Background: Various sterols serve as substrates and allosteric activators for ACAT.
Results: Pregnelonone is the only natural sterol that is an ACAT substrate but not an ACAT activator.
Conclusion: ACAT, along with LCAT contributes to control cellular pregnenolone ester content.
Significance: This work provides a new function for ACAT.
Keywords: Alzheimer Disease, Atherosclerosis, Cholesterol Metabolism, Lipid-binding Protein, Lipid Metabolism, Membrane Enzymes, Steroidogenesis, Sterol, ACAT, Cholesteryl Esters
Abstract
Pregnenolone (PREG) can be converted to PREG esters (PE) by the plasma enzyme lecithin: cholesterol acyltransferase (LCAT), and by other enzyme(s) with unknown identity. Acyl-CoA:cholesterol acyltransferase 1 and 2 (ACAT1 and ACAT2) convert various sterols to steryl esters; their activities are activated by cholesterol. PREG is a sterol-like molecule, with 3-β-hydroxy moiety at steroid ring A, but with much shorter side chain at steroid ring D. Here we show that without cholesterol, PREG is a poor ACAT substrate; with cholesterol, the Vmax for PREG esterification increases by 100-fold. The binding affinity of ACAT1 for PREG is 30–50-fold stronger than that for cholesterol; however, PREG is only a substrate but not an activator, while cholesterol is both a substrate and an activator. These results indicate that the sterol substrate site in ACAT1 does not involve significant sterol-phospholipid interaction, while the sterol activator site does. Studies utilizing small molecule ACAT inhibitors show that ACAT plays a key role in PREG esterification in various cell types examined. Mice lacking ACAT1 or ACAT2 do not have decreased PREG ester contents in adrenals, nor do they have altered levels of the three major secreted adrenal steroids in serum. Mice lacking LCAT have decreased levels of PREG esters in the adrenals. These results suggest LCAT along with ACAT1/ACAT2 contribute to control pregnenolone ester content in different cell types and tissues.
Introduction
Biosynthesis of pregnenolone (PREG)4 occurs in mitochondria, using cholesterol (CHOL) as the precursor (1, 2). Once produced, enzymes in the mitochondria and in the endoplasmic reticulum catalyze the conversions of PREG to various steroid hormones. PREG can also be converted to PREG esters. Early studies showed that crude particulate fraction from bovine adrenal glands was able to catalyze the formation of PREG esters from PREG, with long-chain fatty acid attached at the 3-β-OH moiety of PREG (3). When labeled PREG was added to intact rat fibroblast cells, a significant portion of the label was convert to PREG esters (4). These results show that mammalian cells and tissues can convert PREG to PREG esters. However, the enzyme(s) that catalyzes cellular PREG esterification had not been identified. In addition to cellular esterification, PREG can be converted to PREG esters by the enzyme lecithin:cholesterol acyltransferase (LCAT) present in the plasma (5, 6).
Acyl:coenzyme A:cholesterol acyltransfeases (ACAT) are key cellular enzymes that convert CHOL to cholesteryl esters (CE) for storage, and for providing CE present in lipoproteins. In mammals, there are two isoenzymes ACAT1 and ACAT2. Both ACAT1 and ACAT2 are drug targets for atherosclerosis as reviewed in (7). In addition, ACAT1 is a drug target for Alzheimer disease (8). In most of the tissues examined, ACAT1 is the major isoenzyme, while ACAT2 is mainly expressed in intestinal enterocytes and in hepatocytes, as reviewed in Ref. 7. ACAT1 is homotetrameric and contains nine-TMDs; the first active site His-460 is located within TMD#7, and the second active site Asn-421 is located within the 3rd large cytosolic loop (9). ACAT2 shares high homology with ACAT1 near the C terminus, but not near the N terminus. The ACAT enzymes are founding members of the membrane-bound O-acyltransferase enzyme family (MBOAT), which includes diacylglycerol acyltransferase 1 (DGAT1), ghrelin octanoyl-coenzyme A acyltransferase, and lysophospholipid acyltransferases (LPATs), as reviewed in Ref. 10. We had previously reported (11, 12) that both ACAT1 and ACAT2 are allosteric enzymes; they can use a variety of sterols as substrates, and their activities are significantly activated by a variety of sterols. Among the sterols tested (more than 20), CHOL is the best substrate and the best activator. Epicholesterol, which contains the 3-α-OH at steroid ring A, is neither a substrate nor an activator. The structural feature of the sterol as an ACAT substrate is distinct from that as an activator (12); however, a sterol- like molecule that is only a substrate, but not as an activator has yet to be found.
We note that PREG and its metabolite dihydroepiandrosterone (DHEA) are the only 2 steroids in the steroid hormone biosynthesis pathway that contain a 3-β-OH at its steroid ring A. On the other hand, both PREG and DHEA contain a much shorter side chain at steroid ring D than CHOL. In the current work, we test the possibility that PREG may be an ACAT substrate, and that ACAT1 is the missing enzyme that catalyzes cellular PREG esterification in body cells. We also examine the effect of ACAT deficiency or LCAT deficiency on the PREG ester levels in adrenals, and in the serum-free adrenal steroid levels by using normal (wild-type), Acat1−/−, Acat2−/−, and Lcat−/− mice.
EXPERIMENTAL PROCEDURES
Materials
PREG, DHEA, estradiol, cholestanol, epicholesterol, PREG stearate, PREG sulfate, dehydroergosterol, 24(S)-hydroxycholesterol, 7-ketocholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol, 7α-hydroxycholesterol, and 7β-hydroxycholesterol were from Steraloids. Ent-cholesterol described in Ref. 12 was a research gift from Professor D. Covey at Washington University Medical School, St Louis. Sitosterol, 22(R)-hydroxycholesterol, protease inhibitor mixture, taurocholate, cholesterol, cholesterol oleate, Nile red, oleic acid, acetic anhydride, oleoyl coenzyme A, bovine serum albumin (BSA), fetal bovine serum (FBS), egg phosphatidylcholine, and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), were from Sigma. [3H]cholesterol, [3H]PREG (S.A. 40 μCi/nmol), [3H]DHEA (S.A. 60 μCi/nmol), and [3H]estradiol (S.A. 40 μCi/nmol) were from Perkin Elmer. [3H]Oleoyl CoA was synthesized according to the protocol described (13). All cell culture media were from Cellgro. Penicillin/streptomycin stock solution was from Invitrogen. Newborn calf serum (NBS) was from Atlanta Biologicals. N2a, SH-SY5Y and N9 cells were generous gifts from Professors S. Supattapone, R. Maue, and W. Hickey at Dartmouth Medical School, respectively. Human SW13 adrenal cortical carcinoma cells were from ATCC. All reagent-grade organic solvents were from Fisher Scientific. The isotype nonspecific ACAT inhibitors F12511, CP-113,818, and Dup 128 described in (14) were research gifts from Pierre Fabre. Analytical silica thin-layer chromatography (TLC) plates were from JT Baker. C18 cartridge was from Thermoscientific.
Methods
Separation of Various Steroids using TLC
100 μg of various steroid and lipid standards were loaded onto an analytical TLC plate. The mobile phase used to separate the lipids was 90:10:1 (petroleum ether/ether/acetic acid). Lipids were visualized by spraying the plate with 40 μg/ml Nile red. Rf values were: cholesterol oleate, 0.89; cholesterol, 0.27; trigylceride, 0.54; oleic acid, 0.41; PREG, 0.14; allopregnanolone, 0.13; progesterone, 0.11; DHEA, 0.11; estradiol, 0.12; PREG sulfate, 0; PREG stearate, 0.41. PREG stearate was used as the standard for PREG oleate.
Enzyme Purification
The source of ACAT1 used in kinetic analysis was the recombinant human ACAT1 tagged with 6× histidine at the N terminus (His-ACAT1), expressed in insect Hi5 cells, and partially purified at the nickel column chromatography step by procedures described previously (15). The source of ACAT2 used was the recombinant human ACAT2 tagged with 6× histidine at the N terminus (His-ACAT2), stably expressed in Chinese hamster ovary (CHO) cells as described previously (14). The His-ACAT2 enzyme was partially purified by using the same protocol for purifying recombinant His-ACAT1 using the nickel column chromatography. After purification, the ACAT1 and ACAT2 preparations were stored in 0.5% CHAPS at −80 °C. The source of ACAT1 used in direct binding analysis was the recombinant human ACAT1 tagged with 6× histidine at the N terminus and with a FLAG tag at the C terminus; the enzyme was expressed in H293 cells and purified to homogeneity as described (16). 1 unit of ACAT specific activity is defined as 1 pmol cholesteryl oleate formed/min/μg protein assayed under standard mixed micelle assay condition.
ACAT Activity Assays
Assay with [3H]steroid and with non-radioactive oleyl Co-A as the substrates. The mixed micelles were prepared as described, with 11.2 mm PC/18.6 mm taurocholate (11). Unless stated otherwise, the concentration of [3H]PREG, [3H]DHEA, or [3H]estradiol (S.A. 40 μCi/nmol) in the micelles was 1 μm. Total count per sample was 1 uCi. In activation assays, various concentrations as indicated of a given unlabeled sterol were prepared in PC/taurocholate micelles and mixed with micelles that contained the labeled steroid. The enzyme was then added at 4 °C. To start the enzyme reaction, 10 nmol nonradioactive oleoyl CoA/BSA was added; the reaction was continued at 37 °C for 30 min and terminated by adding 2:1 chloroform/methanol. The lipids were extracted and separated by TLC. The scraped bands were suspended in the scintillation fluid and counted in a scintillation counter. To conduct substrate saturation curves, [3H]steroids in micelles were prepared at varying concentrations as indicated, with 1 μCi total counts per sample.
Assay with nonradioactive steroids and with [3H]oleoyl CoA as the substrates. The ACAT enzyme was pre-incubated with a mixture that contained CHOL with or without PREG at concentrations as indicated in PC/taurocholate micelles for 1 h at 4 °C. The reaction was initiated by adding 10 nmol [3H]oleoyl CoA/BSA (3.0 × 104 dpm/nmol). The reaction was at 37 °C for 10 min. Sterol products were extracted, separated, and counted by the same method as described above.
Binding between ACAT1 and Sterols
Binding was conducted in the same manner as described (16), by monitoring changes in the intrinsic fluorescence of ACAT1 upon ligand binding. The HisACAT1-FLAG protein at 200 ng/ml (in 50 mm potassium phosphate, pH 7.8, 8.13 mm CHAPS, and 0.5 m KCl) was used. Sterol (PREG, or CHOL, or coprostanol, or epicoprostanol) at concentrations as indicated was prepared in CHAPS/PC mixed micelles and stored at room temperature in the dark until use. For each binding assay, 25 μl of ACAT1 protein was added to the cuvette containing 50 μl of mixed micelles with rapid mixing to initiate the binding. Fluorescence was monitored within 15 s of adding the enzyme.
Cell Culture
All cells were grown in 6-well plates at 37 °C with 5% CO2. All media contain and penicillin/streptomycin. SW13 cells were grown in Leibovitz's L-15 medium, 2 mm l-glutamine, and 10% FBS. SH-SY5Y cells were grown in 1:1 Dulbecco's Modified Essential Medium/F-12 with 15% FBS. N2a cells were grown in Minimal Essential Media + 100 μm glutamine. N9 cells were grown in McCoy's 5a Medium Modified plus 10% FBS. All cells were grown to ∼90% confluency before the experiments were initiated.
PREG Esterification in Intact Cells
Cells were grown in the presence or absence of 4 μm of the ACAT inhibitor F12511, or 10 μm of a different ACAT inhibitor CP113,818 or Dup128, for 12 h. The ACAT inhibitor was added from a 1000-fold stock in DMSO. 1 μCi of [3H]PREG or 1 μCi [3H]cholesterol (S.A. 40 μCi/nmol) in DMSO was then added to the 2 ml of tissue culture media in 6-well plates for 6 h. Final concentration of DMSO in media was 0.5%. Cells were washed twice with PBS and once with PBS + 1 mm BSA, then harvested by incubation at room temp with 0.2 m NaOH for 1 h. After aliquoting for protein determination, NaOH was neutralized with 3 m HCl and 100 mm Tris, pH 7.6; lipids were extracted with 2:1 chloroform:methanol and separated by TLC. The PREG, CHOL, PREG stearate, and CHOL oleate bands were scraped and counted in a scintillation counter.
Mice
Normal (WT), Acat1−/−, and Acat2−/− mice maintained at Dartmouth were congenic C57BL6 and are as described previously (8). WT and Lcat1−/− mice maintained at NIH were also congenic C57BL6 as described previously (17). Mice were fed ad libitum with standard chow diet, maintained in a pathogen-free environment and kept on a 12-h light/dark schedule. At Dartmouth, animal procedures were approved by the Animal Care and Use Committee (protocol number 08.05.01). At NIH, animal procedures were approved by an NIH Institutional Animal Care Committee (protocol number H-0050R1).
Mouse Tissue Isolation and Lipid Extraction
Acat1−/− mice, Acat2−/− mice, and WT mice at 2 month of age were killed by CO2 asphyxiation at a time between noon and 2 PM at Dartmouth. Tissue samples were isolated rapidly and frozen on dry ice and stored at −80 °C until use. Lcat−/− mice and WT mice at 3.5 month of age were killed by similar means at the NIH/NHLBI; tissues were isolated rapidly and shipped on dry ice to Dartmouth for processing. The tissue sample (up to 200 mg) was thawed on ice and underwent Folch lipid extraction. The lipid fraction was dried down under nitrogen and re-dissolved in 1 ml methanol, vortexed, and transferred to a 1.5 ml of glass GCMS vial (National Scientific), stored at −20 °C till usage.
Determination of PREG/PREG Ester
PREG and PREG ester were separated by using the reverse phase silica solid phase extraction column based on a published procedure (18) and described as follows. The lipid sample extracted from up to 200 mg tissue was dissolved in 1 ml of methanol and loaded onto a 6-ml size, 500 mg C18 cartridge (Thermoscientific, cat no. 60108-305). The sample tube was washed with 0.5 ml methanol; the wash was loaded onto the cartridge. The cartridge was eluted with 5 ml of methanol/water (85/15). The combined flow-through and the eluate (fraction #1; containing the unesterified forms of PREG and DHEA) was collected, dried, and resuspended in 200 μl of methanol. PREG was analyzed by LC-MS/MS using a published procedure (19) outlined in the next section. The cartridge was next eluted with 5 ml of methanol/water (9/1). This eluate contained most of the oxysterols and up to 10% of CHOL, and was not collected. The cartridge was then eluted with 5 ml of methanol/CHCl3 (1/1), followed with 5 ml of hexane. The combined methanol/CHCl3 (1:1) and the hexane eluate (fraction #2; containing up to 90% of CHOL, all the PREG esters and CHOL esters) were dried; 0.5 ml 1 m NaOH in methanol was added. The sample was heated at 60 °C for 2 h to hydrolyze all the steryl esters. After hydrolysis, 1 ml of water, and 200 μl of 4 m HCl were added per sample. Methanol was removed by evaporation under nitrogen. The PREG released from PREG ester was recovered by extracting with 2× 4 ml of diethyl ether. The ether fraction was dried down under nitrogen, redissolved in 1 ml methanol. The PREG present in methanol was cleaned up by going through the C18 cartridge fractionation as described above and collected as the methanol; water (85:15) eluate (fraction #1). The samples were analyzed by LC-MS/MS in the same manner as the free form of PREG. Recovery of PREG during the alkaline hydrolysis and sample clean up in a set of controls analyzed along with the samples averaged 50%. PREG ester values reported were corrected for procedural losses observed.
Determination of Serum Adrenal-secreted Steroids in Mice by LC-MS/MS
Blood was drawn from 2-month-old male mice between the time of 12 noon and 2 pm using the cardiac puncture method. Serum was isolated after centrifugation at 9,000 RPM for 15 min, and immediately stored at −80 °C until analysis. Samples were transported between the laboratories on dry ice. Quantitation of free (unesterified) PREG, allopregnanolone, and progesterone in serum was performed by a sensitive and specific LC-MS/MS assay (19). Stable isotope labeled internal standards (d4-PREGN, d4-allopregnanolone, d8–17OH progesterone) were added to aliquots of the samples; steroids were extracted from serum by solid-phase extraction, derivatized to form oximes, the derivatives were extracted with methyl t-butyl ether and analyzed. Instrumental analysis was performed on API 4000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex) equipped with HPLC series 1200 (Agilent Technologies), HTC PAL autosampler (LEAP Technologies). Mass transitions monitored in the method were as follows, PREGN m/z 332/86 and 332/300, d4-PREGN m/z 336/90, 336/304, allopregnanolone m/z 334/86, and 334/316, d4-allopregnanolone m/z 338/90, 338/320, progesterone m/z 345/112 and 345/124, d8–17OH progesterone (internal standard for progesterone) m/z 369/128 and 369/315. Identity of each analyte was confirmed by evaluating ratios of two analyte-specific mass transitions. Quantitative calibration was performed with every batch of samples and was used in conjunction with the intensities of the transitions of internal standards to calculate concentrations in the samples; three quality control samples were analyzed with every set of samples. Limit of quantification for the analytes was 0.025 ng/ml, imprecision was <10%.
Determination of CHOL/CHOL Ester
The lipid sample extracted from mouse tissues was redissolved in 2 ml of hexane. Half of the sample was analyzed for free CHOL content. The other half-sample underwent alkaline hydrolysis in the same manner as described above. The total CHOL (free CHOL and CHOL released from chol ester) was recovered by extraction with ethyl acetate, then analyzed for free CHOL content. The free cholesterol contents were analyzed by GC-MS by using a Shimadzu QP2010 instrument, in the same manner as described previously (8). CHOL ester values were derived by subtracting the free CHOL values from the total CHOL values.
Statistical Analyses
When indicated, statistical analyses of results were performed using a two-tailed, unpaired Student's t test. The difference between two sets of values was considered significant when the p value was < 0.05 (*, p < 0.05; **, p < 0.01).
RESULTS
PREG Is an ACAT Substrate but Not an ACAT Activator
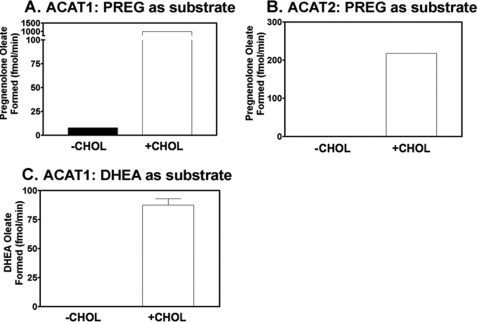
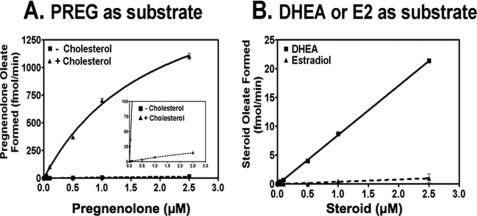
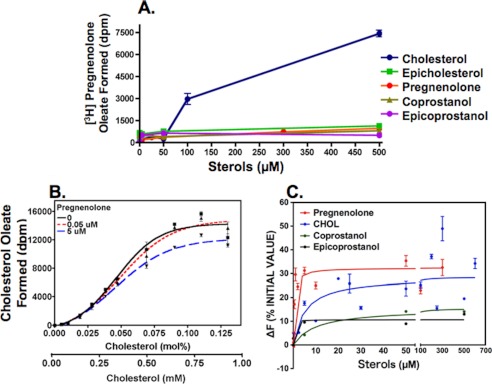
CHOL is converted metabolically to various steroid hormones (Fig. 1). PREG contains the classical A, B, C, D steroid rings, and the 3-β-OH moiety. Based on our previous studies (12), PREG may be an ACAT substrate. We tested this possibility by using tritium-labeled PREG solubilized in mixed micelles as the substrate and partially purified ACAT1 or ACAT2 as the enzyme source. The results show that in the absence of CHOL, PREG is a very poor substrate for ACAT1 (Fig. 2A) or ACAT2 (Fig. 2B). However, in the presence of CHOL, it is a much better substrate for both enzymes. DHEA, an obligatory biosynthetic intermediate between PREG and various sex steroid hormones, is the only other steroid that possesses the same classical A, B, C, D steroid ring and the 3-β-OH moiety. We used ACAT1 as the enzyme source to test whether DHEA might also be an ACAT substrate. Our result (Fig. 2C) shows that, in the absence of CHOL, DHEA is essentially not a substrate, whereas it becomes a substrate in the presence of CHOL. Comparing the results of Fig. 2, A and C reveals that PREG is a much better substrate than DHEA. We next conducted a PREG substrate saturation curve study, with or without CHOL, using ACAT1 as the enzyme source. The results (Fig. 3A) show that CHOL activates PREG esterification by more than 100-fold. The CHOL-dependent activation occurs at two different levels: by reducing the apparent Km for PREG, and by increasing the Vmax for producing PREG ester. Without CHOL, the Km for PREG is too high to be measurable; with CHOL (added at 0.32 mm), the apparent Km for PREG is about 1.2 μm. We next used ACAT1 as the enzyme source and conducted a DHEA substrate saturation curve, with CHOL present. Our results (Fig. 3B) reveal that even with CHOL present, the apparent Km for DHEA is still too high to be measurable; the result of a separate experiment showed that estradiol (E2) is not an ACAT1 substrate. Additional results show that the shape of the CHOL-dependent activation on PREG esterification is sigmoidal (instead of hyperbolic); the result of this experiment also showed that PREG or other steroid analogs tested could act as an activator (Fig. 4A). These results show that CHOL acts as a potent allosteric activator when PREG is used as the substrate. Additional results showed that when CHOL was used as the substrate, PREG added at a high concentration (5 μm; four times the apparent Km value for PREG) only slightly inhibited CHOL esterification (by 10–15%) (Fig. 4B). The inhibitory effect of PREG mainly occurs by decreasing the Vmax for CHOL esterification, not by affecting the apparent Km for CHOL, which is ∼0.3 mm. The results described above suggest ACAT1 can bind to PREG with high affinity. We tested this possibility by comparing the binding affinities of ACAT1 toward PREG and CHOL. The binding assay was based on the fact that ACAT1 is a fluorescent protein; substrate binding causes significant changes in the intrinsic fluorescence of ACAT1 (16). The results show that ACAT1 directly binds to PREG with a Kd = 0.6 μm, which is 58-fold lower than the concentration for half maximal CHOL binding (35 μm) (Fig. 4C; top two curves). The result of a control experiment showed that ACAT1 does not display significant binding to either coprostanol or epicoprostanol, two sterols that contain steroid rings A and B in cis-fused configuration (Fig. 4C; bottom two curves). We had previously shown that ACAT1 does not display significant binding to epicholesterol, which possesses 3-α-OH moiety at ring A (16). The result shown in Fig. 4C confirms our previous finding, indicating that binding between ACAT1 and sterol is sterol structure-specific.
FIGURE 1.
Metabolic conversion of CHOL to various steroids via PREG.
FIGURE 2.
Esterification of PREG and DHEA in the presence or absence of CHOL by ACAT in vitro. ACAT1 (for A and C) or ACAT2 (for B) purified through the Nickel column chromatography step (described under “Experimental Procedures”) was used as the enzyme source. 1 μm [3H] PREG or 1 μm [3H] DHEA was delivered to mixed micelles in the presence (+) or absence (−) of 0.32 mm CHOL. The assay was as described under “Experimental Procedures.” Results shown are representative of at least two separate experiments; error bars represent deviation between duplicates.
FIGURE 3.
Substrate saturation curves for PREG, DHEA, and E2 in the presence or absence of CHOL. ACAT1 was the enzyme source. A. [3H]PREG was delivered to the mixed micelles in the presence or absence of 0.32 mm CHOL. The inset shows a portion of result at a higher magnification. B, [3H]DHEA or [3H]E2 was delivered to mixed micelles in the presence of 0.32 mm CHOL. Assay was as described under “Experimental Procedures.” Results shown are representative of at least two separate experiments; error bars represent deviation between duplicates.
FIGURE 4.
A, activation of PREG esterification by CHOL or by various sterol analogs. ACAT1 was the enzyme source. CHOL or other sterol analogs at various concentrations as indicated was delivered to mixed micelles in the presence of 1 μm [3H]PREG. B, effect of PREG on CHOL esterification. ACAT1 was the enzyme source. [3H] Oleoyl CoA/BSA was the radiolabeled substrate. CHOL was delivered to mixed micelles in the presence of 0, 0.05, or 5 μm of PREG as indicated. The assay was as described under “Experimental Procedures.” C, binding between ACAT1 and PREG or CHOL, or coprostanol, or epicoprostanol as indicated. Changes in intrinsic fluorescence of ACAT1 was monitored to measure binding, using a procedure described under “Experimental Procedures.” The steroid concentration varied from 0.5 to 500 μm as indicated. Results are the composite of 2 separate experiments. The curves were plotted by using the one site-specific binding program. The Prism software program was used for curve plottings.
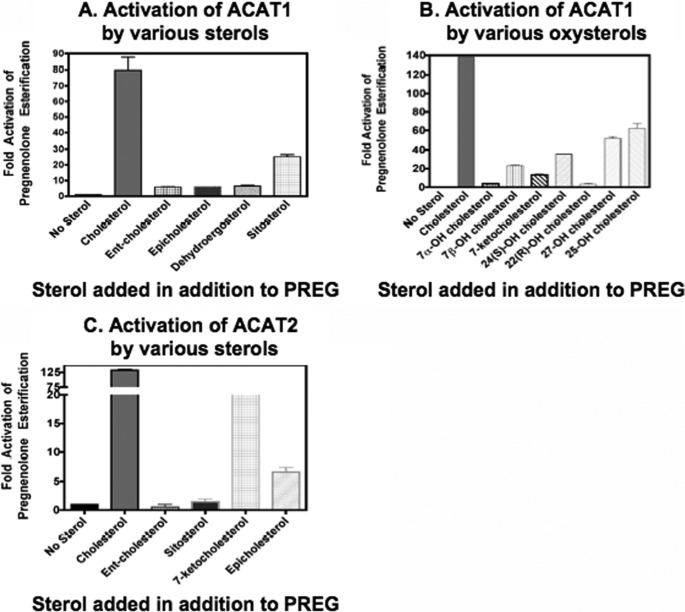
Various Sterols Can All Activate ACAT1 to Catalyze PREG Esterification
The above results show that PREG binds to ACAT1 specifically; it is a substrate but is not an activator for ACAT. This finding provides us the unique opportunity to probe the structural specificity of the activator site, using PREG as the substrate. We first used ACAT1 as the enzyme source to examine the effect of adding various non-oxysterols, all kept at 0.32 mm, on PREG esterification. Our results (Fig. 5A) show that as expected, CHOL provides the maximal activation. Sitosterol, a plant sterol, also activates PREG esterification, although not as efficiently as CHOL. Epi-CHOL, ent-CHOL (the mirror image of CHOL) (12), and dehydroergosterol (a fluorescent CHOL analog) can also slightly activate PREG esterification. In contrast to sitosterol, none of these three latter sterols is an ACAT1 substrate (12). We next tested the effects of various oxysterols, all kept at 0.32 mm, on PREG esterification. Our results (Fig. 5B) show that essentially all the oxysterols tested activate PREG esterification, but at varying degrees. When ACAT2 was used as the enzyme source, we found (Fig. 5C) that CHOL also provides maximal activation of PREG esterification, followed by 7-ketoCHOL, an auto-oxidation product of CHOL. Unlike ACAT1, the plant sterol sitosterol only slightly activated ACAT2 esterification of PREG. These results imply that the mechanisms of sterol-dependent activation of ACAT1 and ACAT2 are similar, but not identical.
FIGURE 5.
Activation of PREG esterification by various sterols. A and B, ACAT1, C, ACAT2. 1 μm [3H]PREG (S.A. 20Ci/mmol) was delivered to micelles in the absence or presence of 0.32 mm of various sterols as indicated. The assay was as described under “Experimental Procedures.” Results shown are representative of at least two separate experiments; error bars represent deviation between duplicates.
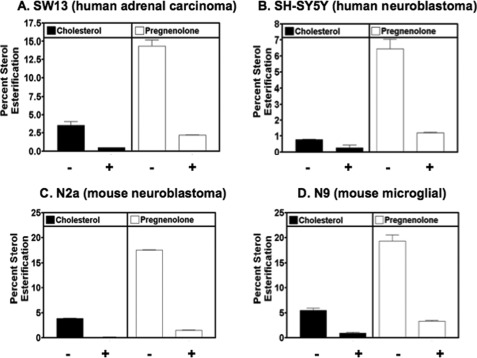
ACAT Carries out PREG Esterification in Various Mammalian Cell Lines
The results described show that ACAT can esterify PREG in vitro. To test the biological significance of these findings, we used human SW13 cells (an adrenal cortical carcinoma cell line) (A), human SH-SY5Y cells (a neuroblastoma cell line) (B), mouse N2a cells (a neuroblastoma cell line) (C), or mouse N9 cells (a microglioma cell line) (D). We pre-incubated these cells with or without the specific ACAT inhibitor F12511 for 12 h, then fed cells with labeled PREG or labeled CHOL for 6 h, and analyzed CHOL esterification and PREG esterification. The results show (Fig. 6, A–D) that all cells tested can esterify both CHOL and PREG, and that pre-incubation of the cells with the ACAT inhibitor F12511 largely abolished both the CHOL esterification and PREG esterification, by 80–90%. In results not shown, we found that in N2a cells, two other isotype nonspecific ACAT inhibitors CP-113,818 and Dup128 added to growth medium at 10 microM for 12 h also inhibited PREG esterification by 70% (with CP-113,818) or by 90% (with Dup128).
FIGURE 6.
PREG esterification and effect of the ACAT inhibitor F12511 in various cell types as indicated. Cells were preincubated for 12 h with 4 μm of the isotype nonspecific ACAT inhibitor F12511 in DMSO (+), or with DMSO only (−). [3H]PREG or [3H]CHOL was delivered to the cells from stock DMSO solutions for 6 h. The labeled cellular lipids were extracted, separated by TLC, and counted as described under “Experimental Procedures.” Results shown are representative of at least two separate experiments; error bars represent deviation between duplicates.
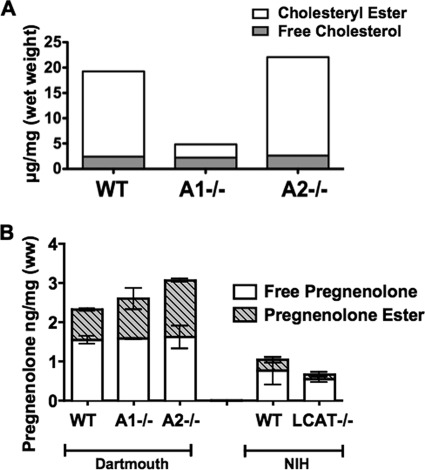
% PREG Esters in Adrenals Are Not Decreased in Either Acat1−/− or Acat2−/− Mice, But Are Decreased in Lcat1−/− Mice
ACAT may play significant role in determining the PREG ester contents in steroidogenic tissues. To test this possibility, in the first experiment, we measured the levels of the unesterified and esterified CHOL as well as the unesterified and esterified PREG in the adrenals isolated from the 2-month-old normal (WT), Acat1−/− and Acat2−/− male mice. The results (Fig. 7A) show that most of the cholesterol present in the adrenals is in esterified form; lacking ACAT1, but not ACAT2, dramatically decreases the % CHOL esters (by approx. 90%) in this tissue. These data confirm an early report (20), and demonstrate that ACAT1 is the major isoenzyme in this tissue. Regarding PREG esterification, in WT mice, ∼33% of the total PREG is esterified; lacking either ACAT1 or ACAT2 does not decrease the % PREG esters present in the adrenals (Fig. 7B; comparing the first three columns). These results suggest that LCAT, instead of ACAT, may play a role in determining the PREG ester content in the adrenals. To test this possibility, in a second experiment, we measured the levels of the unesterified and esterified PREG in the adrenals isolated from normal and Lcat−/− mice, (with comparable numbers in males and females at 3.5 month of age). The results (Fig. 7B; the last two columns) show that in the normal mouse adrenals, 27% of the total PREG is esterified; in the Lcat−/− mouse adrenals, the percent PREG esterification decreases from 27% to 18% (Fig. 7B; comparing 4th and 5th columns), demonstrating that the Lcat−/− mouse adrenals contain reduced but clearly measurable PREG esters. The results shown in Fig. 7 suggest that LCAT plays a role, while ACAT1 may only play auxillilary role in determining the % PREG ester contents in this tissue. We note that in the first experiment, the total pregnenolone content in the WT mouse adrenal was higher than the value found in the second experiment (2.3 ng/mg versus 1.0 ng/mg; (comparing 1st column versus 4th column in Fig. 7B); the % esterified PREG in WT mouse adrenals were however similar between the two experiments (33% versus 27%). For the first experiment, mice were housed at Dartmouth; for the second experiment, mice were housed at NIH. The discrepancy in total adrenal PREG values observed between these two experiments maybe caused by environmental difference(s) in animal facilities used to house the mice.
FIGURE 7.
The CHOL/CHOL ester contents (A) and the PREG/PREG ester contents (B) in adrenals of normal (WT), Acat1−/−, Acat2−/−, and Lcat−/− mice. For adrenal CHOL/CHOL ester measurement, samples were collected from 16 mice/group and analyzed as a single pool. Mice were housed at the Dartmouth animal facility. For PREG/PREG ester measurements, in the first experiment, mice were housed at the Dartmouth animal facility. The adrenals isolated from the 2-month-old normal (WT), Acat1−/− and Acat2−/− male mice were collected at 6 mice/group and analyzed as 2 separate pools, each consisting of tissues from 3 mice. In the second experiment, mice were housed at the NIH animal facility. The samples were analyzed in the same manner as the first experiment. The adrenals were isolated from the 3.5-month-old normal (WT) and Lcat−/− mice, with comparable numbers in males and females. Procedures for lipid extraction, quantitation of PREG/PREG ester by LC-MS/MS, and quantitation of CHOL/CHOL ester by GC-MS are described under “Experimental Procedures.” The error bar indicates differences between the mean.
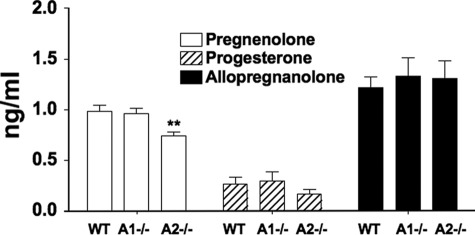
Serum Adrenal Secreted Steroid Levels Are Not Affected by the Lack of ACAT1 or ACAT2 in Male Mice
PREG and its metabolites exhibit a variety of biological activities in vitro and in vivo; reviewed in Refs. 21, 22. PREG fed to animals exhibit beneficial effect on learning and memory behavior; reviewed in Ref. 23. Both ACAT1 are ACAT2 are drug targets for human diseases. Since both enzymes can esterify PREG, it is important to determine whether lacking ACAT1 or lacking ACAT2 affects serum steroid levels in vivo. To address this issue, we extracted the blood from 2-month-old male mice, isolated the serum and subjected the samples to PREG, progesterone, and allopregnanolone analyses by using an established method that involves LC-MS/MS and isotope dilution. The results show (Fig. 8) that, no changes in the serum PREG, progesterone, or allopregnanolone concentrations were seen as a result of Acat1−/− in mice; a slight decrease was seen in the PREG concentration of sera isolated from the Acat2−/− mice.
FIGURE 8.
Serum adrenal-secreted steroids in normal (WT), Acat1−/−, and Acat2−/− mice. Blood was drawn from 2-month-old male mice between the hr of 12 and 2 pm using the cardiac puncture method. Serum was isolated after centrifugation at 9,000 RPM for 15 min., and immediately stored at −80 degree until analysis. Quantitation of free (unesterified) PREG, progesterone, and allopregnanolone in serum by LC-MS/MS are as described under “Experimental Procedures.” n = 15 mice per group. Error bars represent standard errors of the mean. For **, p value = 0.004.
DISCUSSION
Our current results show that ACAT1 uses PREG as a substrate in vitro and in intact cells; in vivo, deficiency in ACAT1 or ACAT2 does not decrease the overall PREG ester content in steroidogenic tissues, nor does it significantly affect the serum levels of several adrenal secreted steroid hormones. Earlier, Vourc'h et al. (24) reported the identification of an acyltransferase activity in rat brain microsomes that esterified several steroids, including PREG and E2; that acyltransferase activity was more active toward E2 than PREG, and was inactive toward CHOL as substrate. Here we show that with CHOL present, ACAT1 can use PREG, but cannot use E2 as the substrate. These results show that ACAT1 is distinct from the acyltransferase activity reported by Vourc'h et al.
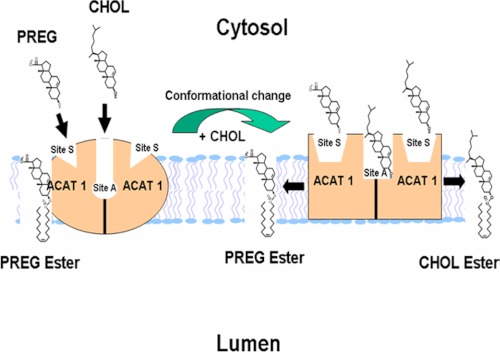
PREG is a sterol-like molecule. Unlike CHOL, PREG does not pack well with phospholipid in the membrane. Our results show that PREG can only be an ACAT1 substrate but not an ACAT1 activator, while many sterols that contain the same iso-octyl side chain at ring D as CHOL does can all serve as an activator (when PREG is used as the substrate). These results support the notion that ACAT1 contains two distinct sterol binding sites, one for an activator; the other for a substrate. The length of the side chain at ring D of the sterol plays more important role to fulfill the activator site than to fulfill the substrate site. ACAT1 binds to PREG much tighter than CHOL. Together, these results indicate that ACAT1's sterol substrate site does not involve extensive interaction between the sterol and phospholipid in the membrane, while its sterol activator site does. Additional results show that, as an activator the major effect of CHOL is to increase the Vmax of the enzyme. Much more work will be needed to understand ACAT1 as an allosteric enzyme. To facilitate further research in this area, we present a working model to explain ACAT1 allosterism (Fig. 9). This model contains the following features: 1) ACAT1 is a homotetramer, although functionally it may act as a dimer (25). Within each dimer, it may contain two identical sterol substrate sites (designated as site S), and one or two sterol activator site(s) (designated as site A). 2) Site S preferentially binds PREG, although it can also bind a variety of sterols including CHOL, sitosterol, and oxysterols. The binding between site S and the steroid is mainly stereospecific and does not involve extensive interaction with phospholipid in membrane. Site S does not bind ent-CHOL, or epi-CHOL. Site A, on the other hand, prefers to bind CHOL but cannot bind PREG; it can also bind a variety of other sterols such as sitosterol, oxysterols, ent-CHOL, and dehydroergosterol, etc. The binding between site A and the steroid may involve the ability of the sterol molecule to interact biophysically with phospholipid in membrane, in addition to its stereospecific structure. 3) When only PREG is present, PREG binds site S, but it fails to trigger appropriate conformational changes, and the enzyme can only catalyze PREG esterification at a very low rate. 4) When PREG and CHOL (or its close analogs) are both present, binding of CHOL at site A causes conformational changes, enabling the enzyme bind the sterol tighter and to catalyze the reaction much more efficiently. CHOL binding at site A may also allow the enzyme to bind and esterify CHOL at site S more efficiently. 5) Site S may exhibit half-of-the-sites reactivity (26) toward PREG or toward oxysterol as a substrate. This feature may explain the finding shown in Fig. 4B: namely, that PREG added at a high concentration (5 μm) can only inhibit 10–15% of CHOL esterification. Previously, we had shown that the addition of 7-keto-CHOL, an oxysterol, could only slightly inhibit CHOL esterification (11). Thus, while the enzyme may contain two identical S sites per dimer, it may only bind one PREG, or one PREG and one CHOL, or one PREG and one oxysterol per dimer.
FIGURE 9.
A working model to explain ACAT allosterism. See “Discussion” for details.
We also show that cells of adrenal cortical origin (SW13) and non-adrenal cells (N2A, SH-SY5Y, and N9) can synthesize PREG esters from PREG, and that this activity is effectively blocked by using various small molecule ACAT inhibitors F12511, CP-113,818, or Dup128, which are isotype-nonspecific inhibitors that inhibit both ACAT1 and ACAT2 with equal potency. Because ACAT1 is ubiquitously expressed while ACAT2 is mainly expressed in the intestines and liver, these results imply that ACAT1 plays a key role in cellular PREG esterification in most of the body cells. Our data in vivo show that lacking either ACAT1 or ACAT2 does not decrease the overall PREG esters levels present in adrenals, suggesting that in adrenals, most of the PREG esters may originate from lipoprotein bound PREG esters. Earlier studies had shown that the plasma enzyme LCAT uses PREG as an excellent substrate, and that PREG esters can be found in high-density lipoproteins (6). To test this interpretation we compared normal mice versus mice lacking LCAT, and found the % PREG ester to be decreased (by 33%) but not completely abolished in the adrenals of these mice. These data suggest that LCAT plays a role, while ACAT1/ACAT2 may play an auxiliary role in determining the PREG ester content in adrenals, perhaps in other tissues as well. In the future, this possibility can be further tested by comparing the PREG ester contents in normal mouse versus single or double KO mouse lacking LCAT, and/or lacking ACAT1, and/or lacking ACAT2; etc. It is possible that, in addition to LCAT and ACAT, other enzymes(s) also participate in esterifying pregnenolone in vivo. As discussed earlier, Vourc'h et al. (24) reported an acyltransferase activity in vitro that esterifies several steroids including PREG, but does esterify CHOL. Based on its substrate specificity, this enzyme is distinct from either LCAT or ACAT; the molecular identity of this acyltransferase remains unknown at present. Our current work along with earlier studies reveals that the enzyme systems involved in determining tissue pregnenolone ester content in vivo are unexpectedly complex. Further investigations are needed in order to identify all the enzymes involved in pregnenolone esterification, and their physiological roles. Other data presented (Fig. 8) show that lacking ACAT2, but not ACAT1, causes a slight but significant decrease in the serum PREG concentration; this decrease might be caused by changes in lipoprotein levels observed in the Acat2−/− mice (27).
Both ACAT1 and ACAT2 are drug targets to treat human diseases including Alzheimer Disease. Over the last 2 decades, numerous small molecule ACAT inhibitors have been synthesized. The kinetic and binding analyses described in this work can be used to characterize and classify the mode of actions of the ACAT inhibitors. The assays described here can also be used as a screen to discover novel ACAT inhibitors. The free, unesterified PREG and its metabolites play important roles in physiology. Our data in animals suggest that inhibiting ACAT1 may not adversely affect the free PREG levels in serum, or the PREG and PREG ester levels in steroidogenic tissues.
Acknowledgments
We thank our colleagues Drs. Larry Myers and Dean Madden for stimulating discussions and Ruhong Dong for assistance during the course of this work.
This work was supported, in whole or in part, by a National Institutes of Health (NIH) NRSA T32 Postdoctoral Fellowship (to M. A. R.), an NIH Institutional Predoctoral Training Grant Fellowship (to J. L.), and NIH Grants R01AG37609 (to T. Y. C.), and HL60306 (to T. Y. C. and C. C. Y. C.). This work was also supported in part by the ARUP Institute for Clinical and Experimental Pathology.
- PREG
- pregnenolone
- LCAT
- lecithin: cholesterol acyltransferase
- ACAT
- acyl-CoA:cholesterol acyltransferase
- CHOL
- cholesterol
- CE
- cholesteryl ester
- DHEA
- dihydroepiandrosterone.
REFERENCES
- 1. Miller W. L. (2008) Steroidogenic enzymes. Endocr. Dev. 13, 1–18 [DOI] [PubMed] [Google Scholar]
- 2. Hu J., Zhang Z., Shen W. J., Azhar S. (2010) Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 7, 47–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mellon-Nussbaum S., Ponticorvo L., Lieberman S. (1979) Characterization of the lipoidal derivatives of pregnenolone prepared by incubation of the steroid with adrenal mitochondria. J. Biol. Chem. 254, 12500–12505 [PubMed] [Google Scholar]
- 4. Damon M., Chavis C. (1982) GLC/MS identification of new pregnenolone metabolites in confluent embryonic rat fibroblast cultures. J. Steroid. Biochem. 16, 771–778 [DOI] [PubMed] [Google Scholar]
- 5. Bélanger B., Caron S., Bélanger A., Dupont A. (1990) Steroid fatty acid esters in adrenals and plasma: effects of ACTH. J. Endocrinol. 127, 505–511 [DOI] [PubMed] [Google Scholar]
- 6. Lavallée B., Provost P. R., Belanger A. (1996) Formation of pregnenolone- and dehydroepiandrosterone-fatty acid esters by lecithin-cholesterol acyltransferase in human plasma high density lipoproteins. Biochim. Biophys. Acta 1299, 306–312 [DOI] [PubMed] [Google Scholar]
- 7. Chang T. Y., Li B. L., Chang C. C., Urano Y. (2009) Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 297, E1–E9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bryleva E. Y., Rogers M. A., Chang C. C., Buen F., Harris B. T., Rousselet E., Seidah N. G., Oddo S., LaFerla F. M., Spencer T. A., Hickey W. F., Chang T. Y. (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc. Natl. Acad. Sci. U.S.A. 107, 3081–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Z. Y., Lin S., Heinen J. A., Chang C. C., Chang T. Y. (2005) The active site His-460 of human acyl-coenzyme A:cholesterol acyltransferase 1 resides in a hitherto undisclosed transmembrane domain. J. Biol. Chem. 280, 37814–37826 [DOI] [PubMed] [Google Scholar]
- 10. Chang C. C., Sun J., Chang T. Y. (2011) Membrane bound O-acyltransferases (MBOAT). Front. Biol. 6, 177–182 [Google Scholar]
- 11. Zhang Y., Yu C., Liu J., Spencer T. A., Chang C. C., Chang T. Y. (2003) Cholesterol is superior to 7-ketocholesterol or 7 alpha-hydroxycholesterol as an allosteric activator for acyl-coenzyme A:cholesterol acyltransferase 1. J. Biol. Chem. 278, 11642–11647 [DOI] [PubMed] [Google Scholar]
- 12. Liu J., Chang C. C., Westover E. J., Covey D. F., Chang T. Y. (2005) Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 391, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bishop J. E., Hajra A. K. (1980) A method for the chemical synthesis of 14C-labeled fatty acyl coenzyme A's of high specific activity. Anal. Biochem. 106, 344–350 [DOI] [PubMed] [Google Scholar]
- 14. Chang C. C., Sakashita N., Ornvold K., Lee O., Chang E. T., Dong R., Lin S., Lee C. Y., Strom S. C., Kashyap R., Fung J. J., Farese R. V., Jr., Patoiseau J. F., Delhon A., Chang T. Y. (2000) Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 275, 28083–28092 [DOI] [PubMed] [Google Scholar]
- 15. Yu C., Chen J., Lin S., Liu J., Chang C. C., Chang T. Y. (1999) Human acyl-CoA:cholesterol acyltransferase-1 is a homotetrameric enzyme in intact cells and in vitro. J. Biol. Chem. 274, 36139–36145 [DOI] [PubMed] [Google Scholar]
- 16. Chang C. C., Miyazaki A., Dong R., Kheirollah A., Yu C., Geng Y., Higgs H. N., Chang T. Y. (2010) Purification of recombinant acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) from H293 cells and binding studies between the enzyme and substrates using difference intrinsic fluorescence spectroscopy. Biochemistry 49, 9957–9963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rousset X., Vaisman B., Auerbach B., Krause B. R., Homan R., Stonik J., Csako G., Shamburek R., Remaley A. T. (2010) Effect of recombinant human lecithin cholesterol acyltransferase infusion on lipoprotein metabolism in mice. J. Pharmacol. Exp. Ther. 335, 140–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liere P., Pianos A., Eychenne B., Cambourg A., Liu S., Griffiths W., Schumacher M., Sjövall J., Baulieu E. E. (2004) Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J. Lipid Res. 45, 2287–2302 [DOI] [PubMed] [Google Scholar]
- 19. Kushnir M. M., Rockwood A. L., Roberts W. L., Pattison E. G., Owen W. E., Bunker A. M., Meikle A. W. (2006) Development and performance evaluation of a tandem mass spectrometry assay for 4 adrenal steroids. Clin. Chem. 52, 1559–1567 [DOI] [PubMed] [Google Scholar]
- 20. Meiner V. L., Cases S., Myers H. M., Sande E. R., Bellosta S., Schambelan M., Pitas R. E., McGuire J., Herz J., Farese R. V., Jr. (1996) Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl. Acad. Sci. U.S.A. 93, 14041–14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baulieu E. E., Robel P. (1990) Neurosteroids: a new brain function? J. Steroid. Biochem. Mol. Biol. 37, 395–403 [DOI] [PubMed] [Google Scholar]
- 22. Mellon S. H., Griffin L. D. (2002) Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 13, 35–43 [DOI] [PubMed] [Google Scholar]
- 23. Roberts E. (1995) Pregneolone–from Selye to Alzheimer and a model of the pregnenolone sulfate binding site on the GABAA receptor. Biochem. Pharmacol. 49, 1–16 [DOI] [PubMed] [Google Scholar]
- 24. Vourc'h C., Eychenne B., Jo D. H., Raulin J., Lapous D., Baulieu E. E., Robel P. (1992) Δ5–3 β-hydroxysteroid acyl transferase activity in the rat brain. Steroids 57, 210–215 [DOI] [PubMed] [Google Scholar]
- 25. Yu C., Zhang Y., Lu X., Chen J., Chang C. C., Chang T. Y. (2002) Role of the N-terminal hydrophilic domain of acyl-coenzyme A:cholesterol acyltransferase 1 on the enzyme's quaternary structure and catalytic efficiency. Biochemistry 41, 3762–3769 [DOI] [PubMed] [Google Scholar]
- 26. Fersht A. (1985) Enzyme Structure and Mechanism, W. H. Freeman and Company, New York [Google Scholar]
- 27. Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr. (2003) Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 100, 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]