Background: Activation of TRPC4/5 channels is mediated by GPCR activation.
Results: TRPC4/5 was activated by the Gαi/o-coupled receptor and the Gαi protein, which interacted directly with each other.
Conclusion: Gαi proteins play an essential role as novel activators of TRPC4/5.
Significance: Our findings provide new insights into the activation mechanism of inhibitory Gα proteins.
Keywords: Calcium, Calcium Channels, Cell Biology, Ion Channels, Membrane Proteins, Signal Transduction, TRP Channels, TRPC, TRPC4, TRPC5
Abstract
The ubiquitous transient receptor potential canonical (TRPC) channels function as non-selective, Ca2+-permeable channels and mediate numerous cellular functions. It is commonly assumed that TRPC channels are activated by stimulation of Gαq-PLC-coupled receptors. However, whether the Gαq-PLC pathway is the main regulator of TRPC4/5 channels and how other Gα proteins may regulate these channels are poorly understood. We previously reported that TRPC4/TRPC5 can be activated by Gαi. In the current work, we found that Gαi subunits, rather than Gαq, are the primary and direct activators of TRPC4 and TRPC5. We report a novel molecular mechanism in which TRPC4 is activated by several Gαi subunits, most prominently by Gαi2, and TRPC5 is activated primarily by Gαi3. Activation of Gαi by the muscarinic M2 receptors or expression of the constitutively active Gαi mutants equally and fully activates the channels. Moreover, both TRPC4 and TRPC5 are activated by direct interaction of their conserved C-terminal SESTD (SEC14-like and spectrin-type domains) with the Gαi subunits. Two amino acids (lysine 715 and arginine 716) of the TRPC4 C terminus were identified by structural modeling as mediating the interaction with Gαi2. These findings indicate an essential role of Gαi proteins as novel activators for TRPC4/5 and reveal the molecular mechanism by which G-proteins activate the channels.
Introduction
Transient receptor potential canonical (TRPC)5 channels are considered the molecular candidates for receptor-operated Ca2+-permeable cation channels. The G-protein-coupled receptor (GPCR)-Gαq-PLC is assumed to be the primary pathway for activation of all TRPC channels, even though the exact mechanism by which the channels are activated remains unknown (1). Several mediators have been proposed to mediate channel activation by stimulation of GPCR. Among them are SESTD1 (2), intracellular Ca2+ (3, 4), lipid metabolites (5, 6), PIP2 (7–9), calmodulin (10, 11), CaM kinase (12), MLCK (13–15), and channel exocytosis (16). In addition, TRPC4 and TRPC5 can be activated by thioredoxin (17) and NO (18).
The physiological role of these channels was established recently, demonstrating that TRPC4 and TRPC6 are the molecular candidates for the non-selective cation channels activated by muscarinic receptor stimulation (mICAT) in visceral smooth muscle cells. mICAT mediates the physiological action of acetylcholine in evoking smooth muscle contraction (19). Activation of muscarinic receptors causes the opening of non-selective cationic channels in smooth muscle cells of the gastrointestinal tract (20, 21). In these cells, PTX-sensitive G-proteins but not Gβγ were suggested to mediate channel activation (22, 23). At the level of M2 or M3 muscarinic receptors, Sakamoto et al. (24) showed three distinct signaling pathways that activate cationic channels in murine gut smooth muscle cells. The three pathways include the M2, M3, and M2/M3 pathways and were demonstrated using M2 KO, M3 KO, and M2/M3 double KO mice, respectively. In addition, the M2/M3 pathway but not the M2 or M3 pathways involves processes in which Ca2+ has a potentiating effect on channel activation, suggesting that the M3 pathway may facilitate the function of the M2/M3 pathway through inositol 1,4,5-trisphosphate-induced Ca2+ release (24). Similarly, activation of mICAT requires the simultaneous activation of both the M2 and M3 muscarinic receptors (20), further suggesting involvement of Gαi in channel activation.
Several studies have also suggested that PTX-sensitive G-proteins play an important role in the activation process of TRPC4 and TRPC5 by GPCR (6, 8, 25). In our previous study, we showed that Gαi2 activates TRPC4β and that PTX inhibits the activation of TRPC4β by stimulation of M2 muscarinic receptors (25). However, the specificity for Gαi subunits and how the PTX-sensitive G-proteins activate the channels are not yet known; it is assumed that the PTX-sensitive G-proteins activate the channels by an indirect mechanism that involves the generation of second messengers. The well known second messenger of PTX-sensitive G-proteins is cyclic AMP (cAMP), found downstream of adenylate cyclase. PTX-sensitive G-proteins inhibit adenylate cyclase, which in turn decreases cAMP concentration. In our recent study, we showed that cAMP inhibits TRPC4 and TRPC5 currents by activating PKA and phosphorylating TRPC4 and TRPC5 channels (26). Thus, it cannot be the mechanism by which Gαi activates the channels.
These findings prompted us to ask whether TRPC4 and TRPC5 are activated by other PTX-sensitive Gαi/o subunits and whether the activation is direct. We were also interested in identifying the TRPC4/5 domain that mediates the interaction with the channels and the activation by Gαi/o subunits, as well as the roles Gαq plays in modulating TRPC4/5. In the present study, we focused on the role of Gαi proteins in regulating TRPC4/5 and report that Gαi subunits specifically activate TRPC4 and TRPC5 by direct interaction with the channels. Moreover, the regulation is specific to Gαi subunits. TRPC4 is mainly activated by Gαi2, whereas TRPC5 is primarily activated by Gαi3. These findings explain how TRPC4 is activated to regulate GI motility. Strategies can now be developed to understand the functional consequences of activation of TRPC4/5 in the central and peripheral nervous systems.
EXPERIMENTAL PROCEDURES
Cell Culture and Transient Transfection, cDNA Clones
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA) were maintained according to the supplier's recommendations. For transient transfection, cells were seeded in 12-well plates. The following day, 0.5 μg/well of pcDNA3 vector containing the cDNA for mouse TRPC4β was mixed with 50–100 ng/well of pEGFP-N1 (Clontech) and transfected using the transfection reagent FuGENE 6 (Roche Molecular Biochemicals), as detailed in the manufacturer's protocol. Human TRPC5-EGFP cDNA and mouse TRPC4β-EGFP cDNA were also transfected in the same way. Coexpression of TRPC channels with G-proteins or receptors was achieved through a channel to G-protein transfection ratio of 1:1. After 30–40 h, the cells were trypsinized and transferred to a small recording chamber (RC-11, Warner Instruments) for whole-cell recording. HEK293 cells stably expressing mouse TRPC4β were established by G418 selection. The cells were cultured as for the transient transfection, except that the medium was supplemented with G418 (400 μg/ml). Human Gαi1Q204L, Gαi2Q205L, Gαi3Q204L, rat Gαi2Q205L, and human GαqQ209L were cloned into pcDNA3.1+. Human Gβ1, Gβ2, Gβ3, Gβ5, and bovine Gγ2 were cloned into pcDNA3.1+ (Invitrogen). Human GαoAQ205L, M2 receptor (the Missouri S&T cDNA Resource Center), and M3 receptor were cloned into pcDNA3.1+. Human Gβ1 was used to insert the Gβ1W99A and Gβ1I80A mutations using the QuikChange site-directed mutagenesis kit (Stratagene).
Western Blotting and Co-immunoprecipitation
Transfected cells were collected and lysed using 300 μl of binding buffer (50 mm HEPES, pH 7.4, 120 mm NaCl, 2 mm EDTA, 2 mm MgCl2, complete protease inhibitor mixture tablet, phosphatase inhibitor mixture tablet (Roche Applied Science), and 0.5% Triton X-100). The lysates were sonicated, and any insoluble material was removed by centrifugation at 13,300 × g for 10 min. For co-immunoprecipitation of TRPC4β-GFP and TRPC5-GFP with Gαi2 and Gαi3, anti-GFP antibody (1 μg, Invitrogen, A11122) was added to 100 μl cell extract and incubated for 12 h at 4 °C. Then, 50 μl of a 1:1 slurry of protein G-Sepharose 4B beads was added to the antibody-extract mix and incubated for 12 h at 4 °C. Beads were washed three times with binding buffer; proteins were released from the beads with 50 μl of 2× SDS-loading buffer and analyzed with 10% or 8% SDS-PAGE. Gαi2 and Gαi3 were co-precipitated with GFP antibody and probed by mouse monoclonal anti-Gαi2 antibody (2 μg, Santa Cruz Biotechnology, sc-13534) and mouse monoclonal anti-EE antibody for Gαi3 (2 μg, Covance, MMS-115P). The mouse monoclonal anti-Gαi2 antibody was used for a reciprocal co-immunoprecipitation with GFP antibody in a sequential experiment. Co-immunoprecipitation of TRPC4β-GFP with Gαq was achieved using the same procedures. Gαq was probed by mouse monoclonal anti-Gαq antibody (Santa Cruz Biotechnology, sc-136181).
Rat brain from day 15 was homogenized on ice using a Dounce homogenizer. The homogenate buffer had the same composition as the binding buffer. Homogenates were centrifuged at 13,000 rpm for 30 min at 4 °C. Supernatants were re-centrifuged at 13,000 rpm at 4 °C for 20 min. Supernatants were pre-cleared with protein G-Sepharose beads for 1 h at 4 °C and centrifuged at 2000 rpm for 2 min at 4 °C. Fifty microliters of a 1:1 slurry of protein G-Sepharose beads was added to the rabbit polyclonal antibody (anti-Gαi2, Santa Cruz Biotechnology, sc-7276 and anti-Gαi3, Santa Cruz Biotechnology, sc-262) extract mix and incubated for 12 h at 4 °C. The omission of primary antibody was used as a control. Beads were washed three times with binding buffer. Immunoprecipitated proteins were probed with anti-TRPC4 (NeuroMab, 75–119) and anti-TRPC5 (NeuroMab, 75–104) on an 8% SDS-PAGE gel. Anti-TRPC4 and Anti-TRPC5 were used for reciprocal pulldowns. Controls were omission of the primary antibody and substitution of normal anti-mouse IgG with non-immune serum (Santa Cruz Biotechnology, sc-2025). Rabbit polyclonal anti-Gαi2 (2 μg, Santa Cruz Biotechnology) and rabbit polyclonal anti-Gαi3 (2 μg, Santa Cruz Biotechnology) antibodies were used to probe co-immunoprecipitation samples on a 10% SDS-PAGE gel.
Surface Biotinylation
Cells were washed with and suspended in PBS. Suspended cells were incubated in 0.5 mg/ml sulfo-NHS-LC-biotin (Pierce) in PBS for 30 min on ice. Free biotin was quenched by the addition of 100 mm glycine in PBS. Lysates were prepared in lysis buffer by being passed 7–10 times through a 26-gauge needle after sonication. Lysates were centrifuged at 13,300 × g for 10 min at 4 °C, and protein concentrations of the supernatants were determined. Forty microliters of a 50% slurry of avidin beads (Pierce) was added to cell lysates equivalent to 400 μg of protein. After incubation for 1 h at room temperature, beads were washed three times with 0.5% Triton X-100 in PBS, and proteins were extracted in sample buffer. Collected proteins were then analyzed by 8% SDS-PAGE gel and probed by anti-GFP antibody (Invitrogen, A11122).
GST Pulldown Assays
The C-terminal domain of TRPC4(621–890) was cloned into BamHI-SalI restriction sites of pGEX4T-1 (Amersham Biosciences Pharmacia) by PCR. The GST fusion constructs were expressed and purified from Escherichia coli (BL21(DE3)). Briefly, E. coli were grown in liquid cultures containing 0.1 mm isopropyl 1-thio-β-d-galactopyranoside with vigorous agitation for 18 h at 20 °C to an A600 of 0.6. Channel protein was purified from the soluble extract using glutathione-agarose beads (Amersham Biosciences). GST fusion proteins appeared to be sensitive to degradation and carefully utilized in subsequent binding assays conducted within 24 h following purification. Histidine-tagged Gαi2Q205L protein was expressed in E. coli (BL21(DE3)) from the pET15b plasmid containing full-length human Gαi2Q205L cDNA. Histidine-tagged forms of Gαi2Q205L were purified using immobilized Ni2+-nitrilotriacetic acid affinity chromatography. Binding between Gαi2Q205L and the GST fusion C-terminal domain of TRPC4 was allowed to occur for 1 h at room temperature on a plate rotator. Each reaction sample was subsequently centrifuged at 500 × g for 5 min. After three washes with 500 μl of PBS with 0.1% Triton X-100, the GST protein-G-protein complexes were eluted with 15 μl of 2× SDS sample buffer, and the entire sample was run on a 10% polyacrylamide-SDS gel. Mouse monoclonal anti-Gαi2 (Santa Cruz Biotechnology) and anti-GST antibodies (Santa Cruz Biotechnology, sc-138) were used for immunoblot analyses. Unless otherwise stated, all pulldown assays were repeated three times for each condition.
Whole-cell Patch Clamp Experiment
The whole-cell configuration was used to measure TRPC channel current in HEK cells as described previously (13, 19, 23, 27). Cells were transferred to a small chamber on the stage of an inverted microscope (TE2000S, Nikon Japan), and attached to coverslips in the small chamber for 10 min prior to patch recording. Currents were recorded using an Axopatch 200B patch clamp amplifier (Axon Instrument). Bath solutions were constantly perfused with a physiological salt solution at a rate of 1–2 ml/min. Glass microelectrodes with 2–4 megohm resistance were used to obtain gigaohm seals. After establishing the whole-cell configuration, the external solution was changed from Normal Tyrode to Cs+-rich external solution. The current was recorded in 500-ms duration RAMPs from +100 to −100 mV and from a holding membrane potential of −60 mV. pCLAMP software (version 10.2) and Digidata 1440A (Axon Instruments) were used for data acquisition and application of command pulses. Data were filtered at 5 kHz and displayed on a computer monitor. Data were analyzed using pCLAMP (version 10.2) and Origin software (Microcal origin, version 7.5).
Solutions and Drugs
For all TRPC channel recordings, physiological salt solution containing 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES. The pH was adjusted to 7.4 using NaOH. Cs+-rich external solution was prepared by replacing NaCl and KCl with equimolar CsCl. The pipette solution contained 140 mm CsCl, 10 mm HEPES, 0.2 mm Tris-GTP, 0.5 mm EGTA, and 3 mm Mg-ATP. The pH was adjusted to 7.3 with CsOH. Pertussis toxin was purchased from Calbiochem (La Jolla, CA), and carbachol, HEPES, and GTPγS were purchased from Sigma.
Modeling of Interaction Between Gαi2 and TRPC4
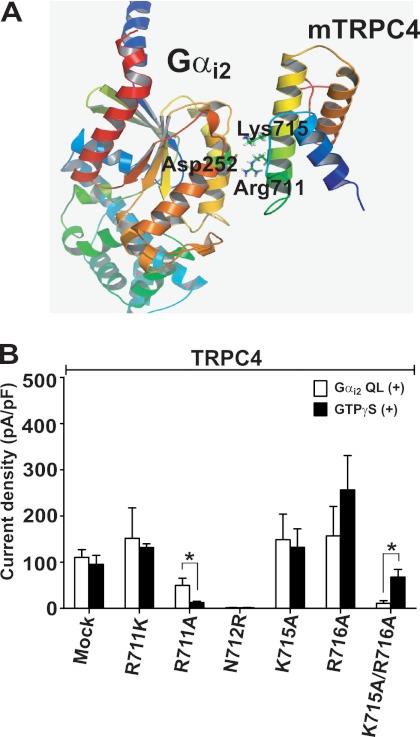
The homology model of Gαi2 structure was constructed using MODELLER 9v8 (28) based on two Gα structures that have high sequence homology with Gαi2 (Protein Data Bank codes 2ODE and 1GP2). Due to the absence of a known protein structure homologous to TRPC4, the structure of TRPC4 C terminus was modeled using I-TASSER, which combines the methods of threading, ab initio modeling, and structural refinement (29, 30). The docking of the TRPC4 C terminus on the Gαi2 was initiated because the putative binding site of TRPC4 (amino acids 701–720) contains positive charges conserved among TRPC channels. An ionic interaction was assumed between the sites of the mTRPC4 and Gαi2. There are nine sites where the Gαi family has negative charges, but the Gαq/11 family does not. Seven crystal structures of protein complexes between Gα and other proteins (Protein Data Bank codes 2RGN, 2ODE, 3CX6, 2G83, 1FQJ, 2BCJ, and 1GP2) were inspected visually, and it was found that only specific areas of Gα participated in interactions with other proteins. Therefore, manual docking was carried out between Gαi2 and the TRPC4 C terminus, emphasizing the close interaction between the two conserved positive charges of TRPC4, Arg-711 and Lys-715, and the conserved positive charges of Gαi2, Asp-252.
Statistics
All data are expressed as means ± S.E. Statistical significance was determined using paired or unpaired Student's t tests. p values of < 0.05 were considered statistically significant. The number of cell recordings is represented by n.
RESULTS
Expression of TRPC4 alone results in a minimal spontaneous current (2.1 ± 1.1 pA/pF; see Fig. 2B, mock, open column, n = 5), compared with TRPC5, which showed a significant basal current (Fig. 2C, 36.6 ± 9.2 pA/pF, n = 14; see also Ref. 26). Although HEK cells endogenously express muscarinic receptors, most likely the M3 subtype (31), the endogenous receptors do not activate TRPC4 (Fig. 1B, mock) (32). In the case of TRPC5, the endogenous muscarinic receptor elicited only small transient TRPC5 activation. Therefore, we analyzed the TRPC4 and TRPC5 currents induced by heterologously expressed muscarinic receptors or by intracellular infusion of GTPγS through the patch pipette. The TRPC maximal inward currents (in Cs+-rich solution) at negative membrane potentials (−60 mV) are represented as a current density (pA/pF). In all cases, maximal peak inward currents were obtained by subtracting the current recorded in bath Cs+.
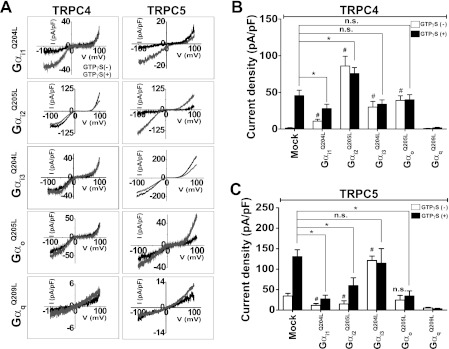
FIGURE 2.
Effect of Gα isoforms on TPRC4 and TRPC5 activity. A, representative I-V relationships of TRPC4 and TRPC5 show the effect of constitutively active Gα QL mutants on the electrophysiological properties of the TRPC4 and TRPC5 channels. B, summary of TRPC4 current density activation by Gα subunits and/or by GTPγS. Note the variable effects of the Gα mutants. All Gα mutants activate TRPC4 channels without an activator (e.g. GTPγS or carbachol (CCh)). Gαi2 provided the most effective activation of TRPC4, whereas Gαq inhibited TRPC4. Current density is represented by maximal current peaks (subtracted Cs+ basal current) at −60 mV in Cs+ solution and is indicated by means ± S.E. Statistical significance was denoted by an asterisk (open column) and number sign (closed column) at p < 0.05. C, summary of TRPC5 activation by Gα subtypes showing that the most effective activator is Gαi3 and that most Gα mutants inhibit the TRPC5 channel, with Gαq inhibiting TRPC5. Current density was obtained by the methods described above. Statistical significance was denoted by an asterisk (open column) and number sign (closed column) at p < 0.05. n. s., not significant.
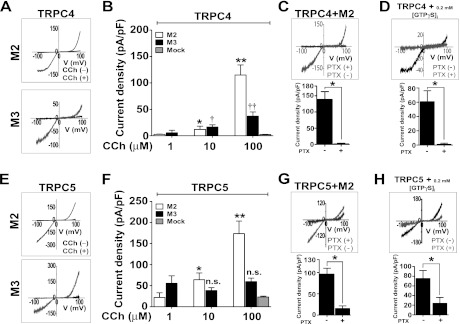
FIGURE 1.
Activation of endogenous Gαi/o by the muscarinic M2 receptor activates TRPC4 and TRPC5. A, carbachol (CCh) activated-TRPC4 current was recorded in HEK cells expressing TRPC4 and either M2 and M3 receptors. The representative I-V relationships of M2- and M3-evoked TRPC4 currents by 100 μm carbachol were recorded by voltage RAMPS of +100 to −100 mV during 500-ms durations, whereas the cells were held at −60 mV. B summarizes the amplitude of M2- and M3-activated TRPC4 currents activated by 1–100 μm carbachol. Current density is represented by maximal current peaks (subtracted Cs+ basal current) at −60 mV in Cs+ solution (changed from Normal Tyrode, Na+) and is indicated by means ± S.E. Statistical significance was denoted by an asterisk (open column, 1 μm versus 10 μm) and double asterisk (open column, 10 μm versus 100 μm), single dagger (closed column, 1 μm versus 10 μm) and double dagger (closed column, 10 μm versus 10 μm) at p < 0.05. C and D, I-V relationship and current densities of M2- and GTPγS-evoked TRPC4 currents show inhibition by PTX pretreatment (100 ng/ml for 16 h). Statistical significance was denoted by an asterisk (p < 0.05). E, the representative I-V relationships of M2- and M3-activated TRPC5 currents were measured in cells stimulated with 100 μm carbachol. F, summary of the M2-activated TRPC5 current at 1–100 μm carbachol. Current density was obtained by the methods described above. Statistical significance was denoted by an asterisk (open column, 1 μm versus 10 μm) and double asterisk (open column, 10 μm versus 100 μm) at p < 0.05. G and H, the representative I-V relationships and current densities of M2- and GTPγS-evoked TRPC5 currents show inhibition by PTX pretreatment (as described under “Experimental Procedures”). Statistical significance was denoted by an asterisk (p < 0.05). n. s., not significant.
Muscarinic Receptor Stimulation Activates TRPC4 and TRPC5 through Endogenous Gαi/o
First, we tested the effects of the expressed Gαi-coupled M2 and the Gαq-coupled M3 muscarinic receptors on the activation of TRPC4 or TRPC5 by endogenous G-proteins in HEK cells (Fig. 1). Activation of the M2 receptors with carbachol elicited 2–3-fold higher TRPC4 and TRPC5 currents than activation of the M3 receptors. The TRPC4 and TRPC5 currents showed a typical doubly rectifying current-voltage relationship (Fig. 1, A and E). The stimulation of the M2 receptor increased both TRPC4 and TRPC5 currents in a dose-dependent manner (Fig. 1, B and F, open column, 1, 10, and 100 μm; n = 5, n = 4, n = 12, and n = 4, n = 4, n = 10), whereas the stimulation of the M3 receptor increased the TRPC4 current dose-dependently and the TRPC5 current dose-independently at the concentration range 1–100 μm carbachol (Fig. 1, B and F, closed column, 1, 10, and 100 μm; n = 5, n = 8, n = 16, and n = 5, n = 7, n = 11). Treatment with PTX markedly inhibited the TRPC4 and TRPC5 currents activated by M2 receptor stimulation and GTPγS (Fig. 1, C, D, G, and H; mock/PTX; n = 8/n = 8, n = 5/n = 5, n = 7/n = 4, and n = 6/n = 3). Thus, the M2 receptor-Gαi/o pathway was more effective than the M3 receptor-Gαq pathway in channel activation by engaging the endogenous PTX-sensitive Gα proteins.
Specific Gα Isoforms Increase TPRC4 and TRPC5 Activity
To determine which Gα isoform is involved in the activation of TRPC4 and TRPC5, we used constitutively active forms of the Gαi subunits (Gα QL mutants). Intracellular application of GTPγS through the patch pipette increased the TRPC4 current to 45.0 ± 7.2 pA/pF (Fig. 2B, mock, closed column, n = 13). The TRPC4 channel was activated to a different extent by all constitutively active Gαi/o subunits, even in the absence of GTPγS. Constitutively active Gαi3 and Gαo mimicked the activation of TRPC4 by GTPγS (open/closed column, n = 10/n = 8 and n = 9/n = 11). Constitutively active Gαi1 activated the TRPC4 channel, whereas application of GTPγS significantly inhibited the TRPC4 current (open/closed column, n = 13/n = 11). Constitutively active Gαi2 (Gαi2Q205L) was the most effective activator among the Gαi subunits tested (Fig. 2B, open columns, and supplemental Fig. S1A, n = 6). Notably, the application of GTPγS had no further effect on the TRPC4 current, indicating that Gαi2 fully activates TRPC4 (closed column, n = 8). Furthermore, stimulation of the M2 receptors (Fig. 1) and Gαi2 (Fig. 2) activated TRPC4 to the same extent. Of particular significance, constitutively active Gαq was unable to activate TRPC4 (Fig. 2B, open/closed column, n = 4/n = 3). Moreover, constitutively active Gαq inhibited the stimulatory effect of GTPγS. This was addressed further below.
Again, TRPC5 showed significant basal activity (36.6 ± 9.2 pA/pF, Fig. 2C, mock, open column). As with TRPC4, application of GTPγS further increased the TRPC5 current to 130.7 ± 16.7 pA/pF (Fig. 2C, mock, closed column, n = 14). Gαi3Q205L was the most effective activator of TRPC5, as all other Gα isoforms tested actually reduced the spontaneous current and GTPγS-induced currents, likely by competing with endogenous Gαi3 (Fig. 2C and supplemental Fig. S1B, open/closed column; Gαi1, Gαi2, Gαi3, and Gαo, n = 12/n = 9, n = 6/n = 4, n = 7/n = 5, and n = 8/n = 9). GTPγS did not increase the TRPC5 current activated by Gαi3Q205L. As was found with TRPC4, constitutively active Gαq was not able to activate TRPC5 and instead inhibited the TRPC5 current (open/closed column, n = 3/n = 3).
Gβγ Isoforms Are Not Required for TPRC4 and TRPC5 Activation
Although Gαi/o subunits are key activators of TRPC4 or TRPC5, Gβγ subunits may also be involved in the activation of the channels, as is the case with GIRK channels, or in the regulation of the channels by altering the availability of activated Gαi. To address these questions, we tested the effects of various Gβγ combinations on TRPC4 and TRPC5 activity. Figs. 3, A–C show that none of the Gβγ combinations tested activated the channels or reduced the activation by GTPγS. The exception is Gβ2γ2, which slightly inhibited the activation of TRPC5, likely by sequestering some of the Gαi3 even in the presence of GTPγS. Moreover, even the free form Gβ1I80A mutant (18, 20, 33, 34) did not activate TRPC4 or TRPC5 (Fig. 3B; open/closed column, n = 4/n = 5, and Fig. 3C; open/closed column; n = 3/n = 14). These results indicate that the PTX-sensitive Gαi2/3 subunits are the activators of TRPC4 and TRPC5.
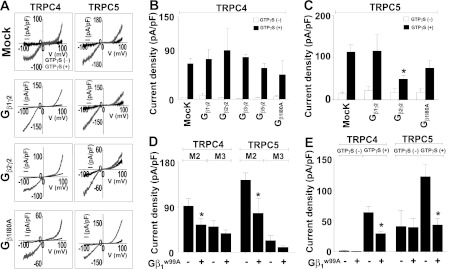
FIGURE 3.
Lack of effect of Gβγ isoforms on TPRC4 and TRPC5 activity. A shows representative I-V curves of TRPC4 and TRPC5 co-expressed with or without the indicated Gβγ isoforms. B and C summarize the current density recorded in cells transfected with the indicated Gβγ subunits and infused with GTPγS (closed columns; mock, Gβ1γ2, Gβ2γ2, Gβ3γ2, and Gβ5γ2 with TRPC4; n = 24, n = 5, n = 3, n = 7, and n = 7; mock, Gβ1γ2, and Gβ2γ2 with TRPC5, n = 10, n = 8, and n = 6) or without (open columns; mock, Gβ1γ2, Gβ2γ2, Gβ3γ2, and Gβ5γ2 with TRPC4, n = 15, n = 5, n = 4, n = 5, and n = 4; mock, Gβ1γ2, and Gβ2γ2 with TRPC5, n = 3, n = 5, and n = 3). D shows the effects of Gβ1W99A on activation of TRPC4 and TRPC5 by muscarinic receptors. The activation of TRPC4 and TRPC5 by the M2 receptor was inhibited by Gβ1W99A, whereas no current was activated by the M3 receptors. E, inhibition by Gβ1W99A of TRPC4 and TRPC5 activated by GTPγS. All current densities represent maximal current peaks (subtracted Cs+ basal current) at −60 mV in Cs+ solution and are indicated by means ± S.E. Statistical significance was denoted by an asterisk (p < 0.05).
As is shown with the use of the Gβ mutant, Gβ1W99A, the major role of Gβ2γ2 in the activation of TRPC4 and TRPC5 is the sequestering of Gαi/o subunits. Gβ1W99A keeps the G-protein as the heterotrimer Gαβγ because Gβ1W99A is unable to support nucleotide exchange on Gα (34). Gβ1W99A inhibited activation of TRPC4 by M2 receptor stimulation to 61.4% ± 7.0% (p = 0.03, without/with Gβ1W99A, n = 10/n = 12), whereas Gβ1W99A did not inhibit the modest M3-induced TRPC4 current (without/with Gβ1W99A, n = 8/n = 10). With TRPC5, Gβ1W99A inhibited M2-induced TRPC5 current to 53.4% ± 16.7% (p = 0.02, without/with Gβ1W99A, n = 11/n = 8), without affecting the minimal M3-induced TRPC5 current (without/with Gβ1W99A, n = 4/n = 6, Fig. 3D). Importantly, Gβ1W99A inhibited GTPγS-induced TRPC4 current to 46.2% ± 4.4% (p = 0.00079, without/with GTPγS, n = 9/n = 8) and GTPγS-induced TRPC5 current to 28.7% ± 7.7% (p = 0.00075, without/with GTPγS, n = 6/n = 6, Fig. 3E).
TRPC4 Is Inhibited by Increased Gαq Activity
Activation of the Gαq-PLC pathway has been shown to modestly activate TRPC4 and TRPC5 (Fig. 1) by an unknown mechanism (1, 7, 8). Hence, the inhibition of TRPC4/5 by the constitutively active Gαq (Fig. 2) was completely unexpected. These results imply that intense overstimulation of a Gαq-activated pathway inhibits TRPC4 and TRPC5. We considered several potential mechanisms, including increased or decreased cytoplasmic Ca2+, interference of channel interaction with Gαi, reduced surface expression of TRPC4, modified cellular PIP2, and channel phosphorylation by PKC, known to induce desensitization of TRPC5 with phosphorylation of residue Thr-972 at the C terminus (35).
Loading the cells with BAPTA-AM recovered only 15 ± 5.6% (mock, 56.7 ± 11.2 pA/pF (n = 6); recovery by BAPTA, 9.8 ± 3.1 pA/pF; n = 6, BAPTA with GTPγS; n = 12, Gαq QL; n = 3, Fig. 4A), and inhibition of PKC with Goe6976 recovered only 13 ± 8.7% (mock, 67.6 ± 12.6 pA/pF (n = 5); recovery by PKC inhibitor, 10.1 ± 5.8 pA/pF (n = 8); Gαq QL (n = 3) Fig. 4B) of the TRPC4 current inhibited by Gαq. The Gαq-TRPC4-inhibited current was recovered to only 27.7 ± 9.2% by 5 μm intracellular Ca2+ (without GαqQ209L, non-buffered/5 μm, n = 7/n = 3; with GαqQ209L, non-buffered/5 μm, n = 3/n = 3; Fig. 4C). Co-IP experiments showed that TRPC4 does not interact directly with Gαq (Fig. 4D). Finally, activated Gαq did not change the surface expression of TRPC4 (Fig. 4F, upper blots). These findings rule out the effects of changes in cytosolic Ca2+, PKC, and altered interaction with Gαi in channel inhibition by Gαq as the major inhibitors of the current induced by activated Gαq. Moreover, they point to an indirect effect of Gαq on channel function.
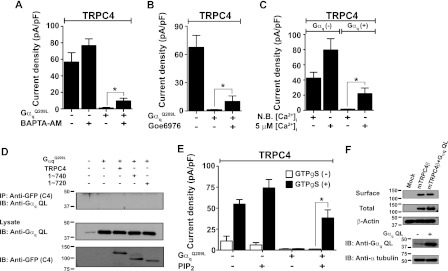
FIGURE 4.
Inhibition of TRPC4 by Gαq is rescued by PIP2. A–C show current densities in HEK293 cells stably expressing mTRPC4β and its inhibition by Gαq. Loading the cells with BAPTA-AM and inhibition of PKC with Goe6976 did not effectively reverse channel inhibition by Gαq. High intracellular Ca2+ (5 μm) reversed channel inhibition by Gαq. D, TRPC4 and C-terminal truncation TRPC4 mutants did not co-IP with Gαq. E, intracellular application of diC8-PIP2 (50 μm) almost recovered the inhibition of TRPC4 by Gαq. All current densities represent subtracted maximal current peaks at −60 mV in Cs+ solution and are indicated by means ± S.E. Statistical significance was denoted by an asterisk (p < 0.05). All whole-cell currents were recorded in the intracellular application of GTPγS. F, surface expression of TRPC4 and Gαq co-expressed TRPC4 in HEK cells. Surface expression of TRPC4 was not altered by Gαq QL as determined by co-expression and surface biotinylation. Immunoblots of surface and total were detected by anti-GFP antibody (upper panel). Expression of endogenous Gαq and transfected Gαq QL were detected by Gαq antibody (bottom panel). IB, immunoblot. N. B., non buffered.
Gαq activates PLC to hydrolyze PIP2, reported to have an effect of TRPC4 and TRPC5 channel activity (7, 8). When diC8-PIP2 (50 μm) was applied via the patch pipette together with GTPγS, it recovered Gαq-induced inhibition of TRPC4 up to 51.83 ± 12.83% of the PIP2 control (open/closed column, mock, PIP2 alone, GαqQ209L, and PIP2 with GαqQ209L; n = 18/26, n = 4/15, n = 3/18, and n = 3/14, Fig. 4E). PIP2 was reported to inhibit TRPC4α but not the TRPC4β isoform (8), the isoform used in the present work. In addition, we reported that PIP2 slows TRPC5 desensitization (7). Thus, the combined results in Fig. 4 indicate that PIP2 and perhaps increased cytoplasmic Ca2+ are required for TRPC4 and TRPC5 activation.
Mechanisms Associated with Interaction between Gαi2 with C Terminus of TRPC4
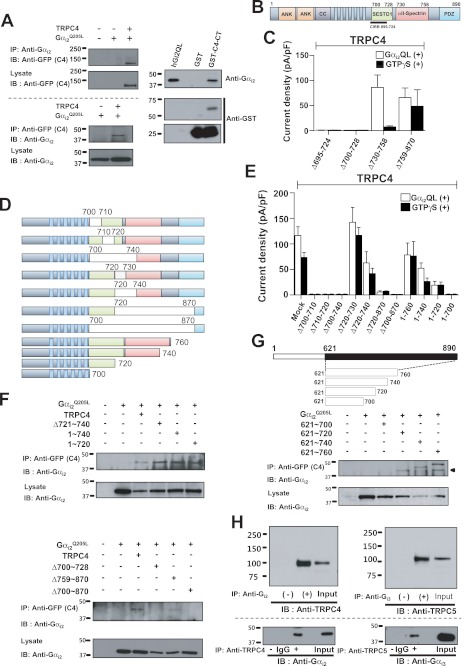
Together, the results in Figs. 1–4 indicate that activation of Gαi subunits by GPCRs is the primary mechanism for activating TRPC4 and TRPC5. This raised the question of whether activation of the channels requires direct interaction with the Gαi subunits, as was shown for other channels regulated by Gα (36) and Gβγ (33, 37) subunits. To address this question, we identified the TRPC4 and TRPC5 domain that might interact with the Gαi subunits. To characterize the association between TRPC4β with Gαi2 in vivo, HEK cells were transfected with TRPC4β-GFP and Gαi2, and their association was analyzed by co-immunoprecipitation. Immunoprecipitation of Gαi2 pulled down TRPC4β-GFP (Fig. 5A, upper panel). Likewise, Gαi2 was present in TRPC4β-GFP immunoprecipitates (Fig. 5A, lower panel). Similar co-immunoprecipitation occurred between TRPC5 and Gαi3 (supplemental Fig. S2A). Pulldown assays were utilized to examine the binding of purified Gαi2Q205L to GST fusion protein containing the C-terminal domain of TRPC4. Gαi2Q205L bound to the C-terminal domain of TRPC4 (Fig. 5A, right panel).
FIGURE 5.
Interaction of Gαi2 with the C terminus of TRPC4. A, TRPC4 co-immunoprecipitates with Gαi2. HEK cells were transfected with empty vector, Gαi2 alone, and Gαi2 with TRPC4-GFP and were used to test reciprocal co-IP of TRPC4 and Gαi2. TRPC4 C-terminal GST fusion protein was used to probe direct binding between Gαi2 and TRPC4. Binding of the TRPC4 C-terminal GST fusion protein with recombinant human Gαi2Q205L protein was shown by Gαi2-antibody in vitro binding assay. B, a schematic of GFP-fused TRPC4. C, summary of the effects of Gαi2 on TRPC4 C-terminal truncation mutants in the presence and absence of GTPγS stimulation. Current densities are represented by subtracted maximal maximal current peaks at −60 mV in Cs+ solution and are indicated by means ± S.E. D, schematic of GFP-fused TRPC4 deletion and truncation mutants used (upper panel). Wild-type TRPC4 and mutants were probed using the GFP antibody in immunoblotting (bottom). E, summary of the effects of Gαi2 on current by TRPC4 deletion and truncation mutants in the presence and absence of GTPγS stimulation. Current density was obtained by the methods described above. F, interaction between Gαi2, TRPC4, and mutants was tested by co-IP, and the interaction site was mapped to the 700∼728 region (the SESTD1 domain of TRPC4β). G, a schematic of GFP-fused C-terminal fragments of TRPC4 (upper panel) and their co-IP with Gαi2. H, the association between Gαi with TRPC4/5 in vivo. Gαi2 and Gαi3 were immunoprecipitated from rat brain extract and were probed for TRPC4 and TRPC5 to show co-IP in vivo (upper panel). TRPC4 and TRPC5 were co-immunoprecipitated reciprocally. Lanes of IgG and control (−) did not show Gαi binding. Input was indicated as 10% input of brain extract. IB, immunoblot.
To map the Gαi2 binding domain in TRPC4β, a series of TRPC4β-GFP truncation or deletion mutants were generated (Fig. 5B). Binding domains for regulatory molecules are clustered in the C-terminal region of TRPC4 and TRPC5. Because considerable evidence suggests that modulation of TRPC4 and TRPC5 function are directed by elements present in this region, we focused on the C-terminal region. We made deletion mutants based on well known binding domains: the CIRB (calmodulin and inositol 1,4,5-trisphosphate receptor binding region) (amino acids 695–724), the SESTD1 (SEC14-like and spectrin-type domain 1) (amino acids 700–728), and the α-spectrin binding domain (amino acids 730–758). Of the truncations shown in Fig. 5C, Δ759–870 and Δ730–758 retained activation by Gαi2Q205L with peak current amplitude similar to that of WT-TRPC4, although TRPC4 (Δ730–758) lost activation by GTPγS (open/closed column, mock, Δ695–724, Δ700–728, Δ730–758, and Δ759–870; n = 8/7, n = 3/5, n = 3/4, and n = 9/6; Fig. 5C). These results implicate amino acids upstream of 730 in channel activation by Gαi. To further narrow the functional site, we examined the function of the 11 deletion or truncation TRPC4 mutants listed in Fig. 5D. Gαi2Q205L did not activate TRPC4β-GFP 1–700, Δ700–710, Δ710–720, Δ700–740, Δ700–870, and Δ720–870, but fully or partially activated the other mutants with the typical doubly rectifying I-V curve (open/closed column, mock, Δ700–710, Δ710–720, Δ700–740, Δ720–730, Δ720–740, Δ720–870, Δ700–870, 1–760, 1–740, 1–720, and 1–700; n = 27/12, n = 3/4, n = 3/3, n = 3/3, n = 10/10, n = 9/7, n = 3/5, n = 3/3, n = 8/9, n = 10/6, n = 5/8, and n = 3/4; Fig. 5E and supplemental Fig. S3). Similarly, Gαi3Q205L did not activate TRPC5-GFP Δ701–733, Δ707–717, Δ707–727, Δ707–735, Δ707–747, and Δ707–954, but fully or partially activated Δ737–765 and Δ764–954 (open/closed column, mock, Δ701–733, Δ707–747, Δ707–954, Δ737–765, and Δ764–954; n = 12/12, n = 3/5, n = 3/3, n = 3/3, n = 3/3, n = 7/3, n = 3/3, n = 3/13, and n = 11/7; supplemental Fig. S2).
The TRPC4β-GFP constructs were also co-expressed with Gαi2 in HEK cells, and their interaction was monitored by co-immunoprecipitation. C-terminal (TRPC4β(1–720) and (1–740) truncations and TRPC4 (Δ721∼740 and Δ759∼870) deletions did not affect binding to Gαi2, whereas deletion of the SESTD1 binding domain (Δ700∼728) or of Δ700∼870 eliminated association with Gαi2 (Fig. 5F). Thus, the Gαi2 binding domain maps to amino acids 700∼728, the SESTD1 domain of TRPC4β.
To further analyze the interaction between the TRPC4 C terminus and Gαi, we prepared C-terminal fragments of TRPC4 and examined their interactions with Gαi. TRPC4β(621-700) did not interact with Gαi, but TRPC4 fragments 621–720, 621–740, and 621–760 did (Fig. 5G), strengthening the conclusion that TRPC4(701–720) mediates the interaction with Gαi2. The TRPC4(701–720) encompass the CaM and IP3R binding region (10) (supplemental Fig. S4). The modeling in Fig. 6A of the interaction between Gαi2 and TRPC4 suggested that the 711RNLVKR716 region is important for binding, and thus, we prepared the mutants R711A, N712R, K715A, and R716A (Fig. 6 and supplemental Fig. S5). Of these mutants, K715A and R716A retained activation by Gαi2Q205L with peak current amplitude similar to that of WT-TRPC4, whereas R711A and N712R lost partial or complete activation by both Gαi2Q205L and GTPγS. However, the double mutant K715A/R716A did not respond to Gαi2Q205L but maintained responsivity to GTPγS (open/closed column, mock, R711K, R711A, N712R, K715A, R716A, and K715A/R716A; n = 6/11, n = 3/3, n = 15/3, n = 3/3, n = 3/9, n = 7/8, and n = 6/6, Fig. 6B). These results implicate amino acids Lys-715 and Arg-716 in channel activation by Gαi. Ordaz et al. (11) showed that a similar sequence in mTRPC5 (718RNLVKR723) is involved in the activation process by calmodulin (11). Thus, we used the mTRPC5 CIRBm1 (R718A/K722A/R723A), CIRBm2 (I717D/L720E/V721 A), and CBII mutants (ΔPro-828∼Asn-854) (11) to test properties of TRPC5 activation by Gαi3 (open/closed column, mock, CIRBm1, CIRBm2, and CBII: n = 7/6, n = 4/6, n = 4/5, and n = 4/3 in absence of Gαi3; supplemental Fig. S6). The constitutively active Gαi3 mutant could not activate the CIRBm1 and CIRBm2 deletion mutants but did activate the CBII mutant (open/closed column, mock, CIRBm1, CIRBm2, and CBII; n = 6/6, n = 5/3, n = 4/4, and n = 6/4; supplemental Fig. S6). Interaction between Gαi and the TRPC channels can also be demonstrated in vivo, as revealed by co-IP of TRPC4 with Gαi2 and of TRPC5 with Gαi3 in brain extract (Fig. 5H).
FIGURE 6.
The interaction modeling of the TRPC4 C terminus (amino acids 701–720) with Gαi2. A, a model of association between Gαi2 and TRPC4. An ionic interaction was assumed between the two proteins. Amino acids from 671 to 758 of TRPC4 are shown. B, summary of effects of Gαi2Q205L on current by mTRPC4 and mTRPC4 mutants in the presence and absence of GTPγS stimulation. mTRPC4 mutants were substituted at CIRB residues (R711K, R711A, N712R, K715A, and R716A). All current densities represent maximal current peaks (subtracted Cs+ basal current) at −60 mV in Cs+ solution and are indicated by means ± S.E. Statistical significance was denoted by an asterisk (p < 0.05).
DISCUSSION
We report here that the primary mechanism for the activation of TRPC4 and TRPC5 in gastric smooth muscle in vivo is through activation of GPCR. TRPC4 and TRPC5 activation appears to require both M2-Gi/o and M3-Gq/11 muscarinic receptors (20). The mechanism involves specific activation of the channels by Gαi subunits. Receptors coupled to Gαi2 primarily activate TRPC4 and receptors coupled to Gαi3 primarily activate TRPC5 to mediate the receptor-stimulated monovalent cation current and the Ca2+ influx.
Several studies have reported modulation of TRPC4 and TRPC5 activity by GTPγS-activated PTX-sensitive G-proteins and Gi/o-coupled receptors (6, 8, 25). Activation of TRPC4 by muscarinic receptor stimulation of GI smooth muscle was inhibited by PTX and was shown to be critical in evoking and regulating GI tract motility (21). Our results provide a molecular mechanism to explain the activation of TRPC4 by the PTX-sensitive G-protein signaling. A unique feature of the mICAT is that it requires simultaneous activation of both the M2 and M3 muscarinic receptors. Conversely, cationic channel activation in murine gut smooth muscle cells involves three separate pathways (24). 1) The M3-Gαq-PLCβ system, which transiently activates the 70-pS and 120-pS cationic channels concurrently with inositol 1,4,5-trisphosphate-induced Ca2+ release. 2) The M2 pathway, which transduces signals from M2 receptors via Gαo to the 70-pS cationic channel and shifts the transient activation toward a longer open mode. 3) The M2/M3 pathway, which transmits M2 signals via Gαo and M3 signals via a Gαq-independent PLC, to the 70-pS cationic channel, resulting in a much longer open mode. The latter pathway does not work well when either the M2 or the M3 receptors are lacking or when either Gαo or PLC are inactivated. In addition, the M2/M3 pathway, but not the M2 or M3 pathway, involves processes in which Ca2+ has a potentiating effect on channel activation, suggesting that the M3 pathway may facilitate the function of the M2/M3 pathway through inositol 1,4,5-trisphosphate-induced Ca2+ release (38). We argue that if the 70-pS channel is mediated by TRPC4, then the pathways involving the M2 receptors use Gαi2 to directly activate the channel.
In addition to TRPC4/5, activation by the Gαi/o subunits have been reported to regulate several other TRP channels. For example, in Go-coupled mGluR6, Gαo closes a downstream nonselective cation channel in ON bipolar cells that is mediated by TRPM1-L (39). Pheromone sensing in the vomeronasal organ is mediated by V1R-Gi and V2R-Go complexes that activate TRPC2 (40). Whether activation of these channels is by direct interaction with Gαi/o subunits as shown here for TRPC4/TRPC5 remains to be determined.
Our findings indicate that regulation of TRPC4 and TRPC5 by G-proteins is more complex than assumed previously. The newly discovered mechanism for activation of TRPC4 and TRPC5 suggests that Ca2+ influx through these channels can be activated by several mediators depending on receptor stimulation. As reported before and shown in Fig. 1, TRPC4 and TRPC5 can clearly be activated by Gαq-coupled receptors and mediated by several of the mechanisms reported before. However, activation by Gαq-coupled receptors appears to be modest (Fig. 1). More significant activation of TRPC4/5 is elicited by stimulation of Gαi-coupled receptors that is mediated by direct activation of the channels by Gαi subunits. The Gαi binding domain in TRPC4 and TRPC5 shares binding motifs with other regulatory molecules (CIRB, SESTD1) in the C-terminal region of TRPC4 and TRPC5. Modeling and mutation analyses indicate that the 711RNLVKR716 region is important for the interaction between Gαi2 and TRPC4 (supplemental Fig. S6). By contrast with GIRK channels, Gβγ subunits do not appear to be involved in the direct activation of TRPC4 or TRPC5 by Gαi. It is likely that the major role of Gβγ is regulating channel function by sequestering Gαi/o subunits in the resting state. The mode of TRPC4/TRPC5 regulation by Gαi/o subunits reported here likely constitutes a major pathway in smooth muscle. It will be interesting to explore the full potential of this form of regulation in other cell types, particularly when the regulation by Gαi/o-coupled receptors involves changes in cellular Ca2+. Examples include acetylcholine-induced vasoregulation, lung microvascular permeability (41, 42) and the increase of 5-hydroxytryptamine 2 receptor-coupled GABA release in thalamic interneurons (43). In summary, the present findings expand knowledge regarding signal transduction by cytoplasmic Ca2+ beyond the Gβγ to the Gα subunits of Gαi-coupled receptors. Moreover, our findings point to a signaling function that is activated rather than inhibited by Gαi proteins and add an important function to the repertoire of functions activated by Gαi subunits.
Supplementary Material
Acknowledgments
We thank Dr. Yong-Sung Juhnn for the human Gαi1Q204L, Gαi3Q204L, rat Gαi2Q205L, and human GαqQ209L; Dr. Seong-Woo Jeong for all the Gβγ; Dr. Michel X. Zhu for the mTRPC5 and CIRB mutants; and Dr. Yasuo Mori for the mTRPC5-GFP and ΔC.
This work was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (MEST) (2008-2005948 and 2010-0019472).

This article contains supplemental Figs. S1–S6.
- TRPC
- transient receptor potential canonical
- GPCR
- G-protein-coupled receptor
- EGFP
- enhanced GFP
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- PTX
- pertussis toxin
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- PLC
- phospholipase C
- GI
- gastrointestinal
- pS
- picosiemen
- n.s.
- not significant
- N.B.
- non-buffered.
REFERENCES
- 1. Schaefer M., Plant T. D., Obukhov A. G., Hofmann T., Gudermann T., Schultz G. (2000) Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 275, 17517–17526 [DOI] [PubMed] [Google Scholar]
- 2. Miehe S., Bieberstein A., Arnould I., Ihdene O., Rütten H., Strübing C. (2010) The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5. J. Biol. Chem. 285, 12426–12434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blair N. T., Kaczmarek J. S., Clapham D. E. (2009) Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J. Gen. Physiol. 133, 525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gross S. A., Guzmán G. A., Wissenbach U., Philipp S. E., Zhu M. X., Bruns D., Cavalié A. (2009) TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J. Biol. Chem. 284, 34423–34432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flemming P. K., Dedman A. M., Xu S. Z., Li J., Zeng F., Naylor J., Benham C. D., Bateson A. N., Muraki K., Beech D. J. (2006) Sensing of lysophospholipids by TRPC5 calcium channel. J. Biol. Chem. 281, 4977–4982 [DOI] [PubMed] [Google Scholar]
- 6. Xu S. Z., Muraki K., Zeng F., Li J., Sukumar P., Shah S., Dedman A. M., Flemming P. K., McHugh D., Naylor J., Cheong A., Bateson A. N., Munsch C. M., Porter K. E., Beech D. J. (2006) A sphingosine 1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ. Res. 98, 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim B. J., Kim M. T., Jeon J. H., Kim S. J., So I. (2008) Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol. Pharm. Bull. 31, 1733–1738 [DOI] [PubMed] [Google Scholar]
- 8. Otsuguro K., Tang J., Tang Y., Xiao R., Freichel M., Tsvilovskyy V., Ito S., Flockerzi V., Zhu M. X., Zholos A. V. (2008) Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 283, 10026–10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trebak M., Lemonnier L., DeHaven W. I., Wedel B. J., Bird G. S., Putney J. W., Jr. (2009) Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 457, 757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang J., Lin Y., Zhang Z., Tikunova S., Birnbaumer L., Zhu M. X. (2001) Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 276, 21303–21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ordaz B., Tang J., Xiao R., Salgado A., Sampieri A., Zhu M. X., Vaca L. (2005) Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J. Biol. Chem. 280, 30788–30796 [DOI] [PubMed] [Google Scholar]
- 12. Shi J., Mori E., Mori Y., Mori M., Li J., Ito Y., Inoue R. (2004) Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J. Physiol. 561, 415–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim M. T., Kim B. J., Lee J. H., Kwon S. C., Yeon D. S., Yang D. K., So I., Kim K. W. (2006) Involvement of calmodulin and myosin light chain kinase in activation of mTRPC5 expressed in HEK cells. Am. J. Physiol. Cell Physiol. 290, C1031–1040 [DOI] [PubMed] [Google Scholar]
- 14. Kim B. J., Jeon J. H., Kim S. J., So I. (2007) Role of calmodulin and myosin light chain kinase in the activation of carbachol-activated cationic current in murine ileal myocytes. Can. J. Physiol. Pharmacol. 85, 1254–1262 [DOI] [PubMed] [Google Scholar]
- 15. Shimizu S., Yoshida T., Wakamori M., Ishii M., Okada T., Takahashi M., Seto M., Sakurada K., Kiuchi Y., Mori Y. (2006) Ca2+-calmodulin-dependent myosin light chain kinase is essential for activation of TRPC5 channels expressed in HEK293 cells. J. Physiol. 570, 219–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. (2004) Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 6, 709–720 [DOI] [PubMed] [Google Scholar]
- 17. Xu S. Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C. J., Bahnasi Y. M., Al-Shawaf E., Porter K. E., Jiang L. H., Emery P., Sivaprasadarao A., Beech D. J. (2008) TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2, 596–607 [DOI] [PubMed] [Google Scholar]
- 19. So I., Kim K. W. (2003) Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle. J. Smooth Muscle Res. 39, 231–247 [DOI] [PubMed] [Google Scholar]
- 20. Lee K. P., Jun J. Y., Chang I. Y., Suh S. H., So I., Kim K. W. (2005) TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol. Cell 20, 435–441 [PubMed] [Google Scholar]
- 21. Tsvilovskyy V. V., Zholos A. V., Aberle T., Philipp S. E., Dietrich A., Zhu M. X., Birnbaumer L., Freichel M., Flockerzi V. (2009) Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137, 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y. C., Kim S. J., Sim J. H., Cho C. H., Juhnn Y. S., Suh S. H., So I., Kim K. W. (1998) Suppression of the carbachol-activated nonselective cationic current by antibody against α subunit of Go protein in guinea pig gastric myocytes. Pflugers Arch. 436, 494–496 [DOI] [PubMed] [Google Scholar]
- 23. Yan H. D., Okamoto H., Unno T., Tsytsyura Y. D., Prestwich S. A., Komori S., Zholos A. V., Bolton T. B. (2003) Effects of G-protein-specific antibodies and G βγ subunits on the muscarinic receptor-operated cation current in guinea pig ileal smooth muscle cells. Br. J. Pharmacol. 139, 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakamoto T., Unno T., Kitazawa T., Taneike T., Yamada M., Wess J., Nishimura M., Komori S. (2007) Three distinct muscarinic signaling pathways for cationic channel activation in mouse gut smooth muscle cells. J. Physiol. 582, 41–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jeon J. P., Lee K. P., Park E. J., Sung T. S., Kim B. J., Jeon J. H., So I. (2008) The specific activation of TRPC4 by Gi protein subtype. Biochem. Biophys. Res. Commun. 377, 538–543 [DOI] [PubMed] [Google Scholar]
- 26. Sung T. S., Jeon J. P., Kim B. J., Hong C., Kim S. Y., Kim J., Jeon J. H., Kim H. J., Suh C. K., Kim S. J., So I. (2011) Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am. J. Physiol. Cell Physiol. 301, C823–832 [DOI] [PubMed] [Google Scholar]
- 27. Kim M. J., Jeon J. P., Kim H. J., Kim B. J., Lee Y. M., Choe H., Jeon J. H., Kim S. J., So I. (2008) Molecular determinant of sensing extracellular pH in classical transient receptor potential channel 5. Biochem. Biophys. Res. Commun. 365, 239–245 [DOI] [PubMed] [Google Scholar]
- 28. Sali A., Blundell T. L. (1993) Comparative protein modeling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 [DOI] [PubMed] [Google Scholar]
- 29. Roy A., Kucukural A., Zhang Y. (2010) I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y. (2008) I-TASSER server for protein three-dimensional structure prediction. BMC Bioinformatics 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller M., Shi J., Zhu Y., Kustov M., Tian J. B., Stevens A., Wu M., Xu J., Long S., Yang P., Zholos A. V., Salovich J. M., Weaver C. D., Hopkins C. R., Lindsley C. W., McManus O., Li M., Zhu M. X. (2011) Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 286, 33436–33446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sung T. S., Kim M. J., Hong S., Jeon J. P., Kim B. J., Jeon J. H., Kim S. J., So I. (2009) Functional characteristics of TRPC4 channels expressed in HEK 293 cells. Mol. Cells 27, 167–173 [DOI] [PubMed] [Google Scholar]
- 33. Ford C. E., Skiba N. P., Bae H., Daaka Y., Reuveny E., Shekter L. R., Rosal R., Weng G., Yang C. S., Iyengar R., Miller R. J., Jan L. Y., Lefkowitz R. J., Hamm H. E. (1998) Molecular basis for interactions of G-protein βγ subunits with effectors. Science 280, 1271–1274 [DOI] [PubMed] [Google Scholar]
- 34. Riven I., Iwanir S., Reuveny E. (2006) GIRK channel activation involves a local rearrangement of a preformed G-protein channel complex. Neuron 51, 561–573 [DOI] [PubMed] [Google Scholar]
- 35. Zhu M. H., Chae M., Kim H. J., Lee Y. M., Kim M. J., Jin N. G., Yang D. K., So I., Kim K. W. (2005) Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am. J. Physiol. Cell Physiol. 289, C591–600 [DOI] [PubMed] [Google Scholar]
- 36. Clancy S. M., Fowler C. E., Finley M., Suen K. F., Arrabit C., Berton F., Kosaza T., Casey P. J., Slesinger P. A. (2005) Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels: Evidence for a heterotrimeric G-protein-channel complex. Mol. Cell Neurosci. 28, 375–389 [DOI] [PubMed] [Google Scholar]
- 37. Finley M., Arrabit C., Fowler C., Suen K. F., Slesinger P. (2004) βL-βM loop in the C-terminal domain of G-protein-activated inwardly rectifying K+ channels is important for Gβγ subunit activation. J. Physiol. 555, 643–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zholos A. V., Bolton T. B. (1997) Muscarinic receptor subtypes controlling the cationic current in guinea pig ileal smooth muscle. Br. J. Pharmacol. 122, 885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koike C., Obara T., Uriu Y., Numata T., Sanuki R., Miyata K., Koyasu T., Ueno S., Funabiki K., Tani A., Ueda H., Kondo M., Mori Y., Tachibana M., Furukawa T. (2010) TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. U.S.A. 107, 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang P., Yang C., Delay R. J. (2010) Odors activate dual pathways, a TRPC2 and an AA-dependent pathway, in mouse vomeronasal neurons. Am. J. Physiol. Cell Physiol. 298, C1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freichel M., Suh S. H., Pfeifer A., Schweig U., Trost C., Weissgerber P., Biel M., Philipp S., Freise D., Droogmans G., Hofmann F., Flockerzi V., Nilius B. (2001) Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat. Cell Biol. 3, 121–127 [DOI] [PubMed] [Google Scholar]
- 42. Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. (2002) Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ. Res. 91, 70–76 [DOI] [PubMed] [Google Scholar]
- 43. Munsch T., Freichel M., Flockerzi V., Pape H. C. (2003) Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc. Natl. Acad. Sci. U.S.A. 100, 16065–16070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.