicating that the 70-kDa fragment contains the entire fourth and fifth FNIII domains and at least most of the third FNIII domain. After longer exposure of the Western blot, significant amounts of the 70-kDa fragment were also detectable in the non-nuclear fraction and in the fraction containing chromatin-associated proteins, whereas it was hardly detectable in the non-nuclear and nuclear fractions of neurons incubated with non-immune control antibody (Fig. 1B). In the cell culture supernatant of neurons treated with the antibody 557, a fragment with an apparent molecular mass of ∼135 kDa was detected by Western blot analysis using a polyclonal L1 antibody against the whole extracellular domain. This fragment was not seen when neurons were treated with a non-immune control antibody or when the 557 antibody was used for detection in Western blot analysis (Fig. 1C). This result indicates that generation of the transmembrane 70-kDa fragment is accompanied by the generation of a corresponding soluble extracellular 135-kDa fragment. When L1-deficient cerebellar neurons were stimulated with the antibody 557, no 135-kDa fragment was detectable in the cell culture supernatant (Fig. 1C), and no 70-kDa fragment was found in the non-nuclear and nuclear fractions (Fig. 1D), confirming that the 70- and 135-kDa fragments are L1-specific. The finding that the 70-kDa fragment, but not the 135-kDa fragment, was detected by the antibody 557 shows that the 70-kDa fragment is distinct from the previously described transmembrane 80-kDa fragment (13, 16), which lacks the epitope recognized by antibody 557 (22), and that the soluble extracellular 135-kDa fragment differs from the well characterized extracellular 140-kDa fragment (13, 16), which is recognized by the antibody 557 (22). The combined results show that exposing cultured neurons to an L1 function-stimulating antibody leads to the generation of a soluble extracellular 135-kDa fragment and a 70-kDa transmembrane fragment that is translocated to the nucleus.
FIGURE 1.
Stimulation of L1 signaling leads to generation and nuclear import of transmembrane 70-kDa L1 fragment. A–F, cerebellar neurons from wild-type (A–F) or L1-deficient mice (C and D) were mock-treated (A), treated with L1 antibody 557 or non-immune rat antibody (Ig) (A–D and F), or treated with Fc or L1-Fc (E). A–E, a non-nuclear fraction (non) (A, B, and D), nuclear fractions containing nucleoplasmic (nuc) or chromatin-associated proteins (DNA) (A, B, D, and E), and the cell culture supernatant (C and E) were isolated and subjected to Western blot (WB) analysis using antibody 172-R (L1-172) directed against an epitope in the intracellular domain (ICD) (A), antibody 557 (L1-557) (B–E), or polyclonal L1 antibody (pL1) (C and E) against epitopes in the extracellular domain (ECD). The different L1 fragments seen on a representative blot after different exposure times are indicated. Identical amounts of protein were loaded, and the HP1γ and actin antibodies were used in Western blot analysis to control loading of non-nuclear or nuclear proteins (A and D). Lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines. The experiments were performed four times (A and B) or two times (C–E) with identical results. F, neurons treated with control antibody (unstimulated) or with antibody 557 (L1-stimulated) were subjected to permeabilization and immunostaining with the antibody 172-R or 555, which is directed to an epitope between the extracellular second and third FNIII domains. Immunostaining by antibody 172-R (L1-172), counterstaining of the nucleus (DRAQ), the phase-contrast image, and the immunostaining by antibody 555 (L1-555) of representative neurons are shown. The results were reproduced in two independent experiments. The bar represents 5 μm, and the nuclei are depicted by dashed lines.
Because L1-Fc also triggers L1 functions, such as neurite outgrowth (28, 29), L1-Fc was used for stimulation of cerebellar neurons. A pronounced increase in the 70-kDa fragment level in the soluble nuclear protein fraction and of the 135-kDa fragment level in the cell-free cell culture supernatant was observed upon application of L1-Fc to neurons when compared with Fc treatment (Fig. 1E). This result shows that L1-Fc triggers the generation of the 70- and 135-kDa fragments in a way similar to that of function-triggering L1 antibody 557, confirming that stimulation of L1 signaling leads to the generation of a soluble extracellular 135-kDa fragment and a corresponding 70-kDa transmembrane fragment.
To verify the nuclear localization of L1 fragments, immunostainings of cerebellar neurons were performed with L1 antibodies 172-R and 555, which are directed against an intracellular epitope and an epitope between the second and third FNIII domain, respectively. A faint nuclear L1 immunoreactivity was observed with these two antibodies in non-stimulated cells, whereas a pronounced increase in nuclear L1 immunoreactivity was observed upon stimulation with antibody 557 (Fig. 1F). Quantification of the area fluorescence intensity within nuclei indicated that 172-R and 555 immunoreactivities were 1.6 ± 0.28 (p > 0.01) and 3.7 ± 0.6 (p > 0.0018) times higher, respectively, in nuclei of stimulated versus non-stimulated neurons. This result confirms that L1 stimulation leads to nuclear import of the 70-kDa fragment.
70-kDa Fragment Is Generated at Plasma Membrane by Serine Protease Activity and Translocated from Plasma Membrane to Nucleus via Late Endosomal Compartment and Cytoplasm
In a previous study, we found that the generation and nuclear import of a transmembrane neural cell adhesion molecule (NCAM) fragment were inhibited by the serine protease inhibitor aprotinin, showing that a serine protease is involved in the generation of the transmembrane NCAM fragment, which is translocated from the plasma membrane to the nucleus via the ER and the cytoplasm (23). To analyze whether the generation and nuclear translocation of the 70-kDa fragment also depend on serine protease-mediated proteolytic processing, cultured cerebellar neurons were treated with antibody 557 in the presence of aprotinin. When compared with the level in the absence of aprotinin, the level of the 70-kDa fragment in the soluble nuclear fraction and the level of the 135-kDa fragment in culture supernatant were reduced when aprotinin was present during stimulation with L1 antibody 557 (Fig. 2A), indicating that the generation of the 70- and 135-kDa fragments depends on a serine protease activity.
FIGURE 2.
L1-triggered generation and nuclear import of transmembrane 70-kDa L1 fragment and L1-induced neurite outgrowth depends on serine protease. Cerebellar neurons from wild-type (A and B) or L1-deficient mice (B) were treated with the L1 antibody 557 or a non-immune rat antibody (Ig) in the absence or presence of aprotinin (apro). A, a nuclear fraction containing nucleoplasm and the cell culture supernatant were subjected to Western blot analysis using antibody 557 (L1-557) or polyclonal L1 antibody (pL1) against the entire extracellular domains. Identical amounts of nucleoplasmic proteins were loaded, and loading was controlled by probing the Western blot (WB) with HP1γ antibody (not shown). The different L1 fragments are indicated. Representative blots from one of three independent experiments with similar results are shown. B, cerebellar neurons were incubated on substrate-coated poly-l-lysine with antibody 557 or a non-immune antibody (Ig) in the absence or presence of aprotinin (apro). Total lengths of neurites were measured, and mean values ± S.E. from three independent experiments are shown (***, p < 0.001).
To show that the serine protease-dependent generation of the 70- and 135-kDa fragments plays a role in regulation of L1 functions, such as neurite outgrowth, we analyzed whether aprotinin affects antibody 557-triggered neurite outgrowth. Neurite outgrowth was promoted in the presence of antibody 557 relative to that determined in the presence of a control antibody, whereas the concomitant presence of aprotinin resulted in a reduction of this antibody-triggered neurite outgrowth to a value observed in the presence of the control antibody (Fig. 2B). Neither aprotinin nor antibody 557 affected neurite outgrowth of L1-deficient neurons (Fig. 2B). This result shows that L1-induced neurite outgrowth depends on the serine protease-mediated cleavage of L1 and suggests that the generation of the 70- and 135-kDa fragments and the nuclear import of the 70-kDa fragment play a role in L1-triggered neurite outgrowth.
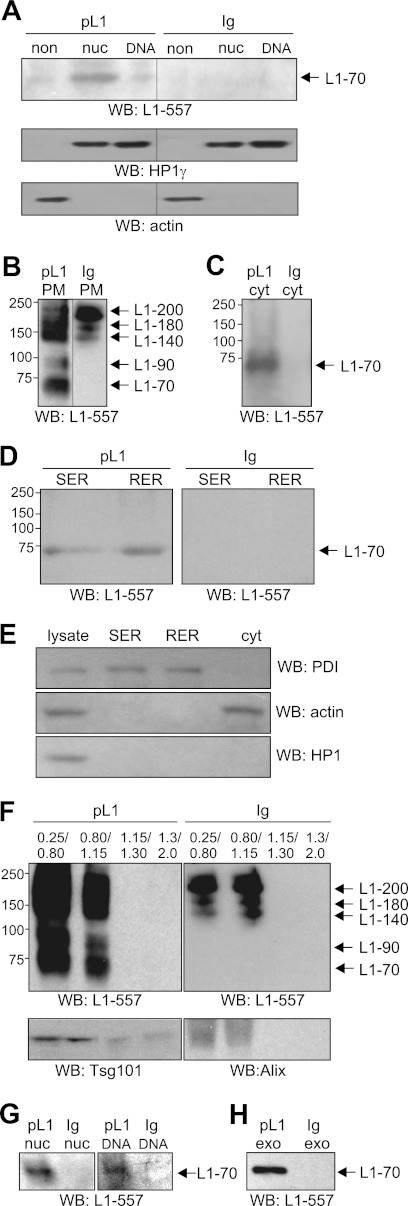
To gain insights into the pathway by which the 70-kDa fragment is transported to the nucleus, we monitored the intracellular trafficking of the fragment by analyzing subcellular fractions after cell surface biotinylation. The highly L1-expressing SH-SY5Y cell line was chosen for this experiment because SH-SY5Y cells are available in large quantities in contrast to cerebellar neurons. Upon stimulation of SH-SY5Y cells with function-triggering polyclonal L1 antibody, the 70-kDa fragment was seen in non-nuclear and nuclear fractions (Fig. 3A). This fragment was not detectable after treatment with the non-immune antibody (Fig. 3A). These results show that L1 stimulation of SH-SY5Y cells leads to the generation and nuclear import of the 70-kDa fragment as seen with cerebellar neurons.
FIGURE 3.
Trafficking of 70-kDa fragment from cell surface via late endosomal compartment, ER, cytoplasm, and exosomes to nucleus. A, non-nuclear (non) and nuclear fractions containing nucleoplasmic (nuc) or chromatin-associated (DNA) proteins were isolated from SH-SY5Y cells treated with polyclonal L1 antibody (pL1) or control antibody (Ig). Identical amounts of protein were loaded, and the HP1γ and actin antibodies were used in Western blot (WB) analysis to control loading of non-nuclear or nuclear proteins. B–H, SH-SY5Y cells were subjected to cell surface biotinylation followed by incubation with polyclonal L1 antibody (pL1) or non-immune control antibody (Ig) and subcellular fractionation. Using streptavidin-coupled beads, biotinylated proteins were isolated from plasma membrane (PM) (B), cytoplasmic (cyt) (C and E), SER and RER (D and E), and endosomal (F) fractions, from nuclear fractions containing nucleoplasmic (nuc) or chromatin-associated (DNA) proteins (G), or from exosomes (exo) (H). Biotinylated L1-immunoreactive bands were detected by Western blot analysis using antibody 557 (L1-557). SER, RER, and cytoplasmic fractions were probed by Western blot analysis with actin, HP1γ, and protein-disulfide isomerase (PDI) antibodies (E), and gradient fractions were probed by Western blot analysis with Tsg101 and Alix antibodies (F). The different L1 fragments are indicated, and lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines. Representative blots from three (A) or two (B–H) independent experiments with similar results are shown. B–G, the protein concentrations in the fractions were determined, and identical amounts of protein from the corresponding fraction were loaded.
Upon cell surface biotinylation and treatment with antibody 557 or control antibody, SH-SY5Y cells were subjected to subcellular fractionation to isolate plasma membrane, SER, RER, cytoplasmic, endosomal, and nuclear fractions. To track the pathway of the biotinylated fragment after its generation at the plasma membrane, biotinylated proteins were isolated from these fractions using streptavidin beads and analyzed for the presence of the biotinylated 70-kDa fragment by performing Western blot analysis using antibody 557. Upon treatment of cells with this antibody, the 70-kDa fragment as well as the 200-kDa full-length L1 and L1 fragments of 90, 140, and 180 kDa were detected as biotinylated proteins in a plasma membrane-enriched fraction, whereas only biotinylated 140-, 180-, and 200-kDa bands, but not the biotinylated 70- and 90-kDa fragments, were detectable in this fraction from cells treated with a non-immune control antibody (Fig. 3B). As seen before with the transmembrane NCAM fragment (23), the biotinylated 70-kDa transmembrane L1 fragment was detected in a cytoplasmic fraction (Fig. 3C) as well as in the RER- and SER-enriched fractions (Fig. 3D) after stimulating cells with the L1 antibody. The fragment was not detectable when cells were incubated with the control antibody (Fig. 3, C and D). The 90-, 140-, 180-, and 200-kDa L1 bands seen in the plasma membrane fraction were not found in the cytoplasmic fraction (Fig. 3C), which was enriched in the cytoplasmic marker protein actin but devoid of the ER marker protein protein-disulfide isomerase and the nuclear marker protein HP1γ (Fig. 3E). These bands were also not found in the ER fractions (Fig. 3D), which were enriched in protein-disulfide isomerase but devoid of actin and HP1γ (Fig. 3E). Analysis of fractions derived from sucrose gradient centrifugation of microsomes revealed that the biotinylated 140-, 180-, and 200-kDa L1 bands were also observed in gradient fractions that were enriched in Alix and Tsg101, two marker proteins of late endosomes and/or multivesicular bodies (MVBs), when cells were treated with L1 or control antibody (Fig. 3F). However, biotinylated 70- and 90-kDa fragments were only seen in those endosomal fractions when cells were incubated with L1 antibody but were not detectable in fractions from cells treated with control antibody (Fig. 3F). Moreover, only the biotinylated 70-kDa fragment was detectable in the nuclear fractions containing soluble or chromatin-associated proteins after stimulation of cells with L1 antibody but not after application of control antibody (Fig. 3G).
Because the 70-kDa fragment was present in an MVB-enriched fraction and because exosomes that mediate the transport of proteins from one cell to another are formed in MVBs (30, 31), we analyzed whether the biotinylated 70-kDa fragment was also present in exosomes. The biotinylated 70-kDa fragment was detectable in exosomes isolated from the cell-free culture supernatant of cells treated with L1 antibody but not in the supernatant of cells treated with control antibody (Fig. 3H). In summary, these results indicate that L1 stimulation leads to generation of the 70-kDa fragment at the plasma membrane and that this fragment is translocated from the plasma membrane to the ER, late endosomal compartment and/or MVBs, cytoplasm, and nucleus.
Release of 70-kDa Fragment from Endosomal Membranes into Cytoplasm Depends on Endosomal Sorting Complex Required for Transport (ESCRT)-III-associated Proteins
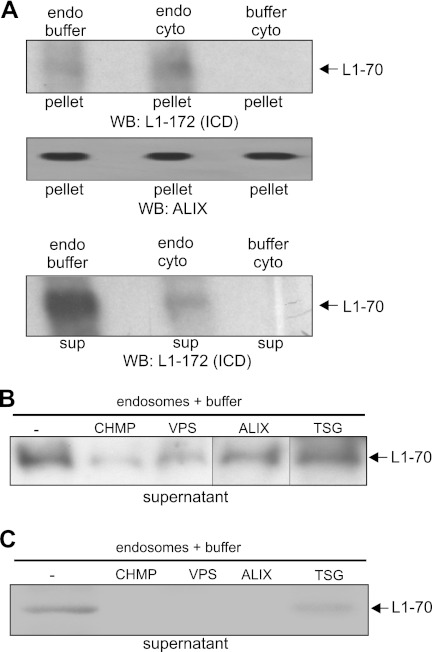
To test whether the 70-kDa fragment is released from the ER and/or the late endosomal compartment into the cytoplasm, ER and endosomal fractions containing this biotinylated fragment were isolated from SH-SY5Y cells after cell surface biotinylation and stimulation with polyclonal L1 antibody. Fractions were incubated at 37 or 4 °C with cytoplasm prepared from untreated SH-SY5Y cells or with buffer used for the isolation of the cytoplasmic fraction. After incubation, ultracentrifugation and isolation of biotinylated proteins from the resulting pellet and supernatant fractions and Western blot analysis with antibody 557 were carried out. The biotinylated 70-kDa fragment was observed only in the pellet fraction but not in the supernatant fraction when ER fractions were used for incubation (data not shown), indicating that this fragment is not released from the ER membranes. After incubation of the endosomal fractions with the cytoplasmic fraction at 37 °C, the biotinylated 70-kDa fragment was no longer detectable either in the pellet or in the supernatant fraction (data not shown), suggesting that it was rapidly degraded at this temperature. However, the biotinylated 70-kDa fragment was detectable in the pellet and supernatant fraction after incubation of the endosomal fraction with buffer or the cytoplasmic fraction at 4 °C (Fig. 4A). Interestingly, the amounts of biotinylated 70-kDa fragment released from the endosomes into the supernatant were higher when the endosomal fraction was incubated with buffer than the amounts seen when the fraction was incubated with cytoplasm (Fig. 4A). This result indicates that cytoplasmic components are not essential for the release of the 70-kDa fragment from endosomal membranes and suggests that this fragment is released from the membrane through endosome-associated proteins.
FIGURE 4.
Release of 70-kDa L1 fragment from endosomal membranes into cytoplasm is mediated by ESCRT-associated proteins bound to endosomes. A and B, SH-SY5Y cells were subjected to cell surface biotinylation followed by incubation with polyclonal L1 antibody and isolation of endosomal fractions (endo) containing biotinylated L1 fragment. This fraction was incubated with a cytoplasmic fraction (cyto) isolated from untreated cells or cells treated with the buffer used for isolation of the fractions in the absence (A and B) or presence of antibodies against CHMP1 (CHMP), Vps4 (VPS), Alix (ALIX), and Tsg101 (TSG) (B). After ultracentrifugation, biotinylated proteins were isolated from the resulting pellet (A) and supernatant (sup) (A and B), and the biotinylated 70-kDa fragment was detected in the pellet and supernatant fractions by Western blot (WB) analysis using L1 antibody 172-R. C, endosomal fractions containing the 70-kDa fragment were isolated from mouse brain homogenate and incubated at 4 °C in the absence or presence of antibody against CHMP1, Vps4, Alix, or Tsg101. After ultracentrifugation, the resulting supernatants were used for immunoprecipitation with antibody 172-R, and immunoprecipitates were subjected to Western blot analysis using antibody 172-R. A, Alix antibody was used in Western blot analysis of the pellet fraction to control that similar amounts of endosomes were used in the experiment and were reisolated after incubation. A and B, lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines. The experiments were performed two times with identical results.
ESCRT proteins and ESCRT-associated proteins adhere to endosomal membranes, mediate the formation of MVBs, and play crucial roles in endosomal protein sorting and intraendosomal trafficking (32–34). We thus investigated whether these endosome-associated proteins are involved in the transfer of the 70-kDa fragment from the endosomal membrane to the cytoplasm. In a first attempt, we tested whether antibodies against distinct ESCRT proteins and ESCRT-associated proteins affect the release of the 70-kDa fragment from the endosomal membrane into the cytoplasm. To this aim, we isolated an endosomal fraction from SH-SY5Y cells upon cell surface biotinylation and antibody stimulation and incubated this fraction, which contained the biotinylated 70-kDa fragment, with buffer in the absence or presence of antibodies against the ESCRT-I subunit Tsg101 or against the ESCRT-III-associated Vps4, which is a key player in membrane abscission reactions during endosomal sorting. In addition, we applied antibodies against the ESCRT-III-associated proteins Alix and CHMP1. After incubation and ultracentrifugation, the biotinylated proteins were isolated from the supernatant fractions and subjected to Western blot analysis with L1 antibody to analyze whether the release of the biotinylated 70-kDa fragment from the endosomal membrane into the supernatant was affected in the presence of the antibodies against Vps4, CHMP1, Alix, and Tsg101. The amount of biotinylated 70-kDa fragment released into the supernatant was reduced in the presence of Vps4, Alix, or CHMP1 antibody when compared with the level obtained in the absence of antibodies or in the presence of Tsg101 antibody, which did not block the release of the 70-kDa fragment from the endosomes (Fig. 4B). This result indicates that Vps4, CHMP1, and Alix antibodies inhibit the release of the 70-kDa fragment from the endosomal membrane.
To further substantiate the notion that antibodies against the endosome-associated proteins Vps4, CHMP1, and Alix block the release of the 70-kDa fragment from endosomal membranes, an endosomal fraction containing the 70-kDa fragment was prepared from mouse brain and incubated at 4 °C in the absence or presence of Vps4, CHMP1, Alix, and Tsg101 antibodies. After ultracentrifugation and immunoprecipitation of L1 from supernatant fractions, Western blot analysis of the immunoprecipitates revealed that the release of the 70-kDa fragment from endosomes was drastically reduced in the presence of Vps4, CHMP1, or Alix antibody relative to the level observed in the absence of antibodies or in the presence of Tsg101 antibody (Fig. 4C). The combined results indicate that blocking the functions of Vps4, CHMP1, or Alix by antibodies inhibits the release of the 70-kDa fragment and suggest that these ESCRT-III-associated proteins are involved in the release of this fragment from the endosomal membrane into the cytoplasm.
Nuclear Import of 70-kDa Fragment Depends on Importin and Is Associated with Import of CHMP1 into Nucleus
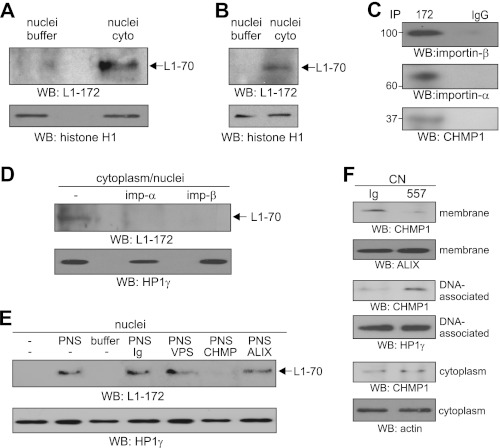
To show that the 70-kDa fragment is imported from the cytoplasm into the nucleus after its release from the endosomal membrane into the cytoplasm, we performed an in vitro nuclear import assay. First, we isolated nuclei from untreated SH-SY5Y cells and a cytoplasmic fraction from SH-SY5Y cells after cell surface biotinylation and L1 stimulation. The cytoplasm containing the biotinylated 70-kDa fragment or the buffer that had been used for the isolation of the cytoplasm was incubated with the nuclei from untreated cells. Biotinylated proteins that had been translocated from the cytoplasmic fraction into the nucleus were then isolated from the nuclei and subjected to Western blot analysis with antibody 172-R. The biotinylated 70-kDa fragment was hardly detectable after incubation with buffer but was detectable after incubation with cytoplasm containing this fragment (Fig. 5A). In a similar experiment, we incubated nuclei from mouse brain with a cytoplasmic fraction isolated from brain homogenate or with buffer used for fractionation. After reisolation of the nuclei, Western blot analysis with antibody 172-R showed that the 70-kDa fragment was imported into the nuclei after incubation with cytoplasm but not after incubation with buffer (Fig. 5B).
FIGURE 5.
Translocation of 70-kDa fragment from cytoplasm to nucleus depends on importins and affects localization and nuclear transport of CHMP1. A and D, SH-SY5Y cells were subjected to cell surface biotinylation followed by incubation with polyclonal L1 antibody and isolation of a cytoplasmic fraction containing biotinylated L1 fragment. A, this fraction or the buffer that was used for the isolation of the fractions was incubated with nuclei isolated from untreated cells. After reisolation of nuclei by centrifugation, biotinylated proteins were isolated from nuclei, and the biotinylated L1 fragment was detected by Western blot (WB) analysis using L1 antibody 172-R (L1-172). B, a cytoplasmic fraction (cyto) and nuclei were isolated from mouse brain, and nuclei were incubated either with the cytoplasmic fraction or with the buffer used for the isolation of the cytoplasmic fraction. After reisolation, nuclei were subjected to Western blot analysis using antibody 172-R. C, the cytoplasmic fraction isolated from L1-stimulated SH-SY5Y cells was subjected to immunoprecipitation (IP) using antibody 172-R (172) or non-immune mouse control antibody (IgG), and immunoprecipitates were probed by Western blot analysis using antibodies against importin-α (imp-α), importin-β (imp-β), and CHMP1. D, a cytoplasmic fraction from L1-stimulated SH-SY5Y cells containing the 70-kDa fragment was incubated with nuclei of untreated SH-SY5Y cells in the absence or presence of importin-α (imp-α) or importin-β antibody (imp-β). After reisolation of nuclei by centrifugation, a nuclear extract was subjected to Western blot analysis using antibody 172-R (L1-172). E, nuclei from mouse brain were incubated either with a postnuclear supernatant (PNS) isolated from mouse brain homogenate and containing the 70-kDa fragment or with the buffer used for isolation of the postnuclear supernatant in the absence or presence of Vps4 (VPS), CHMP1 (CHMP), and Alix (ALIX) antibodies or non-immune control antibody (Ig). After reisolation of the nuclei, a nuclear extract was isolated and subjected to Western blot analysis using antibody 172-R (L1-172). F, cerebellar neurons (CN) were treated with antibody 557 or non-immune antibody (Ig) and subjected to subcellular fractionation. Fractions enriched in membranes and DNA-associated and cytoplasmic proteins were subjected to Western blot analysis using CHMP1 antibody. A, B, D, and E, histone H1 or HP1γ antibody was used in Western blot analysis of the pellet fraction to control that similar amounts of nuclei were used in the experiment and were reisolated after incubation. F, Alix, HP1γ, and actin antibodies were used in Western blot analysis to control loading of membrane, DNA-associated, or cytoplasmic proteins. A–F, the experiments were performed three times with identical results, and representative blots are shown.
Because transport of macromolecules into the nucleus depends on nuclear localization signals and is mediated by importins, which bind to the nuclear localization signals (35, 36), and because L1 contains two putative monopartite nuclear localization signals (K1147RSK and K1235KEK), we investigated whether the import of the 70-kDa fragment into the nucleus was importin-dependent. In parallel, we also investigated whether CHMP1 is involved in the nuclear import of this fragment because CHMP1 recruits the BMI1 polycomb ring finger oncogene to the subnuclear regions of condensed chromatin (37) and may thus act as a nuclear carrier protein. Beforehand, we investigated by immunoprecipitation whether the 70-kDa fragment in the cytoplasmic fraction of SH-SY5Y cells was associated with importin-β and importin-α or CHMP1, respectively. Western blot analysis after immunoprecipitation showed that both importins and CHMP1 were co-immunoprecipitated with L1 from the cytoplasmic fraction of SH-SY5Y cells (Fig. 5C). To test whether the 70-kDa fragment was imported from the cytoplasm into the nucleus in an importin-dependent manner, cytoplasm obtained from SH-SY5Y after L1 stimulation and containing the 70-kDa fragment was incubated with nuclei from non-stimulated cells in the absence and presence of antibodies against importin-α and -β to block the nuclear import. After isolating the nuclei, nuclear extracts were subjected to Western blot analysis using the antibody 172-R. In the absence of antibodies, the 70-kDa fragment was detected after incubation with cytoplasm containing the fragment. The fragment was hardly detectable after incubation with cytoplasm in the presence of the importin antibodies (Fig. 5D). To test whether CHMP1 plays a role in the nuclear import of the 70-kDa fragment, nuclei from mouse brain were incubated with a postnuclear supernatant isolated from mouse brain or with buffer in the absence and presence of CHMP1 antibody or for control in the presence of Vps4, Alix, or non-immune antibody. After isolating the nuclei, nuclear extracts were isolated and subjected to Western blot analysis with antibody 172-R. The 70-kDa fragment was present in the nuclear extract after incubation of nuclei with the postnuclear supernatant in the absence of antibodies and in the presence of the non-immune, Vps4, and Alix antibodies, whereas it was not detectable in untreated nuclei and in nuclei after incubation with buffer or with the postnuclear supernatant in the presence of the CHMP1 antibody (Fig. 5E). These results indicate that the import of the 70-kDa fragment from the cytoplasm into the nucleus is mediated by importin-α/β and CHMP1-dependent mechanisms.
To investigate whether the nuclear import of the 70-kDa fragment triggers or affects nuclear import of CHMP1, cerebellar neurons were treated with the 557 or non-immune antibody, and subcellular fractions were isolated and subjected to Western blot analysis with CHMP1 antibody. Relative to the CHMP1 levels observed upon treatment with the non-immune antibody, the level of membrane-associated CHMP1 was strongly decreased, whereas the level of DNA-associated CHMP1 was strongly increased upon stimulation with L1 antibody (Fig. 5F). The level of cytoplasmic CHMP1 was not significantly altered (Fig. 5F). This result indicates that the nuclear import of the 70-kDa fragment is accompanied by an altered subcellular localization and nuclear import of CHMP1.
Sumoylation of Lys1172 Is Prerequisite for Generation of 70-kDa Fragment, and Nuclear Localization Signal Lys1147RSK Mediates Its Nuclear Import
Analysis of the intracellular trafficking of the 70-kDa fragment in SH-SY5Y cells after cell surface biotinylation and L1 stimulation showed that the fragment is internalized after its generation at the plasma membrane and transported via a late endosomal compartment to the nucleus. Because ubiquitination mediates endocytosis of transmembrane proteins (38, 39), we tested whether the 70-kDa fragment was ubiquitinated. In parallel, we analyzed whether this fragment is sumoylated because the intracellular domain of L1 contains two potential sites for the attachment of SUMO proteins and because sumoylation plays an important role in nucleocytoplasmic transport (40). Using the SUMOsp 2.0 software (41), we identified the non-canonical type II sumoylation site GK1235KE and the type I sumoylation site MK1172DE, which represents a canonical ΨKXE sumoylation motif (Ψ is Ala, Ile, Leu, Met, Pro, Phe, or Val, and X is any amino acid). After immunoprecipitation with L1 or ubiquitin antibody and Western blot analysis with ubiquitin or L1 antibody, respectively, only the full-length 200-kDa band was observed in the immunoprecipitates from mouse brain homogenate, but no L1 fragment was detectable (Fig. 6A). This result indicates that only full-length L1, but not the 70-kDa fragment, is ubiquitinated.
FIGURE 6.
70-kDa fragment is generated at plasma membrane by serine protease and is sumoylated but not ubiquitinated. A, mouse brain homogenate was subjected to immunoprecipitation using antibodies 555 or 172-R (172), ubiquitin antibody (ubi), or non-immune control antibody (IgG). Homogenate (input) and immunoprecipitates were subjected to Western blot (WB) analysis with ubiquitin antibody or antibody 557 (L1-557). B–D, fractions enriched in plasma membranes (B), endosomes (C), or cytoplasm or SER (D) were isolated from mouse brain and subjected to immunoprecipitation using antibody 557 or non-immune control antibody (IgG) followed by Western blot analysis with antibody 557 (L1-557) (B and C) or with a pan-SUMO antibody (sumo) (B–D). E and F, SH-SY5Y cells were treated with polyclonal L1 antibody (pL1) (E and F) or control antibody (Ig) (F), and exosomes isolated from the cell-free cell culture supernatant (E) or nuclear protein extracts (F) were subjected to immunoprecipitation (IP) using antibody 557 or non-immune control antibody (IgG) (E and F). Immunoprecipitates were probed by Western blot analysis with a pan-SUMO antibody. A–E, the full-length L1 and the different L1 fragments are indicated by arrows, and lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines. The experiments were performed three times with identical results, and representative blots are shown.
Western blot analysis with a pan-SUMO antibody showed only a 70-kDa band in the L1 immunoprecipitates of the plasma membrane-enriched mouse brain fraction (Fig. 6B). Not only the 70-kDa but also the 200 kDa full-length L1 and the 140- and 180-kDa L1 fragments were detectable in the L1 immunoprecipitates using the antibody 557 for Western blot analysis (Fig. 6B). In L1 immunoprecipitates of endosomal mouse brain fractions, the 70-kDa fragment was detected with pan-SUMO and L1 antibodies, whereas 140-, 180-, and 200-kDa L1 bands were detected by L1 antibody but not by pan-SUMO antibody (Fig. 6C). After L1 immunoprecipitation from SER or cytoplasmic mouse brain fractions, only the 70-kDa fragment was detected by Western blot analysis with pan-SUMO antibody (Fig. 6D) and antibody 557 (data not shown). When the non-immune antibody was used for immunoprecipitation, no SUMO- or L1-immunoreactive bands were observed in either immunoprecipitates (Fig. 6, B–D).
Next, we addressed the question whether the 70-kDa fragment observed in the nucleus and in exosomes after L1 stimulation of SH-SY5Y cells was also sumoylated. To this aim, SH-SY5Y cells were treated with the polyclonal L1 antibody or with the non-immune antibody, and nuclear fractions and exosomes were isolated and subjected to immunoprecipitation with L1 or non-immune antibody. Western blot analysis of the L1 immunoprecipitates with SUMO antibody showed the 70-kDa fragment in exosomes (Fig. 6E) and in the nuclear fraction upon L1 stimulation (Fig. 6F), whereas it was not seen when the non-immune antibody was used for immunoprecipitation or treatment of the cells (Fig. 6, E and F). The combined results indicate that the sumoylated 70-kDa fragment is present at the plasma membrane and in endosomes, ER, cytoplasm, exosomes, and nuclei.
To investigate whether the sumoylation and nuclear localization signals are required for the nuclear import of the 70-kDa fragment, the potential sumoylation site Lys1172, the nuclear localization signal at Lys1147, and the potential sumoylation site and nuclear localization signal at Lys1235 were subjected to site-directed mutagenesis (Fig. 7A). HEK293 cells, which do not express L1, were mock-transfected or transfected with an empty expression vector or expression vectors encoding full-length non-mutated or mutated L1. After stimulation of the cells with polyclonal L1 antibody, non-nuclear and nuclear fractions were isolated. In parallel, cell surface biotinylation was carried out before L1 stimulation, and biotinylated proteins were isolated from the cell lysates. Full-length L1 was detectable in the non-nuclear fraction and as biotinylated cell surface protein after transfection with non-mutated full-length L1, whereas no L1-positive band was seen in non-transfected or mock-transfected cells (Fig. 7, B and C). Interestingly, the levels of mutated L1 in non-nuclear fractions and at the cell surface were higher than the levels of wild-type non-mutated L1 (Fig. 7, B and C), indicating that the L1 mutants are transported to the cell surface and suggesting that the mutants, in particular upon disruption of the putative sumoylation site at Lys1172, are not removed from the plasma membrane upon L1 stimulation. The 70-kDa fragment was detectable in L1 immunoprecipitates isolated from the non-nuclear fraction of cells expressing wild-type L1 or expressing the L1 mutation of the sumoylation site and the nuclear localization signal at Lys1235 (Fig. 7D). The fragment was not detectable in the L1 immunoprecipitates of cells expressing the mutation of the sumoylation site at Lys1172, whereas the level of the fragment was reduced in the fraction from cells expressing L1 with mutated nuclear localization signal at Lys1147 (Fig. 7D). The 70-kDa fragment was detectable in nuclear fractions containing chromatin-associated proteins from cells expressing wild-type L1 or the mutation of the sumoylation site and the nuclear localization signal at Lys1235, whereas no 70-kDa fragment was found in the nuclear fraction isolated from cells expressing the mutation of the nuclear localization signal at Lys1147 or the sumoylation site at Lys1172 (Fig. 7E). These results suggest that sumoylation of L1 at Lys1172 is a prerequisite for the extracellular proteolytic cleavage of L1 and the generation of the 70-kDa fragment and that the mutation of the nuclear localization signal at Lys1147 has a minor effect on the generation of the 70-kDa fragment but impairs the nuclear import of the fragment. In addition, we show that the motifs at Lys1235 do not affect sumoylation and thus generation and nuclear import of the 70-kDa fragment.
FIGURE 7.
Sumoylation of L1 at Lys1172 is required for generation of L1 fragments, and nuclear import depends on nuclear localization signal Lys1147. A, a schematic presentation of the nuclear localization signals (NLS) and potential sumoylation sites (sumo) in the intracellular domain of mouse L1 at Lys1147, Lys1172, and Lys1235, respectively. Sequences of the wild-type L1 (WT) and L1 mutants (mut) are shown. B–F, untransfected (non) (B–D) or mock-transfected HEK cells (B–D and F), HEK cells transiently transfected with wild-type (WT) or mutated L1 (B–E), and HEK cells transfected with L1 alone or co-transfected with L1 and GFP-tagged SUMO-1, SUMO-2, or SUMO-3 (F) were incubated with polyclonal L1 antibody. C, untransfected and transfected cells were subjected to cell surface biotinylation before treatment with L1 antibody. Biotinylated proteins (C), the non-nuclear fraction (B and D), and the nuclear fraction containing chromatin-associated proteins (E) were isolated from lysates of transfected cells. The non-nuclear fraction (D), nuclear fraction containing chromatin-associated proteins (E), and cell lysates (F) were subjected to immunoprecipitation using antibody 172-R. B–F, Western blot (WB) analysis using antibody 172-R (B–E) or using a GFP antibody (F) is shown. Full-length L1 and the different L1 fragments are indicated by arrows, and lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines. B, actin antibody was used in Western blot analysis to control loading. B–F, the experiments were performed two times with identical results and representative blots are shown.
To verify that the 70-kDa fragment is sumoylated at Lys1172, HEK293 cells were co-transfected with full-length L1 and GFP-tagged SUMO-1, SUMO-2, or SUMO-3. A GFP-positive band of 100 kDa was observed in cell lysates obtained upon L1 stimulation and co-transfection of L1 with the GFP-tagged SUMO isoforms but not when cells were mock-transfected or treated with control antibody (Fig. 7G). Because GFP has a molecular mass of ∼30 kDa and because the attachment of one GFP-SUMO to L1 shifts the molecular mass of the 70-kDa fragment to 100 kDa, this result indicates that the 70-kDa fragment carries only one SUMO molecule.
Generation of 70-kDa Fragment Correlates with Brain Development, Regeneration after Spinal Cord Injury, and Degeneration in Alzheimer Disease Mouse Model
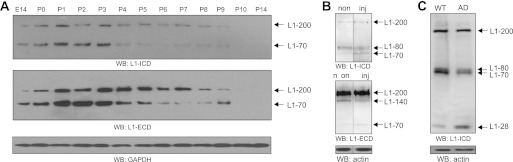
To investigate the functional role(s) of the 70-kDa fragment in vivo, we first measured the levels of this fragment in brain homogenates from mice of different ages. Western blot analysis of brain homogenates using antibody 172-R revealed that the levels of 200-kDa full-length L1 and of a band of ∼70 kDa increased from embryonic day 14 to birth (Fig. 8A). These enhanced levels remained constant until postnatal day 3, thereafter declining (Fig. 8A). Because antibody 172-R does not discriminate between the transmembrane 70-kDa fragment identified in this study and the previously described transmembrane 80-kDa fragment resulting from the cleavage in the third FNIII domain (16), we used L1 antibody 555, which recognizes the 70-kDa but not the 80-kDa fragment, for Western blot analysis to specifically determine the level of the 70-kDa fragment during development. The antibody detected the full-length L1 and the 70-kDa fragment. The levels of full-length L1 and of the 70-kDa fragment increased from embryonic day 14 to postnatal day 3 and then declined (Fig. 8A). These results indicate that the 70-kDa fragment is mainly generated during early postnatal development and suggest that its occurrence may underlie or accompany cellular events that take place between birth and postnatal day 5, such as neuronal migration and differentiation, neuritogenesis, and developmental synaptogenesis.
FIGURE 8.
Levels of 70-kDa fragment are increased during early postnatal development and after spinal cord injury but decreased in mouse model of Alzheimer disease. A, brain homogenates from mice of different ages were subjected to Western blot (WB) analysis with the antibodies 172-R (L1-ICD; upper panel) or 555 (L1-ECD; lower panels). B, 1 week after spinal cord injury, segments of the thoracic spinal cord rostral to the lesion site were taken from injured mice (inj), and corresponding segments were taken from non-injured mice (non). The segments were subjected to Western blot analysis with antibodies 172-R (L1-ICD; upper panel) and 555 (L1-ECD; lower panels). C, homogenates from the frontal cortex of wild-type (WT) and APPPS1–21 (AD) mice were subjected to Western blot analysis using antibody 172-R. A–C, representative results from one of four animals per group are shown after different exposure times of the blots. A GAPDH or actin antibody was used in Western blot analysis to control loading. The experiments were performed two times with identical results, and representative blots are shown.
We also determined the levels of this fragment in spinal cord segments rostral to the lesion site 1 week after spinal cord injury of adult mice. We specifically looked into the expression of L1 in the thoracic spinal cord because this is the segment where the severed axons of the corticospinal tract regrow and strongly express L1 following spinal cord injury (42).
Western blot analysis of thoracic spinal cord segments of non-injured and injured mice 7 days postinjury using antibody 172-R showed that the 70-kDa fragment levels increased in the injured spinal cord relative to the low level observed in spinal cords from non-injured mice, whereas the level of the 80-kDa fragment was reduced (Fig. 8B). Detection with the L1 antibody 555, which only recognizes the 70-kDa fragment and the soluble extracellular 140-kDa fragment but not the transmembrane 80-kDa fragment, showed enhanced levels of the 70-kDa fragment and reduced levels of the 140-kDa fragment after spinal cord injury relative to the levels observed in non-injured animals. The level of full-length L1 detected by both antibodies was not significantly altered after injury when compared with non-injured mice (Fig. 8B). The results indicate that the expression of full-length L1 is not altered by injury, whereas the proteolytic processing of L1 is altered upon spinal cord injury, leading to a decrease in the level of the transmembrane 80-kDa fragment in favor of the generation of the transmembrane 70-kDa fragment.
We further investigated whether the 70-kDa fragment is also seen in a degenerating brain using a mouse model of Alzheimer disease. APPPS1–21 mice develop amyloid plaques in the cortex and hippocampus at 3 months of age due to overexpression of the mutated form of amyloid precursor protein and increased γ-secretase activity (21). Western blot analysis of the homogenates from the frontal cortex of APPPS1–21 mice compared with wild-type mice using antibody 172-R revealed a slight reduction in the amount of the full-length L1 and the 80-kDa fragment, whereas the level of the 70-kDa fragment was markedly reduced in APPPS1–21 mice (Fig. 8C). In addition, highly increased levels of the γ-secretase cleavage product with an apparent molecular mass of 28 kDa (20) were observed in APPPS1–21 mice (Fig. 8C). This result indicates that a higher γ-secretase activity in APPPS1–21 mice leads to increased cleavage of the 70-kDa fragment, whereas only negligible cleavage of full-length L1 and the 80-kDa fragment was observed. Moreover, the generation of the 28-kDa fragment resulting from the predominant cleavage of the 70-kDa fragment suggests that proteolytic processing of L1 may be implicated in the pathogenesis of Alzheimer disease.
DISCUSSION
Generation of L1 Transmembrane Fragment upon Stimulation of L1 Signaling
In the present study, we show that stimulation of neuronal cells by L1 antibody or L1-Fc results in the generation and nuclear import of a 70-kDa L1 transmembrane fragment (Fig. 9). This fragment is recognized by L1 antibodies against distinct intracellular and extracellular epitopes, showing that it consists of the intracellular domain, the transmembrane domain, and part of the extracellular domain including the entire fourth and fifth FNIII domains and at least most of the third FNIII domain. The generation of the 70-kDa transmembrane fragment as well as the concomitant generation of a corresponding soluble extracellular 135-kDa fragment was abolished in the presence of aprotinin, a serine protease inhibitor. Thus, it is likely that cleavage of full-length L1 by a serine protease generates the 70-kDa transmembrane fragment and the extracellular 135-kDa fragment (Fig. 9). Cleavage of full-length L1 by the serine proteases trypsin, plasmin, and PC5a results in the generation of an 80-kDa transmembrane fragment that is not recognized by the antibody 557 and a corresponding 140-kDa fragment that is recognized by this antibody (22). In contrast, the 70-kDa fragment is recognized by antibody 557, whereas the 135-kDa fragment is not recognized by this antibody, clearly demonstrating that these fragments differ from the previously described 80- and 140-kDa fragments. In addition, these findings show that the 70- and 135-kDa fragments are not derived from the cleavage by the serine proteases trypsin, plasmin, and PC5a, which cleave within the third FNIII domain at sites that are distal to the binding site of the antibody 557 at the N terminus of the third FNIII domain (22). Therefore, one has to assume that the 70-kDa fragment is generated via a cleavage that involves a yet unknown serine protease, which is sensitive to aprotinin.
FIGURE 9.
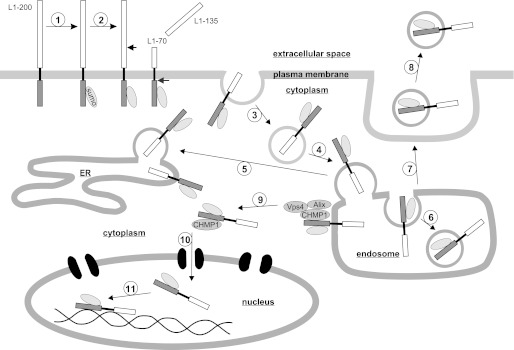
Working model of trafficking of L1 fragments to nucleus upon stimulation of L1 signaling. Stimulation of L1 signaling triggers attachment of one SUMO molecule to the full-length 200-kDa L1 (step 1). Extracellular serine protease-dependent cleavage of the sumoylated L1 results in the generation of a soluble extracellular 135-kDa fragment and a transmembrane 70-kDa fragment containing the intracellular domain and part of the extracellular domain (step 2). The 70-kDa fragment is internalized by endocytosis (step 3) and translocated to endosomal compartments (step 4) from where it is transferred to the ER (step 5). In late endosomes/MVBs, the 70-kDa fragment is packed into intraluminal vesicles (step 6), and after fusion of MVBs with the plasma membrane (step 7), the fragments are released into the extracellular space in association with exosomes (step 8). Through ESCRT-III-associated proteins CHMP1, Alix, and Vps4, the 70-kDa fragment is translocated from the endosomal membrane to the cytoplasm (step 9) followed by import through nuclear pores into the nucleus in association with CHMP1 and in an importin-dependent pathway (step 10). In the nucleus, the 70-kDa fragment may associate with nucleoplasmic proteins or interact with DNA or chromatin-associated proteins (step 11).
Sumoylation of L1 at Plasma Membrane
Here, we showed that the generation and nuclear import of the 70-kDa L1 fragment upon L1 stimulation are accompanied by sumoylation (Fig. 9), which has been shown to take place not only in the nucleus and cytoplasm but also in mitochondria and ER and at the plasma membrane (40, 43). Although we could not detect sumoylation of full-length L1 in our experiments, the finding that generation of the 70-kDa fragment was abolished by disruption of the sumoylation site Lys1172 implies that the extracellular cleavage of full-length L1 occurs immediately after or in parallel with sumoylation of full-length L1 at the plasma membrane and in addition excludes sumoylation of the fragments after cleavage of the full-length L1 (Fig. 9). Because co-transfection experiments revealed that the 70-kDa fragment carries only one SUMO molecule, we conclude that the 70-kDa fragment represents a monosumoylated 55-kDa fragment.
Intracellular Trafficking of Sumoylated L1 Fragment
Our results indicate that the sumoylated 70-kDa fragment is internalized by endocytosis after its generation at the plasma membrane (Fig. 9). After its internalization, the fragment is carried by vesicular transport to the late endosomal or MVB compartment and to the ER probably via endosomes (Fig. 9). In MVBs, the 70-kDa fragment is transferred to exosomes, which are formed in MVBs (30), and upon L1-triggered fusion of MVBs with the plasma membrane, fragment-carrying exosomes are released into the extracellular space (Fig. 9).
EGF receptor and a transmembrane fragment of NCAM are translocated from the plasma membrane to the ER after stimulation and are released from the ER membrane into the cytoplasm by a sec61-dependent (44) or calmodulin-dependent pathway (23), respectively. In contrast to the release of these proteins from the ER membrane, a release of the 70-kDa L1 fragment from the ER membrane was not detected, but this fragment is released from the endosomal membrane by an unknown mechanism involving the ESCRT-III-associated proteins Alix, CHMP1, and Vps4 (Fig. 9).
Nuclear Import of Transmembrane L1 Fragment from Cytoplasm into Nucleus and Possible Functional Roles of This Fragment in Nucleus
After their release from the ER membrane, the EGF receptor and the NCAM fragment are imported from the cytoplasm into the nucleus in an importin-dependent (44) or calmodulin-dependent but importin-independent pathway (23), respectively. Here, we obtained indications that the transmembrane 70-kDa L1 fragment is transferred after its release from the endosomal membrane via the cytoplasm to the nucleus in association with CHMP1 and by an importin-dependent process after its release from the endosomal membrane (Fig. 9). Because the 70-kDa fragment was found in fractions of chromatin-associated proteins, it is likely that it interacts with DNA-binding proteins, such as transcription factors, and/or with DNA and thus could be involved in modulating gene expression. A nuclear translocation of an L1 fragment may represent an alternative or parallel pathway accompanying the classical L1-induced signal transduction pathways. Having shown that L1-induced neurite outgrowth is inhibited by the serine protease inhibitor aprotinin, which reduces the generation and nuclear import of the 70-kDa fragment, we infer that this fragment is involved in regulation of neurite outgrowth. Moreover, the 70-kDa fragment may play an important role during early postnatal development because it is generated in mouse brain during decisive stages of neuronal migration, neuritogenesis, and synaptic targeting. Because increased amounts of soluble L1 fragments in the cerebrospinal fluid are associated with Alzheimer disease and dementia syndromes (45) and because we observed alterations in proteolytic processing of L1 after spinal cord injury and in a mouse model of Alzheimer disease, it is likely that proteolytic processing of L1 is involved in L1-dependent cellular responses to acute and chronic damage of the nervous system.
Sumoylation can modify activity, degradation, localization, and inter- or intramolecular interactions of target proteins; can affect nucleocytoplasmic transport, transcription, and DNA repair; and is associated with diseases, such as various types of cancer and neurodegenerative diseases (40, 43). Because L1 is associated with the congenital L1 and fetal alcohol syndromes, Hirschsprung disease, various types of cancer, neurodegenerative diseases (8, 46), bipolar disorders and schizophrenia (47–49) and because it plays a role in DNA repair (50), the investigation of the functional roles of the sumoylated nuclear L1 fragment in the developing and adult nervous system under physiological and pathological conditions may contribute to the understanding of the pathogenesis of L1-linked disorders.
Acknowledgments
We are grateful to Hans Will for the plasmids encoding GFP-tagged SUMO isoforms, Mathias Jucker for APPPS1 transgenic mice, Eva Kronberg for excellent animal care, and Ute Bork for excellent technical assistance.
Footnotes
- FNIII
- fibronectin type III
- CHMP1
- chromatin-modifying protein 1
- HP1γ
- heterochromatin-associated protein 1-γ
- Alix
- apoptosis-linked gene-2-interacting protein X
- Tsg101
- tumor susceptibility gene 101
- Vps4
- vacuolar protein sorting-associated protein 4
- SUMO
- small ubiquitin-like modifier
- NHS
- N-hydroxysulfosuccinimide
- ER
- endoplasmic reticulum
- SER
- smooth ER
- RER
- rough ER
- NCAM
- neural cell adhesion molecule
- MVB
- multivesicular body
- ESCRT
- endosomal sorting complex required for transport.
REFERENCES
- 1. Kamiguchi H., Lemmon V. (1997) Neural cell adhesion molecule L1: signaling pathways and growth cone motility. J. Neurosci. Res. 49, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Dahme M., Bartsch U., Martini R., Anliker B., Schachner M., Mantei N. (1997) Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 17, 346–349 [DOI] [PubMed] [Google Scholar]
- 3. Cohen N. R., Taylor J. S., Scott L. B., Guillery R. W., Soriano P., Furley A. J. (1998) Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 8, 26–33 [DOI] [PubMed] [Google Scholar]
- 4. Law J. W., Lee A. Y., Sun M., Nikonenko A. G., Chung S. K., Dityatev A., Schachner M., Morellini F. (2003) Decreased anxiety, altered place learning, and increased CA1 basal excitatory synaptic transmission in mice with conditional ablation of the neural cell adhesion molecule L1. J. Neurosci. 23, 10419–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y., Yeh J., Richardson P. M., Bo X. (2008) Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor. Neurol. Neurosci. 26, 81–96 [PubMed] [Google Scholar]
- 6. Guseva D., Zerwas M., Xiao M. F., Jakovcevski I., Irintchev A., Schachner M. (2011) Adhesion molecule L1 overexpressed under the control of the neuronal Thy-1 promoter improves myelination after peripheral nerve injury in adult mice. Exp. Neurol. 229, 339–352 [DOI] [PubMed] [Google Scholar]
- 7. Guseva D., Angelov D. N., Irintchev A., Schachner M. (2009) Ablation of adhesion molecule L1 in mice favours Schwann cell proliferation and functional recovery after peripheral nerve injury. Brain 132, 2180–2195 [DOI] [PubMed] [Google Scholar]
- 8. Katidou M., Vidaki M., Strigini M., Karagogeos D. (2008) The immunoglobulin superfamily of neuronal cell adhesion molecules: lessons from animal models and correlation with human disease. Biotechnol. J. 3, 1564–1580 [DOI] [PubMed] [Google Scholar]
- 9. Maness P. F., Schachner M. (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 [DOI] [PubMed] [Google Scholar]
- 10. Ebinu J. O., Yankner B. A. (2002) A RIP tide in neuronal signal transduction. Neuron 34, 499–502 [DOI] [PubMed] [Google Scholar]
- 11. Lee T. W., Tsang V. W., Birch N. P. (2008) Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biol. 4, 223–234 [DOI] [PubMed] [Google Scholar]
- 12. Brümmendorf T., Kenwrick S., Rathjen F. G. (1998) Neural cell recognition molecule L1: from cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 8, 87–97 [DOI] [PubMed] [Google Scholar]
- 13. Sadoul K., Sadoul R., Faissner A., Schachner M. (1988) Biochemical characterization of different molecular forms of the neural cell adhesion molecule L1. J. Neurochem. 50, 510–521 [DOI] [PubMed] [Google Scholar]
- 14. Silletti S., Mei F., Sheppard D., Montgomery A. M. (2000) Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J. Cell Biol. 149, 1485–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nayeem N., Silletti S., Yang X., Lemmon V. P., Reisfeld R. A., Stallcup W. B., Montgomery A. M. (1999) A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J. Cell Sci. 112, 4739–4749 [DOI] [PubMed] [Google Scholar]
- 16. Kalus I., Schnegelsberg B., Seidah N. G., Kleene R., Schachner M. (2003) The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J. Biol. Chem. 278, 10381–10388 [DOI] [PubMed] [Google Scholar]
- 17. Maretzky T., Schulte M., Ludwig A., Rose-John S., Blobel C., Hartmann D., Altevogt P., Saftig P., Reiss K. (2005) L1 is sequentially processed by two differently activated metalloproteases and presenilin/γ-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol. Cell Biol. 25, 9040–9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutwein P., Mechtersheimer S., Riedle S., Stoeck A., Gast D., Joumaa S., Zentgraf H., Fogel M., Altevogt D. P. (2003) ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J. 17, 292–294 [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto-Miyai K., Ninomiya A., Yamasaki H., Tamura H., Nakamura Y., Shiosaka S. (2003) NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J. Neurosci. 23, 7727–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riedle S., Kiefel H., Gast D., Bondong S., Wolterink S., Gutwein P., Altevogt P. (2009) Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/γ-secretase activity. Biochem. J. 420, 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radde R., Bolmont T., Kaeser S. A., Coomaraswamy J., Lindau D., Stoltze L., Calhoun M. E., Jäggi F., Wolburg H., Gengler S., Haass C., Ghetti B., Czech C., Hölscher C., Mathews P. M., Jucker M. (2006) Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7, 940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Appel F., Holm J., Conscience J. F., von Bohlen und Halbach F., Faissner A., James P., Schachner M. (1995) Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal transducing domain. J. Neurobiol. 28, 297–312 [DOI] [PubMed] [Google Scholar]
- 23. Kleene R., Mzoughi M., Joshi G., Kalus I., Bormann U., Schulze C., Xiao M. F., Dityatev A., Schachner M. (2010) NCAM-induced neurite outgrowth depends on binding of calmodulin to NCAM and on nuclear import of NCAM and fak fragments. J. Neurosci. 30, 10784–10798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makhina T., Loers G., Schulze C., Ueberle B., Schachner M., Kleene R. (2009) Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol. Cell. Neurosci. 41, 206–218 [DOI] [PubMed] [Google Scholar]
- 25. Jakovcevski I., Wu J., Karl N., Leshchyns'ka I., Sytnyk V., Chen J., Irintchev A., Schachner M. (2007) Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J. Neurosci. 27, 7222–7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehanna A., Jakovcevski I., Acar A., Xiao M., Loers G., Rougon G., Irintchev A., Schachner M. (2010) Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol. Ther. 18, 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curtis R., Green D., Lindsay R. M., Wilkin G. P. (1993) Up-regulation of GAP-43 and growth of axons in rat spinal cord after compression injury. J. Neurocytol. 22, 51–64 [DOI] [PubMed] [Google Scholar]
- 28. Loers G., Chen S., Grumet M., Schachner M. (2005) Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J. Neurochem. 92, 14673–14676 [DOI] [PubMed] [Google Scholar]
- 29. Loers G., Schachner M. (2007) Recognition molecules and neural repair. J. Neurochem. 101, 865–882 [DOI] [PubMed] [Google Scholar]
- 30. Lakkaraju A., Rodriguez-Boulan E. (2008) Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 18, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simons M., Raposo G. (2009) Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 32. Slagsvold T., Pattni K., Malerød L., Stenmark H. (2006) Endosomal and non-endosomal functions of ESCRT proteins. Trends. Cell Biol. 16, 317–326 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka N., Kyuuma M., Sugamura K. (2008) Endosomal sorting complex required for transport proteins in cancer pathogenesis, vesicular transport, and non-endosomal functions. Cancer Sci. 99, 1293–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Goot F. G., Gruenberg J. (2006) Intra-endosomal membrane traffic. Trends Cell Biol. 16, 514–521 [DOI] [PubMed] [Google Scholar]
- 35. Chook Y. M., Süel K. E. (2011) Nuclear import by karyopherin-βs: recognition and inhibition. Biochim. Biophys. Acta 1813, 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marfori M., Mynott A., Ellis J. J., Mehdi A. M., Saunders N. F., Curmi P. M., Forwood J. K., Bodén M., Kobe B. (2011) Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta 1813, 1562–1577 [DOI] [PubMed] [Google Scholar]
- 37. Stauffer D. R., Howard T. L., Nyun T., Hollenberg S. M. (2001) CHMP1 is a novel nuclear matrix protein affecting chromatin structure and cell-cycle progression. J. Cell Sci. 114, 2383–2393 [DOI] [PubMed] [Google Scholar]
- 38. Clague M. J., Urbé S. (2010) Ubiquitin: same molecule, different degradation pathways. Cell 143, 682–685 [DOI] [PubMed] [Google Scholar]
- 39. Kirkin V., Dikic I. (2007) Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr. Opin. Cell Biol. 19, 199–205 [DOI] [PubMed] [Google Scholar]
- 40. Geiss-Friedlander R., Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 41. Ren J., Gao X., Jin C., Zhu M., Wang X., Shaw A., Wen L., Yao X., Xue Y. (2009) Systematic study of protein sumoylation: development of a site-specific predictor of SUMOsp 2.0. Proteomics 9, 3409–3412 [DOI] [PubMed] [Google Scholar]
- 42. Jakeman L. B., Chen Y., Lucin K. M., McTigue D. M. (2006) Mice lacking L1 cell adhesion molecule have deficits in locomotion and exhibit enhanced corticospinal tract sprouting following mild contusion injury to the spinal cord. Eur. J. Neurosci. 23, 1997–2011 [DOI] [PubMed] [Google Scholar]
- 43. Scheschonka A., Tang Z., Betz H. (2007) Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci. 30, 85–91 [DOI] [PubMed] [Google Scholar]
- 44. Liao H. J., Carpenter G. (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol. Biol. Cell 18, 1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strekalova H., Buhmann C., Kleene R., Eggers C., Saffell J., Hemperly J., Weiller C., Müller-Thomsen T., Schachner M. (2006) Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol. Aging 27, 1–9 [DOI] [PubMed] [Google Scholar]
- 46. Schäfer M. K., Altevogt P. (2010) L1CAM malfunction in the nervous system and human carcinomas. Cell. Mol. Life Sci. 67, 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurumaji A., Nomoto H., Okano T., Toru M. (2001) An association study between polymorphism of L1CAM gene and schizophrenia in a Japanese sample. Am. J. Med. Genet. 105, 99–104 [PubMed] [Google Scholar]
- 48. Poltorak M., Khoja I., Hemperly J. J., Williams J. R., el-Mallakh R., Freed W. J. (1995) Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp. Neurol. 131, 266–272 [DOI] [PubMed] [Google Scholar]
- 49. Wakabayashi Y., Uchida S., Funato H., Matsubara T., Watanuki T., Otsuki K., Fujimoto M., Nishida A., Watanabe Y. (2008) State-dependent changes in the expression levels of NCAM-140 and L1 in the peripheral blood cells of bipolar disorders, but not in the major depressive disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 50. Cheng L., Wu Q., Huang Z., Guryanova O. A., Huang Q., Shou W., Rich J. N., Bao S. (2011) L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 30, 800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]