Background: This study aimed at developing a new set of maurocalcine-derived cell-penetrating peptides from truncation.
Results: Several truncated peptides were designed and evaluated for Cy5 dye cell penetration.
Conclusion: All truncated peptides are competitive cell-penetrating peptides, many of them comparing favorably well with TAT.
Significance: Maurocalcine-derived truncated cell-penetrating peptides differ in their properties, enlarging the potential fields of applications.
Keywords: Cell-penetrating Peptides, Drug Delivery, Intracellular Trafficking, Membrane Biophysics, Membrane Trafficking, Peptide Chemical Synthesis, Peptides, Protein Motifs, Toxins
Abstract
Maurocalcine is the first demonstrated example of an animal toxin peptide with efficient cell penetration properties. Although it is a highly competitive cell-penetrating peptide (CPP), its relatively large size of 33 amino acids and the presence of three internal disulfide bridges may hamper its development for in vitro and in vivo applications. Here, we demonstrate that several efficient CPPs can be derived from maurocalcine by replacing Cys residues by isosteric 2-aminobutyric acid residues and sequence truncation down to peptides of up to 9 residues in length. A surprising finding is that all of the truncated maurocalcine analogues possessed cell penetration properties, indicating that the maurocalcine is a highly specialized CPP. Careful examination of the cell penetration properties of the truncated analogues indicates that several maurocalcine-derived peptides should be of great interest for cell delivery applications where peptide size matters.
Introduction
Maurocalcine (MCa)3 is a 33-mer peptide that was initially isolated from the venom of a Tunisian chactid scorpion, Scorpio maurus palmatus (1). The toxin belongs to a family of peptide that folds according to an inhibitor cystine knot motif and thus contains three disulfide bridges with a Cys1-Cys4, Cys2-Cys5, and Cys3-Cys6 connecting pattern (2). The solution structure, as defined by 1H NMR, illustrates that MCa contains three β-strands (strand 1 from amino acid residues 9–11, strand 2 from 20–23, and strand 3 from 30–33). One distinctiveness of MCa is the fact that it is greatly enriched in basic amino acid residues. Of the 33 amino acids that compose MCa, 12 of them are basic, most of them represented by Lys residues. Interestingly, the β-strands of MCa encompass most of the basic domains (see Fig. 1A). MCa turned to be of interest to our research group for several reasons. First, it is an exquisite pharmacological activator of the ryanodine receptor type 1 (RyR1) from skeletal muscle because it promotes high Po gating modes and long lasting subconductance states of the ion channel (3, 4). On myotubes, application of MCa rapidly induces Ca2+ release from the sarcoplasmic reticulum (SR) (5), a result further confirmed by positive effect of MCa on the release of Ca2+ from purified SR vesicles (3, 5). The interaction of MCa with RyR1 has been witnessed by increased [3H]ryanodine binding onto purified RyR1 (3, 5). The binding site for MCa on RyR1 has also been mapped and shown to correspond to domain(s) that have a predicted localization within the cytoplasm (6). Second, similarly to imperatoxin A (7) for which this was first noted, MCa has an interesting sequence homology with the II–III loop of the L-type calcium channel Cav1.1 subunit over a domain that is slightly larger than the second β-strand of MCa (see Fig. 1A) (5). This loop is predominantly involved in excitation-contraction coupling through direct molecular interactions with RyR1 (6, 8). This homology has been a source of inspiration to an understanding of how toxins may interfere with the process of excitation-contraction coupling (4, 8, 9). Third, and this is the scope of this paper, MCa has been shown to act as a cell-penetrating peptide (CPP) (10). This discovery stemmed from earlier criticisms that MCa may not be an activator of RyR1 because peptide toxins were not known to cross the plasma membrane, which would be required here to bind to RyR1. Studies that were undertaken to demonstrate the ability of MCa to reach its target showed that (i) MCa triggers Ca2+ release from the SR a few seconds after its application in the extracellular medium (5) and (ii) intracellular accumulation of fluorescent-streptavidin occurs if it incubated first with biotinylated MCa (10). Since these pioneering studies, MCa or analogues thereof proved powerful vectors for the cell entry of proteins, peptides (11), nanoparticles, or drugs such as doxorubicin (12–15). Although the mode of cell penetration of MCa may vary according to cargo nature, cell type, or chemical linkage employed, the data gathered so far suggest that the peptide may enter cells according to two priming steps onto the plasma membrane: first an interaction with proteoglycans with an affinity in the micromolar range, followed by a second interaction with negatively charged lipids which occurs with greater affinity (16, 17). The mode of cell entry of MCa is not altered by the absence of proteoglycans, but simply reduced quantitatively, suggesting that proteoglycans do not orient the mode of cell penetration. Two modes seem to concur to MCa cell entry, as far as observed, one related to macropinocytosis and another to membrane translocation. The balance between both modes of entry was found correlated to cargo nature and the type of MCa analogue used. It is of great interest to pursue the study of MCa as CPP despite the wealth of new CPP sequences that are discovered yearly. Among the competitive advantage of MCa over other CPP sequences are the facts that it has almost no associated toxicity in vitro and in vivo, penetrates into cells at very low concentrations, and is extremely stable in vivo upon intravenous injection (over 24 h).4
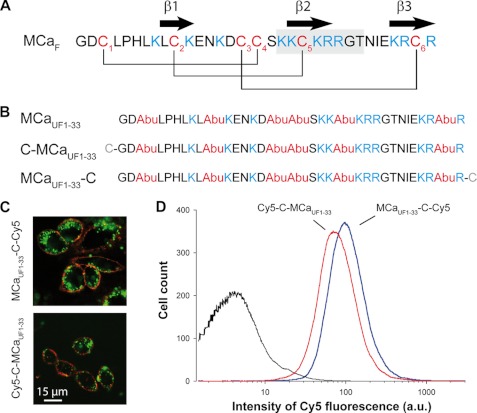
FIGURE 1.
Efficacy of cargo penetration as function of grafting position on MCaUF1–33. A, amino acid sequence of MCaF in single-letter code. The positions of half-cystine residues are highlighted in red. Cys residues are numbered, and basic amino acids are highlighted in blue. Secondary structures (β-strands) are indicated by arrows. The gray box is the sequence of homology of MCa with the dihydropyridine-sensitive Cav1.1 channel. B, amino acid sequences of unfolded MCa analogues in single-letter code. Cys residues are replaced by isosteric 2-aminobutyric acid residues (Abu, in red) to form MCaUF1–33. An additional N-terminal (C-MCaUF1–33) or C-terminal (MCaUF1–33-C) Cys residue was added in two novel analogues competent for cargo grafting (shown in gray). C, confocal microscopy images illustrating cell penetration of Cy5-C-MCaUF1–33 and MCaUF1–33-C-Cy5 (green labeling). Plasma membranes are labeled with concanavalin A-rhodamine (in red). CHO cells were incubated 2 h with 1 μm peptide concentration. D, comparison of cell penetration efficacy between Cy5-C-MCaUF1–33 and MCaUF1–33-C-Cy5 as determined by FACS. CHO cells were incubated for 2 h with 3 μm peptide, washed and treated 5 min by 1 mg/ml trypsin before quantification of intracellular fluorescence. a.u., arbitrary unit.
Although MCa appears as an elaborate and efficient CPP, its pharmacological properties represent a serious hindrance while envisioning in vitro and in vivo applications. In addition, because of its length (33 amino acid residues) and the presence of three disulfide bridges, MCa is a relatively difficult to synthesize CPP, compared with other CPPs, and would benefit from a downsizing approach. Several strategies have been employed successfully in the past to overcome one or both of these issues. The first strategy was based on single-point mutations of the MCa sequence. This strategy preserved the disulfide bridges and the three-dimensional structure of the analogues. Overall, mutations affected more seriously the pharmacology of MCa than the cell penetration properties (18). Many of the amino acids involved in RyR1 binding and pharmacology were located within the cluster of basic amino acids that presented sequence homology with the L-type Cav1.1 channel. Some of these residues, but not all, were also important for cell penetration properties. Hence, several analogues could be defined that kept close to intact cell penetration properties while entirely losing their pharmacological action (MCa R24A for instance). Some other analogues were actually better than MCa itself for cell penetration, suggesting that pairs of mutations, aiming at disrupting pharmacology and improving penetration, may be used in the future to define still better CPP analogues of MCa. The second strategy, which has yield success, is based on the chemical synthesis of d-MCa, an analogue entirely based on the use of d-amino acids. This peptide is a mirror image of the natural l-MCa but, like other d-CPPs, preserves its cell penetration properties while losing entirely its ability to interact with RyR1 (19). This method has several advantages. It no longer is sensitive to proteases that may be an additional advantage for in vivo experiments where the half-life of the circulating peptide matters. It is also possible to improve this analog by introducing point mutations shown previously to improve cell penetration (19). In these two strategies, although being effective, one may argue that (i) the peptides are still among the longest CPP known to date, implying increased costs of production, and (ii) the yield of production of these peptides is hampered by the folding process. Also, the use of peptides with internal disulfide bridges, despite have advantageous features in term of stability in vivo, makes chemical coupling of these CPPs to cargoes more complicated (difficulty to add extra Cys residues to the peptides for instance without interfering with the correct folding process). The third strategy that was used to circumvent one of this criticism was the chemical synthesis of an MCa analog in which all internal Cys residues were replaced by isosteric 2-aminobutyric acid residues (11). The resulting peptide was still 33-mer long but one step in production was saved by avoiding the folding process. In addition, an extra-Cys residue could be added to the N terminus of the peptide to favor simplified cargo grafting on this CPP analogue. This peptide, termed here C-MCaUF1–33 (C for extra-Cys, UF for unfolded, and 1–33 for its length; see Fig. 1B) no longer has any secondary structures, but efficiently penetrates into cells. Interestingly also, the peptide completely lacks pharmacological activity, indicating that folding and secondary structures are essential for binding onto RyR1. Although this peptide is an efficient CPP, it remains less potent than MCa in its folded version, suggesting that further optimization should be brought to this analogue. Such optimization appears feasible on the basis of the fact that MCa fulfills three different functions (pharmacology, obligation to resemble the L-type channel, and cell penetration). We reasoned that because only cell penetration was the quality we searched for, the peptide could be further simplified and novel analogues be designed.
In this study, we undertook to identify new more potent MCa analogues with three criteria in mind. First, these analogues should be shorter than the folded version of MCa (MCaF) or the unfolded version (MCaUF). Second, these peptides should be designed to better delimitate the domains of MCa responsible for cell penetration. Third, we should be able to design analogues with extra free SH functions for cargo grafting. We present several new analogues that have highly potent cell penetration capabilities, while losing pharmacological activity, preserving lack of cell toxicity, and with facilitated cargo grafting. This new generation of MCa analogues is predicted to have bright futures for CPP applications in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Reagents
N-α-Fmoc-l-amino acid, Wang-Tentagel resin, and reagents used for peptide syntheses were obtained from Iris Biotech. Solvents were analytical grade products from Acros Organics. Cy5 maleimide mono-reactive dye was purchased from GE Healthcare.
Solid Phase Peptide Syntheses
Chemical syntheses of MCa analogues were performed as described previously (19). Briefly, analogues of MCa were chemically synthesized by the solid phase method (20) using an automated peptide synthesizer (CEM© Liberty). Peptide chains were assembled stepwise on 0.24 meq of Fmoc-d-Arg-Pbf-Wang-Tentagel resin using 0.24 mmol of Fmoc l-amino acid derivatives. The side chain protecting groups were: Trityl for Cys and Asn; tert-butyl for Ser, Thr, Glu, and Asp; Pbf for Arg; and tert-butylcarbonyl for Lys. Reagents were at the following concentrations: Fmoc-amino acids (0.2 m Fmoc-AA-OH in dimethylformamide), activator (0.5 m 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate in dimethyformamide), activator base (2 m diisopropylethylamine in N-methyl-pyrrolidone) and deprotecting agent (5% piperazine/0.1 m 1-hydroxybenzotriazole in dimethyformamide), as advised by PepDriver (CEM©). After peptide chain assembly, resins were treated 4 h at room temperature with a mixture of TFA/water/triisopropylsilan/dithiothreitol (DTT) (92.5/2.5/2.5/2.5). The peptide mixtures were then filtered, and the filtrates were precipitated by adding cold t-butylmethyl ether. The crude peptides were pelleted by centrifugation (10,000 × g, 15 min), and the supernatants were discarded. MCa analogues were purified by HPLC using a Vydac C18 column (218TP1010, 25 × 10 cm). Elutions of the peptides were performed with a 10–60% acetonitrile linear gradient containing 0.1% TFA. The purified fractions were analyzed by analytical RP-HPLC (Vydac C18 column 218TP104, 25 × 4.6 cm). All analogues were characterized by MALDI-TOF mass spectrometry.
Labeling of Peptide with Cy5
Each peptide was labeled with Cy5 according to the manufacturer's protocol (GE Healthcare). Peptides were dissolved at 1 mg/ml in 0.1 m Na2CO3 buffer, pH 9.3. 300 μl of the solubilized peptides was added to Cy5-maleimide-containing tubes. The mixtures were incubated for 2 h at room temperature and then purified by HPLC using an analytical Vydac C18 column. Elution of the Cy5-labeled peptides was performed with a 10–60% acetonitrile linear gradient containing 0.1% TFA. The pure peak fractions were lyophilized and peptides quantified by UV spectrophotometer at 649 nm.
Cell Culture
Chinese hamster ovary (CHO) and F98 cell lines (from ATCC) were maintained at 37 °C in 5% CO2 in F-12 nutrient medium (Invitrogen) supplemented with 10% (v/v, CHO) or 2% (v/v, F98) heat-inactivated fetal bovine serum (Invitrogen) and 100 units/ml streptomycin and penicillin (Invitrogen).
MTT Assay
Cells were seeded into 96-well micro plates at a density of ∼8 × 104 cells/well. After 2 days of culture, the cells were incubated for 24 h at 37 °C with MCa analogues at a concentration of 10 μm. Control wells containing cell culture medium alone or with cells, both without peptide addition, were included in each experiment. 0.1% saponin was used as toxic agent for comparison. The cells were then incubated with MTT for 30 min. Conversion of MTT into purple colored MTT formazan by the living cells indicates the extent of cell viability. The crystals were dissolved with dimethyl sulfoxide, and the optical density was measured at 540 nm using a microplate reader (Biotek ELx-800; Mandel Scientific Inc.) for quantification of cell viability. All assays were run in triplicate.
Confocal Microscopy
For analysis of the subcellular localization of MCa-Cy5 analogues in living cells, cell cultures were incubated with the fluorescent peptides for 2 h and then washed with phosphate-buffered saline (PBS) alone. The plasma membrane was stained with 5 μg/ml rhodamine-conjugated concanavalin A (Molecular Probes) for 5 min. Cells were washed once more. Live cells were then immediately analyzed by confocal laser scanning microscopy using a Leica TCS-SPE operating system. Rhodamine (580 nm) and Cy5 (670 nm) were sequentially excited, and emission fluorescence was collected in z-confocal planes of 10–15-nm steps.
Fluorescence-activated Cell Sorting
CHO cells were incubated with various concentrations of Cy5-labeled peptides in F-12K culture medium without serum at 37 °C for 2 h. The cells were then washed with PBS to remove excess extracellular peptide and treated with 1 mg/ml trypsin (Invitrogen) for 5 min at 37 °C to detach cells from the surface and centrifuged at 200 × g before suspension in PBS. For experiments with the macropinocytosis inhibitor, amiloride, CHO cells were initially washed with F-12K and preincubated for 30 min at 37 °C with 1 mm amiloride (Sigma). The cells were then incubated for 2 h at 37 °C with 1 μm Cy5-MCa analogues. For all of these experimental conditions, flow cytometry analyses were performed with live cells using a Becton Dickinson FACS LSR II flow cytometer (BD Biosciences). Data were obtained and analyzed using FCS express software (De Novo). Live cells were gated by forward/side scattering from a total of 10,000 events.
Preparation of Heavy SR Vesicles
Heavy SR vesicles were prepared following the method of Kim et al. (21). Protein concentration was measured by the Biuret method.
[3H]Ryanodine Binding Assay
Heavy SR vesicles (1 mg/ml) were incubated at 37 °C for 2 h in an assay buffer composed of 10 nm [3H]ryanodine, 150 mm KCl, 2 mm EGTA, 2 mm CaCl2 (pCa = 5), and 20 mm MOPS, pH 7.4. Truncated MCa analogues were added prior to the addition of heavy SR vesicles. [3H]Ryanodine bound to heavy SR vesicles was measured by filtration through Whatman GF/B glass filters followed by three washes with 5 ml of ice-cold washing buffer composed of 150 mm NaCl, 20 mm HEPES, pH 7.4. [3H]Ryanodine retained on the filters was measured by liquid scintillation. Nonspecific binding was measured in the presence of 80 μm unlabeled ryanodine. The data are presented as mean ± S.E. Each experiment was performed in triplicate.
Statistical Analyses
All data are given as mean ± S.D. for n number of observations, and statistical significance (p) was calculated using Student's t test.
RESULTS
Nonfolded Truncated Maurocalcine Peptides Are Efficient CPPs
Fig. 1A illustrates the primary structure of MCa with its secondary structures (β-strands) and its pattern of disulfide bridges. This peptide will be termed MCaF, for folded (F) MCa. An earlier report has demonstrated that replacing the six internal cysteine residues of MCa by Abu residues results in a pharmacologically inert and unfolded (UF) CPP (MCaUF1–33, Fig. 1B). This peptide loses its secondary structures (11). Because this project aims at identifying shorter CPP sequences based on MCaUF1–33 sequence by the delivery of Cy5 cargo, we first determined where at the N terminus (C-MCaUF1–33) or C terminus (MCaUF1–33-C) the cargo could be best grafted after addition of an extra cysteine residue (C) (Fig. 1B). As shown, both vector-cargo complexes Cy5-C-MCaUF1–33 and MCaUF1–33-C-Cy5 penetrated efficiently within CHO cells, as estimated by confocal microscopy (Fig. 1C) or by FACS (Fig. 1D). At 3 μm, a slightly better cell penetration was observed with Cy5 localized at the C terminus of MCaUF1–33, but this difference was not significant. Because chemical syntheses of truncated MCaUF1–33 analogues was facilitated by adding the extra cysteine residue at the C terminus of the sequence rather than at the N terminus, we kept on working on the basis of MCaUF1–33-C sequence. Nevertheless, these data indicate for the first time that cargo grafting on the CPP MCaUF1–33 can be performed likewise at both extremities of the sequence.
Next, we designed a series of truncated MCaUF-C peptides comprising either a C-terminal truncation (three analogues: MCaUF1–20-C, MCaUF1–15-C, and MCaUF1–9-C), a N-terminal truncation (7 analogues: MCaUF8–33-C, MCaUF11–33-C, MCaUF14–33-C, MCaUF18–33-C, MCaUF20–33-C, MCaUF22–33-C, and MCaUF25–33-C), and Both N- and C-terminal truncations (2 analogues: MCaUF6–25-C and MCaUF14–25-C) (Fig. 2A). All of these analogues were then labeled with Cy5 to investigate their cell penetration properties. Every one of these peptides has been designed in such a way that the cargo would be removed from the peptide upon trypsin cleavage. This was useful for the FACS experiments in which the fluorescence associated to the cells is measured after trypsin treatment, thereby potentially removing the cargo from peptides that would eventually be associated with the outer part of the plasma membrane. The net positive charges of the peptides were drastically different, ranging from 0 (MCaUF1–15-C and MCaUF1–9-C) to +8 (MCaUF8–33-C). However, many of the peptides contained a percentage of positively charged residues equal (MCaUF-25–33-C) or superior to MCaF or MCaUF1–33 (8 of 12 analogues). Three analogues had a lower percentage of basic residues than MCaF (all three C-terminal truncated analogues, MCaUF1–20-C, MCaUF1–15-C, and MCaUF1–9-C).
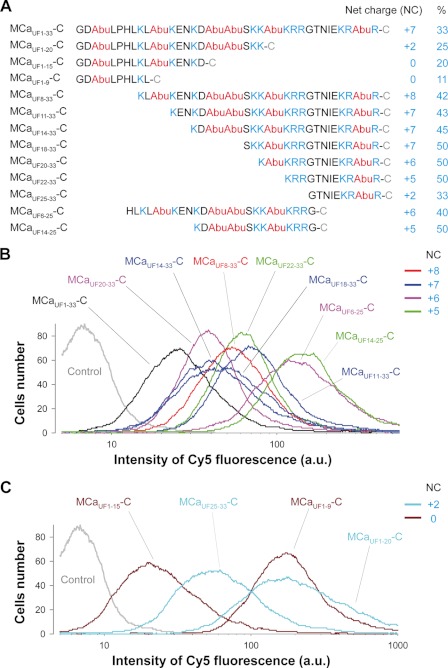
FIGURE 2.
Primary structure of truncated MCaUF analogues and comparison of cell penetration efficacies. A, primary structures of truncated MCaUF-C analogues and determination of their net positive charge and percentage of basic amino acid residues within the sequence. A total of 12 truncated MCaUF-C analogues were produced (three with truncations in C terminus, seven in N terminus, and two in both N and C termini). Positively charged residues are in blue (His residues were not counted), whereas Abu residues that replace Cys residues are in red. B, comparative cell penetration efficacy of all MCaUF-C-Cy5 truncated analogues that possess a net positive charge ≥ +5. Code colors: red (net charge +8), blue (+7), pink (+6), and green (+5). The nontruncated MCaUF1–33-C-Cy5 analogue is shown as reference (black line) for the efficacy of cell penetration of all analogues. Experimental conditions: CHO cell incubation with 1 μm concentration of each analogue for 2 h and fluorescence quantification by FACS. a.u., arbitrary unit. C, same as B but for truncated MCaUF-C-Cy5 analogues with positive net charge ≤ +2.
We first evaluated by FACS the fluorescence accumulation within CHO cells that occurred after a 2 h incubation with 3 μm positively charged MCa peptides (net charge ≥ +5; Fig. 2B). This first study revealed several unexpected findings. First, all of the charged peptides (8 tested) demonstrated CPP properties. These peptides all had the K22R23R24 sequence in common, a cluster of basic amino acid residues shown to contribute to the dose efficacy of cell penetration of MCaF in an earlier study (18). Interestingly, removing the last 8 C-terminal amino acids of MCa had little impact on the cell penetration properties (if one compares MCaUF14–25-C with MCaUF14–33-C). Similarly, the removal of the amino acid region His6-Asn13 did not drastically change cell penetration properties (MCaUF6–25-C versus MCaUF14–25-C). Second, all peptides appeared to behave better than the reference peptide MCaUF1–33-C, suggesting that sequence truncation of MCaUF may represent a potent strategy to define more efficient CPPs. Less positively charged peptides were also tested for their ability to penetrate into CHO cells (Fig. 2C). No less surprisingly, all peptides showed CPP properties, including two peptides with no net positive charge (MCaUF1–9-C and MCaUF1–15-C). MCaUF1–9-C appeared as a better CPP than MCaUF1–15-C, suggesting that the Abu10-Asp15 region introduces no competitive advantage and confirming results shown in Fig. 2B. This may represent an inhibitory region because of the presence of Glu12 and Asp15, two negatively charged residues. The finding that mutation of Glu12 to Ala enhances cell penetration of both MCaF (18) and MCaUF (11) further supports this conclusion. MCaUF25–33-C turned out to have CPP properties also, even though this sequence did not confer a competitive advantage to other MCa CPP analogues as shown in Fig. 2B. Two additional experiments were conducted to confirm the specificity of these findings. First, a truncated charybdotoxin peptide was synthesized (ChTxUF1–12-C) in which the internal Cys residue was replaced by Abu and an additional C-terminal Cys residue added for Cy5 labeling, as for our MCaUF analogues (supplemental Fig. 1A). As shown by confocal microscopy, ChTxUF1–12-C was unable to deliver the Cy5 cargo at a higher concentration of 5 μm. Second, we also designed two MCaUF peptides in which, instead of using Abu derivatives, we mutated internal Cys residues by Ala residues. As shown, both MCaUF1–9(Ala)-C and MCaUF14–25(Ala)-C worked well for Cy5 delivery into CHO cells, demonstrating that Abu residues were not responsible by themselves for the cell penetration properties of the peptides (supplemental Fig. 1, B and C). Ala residues were, however, not fully equivalent to Abu residues as dose-response curve was slightly better for MCaUF14–25(Ala)-C than for MCaUF14–25-C, further arguing that Abu residues were not central to the cell penetration properties of truncated MCaUF peptides (supplemental Fig. 1D). The overall message from this first study is that all truncated MCaUF analogues can behave as CPPs at the concentration tested. The findings suggest that MCa is a peptide fully specialized to achieve cell penetration including in domains that are not highly charged.
Intracellular Distribution of All Truncated MCaUF Analogues Bears Resemblance to That of Full-length MCaUF
Although all truncated derivatives of MCaUF1–33 show cell penetration properties according to the FACS analyses, we examined whether there were differences in intracellular distribution among these peptides. This question was investigated by confocal microscopy after 2 h of peptide accumulation into CHO cells (Fig. 3). Interestingly, all peptides showed very similar intracellular distributions, although the degree of accumulated cell fluorescence varied somewhat with peptide sequences. In confirmation of the FACS results, the peptide that appeared to penetrate the least was the full-length unfolded MCa, MCaUF1–33-C-Cy5 (Fig. 3A). The vast majority of the fluorescence appears in punctuate dots within the cells. In many cases, these dots appear at higher concentrations within one pole of the cell (see labeling of MCaUF8–33-C-Cy5, MCaUF11–33-C-Cy5, MCaUF25–33-C-Cy5, and MCaUF1–9-C-Cy5, for instance). On various occasions also, all of the peptides tend to present a subplasma membrane distribution, forming a rim of smaller circumference than the concanavalin A labeling itself. This subplasma membrane rim localization was more evident for CHO cells labeled with MCaUF14–25-C-Cy5 as illustrated in Fig. 4A. Finally, more rarely, a direct plasma membrane labeling by the peptide-cargo complex was observable (Fig. 4B). This type of labeling could be observed with N-terminal truncated vectors exclusively and was most evident for MCaUF22–33-C-Cy5. The staining of the plasma membrane was always diffuse in contrast to intracellular staining which was mainly punctuated. Diffuse membrane labeling was also observed for MCaUF25–33-C-Cy5 and MCaUF20–33-C-Cy5, two peptides that differ from 2 to 3 amino acids of MCaUF22–33-C-Cy5. It was difficult to evidence for the other vector-cargo complexes. We propose that this staining coincides with an alteration of the duration of peptide plasma membrane residency for these truncated MCaUF analogues. The lower occurrence of this diffuse staining for the other truncated variants may reflect faster internalization by endocytosis and/or membrane translocation. Globally, these effects reflect cell entry and distribution tendencies that were hard to quantify, and they should therefore be interpreted with caution.
FIGURE 3.
All truncated MCaUF-C-Cy5 vector-cargo complexes have resembling intracellular distributions. A, intracellular distribution of N-terminal truncated MCaUF analogues. B, intracellular distribution of C-terminal truncated MCaUF analogues. C, intracellular distribution of N- and C-terminal truncated MCaUF analogues. CHO cells were incubated for 2 h with 3 μm MCaUF-C-Cy5 vector-cargo complexes, before extensive washing, membrane labeling with rhodamine-conjugated concanavalin A, and live cell confocal microscopy imaging. Cy5 is in blue, and rhodamine is in red. White arrows illustrate a tendency for a preferential apical localization of the Cy5 dye. Yellow arrows illustrate the tendency for a subplasma membrane labeling of the Cy5 dye.
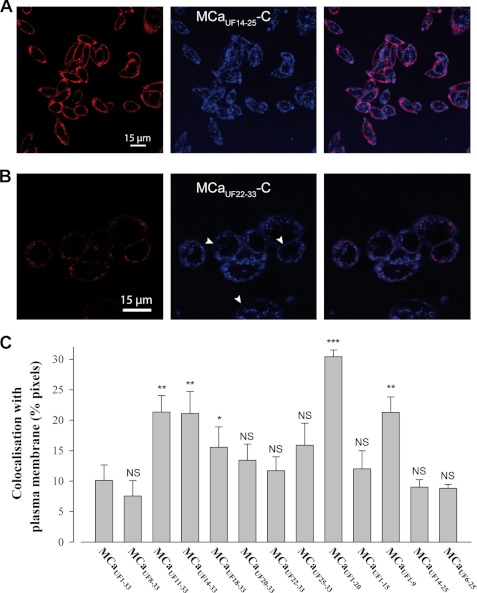
FIGURE 4.
Membrane staining is diffuse whereas intracellular staining is punctuated. A, lower magnification image of CHO cells stained with 3 μm MCaUF14–25-C-Cy5 that illustrates a predominant subplasma membrane rim-like distribution. B, diffuse membrane staining of CHO cells by MCaUF22–33-C-Cy5. White arrows indicate domains of the plasma membrane where the diffuse staining of the peptide-cargo complex is the most evident. C, extent of colocalization of the Cy5-labeled peptides with the rhodamine-labeled plasma membrane. NS, nonsignificant; *, ≤0.1; **, ≤0.05; and ***, ≤0.001.
In an attempt to better apprehend peptide behavior at the plasma membrane, we quantified the extent of Cy5/rhodamine staining colocalization. Rhodamine-positive staining was also Cy5-positive for 63–86% of the pixels (best performing peptides were MCaUF14–33-C-Cy5, MCaUF18–33-C-Cy5, MCaUF20–33-C-Cy5, and MCaUF22–33-C-Cy5; data not shown). This finding indicates that the peptides invade large membrane areas and that membrane interaction is not limited to small specialized surface areas. In contrast, Cy5-positive pixels were rhodamine-positive to far more variable extents (Fig. 4C). For instance, 10.1 ± 2.6% of MCaUF1–33-C-Cy5, the reference compound, was colocalized with the plasma membrane indicator. Despite the fact that short plasma membrane staining times were used (few minutes), a fraction of the colocalization that is quantified also corresponds to intracellular staining following ongoing endocytosis. Nevertheless, this result indicates that this peptide does not remain stuck within the plasma membrane during its 2-h incubation with CHO cells. It thus indicates relatively fast cell penetration. Many of the other peptides, however, behaved differently from MCaUF1–33-C-Cy5. Indeed, several peptides show surprisingly higher colocalization with rhodamine (21.3 ± 2.6% for MCaUF11–33-C-Cy5 and 30.4 ± 1.4% for MCaUF1–20-C-Cy5, for instance). These higher values of colocalization indicate that some peptides remain for longer periods of time or at higher concentration within the plasma membrane. Alternatively, these peptides may rely more heavily on endocytosis for cell penetration and are present within intracellular organelles to which subsequent endocytotic vesicles that contain rhodamine labeling will fuse. Peptides most concerned by these behaviors were MCaUF11–33-C-Cy5 and MCaUF14–33-C-Cy5, which contained two or one of the CPP inhibitory negative charges (Glu12 and Asp15), and MCaUF1–9-C-Cy5 and MCaUF1–20-C-Cy5, which were poorly charged peptides.
Amiloride Sensitivity of Cell Penetration of Truncated MCaUF Analogues
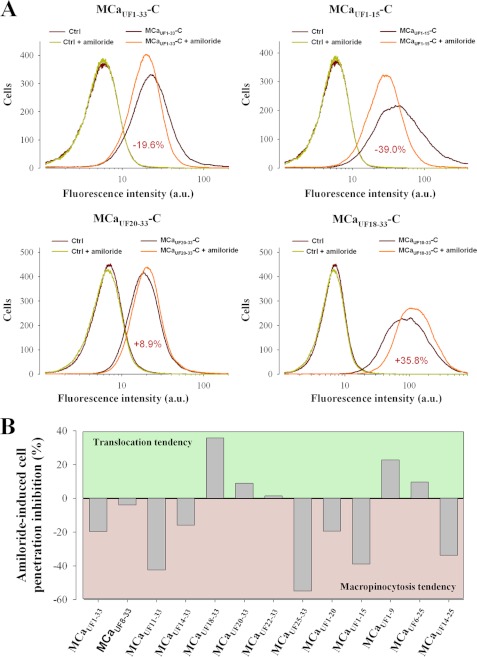
In earlier studies, we have demonstrated that the cell entry of MCaUF1–33 was largely sensitive to amiloride. Because amiloride is an exquisite blocker of macropinosomes (22–24), this suggested a predominant macropinocytosis mechanism for its cell penetration (17). However, it was likely that such a predominant reliance on macropinocytosis was also conferred by the cargo type transported (streptavidin in that report). We therefore conducted an in-depth analysis of the amiloride sensitivity of the various truncated MCaUF peptides with Cy5 as cargo and quantified by FACS the degree of cell penetration inhibition. Fig. 5A illustrates the amiloride sensitivity of four different truncated peptides. As shown, amiloride inhibits the cell penetration of MCaUF1–33-C-Cy5 by 19.6% and of MCaUF1–15-C-Cy5 by 39%. The finding that amiloride blocks to a far lesser extend the penetration of Cy5 compared with that of streptavidin (17) when MCaUF1–33 is the vector indicates the influence of the cargo nature on the mechanism of cell entry. Surprisingly, amiloride was found to enhance rather than inhibit the cell penetration of MCaUF20–33-C-Cy5 and MCaUF18–33-C-Cy5 (Fig. 5A). Preserving the plasma membrane from undergoing macropinocytosis may free surface areas for enhanced peptide translocation through the membrane. The effect of amiloride was always associated with a sharpening of the fluorescence intensity distribution in the x axis (see, for instance MCaUF1–15-C-Cy5), reflecting reduced cell heterogeneity for the mechanisms underlying peptide penetration. The amiloride sensitivity of cell penetration was further investigated for all truncated MCaUF peptides, and the results are presented in Fig. 5B. Four peptides showed higher amiloride sensitivity than MCaUF1–33-C-Cy5 (MCaUF11–33-C-Cy5, MCaUF25–33-C-Cy5, MCaUF1–15-C-Cy5, and MCaUF14–25-C-Cy5). All other peptides showed reduced amiloride sensitivities or a tendency for greater cell penetration under the effect of amiloride. We conclude that the Cy5 cargo does not promote macropinocytosis as the main route of peptide entry and that truncation of MCaUF may lead to analogues that rely to a lesser extent on macropinocytosis for cell entry.
FIGURE 5.
Amiloride sensitivity of truncated MCaUF peptide cell entry. A, representative FACS analyses of the effect of 5 mm amiloride on MCaUF1–33-C-Cy5 (upper left), MCaUF1–15-C-Cy5 (upper right), MCaUF20–33-C-Cy5 (lower left), and MCaUF18–33-C-Cy5 (lower right) entries. Numbers in red represent average decrease or increase in peptide entry upon amiloride treatment. Cells were treated for 2 h with 3 μm peptide concentration with or without 5 mm amiloride. a.u., arbitrary unit. B, average effect of amiloride on mean cell entry of the truncated peptides. Positive values reflect increase in cell entries, whereas negative values indicate reduction in cell penetration.
Comparative Dose-dependent Cell Penetration of MCaUF Analogues
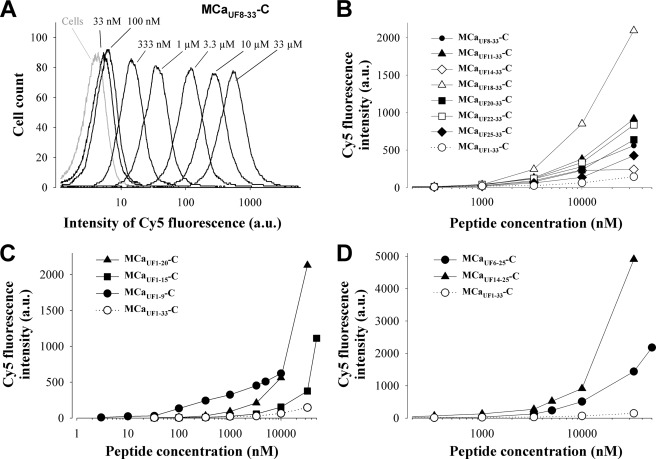
Although we compared the properties of cell penetration of truncated peptides at rather mild concentrations, we also aimed at comparing the dose dependence of cell penetration of these peptides by FACS (Fig. 6). One example of such an analysis is shown for peptide MCaUF8–33-C-Cy5 in Fig. 6A. 33 μm was the highest concentration that could be tested on CHO cells, and obviously cell penetration did not show any sign of saturation for cell incubation times with this peptide of 2 h. The dose-dependent cell penetrations were compared for all N-terminal truncated peptides (Fig. 6B), C-terminal truncated peptides (Fig. 6C), and double truncated analogues (Fig. 6D) with the same settings. These analyses confirm that MCaUF1–33-C-Cy5 is the least performing cell-penetrating peptide. Most truncated peptides show detectable cell penetration at concentrations ≥1 μm. One remarkable exception to this rule was noticeable. MCaUF1–9-C-Cy5 shows an unusual dose-dependent penetration with detectable cell penetration at 10 nm and only small progressive increases in fluorescence intensity with higher peptide concentrations (Fig. 6C). This peptide was therefore the best performing peptide for cell penetration at low concentrations. Finally, additional information that could be taken from these analyses is that the peptides differed significantly with regard to the maximal extent of cell penetration. Among the N-terminal truncated MCaUF analogues, MCaUF18–33-C-Cy5 performed drastically better than the other truncated peptides (Fig. 6B). The difference in cell penetration among MCaUF11–33-C-Cy5 and MCaUF18–33-C-Cy5 resides in the removal of the KENKDAbuAbu sequence which we presume is inhibitory to some extent because of the presence of Glu12 and Asp15. Among the C-terminal truncated peptides, MCaUF1–20-C-Cy5 was performing as well as MCaUF8–33-C-Cy5, and although not tested at higher concentrations, MCaUF1–9-C-Cy5 would be expected to perform still better. Finally, for N- and C-terminal truncated analogues, the best peptide turns out to be MCaUF14–25-C-Cy5, which yields the greatest fluorescence accumulation at 33 μm compared with all other truncated MCaUF analogues. Whereas all of these peptides performed quite well, we were curious to compare them with a classical CPP under similar experimental conditions. Cell penetration was observed for TAT-C in CHO cells, and the dose-response curve was equivalent or even less favorable for TAT than for many MCaUF peptides (supplemental Fig. 1, E and F). This was further largely confirmed when comparing the vector properties of TAT-C and MCaUF1–9-C in yet another cell type, the glioma F98 rat cell line (supplemental Fig. 2). 3 μm MCaUF1–9-C proved better than TAT-C at 3 and 10 μm.
FIGURE 6.
Dose-dependent cell penetration of truncated MCaUF peptides. A, representative example of the dose-dependent cell penetration of MCaUF8–33-C-Cy5 in CHO cells as analyzed by FACS. The peptide was incubated for 2 h with the cells before analyses. There was no saturation of cell entry for a concentration up to 33 μm. B, dose-dependent cell penetration of N-terminal truncated MCaUF peptides compared with MCaUF1–33-C-Cy5 (open circles, dotted line). a.u., arbitrary unit. C, dose-dependent cell penetration of C-terminal truncated MCaUF peptides. D, dose-dependent cell penetration of N- and C-terminal truncated MCaUF peptides. Note the increase in scale for the penetration of these two peptides.
Truncated MCaUF Peptides Lack Pharmacological Effects and Are Predominantly Nontoxic
An earlier report has shown that MCaUF1–33 is unable to interact with the MCa target, RyR1 (11). This is due to the loss of secondary structures because of the lack of internal disulfide bridging. We did therefore expect that truncated analogues of MCaUF should also be pharmacologically inert. This hypothesis was challenged by testing the ability of the Cy5-free peptides to stimulate [3H]ryanodine binding (Fig. 7A). As shown, contrary to MCaF, which contains secondary structures and disulfide bridges, none of the peptides we designed had an effect on [3H]ryanodine binding.
FIGURE 7.
Lack of pharmacological effects of the truncated peptides and reduced cell toxicity. A, effect of MCaF, MCaUF1–33, and truncated MCaUF peptides on [3H]ryanodine binding. Data are expressed as -fold increase in binding induced by the peptides. B, effect of 1 and 10 μm MCaUF1–33 and truncated MCaUF peptides on CHO cell viability. Peptides were incubated for 24 h with the cells in vitro.
Finally, the peptides were challenged for their toxicity by incubating CHO cells with 1 or 10 μm peptide concentrations for an extended duration (24 h) that far exceeds the duration challenged for cell penetration (Fig. 7B). A 10 μm peptide concentration was generally slightly more toxic than 1 μm, except for MCaUF14–25-C. At 1 μm, toxicity never exceeded 8%, and significances of these effects were negligible. In contrast, toxicity could reach 20% at 10 μm peptide concentration, and these effects had higher significance. Most peptides behaved equally well or better than MCaUF1–33-C, indicating that truncation did not enhance cell toxicity of the peptides.
DISCUSSION
MCaF is a rather large and complex CPP with its three disulfide bridges which makes its use in in vitro and in vivo applications more delicate because of production yield (peptide with correct disulfide bridging) and cargo coupling (thiol groups are for the moment difficult to use). One may also argue that the size of the peptide introduces additional synthesis cost compared with other popular in use CPPs such as Tat and penetratin. Whereas size and folding most likely add a competitive advantage for in vivo applications because of peptide stability issues, it may be of interest to derive small CPPs from MCa that could have a broader use than MCa itself. The study we conducted not only aimed at defining these smaller MCa-derived CPP sequences, but also provided interesting clues on how MCa may have evolved for cell penetration. The most surprising finding from this study was therefore that all of the analogues we defined could be considered as CPPs, which suggests that MCa is a highly specialized sequence for the purpose of cell penetration. Although evidently none of the peptides could compete with MCaF itself for cell penetration, it is nevertheless obvious that many of the truncated MCaUF peptides are better CPPs than MCaUF1–33 itself. These findings lead to two general conclusions. First, folding and disulfide bridging appear to be prerequisites to properly optimizing the CPP potential of all of the MCa domains. It is highly likely that the secondary structures triggered by disulfide bridging play a key role in that respect. We therefore believe that it would be worthwhile to design additional MCa analogues presenting both truncations (like in the present study) and 1 to 2 disulfide bridges to regain some of the secondary structures that confer a competitive advantage to MCaF for cell penetration. Second, the fact that all of the truncated sequences are CPPs that possess different properties and efficacies suggests that there is further room for MCa cell penetrating optimization. In this study, our data indicate that the amino acid region Lys11-Ser18 would be an ideal target for mutagenesis. Glu12 mutation has already been shown to improve cell penetration on various analogues (11, 18). We now sense that Asp15 may also represent an excellent target for further optimization of MCa properties. Double mutagenesis may be an option. However, it will be essential to determine whether the disulfide bridging and folding ability of the native peptide is not affected by this procedure. Truncated MCaUF peptides may also benefit from further mutagenesis of negatively charged residues, such as MCaUF1–20. It is noteworthy that another well behaving truncated CPP, MCaUF18–33, also contains a Glu residue at position 29 that could represent another target for mutagenesis and peptide optimization. This is also the case for MCaUF1–9 that contains an Asp residue at position 2.
Several other findings of this report merit some comments. First, we demonstrate that cargo coupling can occur at the N terminus as well as the C terminus of MCa, enhancing the flexibility of cargo coupling to our vectors. Second, although it is obvious that most of our truncated MCaUF analogues remain heavily basic, we also found out that poorly charged MCa peptides could behave as efficient CPPs. This is the case for MCaUF1–9 which is one of our best performing CPPs, especially if one wishes to work with low CPP concentrations. Third, small size CPPs can be derived from MCaUF, MCaUF1–9, and MCaUF25–33 being our smallest peptides. Although we did not test greater truncation, it is not impossible that still smaller CPPs might be designed on the basis of MCa sequence. Fourth, the truncated MCaUF analogues differ somewhat in their mode of cell penetration, some being more prone to enter cells by macropinocytosis than others. Various peptides were even insensitive to amiloride application, suggesting that macropinocytosis did not contribute at all to their entry. We cannot exclude at this stage that these peptides rely on modes of endocytosis other than macropinocytosis. This is suggested by the punctuate nature of intracellular Cy5 fluorescence distribution. How reliably a punctuate distribution reveals endocytosis is however not known, and one cannot rule out also that this distribution is due to peptide aggregation with or without lipid components. When plasma membrane distribution could be observed, large diffuse staining was evident, indicating that when peptides encountered the membrane no such aggregation occurred. In any case, many of the peptides had distribution that mostly differed from plasma membrane labeling (including these membranes that had undergone endocytosis), indicating that peptide intracellular distribution may not necessarily colocalize with the plasma membrane components. Refined studies will be required, however, to define how much of the peptides enter cells by direct membrane translocation versus endocytosis.
In conclusion, we identified several interesting lead CPPs based on MCaUF truncation strategy. This is the case for MCaUF18–33 (macropinocytosis entry-independent), MCaUF1–9 (penetrates better at low concentration), and MCaUF14–25 (yields the greatest cell entry of the dye). These peptides are easy to produce, yield good cell penetration, and we should be able to further improve their cell penetrating characteristics by mutagenesis or by reintroducing one disulfide bridge to restore some of the secondary structures. Although the scope of this study was not to compare our mini-MCa with other popular CPPs, we were surprised to find out that many of the MCaUF vectors behaved far better than TAT for the transport of Cy5, both in CHO and F98 cells. It may be argued that the field does not require many more CPP sequences. However, a significant fraction of the ongoing research is based on the use of TAT, which no longer appears as the most competitive peptide. Also, increasing evidence suggests that CPP sequences differ in their cell type targeting properties, which will undoubtedly represent an important feature to exploit when it comes to developing in vivo applications. Other essential parameters for in vivo applications will be the peptide stability upon intravenous or intraperitoneal injection. In that respect, we found out that MCa is a particularly stable peptide with and without disulfide bridges.4 These still “hidden” aspects of the CPP features may be advantageously exploited later on if the number of known CPPs is high. The advantages of using smaller CPPs over MCaF remains to be investigated as one may of course argue also that they require higher concentrations and possibly longer incubation times for effectiveness, two factors that may lead to spurious signaling events in vivo.
Supplementary Material
This work was supported by grants from Technology pour la Santé (Program TIMOMA2 of the Commissariat à l'Energie Atomique) and from Agence Nationale pour la Recherche PNANO (Programs SYNERGIE and NanoFret). Mass spectrometry analyses were performed by the Centre d'Investigation Clinique of Grenoble under the direction of Dr. Michel Sève.

This article contains supplemental Figs. 1 and 2.
C. Poillot, H. Bichraoui, C. Tisseyre, E. Bahemberae, N. Andreotti, J.-M. Sabatier, M. Ronjat, and M. De Waard, unpublished data.
- MCa
- maurocalcine
- Abu
- 2-aminobutyric acid
- CPP
- cell-penetrating peptide
- F
- folded
- Fmoc
- N-(9-fluorenyl)methoxycarbonyl
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- RyR
- ryanodine receptor
- SR
- sarcoplasmic reticulum
- UF
- unfolded.
REFERENCES
- 1. Fajloun Z., Kharrat R., Chen L., Lecomte C., Di Luccio E., Bichet D., El Ayeb M., Rochat H., Allen P. D., Pessah I. N., De Waard M., Sabatier J. M. (2000) Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+ release channel/ryanodine receptors. FEBS Lett. 469, 179–185 [DOI] [PubMed] [Google Scholar]
- 2. Mosbah A., Kharrat R., Fajloun Z., Renisio J. G., Blanc E., Sabatier J. M., El Ayeb M., Darbon H. (2000) A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. Proteins 40, 436–442 [DOI] [PubMed] [Google Scholar]
- 3. Chen L., Estève E., Sabatier J. M., Ronjat M., De Waard M., Allen P. D., Pessah I. N. (2003) Maurocalcine and peptide A stabilize distinct subconductance states of ryanodine receptor type 1, revealing a proportional gating mechanism. J. Biol. Chem. 278, 16095–16106 [DOI] [PubMed] [Google Scholar]
- 4. Lukács B., Sztretye M., Almássy J., Sárközi S., Dienes B., Mabrouk K., Simut C., Szabó L., Szentesi P., De Waard M., Ronjat M., Jóna I., Csernoch L. (2008) Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor. Biophys. J. 95, 3497–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estève E., Smida-Rezgui S., Sarkozi S., Szegedi C., Regaya I., Chen L., Altafaj X., Rochat H., Allen P., Pessah I. N., Marty I., Sabatier J. M., Jona I., De Waard M., Ronjat M. (2003) Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells. J. Biol. Chem. 278, 37822–37831 [DOI] [PubMed] [Google Scholar]
- 6. Altafaj X., Cheng W., Estève E., Urbani J., Grunwald D., Sabatier J. M., Coronado R., De Waard M., Ronjat M. (2005) Maurocalcine and domain A of the II–III loop of the dihydropyridine receptor Cav 1.1 subunit share common binding sites on the skeletal ryanodine receptor. J. Biol. Chem. 280, 4013–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurrola G. B., Arévalo C., Sreekumar R., Lokuta A. J., Walker J. W., Valdivia H. H. (1999) Activation of ryanodine receptors by imperatoxin A and a peptide segment of the II–III loop of the dihydropyridine receptor. J. Biol. Chem. 274, 7879–7886 [DOI] [PubMed] [Google Scholar]
- 8. Szappanos H., Smida-Rezgui S., Cseri J., Simut C., Sabatier J. M., De Waard M., Kovács L., Csernoch L., Ronjat M. (2005) Differential effects of maurocalcine on Ca2+ release events and depolarization-induced Ca2+ release in rat skeletal muscle. J. Physiol. 565, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pouvreau S., Csernoch L., Allard B., Sabatier J. M., De Waard M., Ronjat M., Jacquemond V. (2006) Transient loss of voltage control of Ca2+ release in the presence of maurocalcine in skeletal muscle. Biophys. J. 91, 2206–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Estève E., Mabrouk K., Dupuis A., Smida-Rezgui S., Altafaj X., Grunwald D., Platel J. C., Andreotti N., Marty I., Sabatier J. M., Ronjat M., De Waard M. (2005) Transduction of the scorpion toxin maurocalcine into cells: evidence that the toxin crosses the plasma membrane. J. Biol. Chem. 280, 12833–12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ram N., Weiss N., Texier-Nogues I., Aroui S., Andreotti N., Pirollet F., Ronjat M., Sabatier J. M., Darbon H., Jacquemond V., De Waard M. (2008) Design of a disulfide-less, pharmacologically inert, and chemically competent analog of maurocalcine for the efficient transport of impermeant compounds into cells. J. Biol. Chem. 283, 27048–27056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aroui S., Brahim S., De Waard M., Bréard J., Kenani A. (2009) Efficient induction of apoptosis by doxorubicin coupled to cell-penetrating peptides compared to unconjugated doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer Lett. 285, 28–38 [DOI] [PubMed] [Google Scholar]
- 13. Aroui S., Brahim S., Hamelin J., De Waard M., Bréard J., Kenani A. (2009) Conjugation of doxorubicin to cell-penetrating peptides sensitizes human breast MDA-MB 231 cancer cells to endogenous TRAIL-induced apoptosis. Apoptosis 14, 1352–1365 [DOI] [PubMed] [Google Scholar]
- 14. Aroui S., Brahim S., Waard M. D., Kenani A. (2010) Cytotoxicity, intracellular distribution and uptake of doxorubicin and doxorubicin coupled to cell-penetrating peptides in different cell lines: a comparative study. Biochem. Biophys. Res. Commun. 391, 419–425 [DOI] [PubMed] [Google Scholar]
- 15. Aroui S., Ram N., Appaix F., Ronjat M., Kenani A., Pirollet F., De Waard M. (2009) Maurocalcine as a nontoxic drug carrier overcomes doxorubicin resistance in the cancer cell line MDA-MB 231. Pharm. Res. 26, 836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boisseau S., Mabrouk K., Ram N., Garmy N., Collin V., Tadmouri A., Mikati M., Sabatier J. M., Ronjat M., Fantini J., De Waard M. (2006) Cell penetration properties of maurocalcine, a natural venom peptide active on the intracellular ryanodine receptor. Biochim. Biophys. Acta 1758, 308–319 [DOI] [PubMed] [Google Scholar]
- 17. Ram N., Aroui S., Jaumain E., Bichraoui H., Mabrouk K., Ronjat M., Lortat-Jacob H., De Waard M. (2008) Direct peptide interaction with surface glycosaminoglycans contributes to the cell penetration of maurocalcine. J. Biol. Chem. 283, 24274–24284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mabrouk K., Ram N., Boisseau S., Strappazzon F., Rehaim A., Sadoul R., Darbon H., Ronjat M., De Waard M. (2007) Critical amino acid residues of maurocalcine involved in pharmacology, lipid interaction and cell penetration. Biochim. Biophys. Acta 1768, 2528–2540 [DOI] [PubMed] [Google Scholar]
- 19. Poillot C., Dridi K., Bichraoui H., Pêcher J., Alphonse S., Douzi B., Ronjat M., Darbon H., De Waard M. (2010) d-Maurocalcine, a pharmacologically inert efficient cell-penetrating peptide analogue. J. Biol. Chem. 285, 34168–34180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merrifield R. B. (1969) Solid-phase peptide synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 32, 221–296 [DOI] [PubMed] [Google Scholar]
- 21. Kim D. H., Ohnishi S. T., Ikemoto N. (1983) Kinetic studies of calcium release from sarcoplasmic reticulum in vitro. J. Biol. Chem. 258, 9662–9668 [PubMed] [Google Scholar]
- 22. West M. A., Bretscher M. S., Watts C. (1989) Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veithen A., Cupers P., Baudhuin P., Courtoy P. J. (1996) v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 109, 2005–2012 [DOI] [PubMed] [Google Scholar]
- 24. Meier O., Boucke K., Hammer S. V., Keller S., Stidwill R. P., Hemmi S., Greber U. F. (2002) Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158, 1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.