Background: Numb/Notch signaling is essential for NSC self-renewal. How Numb works is not well defined.
Results: Numb interacts with α-Adaptin through newly defined domains to regulate the endocytosis of Notch pathway components.
Conclusion: Endocytosis is critically involved in balancing the self-renewal versus differentiation choice of NSCs.
Significance: Aberrant Numb/Notch signaling has been associated with tumorigenesis. Results from this study have important implications for cancer biology.
Keywords: Brain Tumors, Drosophila, Endocytosis, Neural Stem Cell, Notch Pathway, AP-2 Complex, Numb
Abstract
The ability to balance self-renewal and differentiation is a hallmark of stem cells. In Drosophila neural stem cells (NSCs), Numb/Notch (N) signaling plays a key role in this process. However, the molecular and cellular mechanisms underlying Numb function in a stem cell setting remain poorly defined. Here we show that α-Adaptin (α-Ada), a subunit of the endocytic AP-2 complex, interacts with Numb through a new mode of interaction to regulate NSC homeostasis. In α-ada mutants, N pathway component Sanpodo and the N receptor itself exhibited altered trafficking, and N signaling was up-regulated in the intermediate progenitors of type II NSC lineages, leading to their transformation into ectopic NSCs. Surprisingly, although the Ear domain of α-Ada interacts with the C terminus of Numb and is important for α-Ada function in the sensory organ precursor lineage, it was dispensable in the NSCs. Instead, α-Ada could regulate Sanpodo, N trafficking, and NSC homeostasis by interacting with Numb through new domains in both proteins previously not known to mediate their interaction. This interaction could be bypassed when α-Ada was directly fused to the phospho-tyrosine binding domain of Numb. Our results identify a critical role for the AP-2-mediated endocytosis in regulating NSC behavior and reveal a new mechanism by which Numb regulates NSC behavior through N. These findings are likely to have important implications for cancer biology.
Introduction
In a stem cell hierarchy, daughter cells originate from the same mother cell can acquire distinct abilities to self-renew or differentiate. Differential cell-cell signaling activities among daughter cells play key roles in establishing and maintaining the diverse cell fates within the lineage. For example, the Notch (N)2 signaling pathway has been demonstrated to be one of the prime signaling pathways in determining differential cell fates within stem cell lineages in various tissues and species (1–6). However, it remains unclear how differential N signaling is established within a stem cell hierarchy in the first place.
Drosophila neural stem cells (NSCs) called neuroblasts (NBs) in the central brain (CB) or ventral nerve cord (VNC) regions provide excellent model systems for understanding signaling events regulating stem cell behavior. The CB area contains eight type II NB lineages (7–9). Within each type II NB lineage, the NB self-renews while giving rise to immature intermediate progenitors (IPs), which soon become mature IPs. The mature IPs, through self-renewing asymmetric divisions, produce ganglion mother cells that eventually differentiate into pairs of postmitotic neurons and/or glia (Fig. 1A).
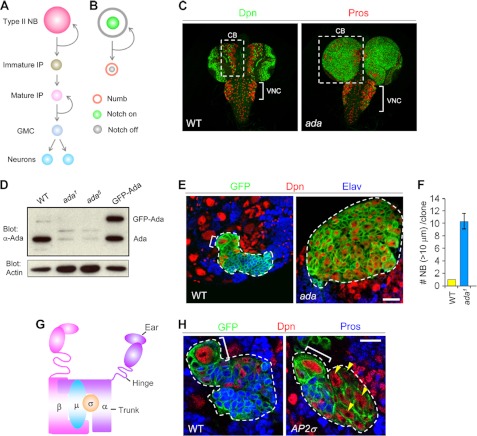
FIGURE 1.
The AP-2 complex acts to prevent NB overproliferation. A, schematic view of a type II NB lineage consists of NB (pink), immature IP (brown), mature IP (light pink), GMC (light blue), and postmitotic neurons (blue). B, during each cycle of NB mitosis, Numb (orange) is asymmetrically segregated into the immature IP. As a consequence, Notch signaling is activated in the NB (green) but inhibited in immature IPs (gray). C, α-ada1 or α-ada5 mutant larval brains stained with the NB marker Deadpan (Dpn) and GMC and neuronal marker Prospero (Pros), containing supernumerary NBs but few neurons in the CB (boxed) and ventral nerve cord (VNC, bracket) areas. D, α-Ada protein levels were greatly reduced in α-ada1 or α-ada5 mutant larval brains, indicating that both are strong hypomorphic alleles. Note that the UAS-EGFP-α-Ada transgene specifically expressed in NBs by the 1407-GAL4 driver showed similar protein level as endogenous α-Ada. E, WT or α-ada1 mutant MARCM clones induced from type II NBs that are marked by CD8-GFP. Neurons are marked with Elav. F, quantification of data from E. G, schematic drawing of the AP-2 complex. H, AP-2σKG02457 mutant MARCM clones contained ectopic NB (Dpn+Pros−, yellow arrowhead). Scale bars: 20 μm (E) and 10 μm (H).
The N signaling pathway is a central pathway governing NB homeostasis. In a type II NB lineage, E(spl)mγ-GFP, a reporter for N activity (10), is highly expressed in the NB but not immature IPs (Fig. 1B), indicating that differential N signaling within the lineage confers distinct cell fates. Indeed, inactivation of N signaling results in the depletion of all type II NBs (7, 11, 12), whereas overactivation of N signaling leads to the dedifferentiation of IPs back to NB cell fate and consequent ectopic NB formation and brain tumor phenotypes (7, 12–14).
One strategy to establish differential N signaling among the daughter cells of a NB lineage is through unequal segregation of signaling modulators such as Numb. In a type II NB lineage, Numb is asymmetrically segregated into the IPs, where it antagonizes N signaling and prevents IPs from acquiring NB fate (Fig. 1B) (7, 11, 15, 16).
How Numb antagonizes N signaling within NB lineages, however, remains enigmatic (17). Various studies indicate that Numb may act as an endocytic protein (17–26), but whether the endocytic pathway is critical for Numb function is uncertain (27). Studies in non-stem cell settings have suggested that N signaling can be modulated by the endocytic regulation of N receptor (28–37). However, the reported effects of these endocytic events on N signaling have been quite divergent and controversial (38–40), partly because of cell type-specific differences in the regulation and function of N signaling (39).
Given the essential roles of N signaling in balancing stem cell self-renewal versus differentiation in diverse organisms, it is imperative to investigate the role of the endocytic machinery in regulating N signaling during stem cell homeostasis and to explore the mode of action of Numb in this process. Here we show that Numb biases N signaling by promoting the endocytosis and down-regulation of both N and Spdo in the IPs, restraining their ability to self-renew and acquire stem cell fate. We show that Numb does so via physical interaction with α-Ada through newly identified interaction motifs.
EXPERIMENTAL PROCEDURES
Fly Genetics
Fly culture and crosses were performed according to standard procedures and were raised at the indicated temperatures. To generate various truncated version of pUAST-GFP-α-Ada, pUAST-Spdo-GFP, and pUAST-Numb-GFP transgenic flies, the corresponding cDNA constructs generated in the pUAST vector were injected into w- embryos to obtain transgenic lines according to established procedures. Drosophila stocks used in this study were numb15 (from W. Chia); Scabous-GAL4, UAS-nb03 (from Y. Jan); 1407-GAL4 (from L. Luo); adaear5 (from F. Roegiers); lgl1 (from F. Matsuzaki); UAS-N (from M. Fortini); spdoG104, AP-2σKG02457, UAS-Tom, UAS-Dl-RFP, P{PZ}α-Adaptin06694 (α-ada1) (from the Bloomington Drosophila stock center); l(2)SH0460 (ada5) (from the Szeged Drosophila Stock Center); UAS-Dl RNAi (TRiP). All other fly stocks were obtained from the Bloomington Drosophila stock center, the Szeged Drosophila Stock Center, and the VDRC.
Clonal Analysis and Temperature Shift Analyses
To generate NB mosaic analysis with a repressible cell marker (MARCM) clones, newly hatched or 24 hours after larval hatching (ACH) larvae were heat-shocked at 37 °C for 90 min and further aged for 3–4 days at 25 °C before dissection. MARCM analyses were performed essentially as described (41).
NB Quantification
Quantification of either total or type II neuroblasts was performed as described previously (12).
Cell Culture, Coimmunoprecipitation, and Western Blot Analyses
HEK293T cells were maintained in DMEM medium (Gibco) supplemented with 10% newborn calf serum (Lonza). For coimmunoprecipitation experiments, HEK293T cells were transfected with FuGENE 6 transfection reagent (Roche) following the protocol of the manufacturer. 48 h after transfection, cells were harvested, washed with ice-cold PBS, and incubated for 20 min with 450 μl of lysis buffer (50 mm Tris-HCl (pH 8.0), 120 mm NaCl, 5 mm EDTA, 1% Triton X-100, 10% glycerol) containing protease inhibitor mixture (Sigma) and phosphatase inhibitor mixture 1 (Sigma). The cell lysate was centrifuged for 5 min at 13,000 rpm, and the supernatant was collected. The lysate was incubated with mouse anti-FLAG M2 antibody coupled to agarose beads (Sigma) with gentle mixing at 4 °C for 3–4 h. Beads were washed with lysis buffer three times for 5 min each. Proteins were eluted from agarose beads by the addition of sample buffer (Bio-Rad), boiled for 5 min, and analyzed by Western blotting with the indicated antibodies.
For Western blot analysis of fly tissues, third instar larval brains from wild-type, ada1 or ada5 mutants or 1407>GFP-Ada animals were dissected in cold 1× PBS and directly homogenized in 25 μl of SDS sample buffer using a motor-driven pestle. After centrifugation at 13,000 rpm for 10 min, the supernatant was used in SDS-PAGE.
Statistical Analysis
Unpaired Student's t-tests were used for statistical analysis between two groups.
Molecular Biology and Protein Analysis
Detailed information is provided in the supplemental data.
RESULTS
Inactivation of AP-2 Complex Subunits Resulted in Ectopic NB Formation in the Drosophila Larval Brain
A key component of the endocytic pathway is the AP-2 complex, which plays a pivotal role in serving the endocytosis of a selective subset of cargoes by linking these protein cargoes to clathrin at the plasma membrane (42). We found that two lethal P-element insertions in the Drosophila AP-2 subunit gene α-adaptin (α-ada), P{PZ}α-Adaptin06694 (α-ada1) (71) and l(2)SH0460 (α-ada5) led to massive overgrowth of the CB areas, marked by supernumerary NBs, but a dramatically reduced number of differentiated neurons (Fig. 1C). Similar NB overproliferation phenotype was observed in trans-heterozygotes between α-ada and Df(2L)al, a chromosomal deficiency that deletes the α-ada locus (data not shown). Importantly, (α-ada1 or (α-ada5 mutations largely eliminated α-Ada protein expression in the larval brains (Fig. 1D). Furthermore, NB-specific expression of GFP-α-Ada through a transgene was able to completely rescue the brain tumor phenotype of (α-ada1 and (α-ada5 (Fig. 5F and data not shown), demonstrating that the observed phenotype was specifically caused by α-Ada loss of function in the NB lineages. Thus, loss of α-Ada function by the mutant alleles analyzed here led to NB overproliferation, indicating that α-Ada normally limits NB self-renewal or promotes NB differentiation. To assess whether α-Ada acts cell-autonomously to inhibit ectopic NB formation, we analyzed α-ada mutant NBs within GFP-marked MARCM clones (43). In type II NB lineages in the CB area, WT clones contained one and only one Dpn+ Elav− primary NB (bracket), which was associated with a few smaller-sized Dpn+ Elav− IPs and numerous Elav+ postmitotic neurons (Fig. 1, E and F). In contrast, α-ada mutant clones contained multiple Dpn+ Elav− NBs but very few differentiated neurons (Fig. 1, E and F). Thus, α-Ada is required cell-autonomously to inhibit ectopic NB formation in the type II NB lineages.
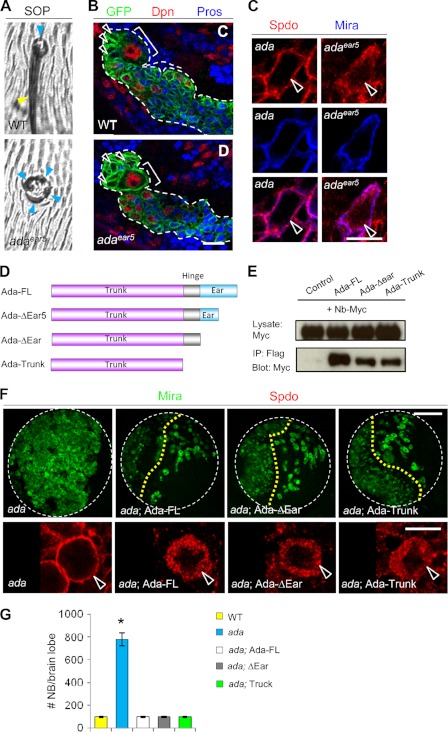
FIGURE 5.
The Ada-Ear domain, important for α-Ada function in the SOPs, is dispensable in the NBs. The α-adaear5 mutant showed cell fate transformation in external sensory (ES) organs (A) but no phenotype in the type II NB lineages (B). Bracket, NB; arrowhead, immature IP. C, Spdo was localized to the cell cortex of α-ada1 NBs but remained at the cytosol of α-adaear5 NBs. D, schematic representation of the α-Ada domain structures and various α-Ada deletion constructs used for generating transgenic lines and for coimmunoprecipitation experiments. E, interaction between Numb and α-Ada Trunk. FLAG-tagged full-length or truncated forms of α-Ada and Myc-tagged full-length Numb were expressed in HEK293T cells. Cell extracts were immunoprecipitated (IP) with anti-FLAG antibody, followed by Western blotting with anti-FLAG or anti-Myc antibodies. F, rescue of the α-ada5 mutant phenotype (H, H') by NB-specific expression (driven by 1407-GAL4) of full-length (Ada-FL) or truncated versions (Ada-ΔEar or Ada-Trunk) of the α-Ada transgenes. G, quantification of data from F. *, p < 0.0001. Scale bars = 10 μm (C) and 100 μm (F).
α-Ada is one of the large subunits of the AP-2 complex, which is composed of another large subunit (β2), an intermediate subunit (μ2), and a small subunit (σ2) (44) (Fig. 1G). Ectopic NBs (yellow arrowheads) were also observed in MARCM clones derived from NBs mutant for the AP-2σ2 subunit (Fig. 1H), supporting that α-Ada exerts its function in NB homeostasis by working together with other subunits of the AP-2 complex.
Mutations in AP-2 Subunits or Numb Affect the Endocytosis of Sanpodo
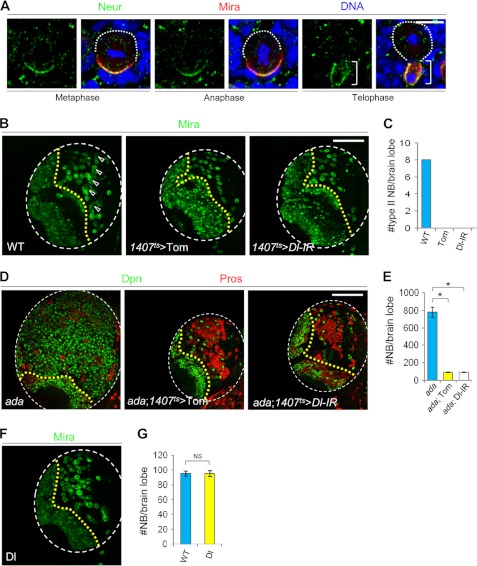
Because α-ada mutant NBs showed aberrant activation of N signaling, we next investigated whether α-Ada regulates N signaling through the endocytic pathway. Spdo, a four-pass transmembrane protein involved in N signaling in Drosophila, is regulated by endocytosis in the SOPs (20, 24, 45–47). Quantification of cortical versus cytoplasmic signals of Spdo showed that in wild-type NBs, Spdo was primarily cytoplasmic at metaphase, and it displayed weak cortical localization in the NB but not IPs at telophase (Fig. 2A and supplemental Fig. S1). In contrast, in α-ada mutant NBs, Spdo was localized predominantly to the cell cortex throughout the cell cycle (Fig. 2A and supplemental Fig. S1). Similar enriched cortical localization of Spdo was also observed in the NBs and IPs of numb or AP2σ mutants (Fig. 2B and supplemental Fig. S2). These results support that the AP-2 complex and Numb regulate N signaling in NB lineages by promoting the endocytosis of Spdo.
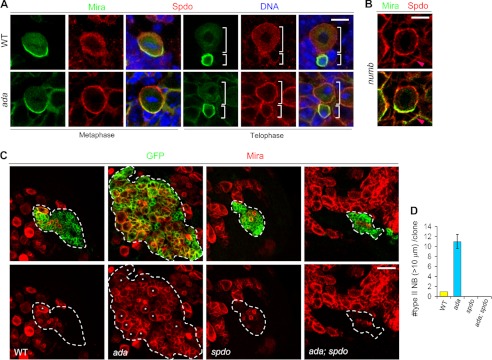
FIGURE 2.
α-Ada and Numb promote Spdo endocytosis. A, WT (G-G”, I-I”) or α-ada1 (H-H”, J-J”) mutant NBs at metaphase or telophase stages triple-labeled with Mira, Spdo, and DNA. B, cortical localization of Spdo in numb15 mutant NBs and adjacent IPs (pink arrowhead). C, MARCM analysis of type II NB lineages of WT control, α-ada1 mutant, spdoG104 mutant, and α-ada1; spdoG104 double mutant genotypes. NBs were marked with stars. The number of type II NBs (> 10 μm) per clone in these various genotypes are quantified in D. Scale bars = 10 μm (A and B) and 20 μm (C).
To investigate the functional significance of endocytic regulation of Spdo, we analyzed NB behavior in spdo mutants. spdo mutant MARCM clones in the type II NB lineages contained fewer IPs and no primary NB (Fig. 2, C and D), indicating that Spdo is required for maintaining NB identity in this lineage. Importantly, a NB depletion phenotype was also observed in the type II NB lineages when spdo mutant clones were induced in the α-ada mutant background (Fig. 2, C and D), demonstrating that Spdo is essential for the formation and/or maintenance of both normal and ectopic type II NBs.
Preventing the Internalization of Spdo Is Not Sufficient to Promote NB Self-Renewal
To investigate the role of Spdo endocytosis in mediating the effect of Numb on NB homeostasis, we tested whether overexpression of a mutant form of Spdo with constitutive cortical localization might be sufficient to promote ectopic NB formation. We found that deleting a short putative PTB domain-interacting motif (YTNPAF) in the N terminus of Spdo (ΔNm-Spdo) effectively abolished its binding to Numb (Fig. 3, A–C), resulting in Spdo accumulating primarily at the cell cortex (D). These findings are consistent with a previous study examining the effect of similar alteration of Numb/Spdo interaction on the endocytosis of Spdo in the SOPs (45). We confirmed that ΔNm-Spdo was fully functional on the basis of its ability to completely rescue the type II NB loss phenotype of the spdo mutant (Fig. 3E).
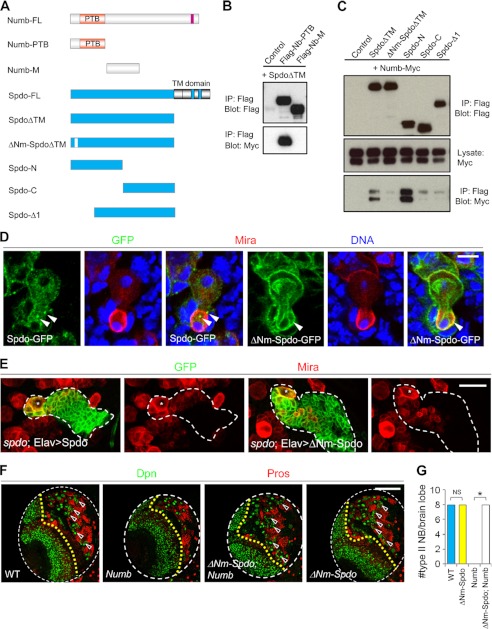
FIGURE 3.
Forced cortical localization of Spdo is not sufficient to promote the formation of ectopic NBs from IPs. A, schematic representation of Numb and Spdo domain structures. These various Numb or Spdo deletion or mutant versions were used for coimmunoprecipitation experiments or in vivo functional assays. B, interaction between Spdo and Numb-PTB. FLAG-tagged PTB or the M domains of Numb and a Myc-tagged Spdo with the C-terminal transmembrane domain deleted (SpdoΔTM) were expressed in HEK-293T cells, and coimmunoprecipitation (IP) experiments were performed as indicated. C, evidence that the Spdo YTNPAF motif is essential for Spdo-Numb interaction. FLAG-tagged full-length or truncated versions of SpdoΔTM and Myc-tagged Numb were expressed in HEK-293T cells, and coimmunoprecipitation experiments were performed as indicated. D, telophase NBs expressing either the Spdo-GFP or ΔNm-Spdo-GFP transgene were triple-labeled with GFP, Mira, and DNA. The arrowheads point to vesicular (upper) versus cortical (lower) GFP localization in future IP daughter cells. Note that ΔNm-Spdo-GFP also localized to some intracellular membrane structures. E, type II lineage NB clones of various genotypes marked with CD8-GFP (encircled by the dashed line). The Mira+ primary NB in each clone is marked with a star. F, the effects of NB-specific overexpression of Nb or ΔNm-Spdo or Nb and ΔNm-Spdo, driven by 1407-GAL4, on type II NB maintenance. Quantification of data from E is shown in F. Note that ΔNm-Spdo overexpression driven by 1407-GAL4 does not change the total NB number per brain lobe. NS, not significant. *, p < 0.0001, Student's t test. Scale bars = 10 μm (D), 20 μm (E), and 100 μm (F).
Interestingly, although NB-specific overexpression of Numb led to a complete depletion of type II lineage NBs, coexpression of ΔNm-Spdo blocked this effect (Fig. 3, F and G), indicating that internalization of Spdo is a critical step through which Numb inhibits NB self-renewal. However, pan-NB overexpression of this cortically localized Spdo was insufficient to cause ectopic NB formation (Fig. 3, F and G). Thus, cortical localization of Spdo appears to be necessary but not sufficient to promote NB self-renewal. This result implicates the involvement of deregulated endocytosis of other key target(s) of Numb in promoting ectopic NB formation.
α-Ada and Numb Promote the Internalization of N in type II NBs and IPs
We next examined whether the N receptor itself might be an endocytic target of Numb. Ectopic NBs induced by overactivation of N signaling have been proposed to originate from the IPs of type II NB lineages through a dedifferentiation process (7, 12, 13). We examined N distribution in α-ada or numb mutant NBs and IPs. α-ada mutant NBs and IPs showed modestly enhanced cortical localization of N (Fig. 4A) compared with the wild-type controls. Furthermore, quantitative analysis of the relative distribution of N showed that the increase in N distribution at the cell cortex was accompanied by its decreased cytoplasmic localization (Fig. 4C). Similar changes in N distribution were also observed in numb mutant NBs and IPs (Fig. 4, B and D), with the effects of numb mutation appeared to be more pronounced. These observations strongly suggest that N endocytosis was impaired in α-ada or numb mutant IPs and NBs. More direct proof of altered N endocytosis in α-ada or numb mutant IPs may come from a live antibody uptake assay, as done in other tissues (34, 37, 48, 49). However, the thickness of whole brain tissues has so far posed a technical challenge for this approach.
FIGURE 4.
The functional relationships among α-Ada, Numb, Spdo, and N. A, distribution of N as detected with the anti-N extracellular domain (NECD) antibody in WT and α-ada5 NBs at the metaphase stage. B, N distribution in a numb15 mutant NB MARCM clone marked with CD8-GFP (red). NBs are labeled with stars. White open or closed arrowheads point to N distribution at the cell cortex of control or numb15 mutant NBs, whereas pink open or closed arrowheads indicate N localization at the cell cortex of control or numb15 mutant IPs, respectively. The dashed line indicates the boundary between control (±, left) and numb15 mutant (right) cells. C and D, quantification of the relative distribution of N immunofluorescence at the cell cortex or cytoplasm of α-ada5 versus control (C) or numb15 mutant versus control (D) NBs or IPs. E, NBs in the signal larval brain lobe of various genotypes were marked with Mira. Data from E were quantified in F. *, p < 0.0001. Scale bars = 10 μm (A and B) and 100 μm (E).
Consistent with the view that Numb acts through α-Ada to promote the endocytosis and down-regulation of N in the IPs, knockdown of N by RNAi but not overexpression of Numb completely suppressed ectopic NB formation in α-ada mutants (Fig. 4, E and F). Furthermore, NB lineage-specific overexpression of a full-length N transgene was sufficient to induce ectopic NB formation (Fig. 4, E and F). Together, these data suggest that the elevated abundance of N at the cell cortex and the consequent N overactivation in α-ada or numb mutant IPs may account for the ectopic formation of NBs.
Ada-Ear, a Domain Important for α-Ada Function in the SOPs, Is Dispensable in the NBs
α-Ada contains an N-terminal Trunk domain, a C-terminal Ear domain, and a flexible Hinge domain in between (Fig. 1G). The Ear domain (Ada-Ear) binds to a large number of accessory proteins and is thought to be important for clathrin-mediated endocytosis (50, 51). Previous studies revealed an Ada-Ear interaction with the Asp-pro-phe (DPF) motif in Numb-C (25, 27). This interaction is critical for proper cell fate determination in the SOPs (19). Indeed, a strong cell fate transformation phenotype in the SOP lineage manifested as multiple sockets was observed in the α-adaear5 mutant (Fig. 5A), which causes a premature truncation within the Ear domain of α-Ada (19). Unexpectedly, the α-adaear5 mutant showed neither the NB overproliferation (Fig. 5B) nor Spdo cortical localization phenotypes (Fig. 5C), indicating that the C-terminal half of Ada-Ear is dispensable for α-Ada function in the NBs.
A New Mode of Protein-Protein Interaction between α-Ada and Numb in Regulating NB Homeostasis
The results described above raised the question as to whether there exists additional interaction between α-Ada and Numb proteins other than the interaction mediated by Ada-Ear and Numb-C. To probe for such an interaction, HEK293T cells were transfected with full-length and various deletion constructs of α-Ada and Numb (Fig. 5D), and coimmunoprecipitation experiments were performed. As expected, Ada-FL but not a control GFP protein efficiently coprecipitated full-length Numb (Nb-FL) (Fig. 5E and supplemental Fig. S3A). Ada-ΔEar or Ada-Trunk also coprecipitated with Numb, albeit with reduced strength than Ada-FL (Fig. 5E). Reciprocally, Numb proteins with its C-terminal domain deleted (Nb-N and Nb-ΔCT) still bound to Ada-FL (supplemental Fig. S3B). These results suggested that a new interaction between Ada-Trunk and Numb-N might be sufficient to regulate type II NB homeostasis.
These results prompted us to further investigate whether there is any functional importance associated with the previously identified interaction between the Ada-Ear domain and Numb-C domain in NBs. Like full-length Ada (Ada-FL), NB-specific expression (driven by 1407-GAL4) of α-Ada proteins with either the C-terminal half of its Ear domain or the entire Ear domain deleted (Ada-ΔEar5 and Ada-ΔEar, respectively) could completely rescue the NB overproliferation and Spdo cortical localization phenotypes in α-ada1 or α-ada5 mutants (Fig. 5, F and G, and data not shown). In fact, an α-Ada deletion form with only the Trunk domain remaining (Ada-Trunk) was still fully functional in rescuing α-ada mutants (Fig. 5, F and G), suggesting that, unlike in the SOPs, the entire Ear domain is dispensable for α-Ada function in the NBs. This result highlights the differential deployment of the endocytic machinery in different cell types and emphasizes the importance of studying the endocytic control of N signaling in multiple tissues.
Consistently, NB lineage-specific expression of Nb-ΔCT or Numb-PTB (supplemental Fig. S3A), which contains the PTB domain that is essential for Numb to interact with N and Spdo (20, 52), effectively suppressed the NB overproliferation phenotypes induced by loss of function of the tumor suppressor gene lethal giant larvae (lgl), which is linked to aberrant Numb/N signaling (14, 53) (supplemental Fig. S3C). Further deletion of the C-terminal portion of the PTB domain rendered the resulting protein, Nb-03 (54), nearly inactive (supplemental Fig. S3, A and C), suggesting that Numb-PTB is the minimal functional unit of Numb in inhibiting ectopic NB formation.
We also generated additional deletions in Ada-Trunk in an effort to further narrow down the minimal domain of α-Ada that is functional in NBs. Deletion of approximately one third of Ada-Trunk from the N terminus or C terminus (Ada-ΔNTΔEar or Ada-N2, respectively) abolished the ability of Ada-Trunk to functionally rescue α-ada mutant phenotypes in terms of NB number and Spdo localization (supplemental Fig. S4), suggesting that the Trunk domain of α-Ada is an indivisible functional unit of the protein.
It was recently shown that NB-specific overexpression of a phospho-mimetic form of Numb (Nb-TS4D), but not its corresponding non-phosphorylatable form (Nb-TS4A), led to a strong NB overproliferation phenotype (supplemental Fig. S5A) (55). The TS4D mutations did not alter the interaction between Numb and its cargo Spdo, but nevertheless led to Spdo cortical localization by unknown mechanisms (55). Given that Numb acts as an adaptor by linking α-Ada with its cargo proteins Spdo and N to regulate NB homeostasis, we investigated the possibility that the TS4D mutations might alter the interaction between Numb and α-Ada. Intriguingly, compared with Nb-WT or Nb-TS4A, Nb-TS4D exhibited a markedly enhanced interaction with α-Ada (supplemental Fig. S5B), suggesting that the dominant-negative effects exerted by Nb-TS4D on endogenous Numb might be due to its deregulated interaction with α-Ada, which could impair the normal activity of the AP-2 complex (see “Discussion”).
A Numb-PTB-α-Ada Chimera Recapitulates the Functional AP-2/Numb Complex
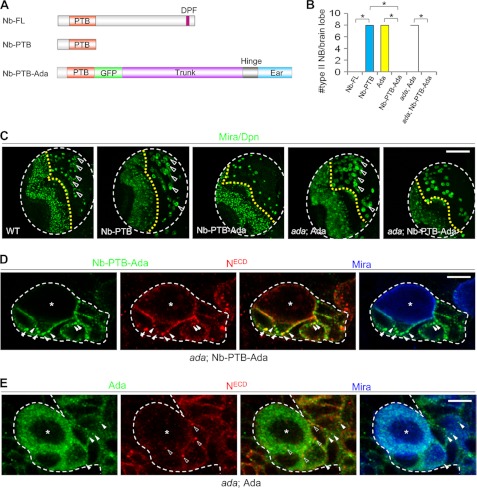
Although overexpression of a full-length Numb transgene led to a complete depletion of type II NB lineages (Fig. 3, F and G, and 6, A and B), overexpression of Numb-PTB showed no effect on normal NB maintenance (Fig. 6, A–C), suggesting that the missing portion of Numb might be required to ensure a tighter interaction with α-Ada and, hence, its full activity in N inhibition. To test this idea, we tethered full-length α-Ada to the C terminus of Nb-PTB-GFP to generate an Nb-PTB-α-Ada chimeric protein (Fig. 6A). Like full-length Numb, expression of Nb-PTB-α-Ada led to a complete depletion of type II NBs (Fig. 6, B and C). Furthermore, although full-length α-Ada rescued NB overproliferation in α-ada mutants and brought NB number back to normal, expression of PTB-α-Ada in the α-ada background further depleted all type II NBs (Fig. 6, B and C). In addition, Nb-PTB-α-Ada, but not full-length α-Ada, completely suppressed the NB overproliferation phenotype of numb mutants (supplemental Fig. S6), whereas full-length Numb was unable to rescue α-ada mutant phenotypes (Fig. 4, E and F). These data provide compelling evidence that most of Numb and α-Ada function in NBs is mediated by the Numb-α-Ada complex formed through direct protein-protein interaction, which might contain other protein components as well. They also offer more molecular insights about how Numb functions in the NB lineages. Through its interaction with N and Spdo cargo proteins and the endocytic protein α-Ada, asymmetrically segregated Numb in the IPs incorporates these NB self-renewal-promoting cargos into the AP-2 complex to promote their internalization and down-regulation, thereby preventing the IPs from acquiring stem cell fate.
FIGURE 6.
An Nb-PTB-α-Ada chimera recapitulates the functional AP-2/Numb complex. A, schematic diagrams of Nb-FL, Nb-PTB, and Nb-PTB-α-Ada chimeric constructs. C, effects of overexpressing Nb-PTB, α-Ada, or Nb-PTB-α-Ada on type II NB number in WT or α-ada1 mutants. Data quantification is shown in B. *, p < 0.0001. D, N and Nb-PTB-GFP-α-Ada vesicles (arrowhead) colocalize (solid arrowheads) in the differentiating daughter cells but not NBs (star), in α-ada1; 1407>Nb-PTB-GFP-α-Ada background. E, in α-ada1; 1407>GFP-α-Ada background, N (open arrowheads) and GFP-α-Ada positive vesicles (solid arrowheads) rarely colocalize in either the NBs (star) or differentiating daughter cells. Scale bars = 100 μm (C) and 5 μm (D and E).
Because the Nb-PTB-α-Ada chimeric protein contains the full activity of both Numb and α-Ada, its distribution could reveal the cellular location of the functional pool of AP-2/Numb-dependent endocytic vesicles. Interestingly, we found that N and Nb-PTB-α-Ada puncta (arrowheads) colocalized in the differentiating daughter cells (arrowheads) but not the NBs (Fig. 6D). In comparison, colocalization of N puncta with either α-Ada-GFP-positive vesicles or Numb protein in the NBs or differentiating daughter cells was rarely detected (Fig. 6E and data not shown), emphasizing the importance of Numb in bringing N and α-Ada together. With the caveat that transgene-derived proteins were used here, the differential colocalization of N with Nb-PTB-α-Ada and α-Ada-GFP still provided strong support for the notion that N receptor is asymmetrically internalized and down-regulated in the IPs by Numb and AP-2-mediated endocytosis.
The N Ligand Delta Is Critically Involved in NB Homeostasis, but the Ectopic NB Formation in α-ada Mutant Is Unlikely due to Altered Delta Trafficking or Function
Although it is well known that N signaling is activated within the NBs to maintain stem cell fate, the source and identity of the N ligand involved in NB regulation remain unresolved (56). To begin to address this issue, we first examined the distribution of the E3 ubiquitin ligase Neuralized (Neur), which is required for the activation of N ligand Delta (Dl) in the signal-sending cell in the SOP lineage (49). Neur was asymmetrically segregated into the IPs during NB division (Fig. 7A), suggesting that the IPs might be the cells that present the Dl signal. Consistent with a unidirectional N signaling from the IPs to the NB, the N reporter is on in the NB but off in the IP (10, 12). Secondly, to evaluate the functional significance of Dl in the NB lineages, we performed either RNAi-mediated knockdown of Dl or overexpression of its inhibitor, Tom (57). Down-regulation of Dl specifically within the NB lineages led to a complete depletion of type II NBs (Fig. 7, B and C), demonstrating that Dl is absolutely required for NB formation or maintenance. Furthermore, NB lineage-specific inactivation of Dl also completely suppressed NB overproliferation in α-ada mutants (Fig. 7, D and E), indicating that Dl is also needed for N activation in the formation or maintenance of ectopic NBs.
FIGURE 7.
The function of N ligand Delta in regulating stem cell homeostasis in the type II NB lineages. A, WT NBs at metaphase, anaphase, or telophase stages were triple-labeled with Neur, Mira, and DNA. B–E, NB lineage-specific overexpression of the Tom or Dl RNAi transgenes led to a complete depletion of type II NBs (B) and complete suppression of NB overproliferation in α-ada1 mutants (D). Arrowheads in B indicate type II NB lineages. Quantification of NB number is shown in C and E. *, p < 0.0001. F, NB lineage-specific overexpression of a full-length Dl transgene had no effect on NB number. Quantification of NB number is shown in G. Scale bars = 10 μm (A) and 100 μm (B and D).
The demonstration that Dl is required within the type II NB lineages for the activation of N signaling required for NB self-renewal and maintenance, and the fact that endocytosis is a critical step in the activation of Dl (57–62), raised the possibility that impairment of the endocytosis of Dl might account for the NB overproliferation phenotype seen after the inactivation of α-Ada. However, such a scenario seemed unlikely, on the basis of the following observations. We did not observe obvious change of Dl protein distribution in a-ada mutant NB lineages compared with control wild-type NB lineages (supplemental Fig. S7). Unlike the overexpression of N, overexpression of a Dl transgene (63) within the NB lineages had no obvious effect on NB number (Fig. 7, F and G). These data support the notion that ectopic NB formation seen in α-ada mutants is largely attributable to altered trafficking and activity of N instead of Dl.
DISCUSSION
Understanding how differential cell fates were established and maintained within a stem cell lineage is fundamental to stem cell biology and has important implications for cancer research. Our results uncover a critical role for the cell fate determinant Numb in regulating NSC homeostasis by preventing ectopic NSC formation through interaction with the endocytic protein α-Ada. This interaction serves to down-regulate N signaling by promoting the internalization of both N and Spdo, ensuring that the IP that receives the majority of the Numb protein will not acquire the NB fate.
Previous studies indicated that Numb uses its C-terminal domain to interact with the α-Ada Ear domain and that such an interaction is essential for asymmetric cell fates in the SOP lineage (19). Surprisingly, we found that neither Numb-C nor Ada-Ear is required for NB lineage homeostasis regulation. Instead, a novel interaction between Numb-N and α-Ada Trunk domain is sufficient to exert their functions in NBs. This is one of the several differences between the NBs and SOPs we have uncovered in this study in terms of the regulation and function of the endocytic machinery, and it highlights the need to further study the endocytic regulation of N signaling in NSCs, even though much has been done in non-stem cell settings. Interestingly, the protein sequences of both the Numb-N and α-Ada Trunk domain are highly conserved in vertebrates (supplemental Fig. S8). Whether mammalian Numb and α-Ada utilize a similar mode of protein interaction to exert their function in inhibiting ectopic NSC formation within vertebrate warrants future investigation.
Our structure-function studies identify the Trunk domain of α-Ada as the minimal functional unit in mediating α-Ada/Numb interaction. It is kind of surprising that the Ear domain of α-Ada, which binds to a large number of accessory proteins important for endocytosis, is dispensable for α-Ada function in the NBs. One possibility is that through interaction with β-Ada, which also contains an Ear domain, α-Ada-Trunk may still be able to recruit the accessory proteins to the AP-2 complex. Consistent with this notion, the C-terminal 1/3 of α-Ada-Trunk, which may mediate the interaction between α-Ada and β-Ada (44), is indispensable for α-Ada-Trunk function. The N-terminal 1/3 of α-Ada-Trunk, which may be required for interaction with the phospholipids PIP2/PIP3 and other subunits of the AP-2 complex (44, 64, 65), is also indispensable for α-Ada-Trunk domain function. The Trunk domain of α-Ada thus represents one indivisible functional unit.
Despite the fact that Numb plays an evolutionary conserved role in inhibiting N signaling (52, 66–69), it remains unclear how it exerts such a function. Previous studies in other systems have led to divergent proposals of Numb regulation of N signaling, including endocytosis- and proteasome-independent mechanism (27), ubiquitination and degradation of N (70), or promotion of Spdo endocytosis (20, 24). It is worth noting that all of these previous studies were done in non-stem cell settings. Here we provide compelling evidence that in Drosophila NBs, Numb inhibits N signaling specifically in the IPs of the type II NB lineages through promoting the endocytosis of N and Spdo. Our finding that the expression of a mutant form of Spdo, which no longer binds to Numb and thus escapes the regulation by Numb, is sufficient to rescue the Numb overexpression-induced type II NB loss provides compelling evidence that Spdo is an important downstream mediator of Numb function in regulating NB self-renewal.
To directly test the model that Numb primarily acts as an adaptor protein to link N and Spdo to the α-Ada-containing AP-2 complex, we fused α-Ada to the C terminus of the Numb-PTB domain. This fusion protein possesses the full activities of both Numb and α-Ada, thus recapitulating the function of the active Numb/α-Ada complex. Interestingly, the resulting Nb-PTB-α-Ada chimeric protein displayed stronger activity than α-Ada protein alone in inhibiting the NB overproliferation phenotype of α-ada mutants. One plausible explanation is that α-Ada normally interacts with many cargo proteins directly or indirectly in vivo. Hence, only a small portion of AP-2 endocytic vesicles contains N and/or Spdo under normal condition. However, when α-Ada is replaced by Nb-PTB-α-Ada, more AP-2 endocytic vesicles can now gain access to N and/or Spdo, resulting in more efficient inhibition of N signaling and NB self-renewal. Interestingly, we observed colocalization between N and Nb-PTB-α-Ada vesicles in the differentiating daughter cells but not NBs. Although this observation is on the basis of transgene-derived Nb-PTB-α-Ada, it nevertheless provides compelling evidence that N is asymmetrically internalized in AP-2-dependent endocytic vesicles and is down-regulated in the differentiating daughter cells. Under normal physiological conditions, this process is likely facilitated by Numb, which is preferentially segregated into the differentiating daughter cell after stem cell division.
Although the interaction between Numb and α-Ada is absolutely critical for the endocytic regulation of N signaling in NB lineages, our data also suggested that such interaction might be delicately and dynamically regulated in vivo. It is possible that the N/Spdo cargo-Numb-AP-2 complex goes through cycles of association and dissociation, and this dynamic regulation might be partly mediated by phosphorylation/dephosphorylation cycles of Numb, as in the case of Numb regulation of integrin endocytosis during directional cell migration (23). We found that the phospho-mimetic form of Numb (Nb-TS4D) binds to α-Ada protein with an unusually high affinity. This tight binding might hinder the recruitment of other important endocytic proteins by α-Ada and disrupt the endocytic cycles, resulting in deregulated protein trafficking, overactivation of N signaling, and tumorigenesis. The detailed molecular mechanisms underlying the effects of Nb-TS4D on the endocytic regulation in the NB lineages warrant further investigations.
Furthermore, our results offer the first evidence that the N ligand Dl is required for type II NB self-renewal and/or maintenance and that it acts within the NB lineages. We propose that Dl, likely coming from the immature IP, activates N in the type II NB to maintain its NSC fate. This cell-cell interaction mediated by Dl-N signaling could happen between two immediate daughter cells of a NB division or between the NB and some earlier born IPs, which tend to cluster around the parental NBs. In other cell types, Dl has been shown to require an endocytosis step in the signal-sending cell to become competent for activating N in neighboring signal-receiving cells (57–62). Our data suggest that the overall distribution of Dl was not as significantly altered as N by the inactivation of α-Ada, and unlike overexpression of N, which causes ectopic NB formation, the overexpression of Dl did not affect NB number. Thus, alteration of Dl trafficking is unlikely to be a major contributor of the ectopic NB phenotype seen in α-ada mutants.
In summary, our studies unveil new insights into the modes of action of Numb in antagonizing N signaling within the NB lineages. Through interacting with α-Ada, Numb bridges N and Spdo with the AP-2-dependent endocytic machinery, promotes N and Spdo internalization, and directs these stem cell fate-promoting factors to a presumably degradative route. Our results also reveal that there exist dual modes of interaction between α-Ada and Numb and that stem cells and non-stem cells may exploit these interactions in qualitatively different ways, highlighting the need to study the endocytic regulation of N signaling specifically in a stem cell setting to better understand the general involvement of N signaling in stem cell homeostasis regulation.
Supplementary Material
Acknowledgments
We are grateful to Drs. H. Bellen, D. Bilder, S. Bray, E. Chen, W. Chia, C. Doe, J. Fischer, M. Fortini, N. Gay, Y. Jan, T. Lee, L. Luo, F. Matsuzaki, A. Nakamura, D. Ready, F. Roegiers, J. Skeath, and W. Zhong, the University of Iowa Developmental Studies Hybridoma Bank; the Bloomington Drosophila Stock Center; VDRC, the TRiP at Harvard Medical School, and Szeged Drosophila Stock Centre for fly stocks and reagents. We also thank Dr. H. Bellen for insightful suggestions; Dr. S. Guo for reading the manuscript; Dr. J. Zhang for help with notum preparation; A. Gatum for help with microinjection; J. Gaunce, W. Lee, and G. Silverio for technical assistance; and members of the Lu lab for discussions and help.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 NS043167 (to B. L.). This work was also supported by a Stanford University School of Medicine Dean's postdoctoral fellowship (to Y. S.).

This article contains supplemental Figs. S1–S8, experimental procedures, and references.
- N
- Notch
- NSC
- neural stem cell
- NB
- neuroblast
- CB
- central brain
- IP
- intermediate progenitor
- Spdo
- Sanpodo
- α-Ada
- α-Adaptin
- SOP
- sensory organ precursor
- PTB
- phospho-tyrosine binding
- Neur
- Neuralized
- Dl
- Delta
- MARCM
- mosaic analysis with a repressible cell marker.
REFERENCES
- 1. Varnum-Finney B., Xu L., Brashem-Stein C., Nourigat C., Flowers D., Bakkour S., Pear W. S., Bernstein I. D. (2000) Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 6, 1278–1281 [DOI] [PubMed] [Google Scholar]
- 2. Yu X., Zou J., Ye Z., Hammond H., Chen G., Tokunaga A., Mali P., Li Y. M., Civin C., Gaiano N., Cheng L. (2008) Notch signaling activation in human embryonic stem cells is required for embryonic, but not trophoblastic, lineage commitment. Cell Stem Cell 2, 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mizutani K., Yoon K., Dang L., Tokunaga A., Gaiano N. (2007) Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 [DOI] [PubMed] [Google Scholar]
- 4. Ohlstein B., Spradling A. (2007) Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science 315, 988–992 [DOI] [PubMed] [Google Scholar]
- 5. Luo D., Renault V. M., Rando T. A. (2005) The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin. Cell Dev. Biol. 16, 612–622 [DOI] [PubMed] [Google Scholar]
- 6. Harrison H., Farnie G., Howell S. J., Rock R. E., Stylianou S., Brennan K. R., Bundred N. J., Clarke R. B. (2010) Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 70, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowman S. K., Rolland V., Betschinger J., Kinsey K. A., Emery G., Knoblich J. A. (2008) The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev. Cell 14, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boone J. Q., Doe C. Q. (2008) Indentification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev. Neurobiol. 68, 1185–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bello B. C., Izergina N., Caussinus E., Reichert H. (2008) Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almeida M. S., Bray S. J. (2005) Regulation of post-embryonic neuroblasts by Drosophila Grainyhead. Mech. Dev. 122, 1282–1293 [DOI] [PubMed] [Google Scholar]
- 11. Wang H., Somers G. W., Bashirullah A., Heberlein U., Yu F., Chia W. (2006) Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 20, 3453–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song Y., Lu B. (2011) Regulation of cell growth by Notch signaling and its differential requirement in normal vs. tumor-forming stem cells in Drosophila. Genes Dev. 25, 2644–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weng M., Golden K. L., Lee C. Y. (2010) dFezf/Earmuff maintains the restricted developmental potential of intermediate neural progenitors in Drosophila. Dev. Cell 18, 126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wirtz-Peitz F., Nishimura T., Knoblich J. A. (2008) Linking cell cycle to asymmetric division. Aurora-A phosphorylates the Par complex to regulate Numb localization. Cell 135, 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee C. Y., Andersen R. O., Cabernard C., Manning L., Tran K. D., Lanskey M. J., Bashirullah A., Doe C. Q. (2006) Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 20, 3464–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H., Ouyang Y., Somers W. G., Chia W., Lu B. (2007) Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature 449, 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pece S., Confalonieri S., R Romano P., Di Fiore P. P. (2011) NUMB-ing down cancer by more than just a NOTCH. Biochim. Biophys. Acta 1815, 26–43 [DOI] [PubMed] [Google Scholar]
- 18. Gulino A., Di Marcotullio L., Screpanti I. (2010) The multiple functions of Numb. Exp. Cell Res. 316, 900–906 [DOI] [PubMed] [Google Scholar]
- 19. Berdnik D., Török T., González-Gaitán M., Knoblich J. A. (2002) The endocytic protein α-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3, 221–231 [DOI] [PubMed] [Google Scholar]
- 20. Hutterer A., Knoblich J. A. (2005) Numb and α-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep. 6, 836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kyriazis G. A., Wei Z., Vandermey M., Jo D. G., Xin O., Mattson M. P., Chan S. L. (2008) Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner. Implications for Alzheimer disease pathogenesis. J. Biol. Chem. 283, 25492–25502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGill M. A., Dho S. E., Weinmaster G., McGlade C. J. (2009) Numb regulates post-endocytic trafficking and degradation of Notch1. J. Biol. Chem. 284, 26427–26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishimura T., Kaibuchi K. (2007) Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell 13, 15–28 [DOI] [PubMed] [Google Scholar]
- 24. O'Connor-Giles K. M., Skeath J. B. (2003) Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5, 231–243 [DOI] [PubMed] [Google Scholar]
- 25. Santolini E., Puri C., Salcini A. E., Gagliani M. C., Pelicci P. G., Tacchetti C., Di Fiore P. P. (2000) Numb is an endocytic protein. J. Cell Biol. 151, 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlüter T., Knauth P., Wald S., Boland S., Bohnensack R. (2009) Numb3 is an endocytosis adaptor for the inflammatory marker P-selectin. Biochem. Biophys. Res. Commun. 379, 909–913 [DOI] [PubMed] [Google Scholar]
- 27. Tang H., Rompani S. B., Atkins J. B., Zhou Y., Osterwalder T., Zhong W. (2005) Numb proteins specify asymmetric cell fates via an endocytosis- and proteasome-independent pathway. Mol. Cell Biol. 25, 2899–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaccari T., Lu H., Kanwar R., Fortini M. E., Bilder D. (2008) Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sasaki N., Sasamura T., Ishikawa H. O., Kanai M., Ueda R., Saigo K., Matsuno K. (2007) Polarized exocytosis and transcytosis of Notch during its apical localization in Drosophila epithelial cells. Genes Cells 12, 89–103 [DOI] [PubMed] [Google Scholar]
- 30. Jaekel R., Klein T. (2006) The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of endosomal trafficking. Dev. Cell 11, 655–669 [DOI] [PubMed] [Google Scholar]
- 31. Gallagher C. M., Knoblich J. A. (2006) The conserved c2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev. Cell 11, 641–653 [DOI] [PubMed] [Google Scholar]
- 32. Childress J. L., Acar M., Tao C., Halder G. (2006) Lethal giant discs, a novel C2-domain protein, restricts notch activation during endocytosis. Curr. Biol. 16, 2228–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaccari T., Bilder D. (2005) The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687–698 [DOI] [PubMed] [Google Scholar]
- 34. Lu H., Bilder D. (2005) Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232–1239 [DOI] [PubMed] [Google Scholar]
- 35. Klein T. (2003) The tumour suppressor gene l(2)giant discs is required to restrict the activity of Notch to the dorsoventral boundary during Drosophila wing development. Dev. Biol. 255, 313–333 [DOI] [PubMed] [Google Scholar]
- 36. Fortini M. E. (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16, 633–647 [DOI] [PubMed] [Google Scholar]
- 37. Couturier L., Vodovar N., Schweisguth F. (2012) Endocytosis by Numb breaks Notch Symmetry at cytokinesis. Nat. Cell Biol. 14, 131–139 [DOI] [PubMed] [Google Scholar]
- 38. Giebel B., Wodarz A. (2006) Tumor suppressors. Control of signaling by endocytosis. Curr. Biol. 16, R91–92 [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto S., Charng W. L., Bellen H. J. (2010) Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top Dev. Biol. 92, 165–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kopan R., Ilagan M. X. (2009) The canonical Notch signaling pathway. Unfolding the activation mechanism. Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee T., Luo L. (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 [DOI] [PubMed] [Google Scholar]
- 42. Robinson M. S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 43. Lee T., Lee A., Luo L. (1999) Development of the Drosophila mushroom bodies. Sequential generation of three distinct types of neurons from a neuroblast. Development 126, 4065–4076 [DOI] [PubMed] [Google Scholar]
- 44. Collins B. M., McCoy A. J., Kent H. M., Evans P. R., Owen D. J. (2002) Molecular architecture and functional model of the endocytic AP2 complex. Cell 109, 523–535 [DOI] [PubMed] [Google Scholar]
- 45. Tong X., Zitserman D., Serebriiskii I., Andrake M., Dunbrack R., Roegiers F. (2010) Numb independently antagonizes Sanpodo membrane targeting and Notch signaling in Drosophila sensory organ precursor cells. Mol. Biol. Cell 21, 802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langevin J., Le Borgne R., Rosenfeld F., Gho M., Schweisguth F., Bellaïche Y. (2005) Lethal giant larvae controls the localization of Notch signaling regulators numb, neuralized, and Sanpodo in Drosophila sensory organ precursor cells. Curr. Biol. 15, 955–962 [DOI] [PubMed] [Google Scholar]
- 47. Roegiers F., Jan L. Y., Jan Y. N. (2005) Regulation of membrane localization of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs. Mol. Biol. Cell 16, 3480–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benhra N., Lallet S., Cotton M., Le Bras S., Dussert A., Le Borgne R. (2011) AP-1 controls the trafficking of Notch and Sanpodo toward E-cadherin junctions in sensory organ precursors. Curr. Biol. 21, 87–95 [DOI] [PubMed] [Google Scholar]
- 49. Le Borgne R., Schweisguth F. (2003) Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell 5, 139–148 [DOI] [PubMed] [Google Scholar]
- 50. Owen D. J., Vallis Y., Noble M. E., Hunter J. B., Dafforn T. R., Evans P. R., McMahon H. T. (1999) A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell 97, 805–815 [DOI] [PubMed] [Google Scholar]
- 51. Praefcke G. J., Ford M. G., Schmid E. M., Olesen L. E., Gallop J. L., Peak-Chew S. Y., Vallis Y., Babu M. M., Mills I. G., McMahon H. T. (2004) Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 23, 4371–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guo M., Jan L. Y., Jan Y. N. (1996) Control of daughter cell fates during asymmetric division. Interaction of Numb and Notch. Neuron 17, 27–41 [DOI] [PubMed] [Google Scholar]
- 53. Lee C. Y., Robinson K. J., Doe C. Q. (2006) Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature 439, 594–598 [DOI] [PubMed] [Google Scholar]
- 54. Knoblich J. A., Jan L. Y., Jan Y. N. (1997) The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc. Natl. Acad. Sci. U.S.A. 94, 13005–13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ouyang Y., Petritsch C., Wen H., Jan L., Jan Y. N., Lu B. (2011) Dronc caspase exerts a non-apoptotic function to restrain phospho-Numb-induced ectopic neuroblast formation in Drosophila. Development 138, 2185–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doe C. Q. (2008) Neural stem cells. Balancing self-renewal with differentiation. Development 135, 1575–1587 [DOI] [PubMed] [Google Scholar]
- 57. Bardin A. J., Schweisguth F. (2006) Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell 10, 245–255 [DOI] [PubMed] [Google Scholar]
- 58. Wang W., Struhl G. (2004) Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131, 5367–5380 [DOI] [PubMed] [Google Scholar]
- 59. Eun S. H., Banks S. M., Fischer J. A. (2008) Auxilin is essential for Delta signaling. Development 135, 1089–1095 [DOI] [PubMed] [Google Scholar]
- 60. Rajan A., Tien A. C., Haueter C. M., Schulze K. L., Bellen H. J. (2009) The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat. Cell Biol. 11, 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Emery G., Hutterer A., Berdnik D., Mayer B., Wirtz-Peitz F., Gaitan M. G., Knoblich J. A. (2005) Asymmetric Rab 11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell 122, 763–773 [DOI] [PubMed] [Google Scholar]
- 62. Jafar-Nejad H., Andrews H. K., Acar M., Bayat V., Wirtz-Peitz F., Mehta S. Q., Knoblich J. A., Bellen H. J. (2005) Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev. Cell 9, 351–363 [DOI] [PubMed] [Google Scholar]
- 63. Hagedorn E. J., Bayraktar J. L., Kandachar V. R., Bai T., Englert D. M., Chang H. C. (2006) Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J. Cell Biol. 173, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Page L. J., Robinson M. S. (1995) Targeting signals and subunit interactions in coated vesicle adaptor complexes. J. Cell Biol. 131, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gaidarov I., Chen Q., Falck J. R., Reddy K. K., Keen J. H. (1996) A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 α subunit. Implications for the endocytic pathway. J. Biol. Chem. 271, 20922–20929 [DOI] [PubMed] [Google Scholar]
- 66. Endo K., Aoki T., Yoda Y., Kimura K., Hama C. (2007) Notch signal organizes the Drosophila olfactory circuitry by diversifying the sensory neuronal lineages. Nat. Neurosci. 10, 153–160 [DOI] [PubMed] [Google Scholar]
- 67. Pece S., Serresi M., Santolini E., Capra M., Hulleman E., Galimberti V., Zurrida S., Maisonneuve P., Viale G., Di Fiore P. P. (2004) Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J. Cell Biol. 167, 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Conboy I. M., Rando T. A. (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409 [DOI] [PubMed] [Google Scholar]
- 69. Wakamatsu Y., Maynard T. M., Jones S. U., Weston J. A. (1999) NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron 23, 71–81 [DOI] [PubMed] [Google Scholar]
- 70. McGill M. A., McGlade C. J. (2003) Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J. Biol. Chem. 278, 23196–23203 [DOI] [PubMed] [Google Scholar]
- 71. Gonzalez-Gaitan M., Jackle H. (1997) Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell 88, 767–776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.