Background: Little is known about cellular mechanisms that actively lower the levels of the proinflammatory enzyme COX-2.
Results: An interaction between COX-2 and the prostaglandin receptor EP1 facilitates the degradation of COX-2 through the proteasome.
Conclusion: EP1 negatively regulates COX-2 expression.
Significance: Receptor-mediated regulation of COX-2 presents an important means of moderating inflammation and resolution of pathophysiological events.
Keywords: Cell Surface Receptor, Cyclooxygenase (COX) Pathway, Enzyme Degradation, G Protein-coupled Receptors (GPCR), Ubiquitination
Abstract
The enzyme cyclooxygenase-2 (COX-2) is rapidly and transiently up-regulated by a large variety of signals and implicated in pathologies such as inflammation and tumorigenesis. Although many signals cause COX-2 up-regulation, much less is known about mechanisms that actively down-regulate its expression. Here we show that the G protein-coupled receptor prostaglandin E1 (EP1) reduces the expression of COX-2 in a concentration-dependent manner through a mechanism that does not require receptor activation. The reduction in COX-2 protein is not due to decreased protein synthesis and occurs because of enhancement of substrate-independent COX-2 proteolysis. Although EP1 does not interfere with the entry of COX-2 into the endoplasmic reticulum-associated degradation cascade, it facilitates COX-2 ubiquitination through complex formation. Blockade of proteasomal activity results in degradation of the receptor and concomitant recovery in the expression of COX-2, suggesting that EP1 may scaffold an unknown E3 ligase that ubiquitinates COX-2. These findings propose a new role for the EP1 receptor in resolving inflammation through down-regulation of COX-2.
Introduction
Prostanoids are bioactive lipids that play important roles in several key processes including immunity, inflammation, and cardiovascular homeostasis. Among them, prostaglandin E2 (PGE2)2 is the most common across species and the most versatile in its functions (1). The actions of PGE2 are mediated through four members of the G protein-coupled receptor (GPCR) superfamily, designated EP1–4. Of those, the EP1 receptor is the least characterized in terms of G protein coupling, signaling, and trafficking. EP1 belongs to a unique subgroup of GPCRs that are present not only on the plasma membrane but also on the inner and outer membrane of the nuclear envelop (2).
The lipid ligands that activate EP1 and other prostanoid receptors are formed by the catalysis of arachidonic acid (AA) into the metabolite prostaglandin H2 by the action of COX. Because prostaglandin H2 is a single substrate that gives rise to all of the biologically active prostanoids, COX is the rate-limiting enzyme that controls the signaling of many different receptors at the ligand level. COXs exist in two main isoforms, COX-1 and COX-2, both of which reside on the luminal surfaces of the endoplasmic reticulum and the inner and outer membranes of the nuclear envelope (3). They display similar catalytic mechanisms but differ in their expression patterns. COX-1 is a relatively stable protein expressed almost ubiquitously and fulfills many housekeeping functions (4). Conversely, COX-2 expression undergoes rapid and transient increase by a broad range of pathological stimuli, and it is implicated in many pathologies.
One of the important features of COX-2 is its relatively short half-life, which is thought to result from a combination of decreased translation and rapid degradation (5). COX-2 is degraded via two pathways; that is, a substrate-dependent pathway of suicide inactivation and an activity-independent pathway that involves shuttling of the mature, N-glycosylated protein from the endoplasmic reticulum to the cytosol and on to the 26 S proteasome in the endoplasmic reticulum-associated degradation (ERAD) pathway (6, 7). Application of a mannosidase inhibitor or deletion of a 19 amino acid sequence at the C-tail, which contains an N-glycosylation site unique to COX-2, stabilizes the enzyme and prolongs its half-life (6, 7). From the endoplasmic reticulum, the enzyme is transported to the cytosol, where it is ubiquitinated (8) and degraded by the proteasome (7) in a partially understood pathway.
Given the central role of COX-2 in pathogenic processes such as inflammation and cancer, a large body of work focuses on either targeting the cellular pathways that up-regulate the levels of the enzyme or on the use of non steroidal anti-inflammatory drugs (NSAIDs) as a means of inhibiting enzymatic activity. On the other hand, much less is known of cellular mechanisms that may actively promote the degradation of COX-2. Such a role has been recently suggested for caveolin-1, which was found to accelerate degradation of COX-2 through ERAD (9). Here, we propose that the expression of COX-2 may be actively regulated by one of the very receptors that is activated by the products of its catalysis. We set out to determine the relationship between the expression and signaling of the prostaglandin EP1 receptor and COX-2 in an attempt to unravel the possible mechanism by which a GPCR may regulate the expression of COX-2.
EXPERIMENTAL PROCEDURES
Unless indicated otherwise, each experiment was repeated at least three times in triplicate with comparable results.
Materials
Goat polyclonal anti-COX-2 (human) and goat polyclonal anti-actin (human) were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Rabbit polyclonal EP1 receptor (human) was from Cayman Chemical (Ann Arbor, MI). Rabbit polyclonal phospho-eEF2 (Thr-56) and eEF2 (human) were from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated bovine anti-goat IgG, goat anti-rabbit IgG, and goat anti-mouse IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) and were used at a dilution of 1:10,000. PGE2 rabbit antiserum for radioimmunoassays was purchased from Sigma, and tritium-labeled PGE2 (190 Ci/mmol) was obtained from PerkinElmer Life Sciences. Ibuprofen, arachidonic acid, kifunensine, and (s)-MG132 were from Cayman Chemical (Ann Arbor, MI). PGE2, 17-phenyl-trinor prostaglandin E2, and methyl arachidonyl fluorophosphonate were from Biomol-Enzo Life Sciences (Pharmingdale, NY). N-Methylmaleimide was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). All other reagents were standard laboratory grade.
Cell Culture and Transfection
HEK-293 and the breast cancer T47D cell lines were obtained from the ATCC and grown in minimum Eagle's medium and DMEM, respectively. Normal human dermal fibroblasts (NHDFs) were a gift from Prof. Shulamit Levenberg, from the Technion, Israel, and were grown in DMEM.
All media were supplemented with 10% fetal bovine serum and 100 units/ml penicillin and streptomycin. Transient transfections were carried out in subconfluent monolayers (70–80%) using PolyJet (SignaGen Laboratories) at a ratio of 1:3 cDNA:PolyJet, according to the manufacturer's instructions, except for transfections of NHDFs, which were done at a ratio of 1:4. All samples contained the same amount of total cDNA. Transfections in 12-well dishes and 100-mm dishes were done using a total of 0.9 and 2 μg of cDNA, respectively.
cDNA Constructs
EP1 cDNA (gift of Prof. Barry Ashby, Temple University School of Medicine) was PCR-amplified from the pcDNA3.1 expression plasmid using the forward primer 5′-GCCCTTGCGGG-3′ and the reverse primer 5′-ATAAAGCTTGAAGTGGCTGAGGCC-3′. The resulting product was inserted into the multiple cloning site of pECFP-N1 (Clontech) between the Xhol and Hindlll sites. pcDNA5/FRT/TO encoding human COX-2, G533ACOX-2, and Del597–612 COX-2 were gifts from Prof. William L. Smith, University of Michigan. COX-2 was PCR-amplified using the forward primer 5′-ATACTCGAGGTCTCGCCCGCGCCCTG-3′ and the reverse primer 5′-ATAGAATTCCTACAGTTCAGTCGAACG-3′ and inserted into the MCS of the pEYFP-C1 (Clontech) plasmid between Xhol and EcoRl. G533ACOX-2 was amplified using the forward primer 5′-ATACTCGAGATGCTCGCCCGCGCCCT-3′ and the reverse primer 5′-ATAAAGCTTCAGTTCAGTCGAACG-3′ and inserted into the pEYFP-N1 (Clontech) between Xhol and Hindlll. Sequences of all constructs were confirmed by restriction digestion analysis and sequencing at the core facilities of the Technion Israel Institute of Technology and Hylabs (Rehovoth, Israel).
shRNA Sequence and Transfection
The shRNA sequence for human EP1 was 5′-CCGGGCTTGTCGGTATCATGGTGGTCTCGAGACCACCATGATACCGACAAGCTTTTT-3′. 4 μg of PLK0.1-puro vector carrying EP1 shRNA or negative control vector (Sigma) were transfected into NHDF cells (2 million cells in 6-well dishes) using Polyjet. The cells were assayed by Western blot 24 h post-transfection as described below.
Immunoprecipitation and Immunoblotting
Monolayers in 100-mm culture dishes were washed twice with ice-cold PBS and lysed in RIPA/SDS buffer (50 mm Tris, pH 8, 150 mm NaCl, 5 mm EDTA, 1% v/v Nonidet P-40, 0.5%, 0.5% w/v deoxycholic acid, 0.1% w/v SDS, 10 mm NaF). 0.1 mm PMSF and Complete Protease Inhibitor mixture tablets (Roche Applied Science) were added immediately before lysis. In the ubiquitination experiments, the buffer was supplemented with 10 mm N-ethylmaleimide. Aliquots of 100 μl were removed for total fraction assays, and 40 μg of total protein were loaded in all experiments. Samples were centrifuged at 1000 × g at 4 °C for 2 min, and the supernatants were collected. Protein concentrations were determined with the Bradford Assay (Bio-Rad) and equal amounts of total protein were used for immunoprecipitation. Immunoprecipitations were performed using 2 μg of COX-2 antibody and 30 μl of a 50% slurry of protein A/G plus-agarose immunoreagent (Santa Cruz, CA) and agitated overnight at 4 °C. Immune complexes were washed 3 times with ice-cold RIPA/SDS, diluted in 30 μl of 2× Laemmli sample buffer, and resolved by SDS-PAGE. Nitrocellulose membranes containing the immuno-complexes or total cell lysate proteins were incubated with primary antibodies at a dilution of 1:500 (COX-2 and EP1) and 1:250 for ubiquitin. Proteins were visualized by a chemiluminescence detection kit for HRP (EZ ECL, Biological Industries) and quantified using a CCD camera and Quantity One software (XRS, Bio-Rad).
Radioimmunoassay
140,000 cells were seeded per well in 12-well dishes and transfected as above, with each experimental point performed in triplicate. 24 h after transfection, the growth media was siphoned, and samples were washed twice with PBS and stimulated with 1 ml of 50 μm AA in DMEM for 30 min at 37 °C. Reactions were terminated by collecting the supernatant of each well followed by flash-freezing in liquid N2. 50–100 μl of the supernatant was used for the analysis. PGE2 levels were measured by single antibody radioimmunoassay with dextran-coated charcoal precipitation as previously described (10). PGE2 levels were calculated as ng/μg of total protein in each sample.
Flow Cytometry
Cells were washed twice with PBS, trypsinized, and resuspended in 150–200 μl of minimum Eagle's medium for cytometric analysis. The samples were analyzed using BD FACSCanto II flow cytometer with DACSDiva software (BD Biosciences). Yellow fluorescence was measured using a 530/30-nm band pass filter, and cyan fluorescence was determined with a 510/50 band pass filter. Gates were set to exclude necrotic cells and cellular debris, and the fluorescence intensity of events within the gated regions was quantified. Data were collected from 10,000 events for each sample.
RNA Isolation and Quantitative PCR
Total RNA was isolated using the AurumTM Total RNA Fatty and Fibrous Tissue kit (Bio-Rad) according to manufacturer's protocol. cDNA was prepared from 200 ng of total RNA with A260/A280 >1.8 using the High Capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed with a StepOnePlusTM Real-time PCR System using Fast SYBR® Green technology (Applied Biosystems). Reactions were performed using specific primers: for COX-2 the forward primer was 5′- GCTTTATGCTGAAGCCCTATGA-3′, and the reverse primer was 5′-TCCAACTCTGCAGACATTTCC-3′ (PCR cycles 23.7), and for the endogenous control hypoxanthine-guanine phosphoribosyltransferase the forward primer was 5′-TGACCTTGATTTATTTTGCATACC-3′ and the reverse primer was 5′-CGAGCAAGACGTTCAGTCCT-3′ (PCR cycles 21.6). Real-time PCR results were analyzed using StepOne software (Applied Biosystems).
Statistical Analysis
All bars represent the mean ± S.E. Statistical analyses were done using the GraphPad Prism Software. Unless otherwise stated, statistical significance was determined by one-way analysis of variance. Post-hoc analysis was performed with Tukey multicomparison test when appropriate. p values <0.05 were considered significant.
RESULTS
Prostaglandin Receptor EP1 Decreases Expression of COX-2
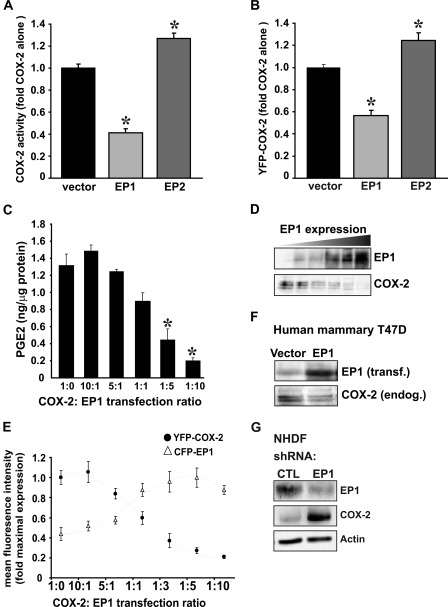
To test whether expression of different prostaglandin EP receptors affects COX-2 activity, HEK293 cells were co-transfected with either COX-2 alone or together with EP1 or EP2, and enzymatic activity was assessed by measuring PGE2 generation. Only cells that were transfected with COX-2 and stimulated exogenously with AA showed any detectable COX-2 activity, suggesting that the data reflect the activity of transfected COX-2. As shown in Fig. 1A, co-expression of EP1 with COX-2 caused a marked reduction in the activity of the enzyme, whereas EP2 caused a small but significant increase in activity. Co-expression of YFP-tagged COX-2 together with EP1 or EP2 showed similar results in flow cytometry experiments, suggesting that the reduction in COX-2 activity is due to decreased levels of the enzyme (Fig. 1B). We next tested whether the effect of EP1 on COX-2 expression is dependent upon the amount of EP1 receptor present. For this, we expressed COX-2 at a constant level with increasing concentrations of EP1. The amount of total cDNA was maintained constant by adding a cDNA of empty vector. Measurements of COX-2 activity in these experiments confirmed that a gradual increase in EP1 levels results in a dose-dependent decrease of COX-2 activity (Fig. 1C). Protein samples from the activity dose-dependent experiment in Fig. 1C were analyzed for levels of COX-2 and EP1 and confirmed that the increase in EP1 levels results in a gradual decrease in the expression of COX-2 protein (Fig. 1D). A similar dose-dependent effect was observed when co-transfecting YFP-COX-2 with increasing concentrations of CFP-EP1, confirming that the expression of YFP-COX-2 is in reverse correlation with CFP-EP1 expression (Fig. 1E). Next, we tested whether elevation of EP1 levels can affect endogenous COX-2 levels. As depicted in Fig. 1F, overexpression of EP1 in T47D human mammary gland cells, which express COX-2 endogenously, caused a significant reduction in the levels of the enzyme. Finally, knockdown of endogenous EP1 expression in NHDF cells yielded an opposite effect and showed a significant increase in levels of endogenous COX-2 (Fig. 1G).
FIGURE 1.
EP1 receptor lowers COX-2 expression. A, HEK 293 cells transfected with 0.15 μg of COX-2 with 0.75 μg of either empty vector, EP1, or EP2 were incubated with 50 μm AA for 30 min. The culture media was collected and probed for PGE2 production by RIA. *, p < 0.001 versus empty vector. B, HEK 293 cells transfected with YFP-COX-2 and either empty vector, CFP-EP1, or EP2 were probed by flow cytometry 16 h after transfection. *, p < 0.001 versus empty vector. C, the effect of EP1 on COX-2 was dose-dependent. HEK 293 cells with a constant amount of COX-2 were transfected with increasing levels of EP1 at the ratios indicated in the graph. COX-2 activity was measured by RIA. *, p < 0.001 versus empty vector. D, Western blots of cell lysates from the experiment in C were probed for EP1 (top panel) and COX2 (bottom panel). E, HEK 293 cells with a constant amount of YFP-COX-2 were transfected with increasing levels of CFP-EP1 at the ratios indicated in the graph and analyzed by flow cytometry. F, human mammary T47D cells with endogenous expression of COX-2 were transfected with 0.75 μg EP1, and levels of COX-2 and EP1 were assayed 16 h after transfection. G, NHDF cells were transfected with EP1 siRNA or negative control. Cells were harvested and probed 24 h post-transfection.
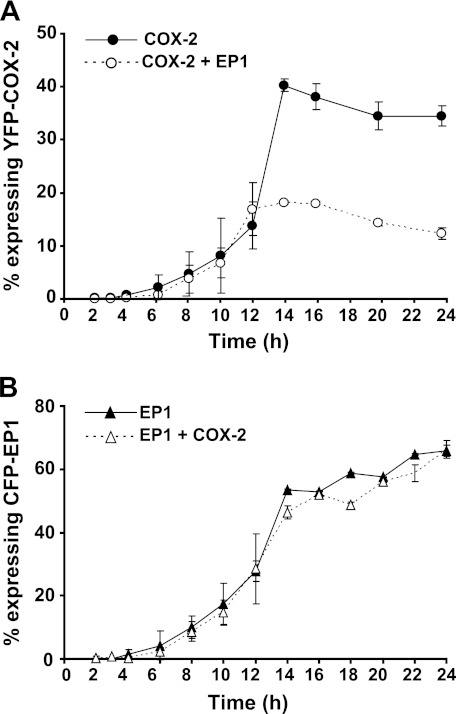
To further characterize the effect of EP1 on COX-2, we performed a detailed time-course analysis of YFP-tagged COX-2 expression in the presence or absence of CFP-EP1 at a ratio of 1:5, which we found reduced COX-2 levels by 40–60% (Fig. 1, C and E). As shown in Fig. 2A, the expression of YFP-COX-2 became detectable 6 h after transfection and reached steady state within 14 h (closed circles). Conversely, when COX-2 was co-expressed together with CFP-EP1, the levels of YFP-COX-2 were lower by ∼50% compared with COX-2 alone (Fig. 2A, open circles), and steady state was reached earlier than in the absence of EP1. EP1 levels were not affected by the presence of COX-2, and showed similar kinetics and levels in the presence or absence of the enzyme (Fig. 2B), suggesting that the effect of EP1 on COX-2 is unidirectional.
FIGURE 2.
Time course of the EP1 effect on COX-2 levels. A, HEK 293 cells were transfected with YFP- COX-2 and either empty vector (closed circles) or CFP-EP1 (open circles). Samples were collected from 2–24 h after transfection and analyzed by flow cytometry. A, the presence of EP1 reduces COX-2 levels as early as 12 h from transfection. B, COX-2 does not affect the expression of CFP-EP1.
Receptor Activation Is Not Required for EP1-mediated Reduction in COX-2
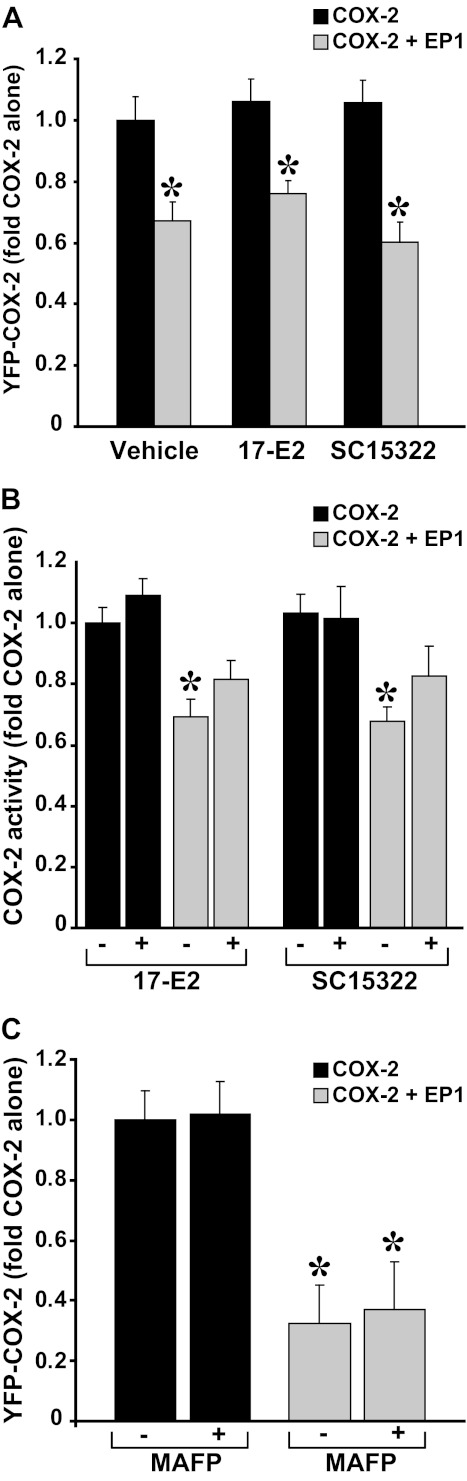
To determine whether the effect of EP1 on COX-2 involves receptor signaling, we expressed YFP-COX-2 with or without CFP-EP1 and treated the samples with either the selective EP1 receptor agonist 17-phenyl-trinor prostaglandin E2 or with the selective antagonist SC51322. Probing the samples for COX-2 and EP1 levels revealed that the decrease elicited by the receptor on COX-2 expression was not relieved by either ligand (Fig. 3A). CFP-EP1 levels, either alone or in the presence of COX-2, were unaffected by the different treatments (not shown). Assays of COX-2 activity in the presence of absence of EP1 ligands revealed that prolonged exposure to the ligands does not change the EP1-mediated reduction in COX-2 activity (Fig. 3B). Furthermore, the effect of the receptor on COX-2 was not mediated by liberation of AA by PLA2 because the use of the selective PLA2 inhibitor methyl arachidonyl fluorophosphonate did not reverse the effect of EP1 (Fig. 3C). Together these results imply that the effect of EP1 on COX-2 levels does not require activation of the EP1 receptor.
FIGURE 3.
The effect of EP1 on COX-2 does not require receptor activation. A, cells were transfected with YFP- COX-2 and either empty vector or CFP-EP1 and treated with vehicle or EP1 agonist 17-phenyl-trinor prostaglandin E2 (17-E2; 10 μm) or the EP1 antagonist SC51322 (10 μm) for the full duration of transfection (16–24 h). YFP COX-2 levels were assessed by flow cytometry, COX-2 activity was assessed by RIA (B). C, cells were transfected as above and treated with the PLA2 inhibitor methyl arachidonyl fluorophosphonate (MAFP, 50 μm) for the full duration of the experiment. YFP-COX-2 levels were assessed by flow cytometry.
EP1 Increases COX-2 Transcription and Enhances General Protein Translation
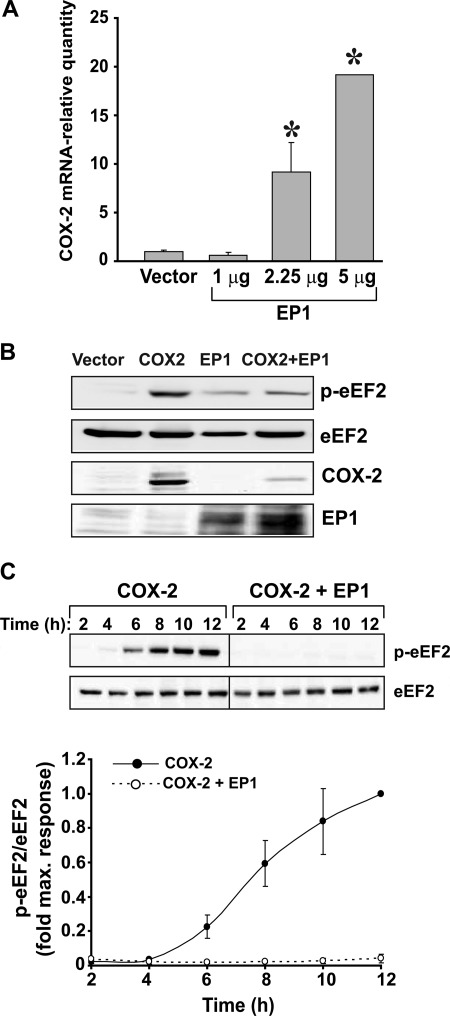
To check whether transfection of cells with EP1 affects transcription of COX-2, we expressed either an empty vector or different amounts of EP1 in cells and quantified their effect on levels of endogenous COX-2 mRNA. Transfection of different amounts of EP1 into HEK 293 cells without COX-2 resulted in a gradual increase in COX-2 mRNA (Fig. 4A). Because the levels of COX-2 transcript were elevated but the levels of the expressed protein were decreased, we examined whether the presence of EP1 interferes with general protein translation in the cell. Because peptide translation involves cellular machinery that is common to all other proteins, we tested how COX-2 expression affects the activity of endogenous eEF2, a key regulating enzyme of elongation that is activated in its dephosphorylated form. As depicted in Fig. 4B, expression of COX-2 at steady state resulted in increased eEF2 phosphorylation, whereas expression of EP1 alone or together with COX-2 did not. In a time-dependent study, expression of COX-2 alone caused a gradual increase in phosphorylation of eEF2, suggesting that its presence attenuates total protein synthesis over time. However, in the presence of EP1 we found that the effect of COX-2 on eEF2 was completely abolished, rendering eEF2 fully active (Fig. 4C). Together the above data suggest that the EP1-mediated reduction in COX-2 is not due to a reduction in protein synthesis. On the contrary, both COX-2 mRNA transcription and general cellular translation are enhanced by EP1.
FIGURE 4.
Overexpression of EP1 elevates COX-2 mRNA levels. A, HEK 293 cells were transfected with 1, 2.25, and 5 μg of EP1 or empty vector, and samples were analyzed for content of endogenously expressing COX-2 mRNA using real-time PCR. *, p < 0.001 versus empty vector. B, EP1 and COX-2 affect activation of eEF2. Shown is a representative immunoblot of cells transfected overnight with each protein alone or both and tested for expression of the different proteins using specific antibodies. C, shown is the time course effect of EP1 and COX-2 on activation of eEF2. Cells were transfected with either COX-2 and empty vector (left panel and closed circles in graph) or COX-2 and EP1 (right panel and open circles in graph) at a ratio of 1:5. Samples were collected 2–12 h post-transfection and analyzed for levels of phosphorylated and total levels of eEF2.
EP1 Enhances Degradation of COX-2
The data presented above show that in the presence of EP1, COX-2 expression is markedly reduced and seems to reach steady state earlier than in the absence of the receptor, two observations that point to the possibility that EP1 enhances COX-2 degradation (11). We therefore tested the effect of EP1 on the two known pathways of COX-2 degradation, ERAD-mediated proteasomal proteolysis and activity-induced suicide inactivation.
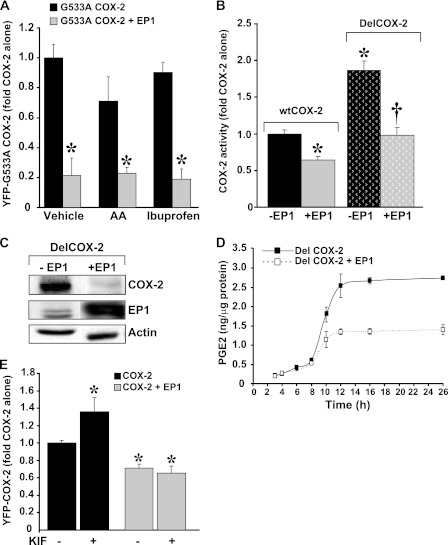
If the observed effect of EP1 is due to substrate-dependent activation of COX-2 followed by its suicide inactivation (7), then we expect that a COX-2 mutant, which is impaired in its ability to bind and catalyze AA, will not be affected by EP1. To test this we co-expressed the YFP-G533A COX-2 catalytically impaired mutant (12) with or without CFP-EP1 and measured the effect on G533A COX-2 expression. As shown in Fig. 5A, EP1 markedly decreased the expression of the catalytically impaired enzyme. Chronic treatment of G533A COX-2 with AA caused an insignificant reduction in the levels of the enzyme, probably because of residual enzymatic activity (12). However, both AA and the non-selective COX inhibitor ibuprofen failed to reverse the EP1 effect.
FIGURE 5.
The EP1 effect on COX-2 is not through suicide inactivation. A, cells were transfected with the YFP-G533A COX-2 mutant with or without CFP-EP1 and treated with arachidonic acid (50 μm) or ibuprofen (200 μm) throughout the experiment. *, p < 0.05 versus COX-2 alone. EP1 did not affect the entry of COX-2 into ERAD. B, cells were transfected with wtCOX-2 or the DelCOX-2 construct with or without EP1 at a ratio of 1:5. COX-2 activity was assessed 24 h later by RIA. *† p < 0.05 versus COX-2 or DelCOX-2 alone C, shown is a representative immunoblot of DelCOX-2 (upper panel) and EP1 levels (middle panel) 26 h after transfection. D, shown is time course of DelCOX-2 activity, performed in triplicate. Samples were collected from 2–26 h after transfection and analyzed by RIA. E, cells were transfected with YFP- COX-2 and either empty vector or CFP-EP1 and treated with the mannosidase inhibitor kifunensine (KIF, 10 μm) for 24 h. *, p < 0.05 versus COX-2 alone.
Because the effect of EP1 on COX-2 is not mediated through suicide inactivation, we examined the possibility that it exerts its effect by accelerating the entry of COX-2 into the ERAD system and/or its degradation in the proteasome. First, we tested if delaying the entry of COX-2 into ERAD reverses the effect of EP1. For this, we used a COX-2 deletion mutant (Del597–612 COX-2) that lacks a 19-amino acid cassette at its carboxyl terminus, a deletion that delays the entry of COX-2 into ERAD and prolongs its turnover rate (6). We found that DelCOX-2 displays a significantly higher level of activity compared with the wild type enzyme. However, EP1 affected the deletion mutant to the same extent as the wild type enzyme, significantly lowering its activity and expression (Fig. 5, B and C). A time course analysis of DelCOX-2 activity in the presence or absence of receptor revealed similar kinetics to those obtained for the wild type enzyme (Fig. 5D). These results were also supported by the use of the mannosidase inhibitor kifunensine, which inhibits N-glycosylation of COX-2 and retards its turnover rate (6). In accordance with previous studies, kifunensine treatment caused an increase in the levels of COX-2, but this effect was only evident in samples that did not contain EP1 (Fig. 5E). Together, the above data suggest that the effect of EP1 on COX-2 is not mediated by accelerating its entry into ERAD.
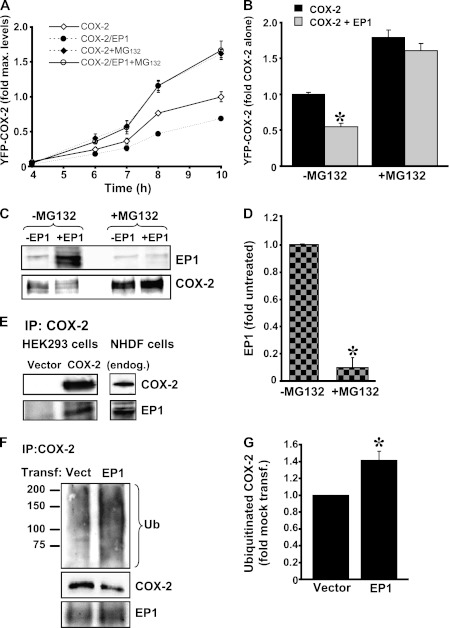
To test if proteasomal degradation is involved in the effect of EP1 on COX-2, cells expressing either YFP-COX-2 or YFP-COX-2 together with CFP-EP1 were treated with or without the specific proteasome inhibitor MG132 (Fig. 6A). A time-course experiment showed that the effect of EP1 on COX-2 expression is already apparent less than 12 h from the beginning of transfection. Strikingly, however, the presence of MG132 completely abolished the effect of EP1, and the levels of COX were doubled in both control and EP1-expressing cells (Fig. 6A). The same effect was observed at steady state, 16 h after transfection (Fig. 6B). Because prolonged inhibition of the proteasome is known to increase cell death, we measured the number of dead cells in cells transfected with either YFP-COX-2, CFP-EP1, or both with or without MG132 for 16 h. Flow cytometry experiments showed that the amount of cells positive for propidium iodide in untreated samples was 21.7 ± 0.7% compared with 39.3 ± 0.3% after overnight treatment with MG132. However, no differences in the rate of cell death were observed among the different transfections in each treatment group, indicating that the obtained results are not an artifact of differential cell death (supplemental Fig. 1). Analysis of receptor levels in the MG 132-treated samples showed that whereas inhibition of the proteasome markedly elevated COX-2 levels, it also caused a dramatic decrease in the total cellular levels of EP1 (Fig. 6, C and D).
FIGURE 6.
EP1 enhances COX-2 ubiquitination and proteasomal degradation. A, HEK 293 cells transfected with YFP-COX-2 and either empty vector or CFP-EP1 at a ratio of 1:5 were treated with or without 10 μm MG132 for the indicated time points. B, shown is a summary of the effect of overnight treatment with MG132 on COX-2 levels in the presence or absence of EP1. *, p < 0.001 versus COX-2. C, inhibition of the proteasome causes degradation of EP1. A representative immunoblot shows that overnight treatment of cells with MG132 abolished the expression of EP1. At the same time, the levels of COX-2 are similar to those in cells transfected with COX-2 alone. D, shown is a summary graph of the effect of overnight treatment MG132 on EP1 levels from six different experiments. *, Student's t test p < 0.05. E, COX-2 and EP1 interact with each other. COX-2 was immunoprecipitated (IB) from HEK 293 cells transfected with empty vector or COX-2 (left panel) and from NHDF cells (right panel). Blots were probed for COX-2 and EP1 using specific antibodies. F, EP1 increases the ubiquitination (Ub) of COX-2. NHDF cells were transfected with either 2 μg of empty vector or EP1, COX-2 was precipitated 12 h later, and blots were analyzed by immunoblotting first with anti-ubiquitin followed by COX-2 and EP1. G, shown are levels of ubiquitinated COX-2 in NHDF cells with or without EP1 overexpression.
The reverse correlation between EP1 and COX-2 expression in the presence of MG132 suggested that EP1 might serve as a scaffold for a protein/proteins that accelerates the entry of COX-2 into the proteasomal pathway, possibly by increasing its ubiquitination levels. In that case, EP1 and COX-2 may be at close proximity with each other, and the ubiquitination content of COX-2 should increase in the presence of the receptor. To test for a possible interaction between COX-2 and EP1, HEK 293 cells, which express EP1 endogenously, were transfected with either mock or EP1 cDNA, and samples were subject to immunoprecipitation with anti-COX-2. Only cells that expressed both proteins showed the presence of EP1 in COX-2 precipitates (Fig. 6E, left panel). The co-immunoprecipitation of COX-2 was also performed in NHDF cells, which express both proteins endogenously, and a similar result was obtained (Fig. 6E, right panel), suggesting that the two proteins form a complex. We next tested whether the levels of COX-2 ubiquitination are altered in the presence of EP1. For this, we transfected NHDF cells with an empty vector or EP1 cDNA, precipitated COX-2 12 h after transfection, and probed samples first for total ubiquitin and again for COX-2 and EP1 levels. As depicted in Fig. 6F, transfection caused a moderate increase in EP1, which was accompanied by a fall in COX-2 levels and an increase in the staining of a smeared band beginning at ∼72 kDa. This effect was clear despite the fact that COX-2 levels were already reduced in the presence of EP1. Quantification of five independent experiments showed that an elevation in EP1 levels increases ubiquitination of COX-2 by ∼40% (Fig. 6G).
DISCUSSION
The main observation of this study is that COX-2 levels are down-regulated by the prostaglandin EP1 receptor, suggesting that other than its classical roles as a relay system for prostaglandin signaling, this receptor may fulfill additional cellular functions that do not necessarily involve activation by ligand. This finding is of particular importance because COX-2 is highly abundant in pathologies of cell growth and inflammation, and much effort is invested in an attempt to lower its levels as a means of treating these ailments. Because of its highly inducible nature, the focus of investigations has been on targeting signaling pathways of growth factors, cytokines, and other GPCRs that up-regulate COX-2 expression. Indeed, activation of numerous GPCRs leads to an increase in COX-2 mRNA and protein expression through intracellular pathways that activate mitogenic cascades (13–16), and elevation of COX-2 may even modulate the levels of the initiating receptor itself (17, 18). However, there is much less evidence of proteins such as caveolin (9) that down-regulate COX-2 expression. One such hint comes from a study where knock-out of the angiotensin II type 1 receptor caused a massive increase in COX-2 expression (19), which presents the mirror image of what we observe with EP1.
At the mechanistic level, we showed that EP1 facilitates the degradation of COX-2. It may be argued that receptor overexpression may cause an increase in the basal activity of endogenous enzymes (e.g. PLA2) that in turn may activate intracellular pathways and lead to a reduction in COX-2 levels through suicide inactivation. However, this explanation for the effect of EP1 on COX-2 is excluded for several reasons. First, the heterologous system of HEK 293 cells lacks significant levels of endogenous PLA2 or AA that may activate COX-2 (7, 20). These findings are supported by our own functional COX-2 assays, which did not show any detectable levels of PGE2 in COX-2-transfected cells before stimulation with AA. Second, whereas suicide-dependent inactivation of COX-2 is largely attenuated by COX inhibitors (7), the use of ibuprofen did not reverse the effect of EP1 (Fig. 5A). Finally, we found that the inhibitory effect of EP1 on COX-2 occurred also when using G533A COX-2, the enzyme mutant that has a basal degradation half-life similar to the wild type enzyme but is protected from substrate-dependent degradation (7, 12).
Targeting proteins for degradation in either proteasomal or lysosomal pathways requires their covalent tagging with ubiquitin by a large group of ubiquitin-protein E3 ligases (21). Although this process may be well defined for many cellular proteins, evidence of COX-2 ubiquitination and its regulation are scarce. In accordance with our own results, inhibition of the proteasome in neuronal cells causes the accumulation of high molecular weight ubiquitin-COX-2 (22). Furthermore, COX-2 ubiquitination in HeLa cells was found to depend on its association with large complexes COP9 signalsomes consisting of cullin proteins that scaffold unidentified E3 ligases (8). Given our evidence for the existence of an EP1-COX-2 complex as well as the fact that they share similar cellular locations (2, 3), it is tempting to speculate that the EP1 receptor or associated accessory proteins may scaffold a currently unrecognized E3 ligase or other proteins that modulate COX-2 degradation. This possibility is supported by the fact that some GPCRs are ubiquitinated after activation through complex formation between the receptor, an E3 ligase, and an adaptor protein such as dioxygenase EGLN3 (23) or β arrestin (23–26). Some GPCRs such as opioid, ocular albinism type 1 (OA1) and adenosine A2A receptors, are also ubiquitinated in an activation-independent manner (27–30), suggesting the constitutive presence of E3 ligases in their vicinity. Currently our results point to EP1-facilitated degradation of COX-2. The identities of possible E3 ligases and accessory proteins that may regulate the effect of EP1 on COX-2 are currently under investigation.
Most studies that examine ubiquitination levels of a desired protein use proteasome inhibitors to detect differences in ubiquitination levels. However, here the presence of the proteasome inhibitors not only elevates COX-2 but also inversely affects EP1, lowering its levels substantially and abolishing its effect on COX-2 (Fig. 6, C and D). If in fact EP1 scaffolds an unknown COX-2 E3 ligase, a reduction in its levels may result in decreased ubiquitination of COX-2 and less protein degradation. The observation that MG132 decreases EP1 levels suggests that whereas the receptor itself may not be degraded through the proteasome, its levels may be affected by the disappearance of unidentified protein(s) that protect it from proteolysis or the accumulation of proteins that promote its degradation. Some candidates for this unknown protein(s) include G protein-coupled receptor kinase 2 (GRK2), which phosphorylates the receptor and accumulates when the proteasome is inhibited (31), or the Golgi chaperone protein RTP4, which protects μ-δ opioid receptor dimers from ubiquitination and degradation (32).
In summary, we found that in addition to its classical role a mediator of prostaglandin signaling, the EP1 receptor plays an important role in facilitating COX-2 degradation. The identity of the E3 ligase that may mediate this effect and whether it interacts with the receptor directly or through scaffold proteins remains to be determined. Nonetheless, these findings point to the existence of a previously unrecognized negative feedback regulation of COX-2, which may help moderate its function and contributed to the self-resolution of inflammatory and tumorigenic processes.
Supplementary Material
Acknowledgments
We thank Dr. Sagie Schif-Zuck for assistance in the flow cytometry analyses and Dr. Niva Shraga-Heled for help with siRNA experiments.

This article contains supplemental Fig. 1.
- PGE2
- prostaglandin E2
- COX-2
- cyclooxygenase-2
- EP1
- prostaglandin E1 receptor
- AA
- arachidonic acid
- ERAD
- endoplasmic reticulum-associated degradation
- eEF2
- eukaryotic elongation factor-2
- PLA2
- phospholipase A2
- NHDF
- normal human dermal fibroblast.
REFERENCES
- 1. Breyer R. M., Bagdassarian C. K., Myers S. A., Breyer M. D. (2001) Prostanoid receptors. Subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41, 661–690 [DOI] [PubMed] [Google Scholar]
- 2. Bhattacharya M., Peri K. G., Almazan G., Ribeiro-da-Silva A., Shichi H., Durocher Y., Abramovitz M., Hou X., Varma D. R., Chemtob S. (1998) Nuclear localization of prostaglandin E2 receptors. Proc. Natl. Acad. Sci. U.S.A. 95, 15792–15797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spencer A. G., Woods J. W., Arakawa T., Singer I. I., Smith W. L. (1998) Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J. Biol. Chem. 273, 9886–9893 [DOI] [PubMed] [Google Scholar]
- 4. Harker L. A. (1986) Clinical trials evaluating platelet-modifying drugs in patients with atherosclerotic cardiovascular disease and thrombosis. Circulation 73, 206–223 [DOI] [PubMed] [Google Scholar]
- 5. Mbonye U. R., Song I. (2009) Posttranscriptional and posttranslational determinants of cyclooxygenase expression. BMB Rep 42, 552–560 [DOI] [PubMed] [Google Scholar]
- 6. Mbonye U. R., Wada M., Rieke C. J., Tang H. Y., Dewitt D. L., Smith W. L. (2006) The 19-amino acid cassette of cyclooxygenase-2 mediates entry of the protein into the endoplasmic reticulum-associated degradation system. J. Biol. Chem. 281, 35770–35778 [DOI] [PubMed] [Google Scholar]
- 7. Mbonye U. R., Yuan C., Harris C. E., Sidhu R. S., Song I., Arakawa T., Smith W. L. (2008) Two distinct pathways for cyclooxygenase-2 protein degradation. J. Biol. Chem. 283, 8611–8623 [DOI] [PubMed] [Google Scholar]
- 8. Neuss H., Huang X., Hetfeld B. K., Deva R., Henklein P., Nigam S., Mall J. W., Schwenk W., Dubiel W. (2007) The ubiquitin- and proteasome-dependent degradation of COX-2 is regulated by the COP9 signalosome and differentially influenced by coxibs. J. Mol. Med. 85, 961–970 [DOI] [PubMed] [Google Scholar]
- 9. Chen S. F., Liou J. Y., Huang T. Y., Lin Y. S., Yeh A. L., Tam K., Tsai T. H., Wu K. K., Shyue S. K. (2010) Caveolin-1 facilitates cyclooxygenase-2 protein degradation. J. Cell Biochem. 109, 356–362 [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock M., Danon A., Rubin M., Rimon G. (2001) Prostaglandin H synthase-2 inhibitors interfere with prostaglandin H synthase-1 inhibition by nonsteroidal anti-inflammatory drugs. Eur. J. Pharmacol. 412, 101–108 [DOI] [PubMed] [Google Scholar]
- 11. DiPiro J. T. S. W., Wade W. E., Blouin R. A., Pruemer J. A. (2010) Concepts in Clinical Pharmacokinetics, pp. 19–58, American Society of Health Systems Pharmacists, Bethesda, MD [Google Scholar]
- 12. Rowlinson S. W., Crews B. C., Lanzo C. A., Marnett L. J. (1999) The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2). A putative L-shaped binding conformation utilizing the top channel region. J. Biol. Chem. 274, 23305–23310 [DOI] [PubMed] [Google Scholar]
- 13. Neaud V., Duplantier J. G., Mazzocco C., Kisiel W., Rosenbaum J. (2004) Thrombin up-regulates tissue factor pathway inhibitor-2 synthesis through a cyclooxygenase-2-dependent, epidermal growth factor receptor-independent mechanism. J. Biol. Chem. 279, 5200–5206 [DOI] [PubMed] [Google Scholar]
- 14. Rösch S., Ramer R., Brune K., Hinz B. (2005) Prostaglandin E2 induces cyclooxygenase-2 expression in human non-pigmented ciliary epithelial cells through activation of p38 and p42/44 mitogen-activated protein kinases. Biochem. Biophys. Res. Commun. 338, 1171–1178 [DOI] [PubMed] [Google Scholar]
- 15. Syeda F., Grosjean J., Houliston R. A., Keogh R. J., Carter T. D., Paleolog E., Wheeler-Jones C. P. (2006) Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-κB pathways. J. Biol. Chem. 281, 11792–11804 [DOI] [PubMed] [Google Scholar]
- 16. Wen X., Chao C., Ives K., Hellmich M. R. (2011) Regulation of bombesin-stimulated cyclooxygenase-2 expression in prostate cancer cells. BMC Mol. Biol. 12, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hinz B., Brune K., Pahl A. (2000) Prostaglandin E2 up-regulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 272, 744–748 [DOI] [PubMed] [Google Scholar]
- 18. Sokolova E., Grishina Z., Bühling F., Welte T., Reiser G. (2005) Protease-activated receptor-1 in human lung fibroblasts mediates a negative feedback down-regulation via prostaglandin E2. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L793–L802 [DOI] [PubMed] [Google Scholar]
- 19. Cheng H. F., Wang J. L., Zhang M. Z., Miyazaki Y., Ichikawa I., McKanna J. A., Harris R. C. (1999) Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J. Clin. Invest. 103, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murakami M., Shimbara S., Kambe T., Kuwata H., Winstead M. V., Tischfield J. A., Kudo I. (1998) The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 273, 14411–14423 [DOI] [PubMed] [Google Scholar]
- 21. Hochstrasser M. (2009) Origin and function of ubiquitin-like proteins. Nature 458, 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Figueiredo-Pereira M. E., Li Z., Jansen M., Rockwell P. (2002) N-Acetylcysteine and celecoxib lessen cadmium cytotoxicity, which is associated with cyclooxygenase-2 up-regulation in mouse neuronal cells. J. Biol. Chem. 277, 25283–25289 [DOI] [PubMed] [Google Scholar]
- 23. Xie L., Xiao K., Whalen E. J., Forrester M. T., Freeman R. S., Fong G., Gygi S. P., Lefkowitz R. J., Stamler J. S. (2009) Oxygen-regulated β2-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL. Sci. Signal. 2, ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shenoy S. K., McDonald P. H., Kohout T. A., Lefkowitz R. J. (2001) Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science 294, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 25. Wang P., Gao H., Ni Y., Wang B., Wu Y., Ji L., Qin L., Ma L., Pei G. (2003) β-Arrestin 2 functions as a G protein-coupled receptor-activated regulator of oncoprotein Mdm2. J. Biol. Chem. 278, 6363–6370 [DOI] [PubMed] [Google Scholar]
- 26. Bhandari D., Trejo J., Benovic J. L., Marchese A. (2007) Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J. Biol. Chem. 282, 36971–36979 [DOI] [PubMed] [Google Scholar]
- 27. Chaturvedi K., Bandari P., Chinen N., Howells R. D. (2001) Proteasome involvement in agonist-induced down-regulation of μ and δ opioid receptors. J. Biol. Chem. 276, 12345–12355 [DOI] [PubMed] [Google Scholar]
- 28. Giordano F., Simoes S., Raposo G. (2011) The ocular albinism type 1 (OA1) GPCR is ubiquitinated, and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc. Natl. Acad. Sci. U.S.A. 108, 11906–11911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petaja-Repo U. E., Hogue M., Laperriere A., Bhalla S., Walker P., Bouvier M. (2001) Newly synthesized human δ opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J. Biol. Chem. 276, 4416–4423 [DOI] [PubMed] [Google Scholar]
- 30. Milojevic T., Reiterer V., Stefan E., Korkhov V. M., Dorostkar M. M., Ducza E., Ogris E., Boehm S., Freissmuth M., Nanoff C. (2006) The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol. Pharmacol. 69, 1083–1094 [DOI] [PubMed] [Google Scholar]
- 31. Salcedo A., Mayor F., Jr., Penela P. (2006) Mdm2 is involved in the ubiquitination and degradation of G protein-coupled receptor kinase 2. EMBO J. 25, 4752–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Décaillot F. M., Rozenfeld R., Gupta A., Devi L. A. (2008) Cell surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc. Natl. Acad. Sci. U.S.A. 105, 16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.