Background: Alum and stress agents induce inflammasomes. The hypothesis was examined whether HSP70, a hallmark of cell stress, is involved both in alum and stress-mediated adjuvanticity.
Results: Alum induced HSP70-dependent adjuvanticity as did the three stress agents.
Conclusion: Inducible HSP70 is involved both in stress and alum-mediated adjuvant functions.
Significance: Stress agents may provide an alternative strategy in developing novel adjuvants enhancing immunity.
Keywords: Caspase, Heat Shock Protein, Immunology, Stress, T Cell Biology
Abstract
The efficacy of a vaccine is generally dependent on an adjuvant, which enhances the immune functions and alum has been widely used in human immunization. Alum activates the intracellular stress sensors inflammasomes, but whether these are responsible for the adjuvanticity is controversial. The objectives of this investigation were to examine the hypothesis that alum-mediated adjuvanticity is a function of stress and conversely that stress agents will elicit adjuvanticity. The investigation was carried out in BALB/c mice by SC immunization with ovalbumin (OVA) mixed with alum. This elicited inflammasomes, with significant activation of caspase 1, production of IL-1β, and adjuvanticity, demonstrated by enhancing OVA-specific serum IgG antibodies, CD4+ T cells, and proliferation. The novel finding that alum induced HSP70 suggests that stress is involved in the mechanism of adjuvanticity. This was confirmed by inhibition studies with PES (phenylethynesulfonamide), which disrupts inducible HSP70 function, and inhibited both inflammasomes and the adjuvant function. Parallel studies were pursued with an oxidative agent (sodium arsenite), K-releasing agent (Gramicidin) and a metal ionophore (dithiocarbamate). All 3 stress agents induced HSP70, inflammasomes, and the adjuvant functions. Furthermore, up-regulation of membrane associated IL-15 on DC and CD40L on T cells in the animals treated with alum or the stress agents mediate the interactions between splenic CD11c DC and CD4+ or CD8+ T cells. The results suggest that the three stress agents elicit HSP70, a hallmark of stress, as well as inflammasomes and adjuvanticity, commensurate with those of alum, which may provide an alternative strategy in developing novel adjuvants.
Introduction
Adjuvants are agents that enhance immune functions and they have long been recognized as critical components of vaccines. Alum (aluminum hydroxide)2 has been the only clinically approved adjuvant and only recently has it been subjected to critical investigation. There is a consensus that alum activates the intracellular stress sensors inflammasomes, producing caspase-1 and IL-1β in DC and monocytes (1–6). Although some immunization studies in mice deficient in inflammasomes have demonstrated that they are critical for adjuvanticity, other studies failed to confirm this (5–7). It is of interest that the MF59 oil-in water emulsion (squalene), which has been approved in Europe for clinical use in vaccination and the classical Freund's complete adjuvant used in animals, both activate Nlrp3 inflammasomes, yet their adjuvanticity is inflammasome independent (6).
Inflammasomes are also elicited by stress agents such as thermal or oxidative stress (8), which induce cell-surface and intracellular HSP70. HSP70 expression is the hallmark of a stress response, which functions as an endogenous danger signal to the immune system (9, 10). A variety of biological, physical, chemical, and metabolic agents induce extracellular and intracellular stress affecting the immune system. Oxidative stress is induced by free radicals of ROS (reactive oxygen species), that leads to a state of redox disequilibrium in cellular glutathione and its disulfide ratio (11). It serves as a regulator of redox balance and functions as a sensor that triggers the stress response (12), which may elicit IL-1β, Il-8, TNF-α, and nitric oxide, and cause tissue damage (13). ROS stimulates the P13K (phosphatidylinositol 3-kinase) pathway, induce downstream ERK 1/2 activation (14), and up-regulates CD40, CD86, and other maturation markers of DC (15).
Homeostatic DC-CD4+ T cell interactive memory circuit in human cells can be stimulated by heat or oxidative stress agents, which may elicit K+ efflux, Ca2+ influx, and ROS in DC, leading to NFκB and maIL-15 expression (8, 16). Membrane-associated (ma)IL-15 ligates IL-15R complex on CD4+ T cells and induces CD40L expression, T-cell proliferation, and IFN-γ production (8). Intracellular stress sensors activate inflammasomes and the production of IL-1β (17). Metabolic stress is also associated with NLRP3 inflammasomes in type 2 diabetes (18) but can be independent of inflammasomes (19). Intracellular sensors involve NLR (nucleotide-binding oligomerization domain-like receptors), which sense endogenous danger signals (20).
These observations led to the hypothesis that Alum may function as a stress agent, which induces HSP70. We examined this paradigm in vivo and tested the requirement of inducible HSP70 for expression of inflammasome and adjuvanticity by inhibition with PES (phenylethynesulfonamide), specific for inducible HSP70 (21). This in turn led us to investigate in vivo 3 diverse stress agents to ascertain if they function as adjuvants and express the immune functions demonstrated with alum. We selected an oxidative agent (sodium arsenite), K releasing (Gramicidin), and a heavy metal ionophore (dithiocarbamate) for their potential adjuvanticity in comparison with alum. These differ in their properties, they act as stress agents that reactivate quiescent HSV-1 viral genomes (22) and they have been used in clinical practice. Gramicidine is an antibiotic, which functions as an ionophore, penetrating cell membranes, causing K+ efflux (23) and is effective against Gram-positive bacteria, fungi, protozoa, and viruses (24). It has been used clinically as an ophthalmic antimicrobial (Neoporin) and for genital ulcers (25). Dithiocarbamate is a fungicide, widely used in agriculture against a broad-spectrum of fungal plant diseases (26). It is a metal ionophore, which interferes in vitro with vesicular transport of glutamate (27). Sodium arsenite is an oxidative stress agent (28, 29), and although it is a toxin that inhibits many enzymes it is commonly used in the treatment of acute promyelocytic leukemia (30).
The investigation was carried out in vivo in 4 groups of BALB/c mice by SC immunization with ovalbumin mixed with each of the 3 stress agents and alum. Two additional control groups consisted of untreated and OVA-treated mice. The results suggest remarkable consistency in most of the stress responses, inducing HSP70 and maIL-15 in DC and CD40L with CD44 memory in CD4+ T cells. The stress agents and alum activate caspase-1, production of IL-1β, and function as adjuvants by enhancing OVA-specific IgG antibodies, generation of OVA-specific CD4+ T cells and proliferation. Altogether, the 3 stress agents elicit inflammasomes and adjuvanticity commensurate with that of alum.
EXPERIMENTAL PROCEDURES
Reagents
Goat anti-mouse IL-15 antibodies were obtained from R&D (Abingdon, UK), anti-HSP70 antibodies from Stressgen (US) and antibodies to mouse CD11c, CD4, CD8, CD44, CD40L and CD62L were purchased from BD Biosciences (Oxford, UK). Sodium arsenite, dithiocarbamate, and Gramicidin were obtained from Sigma-Aldwich. Alum-Gel-S which contains 2% AL(OH)3 was obtained from Serva (Serva Electrophoresis GmbH). 2-phenylethynesulfonamide (PES) was obtained from Calbiochem (Merck, UK). APC-conjugated OVA-tetramer I-A(g7) (141–160 CARELINSWVESQTNGIIRN) and a negative human CLIP-tetramer I-A (g7) was kindly provided by NIH Tetramer Core Facility at Emory University Atlanta, GA.
Animals and Immunization
8-week-old BALB/c mice were divided into 7 groups of 6–10 mice per group. Except for group 1 which was unimmunized all the other groups were injected s.c. with 100 μl saline containing 10 μg OVA as in group 2 and with added 5 μg of arsenite in group 3, 10 μg of Gramicidin in group 4, 20 μg of dithiocarbamate in group 5, and 10% alum gel-S in group 6. The optimum concentration of each stress agent was determined by prior assay of different concentrations of these agents. An additional group 7 was immunized with alum and 10 μg of PES per mouse. Immunization was carried out s.c at the base of tail 3 times at 2-week intervals. One week after final injection, sera were collected. Mononuclear cells from the spleens were isolated and counted, and the viability of the cells was determined by the trypan blue exclusion.
Assay of HSP70 and maIL-15 in Murine CD11c+ and CD40L in Splenic T Cells
HSP70 expression in murine splenic CD11chigh cells are predominatly DC (>90%) (31). These cells were identified with PE-labeled anti-CD11c mAb. The cells (3 × 105) were incubated with 10 μl (10 μg/ml) of anti-HSP70 mAb for 30 min, followed by FITC labeled secondary antibody. Intracellular HSP70 staining was carried out following treatment of cells with fixation and permeabilization buffer (eBiosciences, London). maIL-15 was assayed by incubating splenic cells with 5 μl (10 μg/ml) of APC-conjugated anti-mouse IL-15 mAb. To detect CD40L expression 3 × 105 splenic cells were incubated with 4 μl of PE-conjugated anti-murine CD40L and the isotype control antibody (BD Biosciences) for 4 h at 37 °C. After washing, cells were labeled with CD4 or CD8 mAb. IL-15 and CD40L were analyzed within the gated CD11c+ and CD4+, CD8+ cells, respectively. The cells were analyzed by the BD FACSCanto II flow cytometer, using Diva software. The profiles were presented using the Winmdi software.
Assays for Murine Caspase-1 and IL-1β
Caspase-1 activation in mouse splenic cells was identified by using FAM-FLICA caspase-1 kit (ABD serotec). Murine splenic cells were incubated with 20 μl of 1:300 diluted FAM-YVAD-FMK for 1 h in 96-well round bottom plates at 37 °C. After washing, the cells were treated with PE-labeled anti-murine CD11c mAb and analyzed by flow cytometry. For IL-1β production mouse splenic cells (100 μl of 3 × 106 cells) were plated onto 96-well plates, and 10 ng/ml of LPS was added. After 18 h of incubation, the supernatants were collected, and IL-1β was assayed using mouse IL-1β ELISA set (BD OptEIATM)
Serum IgG Antibody Assay
Murine serum IgG antibodies to OVA were assayed by ELISA. Briefly, plates were coated with a predetermined optimal concentration of OVA (1 μg/ml) and incubated with double dilution of serum (starting dilution of 1:100). Bound antibody was detected by incubation with rabbit IgG anti-mouse IgG (2 μg/ml; Sigma-Aldrich) antibodies, followed by affinity-purified goat anti-rabbit IgG-alkaline phosphatase conjugate (Sigma-Aldwich). OD values were determined by an ELISA reader.
Statistical Analysis
Antibody production was expressed as the total OD of the serially double diluted sera (up to 1:64,000) by calculating the titration area-under curve (32). The statistical analyses were determined at the planning stage of the design of the investigations. The significance between groups was analyzed by ANOVA, followed by comparison with selected groups, using the GraphPad Prism 5 Software.
RESULTS
Cell Surface and Intracellular HSP70 Induced by Alum
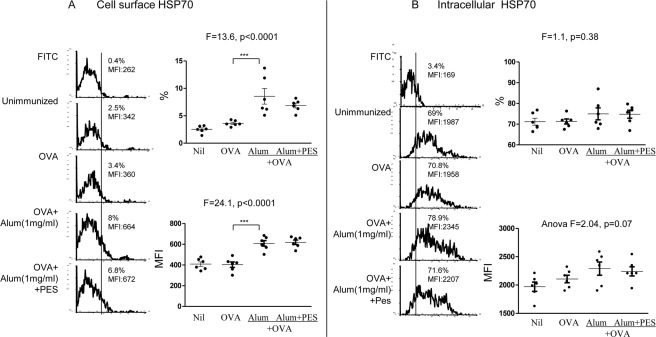
Heat and oxidative stress up-regulates in vitro cell-surface and intracellular HSP70 in human DC (8). In this investigation we examined HSP70 expression in vivo, following co-administration of OVA with alum in 4 groups of 6–10 BALB/c mice per group, with an untreated and OVA-treated control group. Flow cytometry examination of splenic CD11chigh cells, which are predominantly DC, excluding the CD11clow plasmablasts, but not monoctyes (<10%), showed significant increase in the cell-surface HSP70, both in the proportion (p < 0.0001) and MFI (p < 0.0001) of the 4 groups, as well as separately the alum-treated animals (p < 0.01; Fig. 1A). With the intracellular HSP70, however the proportion of cells showed no change, and the MFI showed a near significant increase (p = 0.07; Fig. 1B). These findings demonstrate that alum up-regulates HSP70 in splenic CD11c+ cells, suggesting a stress response.
FIGURE 1.
In vivo effect of alum on stimulation of cell surface and intracellular HSP70 in CD11c+ splenic DC in 4 groups of mice. In vivo effect of alum on the expression of (A) cell surface and (B) intracellular HSP70 in mouse CD11c+ splenic cells, following subcutaneous immunization with 20 μg/ml OVA and alum, and the effect of 10 μg of PES, with representative illustrations. *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
Because inducible HSP70 has not been reported to be involved in the mechanism of alum-induced functions, we used PES (phenylethynesulfonamide), which interacts with the inducible HSP70 peptide-binding domain and leads to disruption of HSP70 co-chaperones and substrate proteins (21). To this end we co-administered PES with alum in a further group of mice and compared the results with those treated with alum alone. As expected PES had no significant effect on HSP70 expression because it affects only the function of HSP70 (Fig. 1).
MaIL-15 on DC and CD40L Expression in CD4+ and CD8+ T Cells
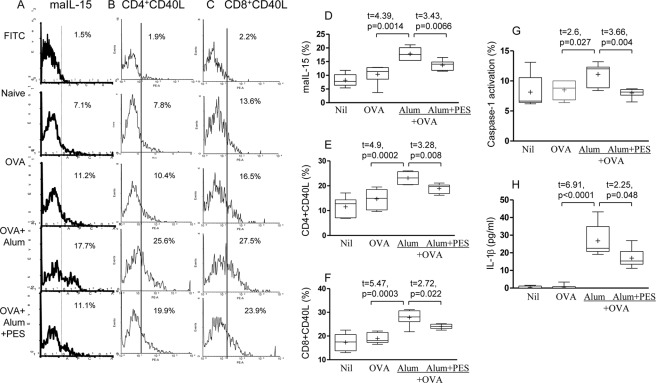
Stress may upregulate maIL-15 in vitro in human DC (8) and we have evaluated murine splenic CD11c+ DC following immunization with OVA and alum. OVA alone had no significant effect on maIL-15, from 8.2 ± 0.9% to 10.4 ± 1.4% but with alum maIL-15 was up-regulated to 17.8 ± 0.9% (p = 0.001; Fig. 2, A and D). PES significantly inhibited maIL-15 from 17.8 ± 0.9% with alum + OVA to 13.7 ± 0.7% with added PES (p < 0.01, Fig. 2D).
FIGURE 2.
Alum induced up-regulation of mIL-15 in DC and CD40L in CD4+ T cells, activation of caspase-1, and production of IL-1β is dependent on HSP70. In vivo effect of alum and the effect of PES on the expression of ma IL-15 in mouse CD11c+ splenic cells (A, D), CD40L in CD4+ T cells (B, E), and CD8+ T cells (C, F), following immunization with OVA and alum. Also, the effect on caspase activation (G) and IL-1β production (H) are presented.
Expression of CD40L was evaluated in CD4+ T cells as maIL-15 of DC interacts with IL-15 receptor complex on CD4+ T cells and stimulates the CD40L activation marker (8). Indeed, CD40L was significantly up-regulated in mice immunized with alum (23.1 ± 1.0), compared with the OVA-immunized mice (14.7 ± 1.2; Fig. 2, B and E). CD40L in CD4+ T cells was significantly inhibited with PES from 23.1 ± 1.0% to 18.9 ± 0.8% (p < 0.01, Fig. 2E and illustration, Fig. 2B). Similarly, CD40L expression was increased significantly in CD8+ T cells by treatment with alum (p < 0.001; Fig. 2, C and F) and inhibited by PES (p = 0.022). Thus, alum significantly up-regulated maIL-15 in CD11c+ DC and CD40L in CD4+ and CD8+ T cells, which may re-engage CD40 on DC and B cells and stimulate their functions. Both maIL-15 and CD40L expression were at least partly dependent on HSP70, as demonstrated by the significant inhibition with PES.
Activation of Inflammasomes Demonstrated by Caspase-1 and IL-1β
Caspase-1 is an integral part of the multiprotein NLRP3 inflammasome complex and was activated by alum (p < 0.05; Fig. 2G). Production of IL-1β was also significantly up-regulated (p < 0.0001; Fig. 2H). PES significantly decreased caspase-1 activity from 11.1 ± 0.8% to 8.0 ± 0.3% (p = 0.004; Fig. 2G) and IL-1β expression (from 26.8 ± 3.7 pg/ml to 17.0 ± 2.2 pg/ml (p < 0.05; Fig. 2H). Thus, alum activates caspase-1, which converts pro-IL-1β to the active form involved in stimulating adaptive immune responses. Both functions were partly inhibited with PES, consistent with HSP70 involvement in inflammasomes.
Alum Induces Adjuvanticity
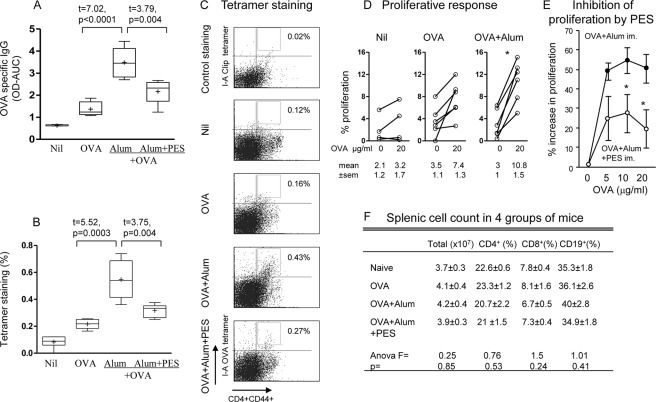
Adjuvant function of alum has been amply demonstrated but here we use it as a baseline to compare with the stress agents. OVA administered with alum elicited a very significant increase in serum IgG OVA specific antibody production from 1.37 ± 0.13 in the OVA immunized to 3.49 ± 0.27 (p < 0.0001) in the OVA + alum immunized mice, assayed 1 week after the 3rd and last immunization (Fig. 3A). IgG antibodies elicited with OVA and alum were significantly inhibited from 3.49 ± 0.27 with alum alone to 2.16 ± 0.2 (p < 0.01) with PES and alum immunized mice (Fig. 3A). Surprisingly, analysis of the effect of PES on the 3 IgG subtypes showed that only IgG2a was significantly inhibited, which is associated with Th1 response (data not presented). The antibodies are expressed as total absorbance (OD) of the serially diluted sera, calculated by the area under the curve.
FIGURE 3.
HSP70-dependent adjuvant function of alum eliciting OVA-specific antibody production and CD4+ T cells responses. Serum IgG antibodies to OVA (A), induction of OVA-specific CD4+ T cells (B) and representative flow cytometry (C), and CD4+ T cell proliferative responses after in vitro restimulation with 20 μg/ml OVA (D), in mice following immunization with OVA and alum without and with PES (E). The viability of CD4+, CD8+, and CD19+ cells are presented in F; *, p < 0.05.
CD4+ T cell responses to OVA were determined first by the OVA-I-A tetramer based assay of CD4+ T cell, which showed significant increase in OVA-specific CD4+ T cells in the alum immunized groups (0.55 ± 0.06%, Fig. 3B), compared with OVA alone immunized mice (0.22 ± 0.02%, p < 0.001). The corresponding flow cytometry illustrations are presented (Fig. 3C). Proliferative responses of OVA-specific CD4+ T cells were assayed after restimulation in vitro with 20 μg OVA. Significant increase in proliferation was found in OVA-immunized mice treated with alum (from 3.0 ± 1.0 to 10.8 ± 1.5%, p < 0.05), but not with OVA alone (Fig. 3D).
Inhibition with PES again demonstrated significant decrease in OVA-specific CD4+ T cells (p < 0.01; Fig. 3, B and C), as well as in the proliferation of splenic cells (p < 0.05, Fig. 3E). These inhibition studies argue in favor of the adjuvanticity of alum in murine OVA-specific B and T cell responses being at least partly dependent on inducible HSP70. It is important to note that these effects were observed in vivo, in the absence of any changes in the proportion of viable CD4+ and CD8+ T cells, CD19+ B cells and total splenic cell population after administration of PES (Fig. 3F), ruling out PES induced cytotoxicity. Altogether, the mechanism of CD11c+ DC-CD4+ T cell interaction, manifested in the serial expression of maIL-15, CD40L respectively, activation of caspase-1, IL-1β, inflammasome, and adjuvant functions appears to be partly dependent on alum stimulated inducible HSP70.
Cell Surface and Intracellular HSP70 Induced by the Stress Agents
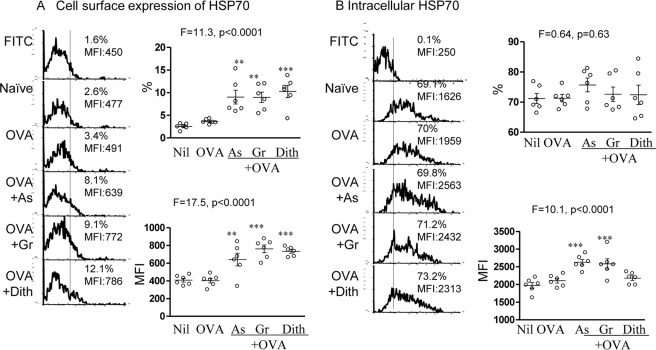
We examined HSP70 expression in vivo, following co-administration of OVA with sodium arsenite, Gramicidin, or dithiocarbamate in 3 groups of 6–10 BALB/c mice per group, with an untreated and OVA-treated control groups as described above for alum. Flow cytometry examination of splenic CD11chigh cells, showed significant difference in the cell-surface HSP70, both in the proportion (ANOVA, p < 0.0001) and MFI (ANOVA, p < 0.0001) among all groups of mice. Each of the stress agent treated group of animals showed significant up-regulation of cell-surface HSP70 (p < 0.01 or <0.001; Fig. 4A). With the intracellular HSP70, however only MFI increased (ANOVA, p < 0.0001) and all but dithiocarbamate were significantly up-regulated (p < 0.001; Fig. 4B). These findings demonstrate that cell-surface and intracellular HSP70 in the splenic CD11c+ cells are up-regulated by the 3 diverse stress agents consistent with that of alum.
FIGURE 4.
In vivo effect of stress inducing agents on the expression of (A) cell surface and (B) intracellular HSP70 in mouse CD11c+ splenic cells, following subcutaneous immunization with 20 μg/ml OVA and sodium arsenite (As), Gramicidin (Gr), or dithiocarbamate (Dith) with representative illustrations.
Membrane-associated (ma) IL-15 on DC and CD40L Expression in CD4+ and CD8+ T Cells
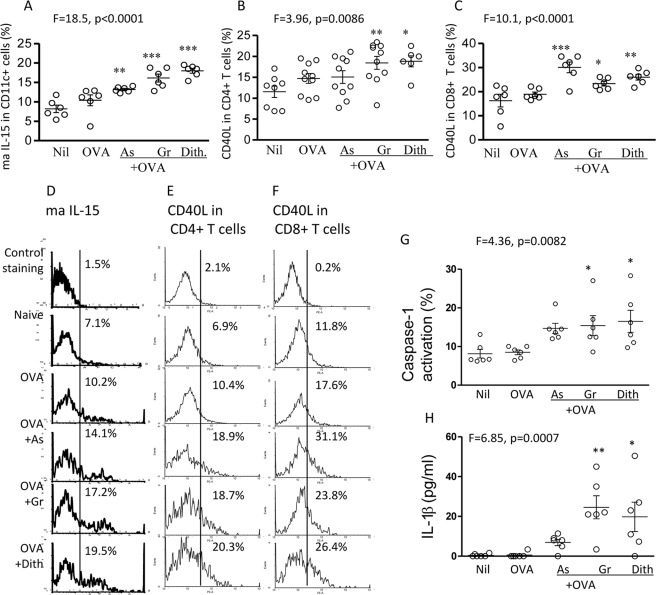
All 3 stress agents significantly up-regulated maIL-15 (ANOVA, p < 0.0001). OVA alone had no significant effect, (from 8.2 ± 0.9% to 10.4 ± 1.4%) but a significant increase was found after immunization with sodium arsenite 13.2 ± 0.3% (p < 0.01), Gramicidin 16.1 ± 1% (p < 0.01), or dithiocarbamate 18 ± 0.6% (p < 0.001; Fig. 5A), as was shown above with alum 17.8 ± 0.9%. The corresponding flow cytometry illustrations are also presented (Fig. 5D).
FIGURE 5.
In vivo effect of stress-inducing agents on the expression of ma IL-15 in mouse CD11c+ splenic cells (A), CD40L in CD4+ T cells (B), and in CD8+ T cells (C), following immunization with OVA and the 3 stress-inducing agents. The corresponding flow cytometry profiles are shown in D, E, and F. Caspase-1 activation (G) and IL-1β production (H) with OVA and the same stress agents are also presented. *, p < 0.05; **, p < 0.01; and ***, p < 0.001, compared with naïve mice.
Significant differences of CD40L expression in CD4+ T cells were found in the entire cohort of mice (ANOVA, p < 0.01). CD40L in CD4+ T cells was up-regulated in mice immunized with Gramicidin (18.4 ± 1.5%, p < 0.01), dithiocarbamate (18.8 ± 1.3, p < 0.05) and sodium arsenite (15.1 ± 1.5%), but the latter failed to reach significant value as compared with the OVA-immunized mice (14.7 ± 1.2; Fig. 5, B and E). CD40L expression was also increased significantly in CD8+ T cells (ANOVA, p < 0.0001), with up-regulation by each stress agent (p < 0.05; p < 0.001; Fig. 5, C and F). Thus, all stress agents significantly up-regulated CD40L of CD4+ and CD8+ T cells (with one exception), as was found with alum.
Activation of Inflammasomes Demonstrated by Caspase-1 and IL-1β
Activation of caspase 1 and production of IL-1β were demonstrated with each test agent (p < 0.05–0.01, Fig. 5, G and H), except sodium arsenite, though IL-1β was up-regulated from 0.4 ± 0.3pg/ml to 6.9 ± 1.6 pg/ml. Thus, both alum and the stress agents activate caspase-1, which converts pro-IL-1β to the active form involved in stimulating adaptive immune responses.
Stress-induced Adjuvanticity
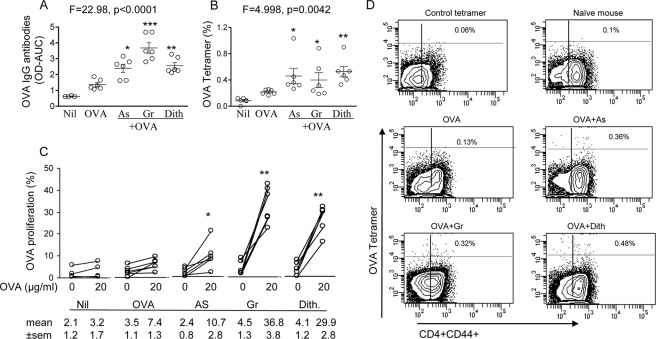
Adjuvant function of stress-inducing agents has received limited attention in the past. Here we have examined the effect of administering the stress agents with OVA on antibody and CD4+ T cell responses, as was done with alum. Serum IgG OVA specific antibody production assayed 1 week after the 3rd immunization showed significant difference in the 5 groups of mice (ANOVA, p < 0.0001; Fig. 6A). Significant increase in OVA IgG antibodies were recorded in mice immunized with OVA and sodium arsenite (2.39 ± 0.26, p < 0.05), Gramicidin (3.68 ± 0.33, p < 0.001) and dithiocarbamate (2.55 ± 0.2, p < 0.001), compared with OVA-immunized controls (1.37 ± 0.13, Fig. 6A). Mice immunized with OVA alone showed no significant increase. The antibodies are expressed as total absorbance of the serially diluted sera, calculated by the area under the curve.
FIGURE 6.
Serum IgG antibodies to OVA (A), induction of OVA-specific CD4+ T cells (B), representative flow cytometry (D), and CD4+ T cell proliferative responses (C), in mice following immunization with OVA and the stress-inducing agents; the responses before and after in vitro restimulation with 20 μg/ml OVA are presented. In A and C Nil group (n = 4). *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
The OVA-I-A tetramer-based assay of CD4+ T cell responses to OVA, showed significant difference in OVA specific CD4+ T cells in the 5 groups (ANOVA p < 0.01). Significant increases were also found separately with sodium arsenite (0.46 ± 0.12%, p < 0.05), Gramicidin (0.4 ± 0.11%, p < 0.05) and dithiocarbamate (0.53 ± 0.08%, p < 0.01) immunized, compared with OVA-immunized mice (0.22 + 0.01%, Fig. 6B) and the corresponding flow cytometry illustrations (Fig. 6D). Proliferative responses of OVA specific CD4+ T cells were then assayed after restimulation in vitro with 20 μg/ml OVA (Fig. 6C). Significant increases in proliferation were found in OVA immunized mice treated with Na arsenite (from 2.4 ± 0.8 to 10.7 ± 2.8%, p < 0.05), Gramicidin (from 4.5 ± 1.3% to 36.8 ± 3.8%, p < 0.01), and dithiocarbamate (from 4.1 ± 1.2% to 29.9 ± 2.8%, p < 0.01) (Fig. 6C). Thus, adjuvanticity of 3 separate stress agents were comparable with those recorded above for alum.
DISCUSSION
Alum has been extensively investigated recently and there is a consensus that it induces inflammasomes (1–4, 6, 33). However, the critical role of inflammasomes in the adjuvanticity of alum has been disputed (5, 6, 33), as mice deficient in inflammasomes can manifest adjuvanticity when treated by alum. This view has been strengthened by recent studies showing that MF59 adjuvant and Freund's complete adjuvant are inflammasome independent (6). The disputed involvement of inflammasomes in the adjuvanticity of alum was examined in the present work by its potential dependence on stimulation of inducible HSP70. Indeed, PES a functional inhibitor of HSP70 (21), when co-administered with alum to BALB/c mice significantly inhibited maIL-15 in DC, CD40L in CD4+ T cells, and the inflammasome-mediated caspase-1 conversion to functional IL-1β. Importantly, OVA-specific IgG antibodies, CD4+ tetramer binding and proliferation were also significantly inhibited by PES. These data are consistent with alum induced adjuvanticity being at least partly dependent on inducible HSP70, which has not been reported previously.
Because up-regulation of HSP70 is the hallmark of a stress response (9, 10), these data suggest that alum acts as a stress agent. Although it does not resolve the question whether inflammasomes are required for the adjuvant function, both in vitro and in vivo evidence points to the importance of inducible HSP70 in IL-1β expression stimulated by the stress agents and alum. This is supported by a previous report that inhibition of another HSP (HSP90) abrogated inflammasome formation (34). It is noteworthy that there is a great deal of evidence that exogenous HSP70 acts as an adjuvant (35–38), which has been marred by criticism that the HSP70 used may have been contaminated with LPS, flaggelin, or nucleotides (39). This critique cannot be applied to the present work as exogenous HSP70 has not been used, but endogenous, inducible HSP70 was up-regulated by alum.
Previous in vitro investigations of the homeostatic stress-activated memory circuit (8) has now been advanced to in vivo studies. In addition to an oxidative agent (sodium arsenite), we have now studied in BALB/c mice OVA-stimulated stress agents, functioning by inducing K+ efflux (Gramicidin) and a metal ionophore (dithiocarbamate). The immunological and adjuvant properties of these stress agents were compared with those elicited by alum. We have confirmed that cell surface HSP70 were up-regulated by all three stress agents as well as by alum and intracellular by all except dithiocarbamate. In vitro studies using human DC (8), HSP70 activates transcription by NFkB of maIL-15, which was also significantly up-regulated in splenic CD11c+ cells. This in turn activated CD40L expression in CD4+ and CD8+ T cells by the stress agents (except sodium arsenite in CD4+ T cells).
Endogenous stress stimulates the development of inflammasomes (reviewed in (17)). Potassium efflux (danger signal) is essential for Nlrp3 activation (40), caspase-1, IL-1β, and IL-18 production (17). SC immunization in BALB/c mice reported in the present work elicited caspase-1 stimulated with all 3 stress agents and IL-1β with all except sodium arsenite. The discrepancy with arsenite might be accounted for by a failure of proteolytic processing of IL-1β by the caspase as reported by others (17). The effects of 3 stress inducing agents in vivo were remarkably similar to that observed with alum, which suggest that the stress response may be involved in adjuvant function. This paradigm was confirmed by demonstrating that stress can enhance antigen specific T and B cell immune responses. Immunization of mice with the three stress-inducing agents elicited OVA-specific CD4+ T cells responses and antibody production. These findings are consistent with stress exerting adjuvanticity, as has also been reported recently with an antibiotic (Oligomycin), which elicits metabolic stress and adjuvanticity (19).
The investigation raises the question whether the stress agents exert only enhancing (positive) signals or also inhibitory (negative) signals elicited by GCN2, a stress responding protein kinase, which may interfere with the T cell cycle (41). Indeed, indoleamine 2, 3-dioxygenase (IDO), which is expressed in some DC and macrophages and play an important role in promoting the formation of T regulatory cells. IDO induces tryptophan depletion and cell stress, which activates the general control nonrepressed 2 (GCN2) protein kinase-dependent pathway and has direct regulatory effect on cell survival and function of immune responses (41–43). Preliminary studies suggest that human monocyte derived DC treated with the stress inducing agents used in this study inhibit Treg formation. Further work will be pursued to find out if the stress inducing agents can also inhibit IDO activity in DC and GCN2 protein kinase pathway in T cells and thereby enhance immune responses.
The proposed mechanism of stress activated DC based on the present data suggests that they up-regulate inducible HSP70, activating the NF-κB signaling pathway (8, 44) that leads to maIL-15 expression. This binds the IL-15 receptor complex on CD4+ T cells activating JAK3, STAT5 phosphorylation to induce CD40L expression in CD4+ memory T cells and IFNγ production, as we have demonstrated previously (8). CD40L also activates CD40 molecules on B cells to boost adaptive immune responses by phosphorylation of IκB kinase complex and nuclear translocation of NF-κB, which initiates class-switch recombination (CSR) by binding to κB site on IH promoters (45, 46). Stress-activated DC also stimulate intracellular stress sensors, which activate Nlrp3 inflammasomes and caspase 1 and converts the IL-1β precursor to its active form. This in turn binds IL-1β receptors on CD4+ T cells and elicits IFN-γ and IL-17 expression (17, 40). The dual stress-induced and inflammasome pathways may contribute to the adjuvanticity of these stress agents. However, Th1 responses can be elicited by TLR4-mediated HSP70-like protein activating DC as reported recently (47).
This work was supported by the European Union Network of Excellence, Europrise (LSHP-CT-2006-037611C) and ADITEC (280873).
- alum
- aluminium hydroxide
- DC
- dendritic cells
- HSP70
- 70kD heat shock protein
- L
- ligand
- ma
- membrane-associated
- PES
- phenylethynesulfonamide.
REFERENCES
- 1. Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., Flavell R. A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li H., Nookala S., Re F. (2007) Aluminium hydroxide adjuvants activate caspase-1 and induce IL-1β and IL-18 release. J. Immunol. 178, 5271–5276 [DOI] [PubMed] [Google Scholar]
- 3. Li H., Willingham S. B., Ting J. P., Re F. (2008) Cutting Edge: Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 181, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kool M. (2008) Cutting Edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 [DOI] [PubMed] [Google Scholar]
- 5. Franchi L., Núñez G. (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seubert A., Calabro S., Santini L., Galli B., Genovese A., Valentini S., Aprea S., Colaprico A., D'Oro U., Giuliani M. M., Pallaoro M., Pizza M., O'Hagan D. T., Wack A., Rappuoli R., De Gregorio E. (2011) Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc. Natl. Acad. Sci. U.S.A. 108, 11169–11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrack P., McKee A. S., Munks M. W. (2009) Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 9, 287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y., Seidl T., Whittall T., Babaahmady K., Lehner T. (2010) Stress-activated dendritic cells interact with CD4+ T cells to elicit homeostatic memory. Eur. J. Immunol. 40, 1628–1638 [DOI] [PubMed] [Google Scholar]
- 9. Lindquist S., Craig E. A. (1988) The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 [DOI] [PubMed] [Google Scholar]
- 10. Matzinger P. (2002) The danger model: a renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 11. Hirst J., King M. S., Pryde K. R. (2008) The production of reactive oxygen species by complex I. Biochem. Soc Trans. 36, 976–980 [DOI] [PubMed] [Google Scholar]
- 12. Li N., Hao M., Phalen R. F., Hinds W. C., Nel A. E. (2003) Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 109, 250–265 [DOI] [PubMed] [Google Scholar]
- 13. Greten F. R., Arkan M. C., Bollrath J., Hsu L. C., Goode J., Miething C., Göktuna S. I., Neuenhahn M., Fierer J., Paxian S., Van Rooijen N., Xu Y., O'Cain T., Jaffee B. B., Busch D. H., Duyster J., Schmid R. M., Eckmann L., Karin M. (2007) NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz C. M., Rinna A., Forman H. J., Ventura A. L., Persechini P. M., Ojcius D. M. (2007) ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rutault K., Alderman C., Chain B. M., Katz D. R. (1999) Reactive oxygen species activate human peripheral blood dendritic cells. Free Rad. Biol. Med. 26, 232–238 [DOI] [PubMed] [Google Scholar]
- 16. Petrilli V., Dostert C, Mayor A., Martinon F., Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Diff. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 17. Martinon F., Mayor A., Tschopp J. (2009) The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 [DOI] [PubMed] [Google Scholar]
- 18. Schroder K., Zhou R., Tschopp J. (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 [DOI] [PubMed] [Google Scholar]
- 19. Andris F., Denanglaire S., Baus E., Rongvaux A., Steuve J., Flavell R. A., Leo O. (2011) Metabolic stress boosts humoral responses in vivo independently of inflammasome and inflammatory reaction. J. Immunol. 186, 2245–2253 [DOI] [PubMed] [Google Scholar]
- 20. Mariathasan S., Monack D. M. (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7, 31–40 [DOI] [PubMed] [Google Scholar]
- 21. Leu J. I., Pimkina J., Frank A., Murphy M. E., George D. L. (2009) A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell 36, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Preston C. M., Nicholl M. J. (2008) Induction of cellular stress overcomes the requirement of herpes simplex virus type 1 for immediate-early protein ICP0 and reactivates expression from quiescent viral genomes. J. Virol. 82, 11775–11783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelkar D. A. (2007) The gramicidin ion channel: a model membrane protein. Biochim. Biophys. Acta 1768, 2011–2025 [DOI] [PubMed] [Google Scholar]
- 24. Dubos R. J., Hotchkiss R. D. (1941) The production of bactericidal substances by aerobic sporulating Bacilli. J. Exp. Med. 73, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bourinbaiar A. S. (1997) The effect of gramicidin, a topical contraceptive and antimicrobial agent with anti-HIV activity, against herpes simplex viruses type 1 and 2 in vitro. Arch. Virol. 142, 2225–2235 [DOI] [PubMed] [Google Scholar]
- 26. Tomlin C. (1994) The Pesticide Manual, 10th Ed. Crop Protection Publications, Farnham and Cambridge [Google Scholar]
- 27. Vaccari A., Saba P., Mocci I., Ruiu S. (1997) Dithiocarbamate pesticides affect glutamate transport in brain synaptic vesicles. J. Pharmacol. Exp. Therap. 288, 1–5 [PubMed] [Google Scholar]
- 28. Lee P. C., Ho I. C., Lee T. C. (2005) Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1 and interleukin-6 in vascular smooth muscle cells. Toxicol. Sci. 85, 541–550 [DOI] [PubMed] [Google Scholar]
- 29. Lemarie A., Bourdonnay E., Morzadec C., Fardel O., Vernhet L. (2008) Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway. J. Immunol. 180, 6010–6017 [DOI] [PubMed] [Google Scholar]
- 30. Soignet S. L., Maslak P., Wang Z-G, Jhanwar S., Calleja E., Dardashti L. J., Corso D., DeBlasio A., Gabrilove J., Scheinberg D. A., Pandolfi P. P., Warrell R. P. (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Eng. J. Med. 339, 1341–1348 [DOI] [PubMed] [Google Scholar]
- 31. Racine R., Chatterjee M., Winslow G. M. (2008) CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection. J. Immunol. 181, 1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gilbert P., Wang M., Wrin T., Petropoulos C., Gurwith M., Sinangil F., D'Souza P., Rodriguez-Chavez I. R., DeCamp A., Giganti M., Berman P. W., Self S. G., Montefiori D. C. (2010) Magnitude and breadth of a nonprotective neutralizing antibody response in an efficacy trial of a candidate HIV-1 gp120 vaccine. J. Infect. Dis. 202, 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McKee A. S., Munks M. W., MacLeod M. K., Fleenor C. J., Van Rooijen N., Kappler J. W., Marrack P. (2009) Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 8, 497–503 [DOI] [PubMed] [Google Scholar]
- 35. Lussow A. R., Barrios C., van Embden J., Van der Zee R., Verdini A. S., Pessi A., Louis J. A., Lambert P. H., Del Giudice G. (1991) Mycobacterial heat-shock proteins as carrier molecules. Eur. J. Immunol. 21, 2297–2302 [DOI] [PubMed] [Google Scholar]
- 36. Barrios C., Lussow A. R., Van Embden J., Van der Zee R., Rappuoli R., Costantino P., Louis J. A., Lambert P. H., Del Giudice G. (1992) Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guerin priming. Eur. J. Immunol. 22, 1365–1372 [DOI] [PubMed] [Google Scholar]
- 37. Ciupitu A. M., Petersson M., O'Donnell C. L., Williams K., Jindal S., Kiessling R., Welsh R. M. (1998) Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J. Exp. Med. 187, 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehner T., Bergmeier L. A., Wang Y., Tao L., Sing M., Spallek R., van der Zee R. (2000) Heat shock protein generates b-chemokines which function as innate adjuvants enhancing adaptive immunity. Eur. J. Immunol. 30, 594–603 [DOI] [PubMed] [Google Scholar]
- 39. Henderson B., Calderwood S. K., Coates A. R., Cohen I., van Eden W., Lehner T., Pockley A. G. (2010) Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones 15, 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munn D. H., Sharma M. D., Baban B., Harding H. P., Zhang Y., Ron D., Mellor A. L. (2005) GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 [DOI] [PubMed] [Google Scholar]
- 42. Manlapat A. K., Kahler D. J., Chandler P. R., Munn D. H., Mellor A. L. (2007) Cell-autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur. J. Immunol. 37, 1064–1071 [DOI] [PubMed] [Google Scholar]
- 43. Yan Y., Zhang G. X., Gran B., Fallarino F., Yu S., Li H., Cullimore M. L., Rostami A., Xu H. (2010) IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185, 5953–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghosh S., May M. J., Kopp E. B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 45. Karin M., Ben-Neriah Y. (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18, 621–663 [DOI] [PubMed] [Google Scholar]
- 46. Manis J. P., Tian M., Alt F. W. (2002) Mechanism and control of class-switch recombination. Trends Immunol. 23, 31–39 [DOI] [PubMed] [Google Scholar]
- 47. Fang H., Wu Y., Huang X., Wang W., Ang B., Cao X., Wan T. (2011) Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 286, 30393–30400 [DOI] [PMC free article] [PubMed] [Google Scholar]