Background: LPA-LPA1 signaling was shown to be required in nerve injury-induced neuropathic pain development.
Results: LPA5-deficient mice are protected from injury-induced neuropathic pain with decreased pCREB expression in spinal cord dorsal horn neurons.
Conclusion: LPA5 contributes to neuropathic pain development through central pCREB signaling.
Significance: A novel contribution of LPA to neuropathic pain, distinct from LPA1, is revealed through the generation and use of LPA5 null mice.
Keywords: Cyclic AMP (cAMP), G Protein-coupled Receptors (GPCR), Lysophospholipid, Mouse, Pain, DRG, LPA1, PSNL, pCREB, Spinal Cord
Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid that serves as an extracellular signaling molecule acting through cognate G protein-coupled receptors designated LPA1–6 that mediate a wide range of both normal and pathological effects. Previously, LPA1, a Gαi-coupled receptor (which also couples to other Gα proteins) to reduce cAMP, was shown to be essential for the initiation of neuropathic pain in the partial sciatic nerve ligation (PSNL) mouse model. Subsequent gene expression studies identified LPA5, a Gα12/13- and Gq-coupled receptor that increases cAMP, in a subset of dorsal root ganglion neurons and also within neurons of the spinal cord dorsal horn in a pattern complementing, yet distinct from LPA1, suggesting its possible involvement in neuropathic pain. We therefore generated an Lpar5 null mutant by targeted deletion followed by PSNL challenge. Homozygous null mutants did not show obvious base-line phenotypic defects. However, following PSNL, LPA5-deficient mice were protected from developing neuropathic pain. They also showed reduced phosphorylated cAMP response element-binding protein expression within neurons of the dorsal horn despite continued up-regulation of the characteristic pain-related markers Caα2δ1 and glial fibrillary acidic protein, results that were distinct from those previously observed for LPA1 deletion. These data expand the influences of LPA signaling in neuropathic pain through a second LPA receptor subtype, LPA5, involving a mechanistically distinct downstream signaling pathway compared with LPA1.
Introduction
Neuropathic pain is a chronic pain state initiated by injury or perturbation of the peripheral nervous system or the central nervous system. In developed countries, ∼7–8% of the population is affected by neuropathic pain, and the limited treatment options are often ineffectual (1, 2). One of the animal models developed to study neuropathic pain is partial sciatic nerve ligation (PSNL),2 which mimics at least some of the major end points observed in human neuropathic pain (3). These models led to the identification of a range of different neuropathic pain associated stimuli (4–6), one of which is an extracellular signaling lipid known as lysophosphatidic acid (LPA), a lysophospholipid that normally participates in the regulation of diverse cellular activities (7).
LPA is involved in various pathological conditions including cardiovascular diseases (8), fibrosis (9), cancer (10), infertility (11), and central nervous system disorders such as hydrocephalus (12) and neuropathic pain (7). These effects are produced through one or more of the six confirmed G protein-coupled receptors, LPA1–6 (gene names Lpar1-Lpar6), that couple to different combinations of heterotrimeric G proteins (13, 14). Insights into the biological roles for LPA signaling have come from studies of LPA receptor null mutant mice (15–17); however, mice deficient for Lpar5 have not been reported (18).
Using Lpar1 null mutants, prior studies identified LPA1 as an essential receptor for the initiation of neuropathic pain induced by both intrathecal injection of LPA and by PSNL (7). Surrogate markers of neuropathic pain including demyelination of the dorsal root, PKCγ expression, and calcium channel Caα2δ1 expression were all significantly reduced in Lpar1 null mice challenged with PSNL (7). Related studies supported the involvement of LPA1 in neuropathic pain (7, 19, 20). These reports also raised the possibility that other LPA receptors might contribute to aspects of neuropathic pain.
LPA5, previously known as orphan receptor GPR92, was identified as a fifth LPA receptor in 2006 (21). LPA5 activates Gα12/13 and Gαq signaling responses and can also increase intracellular cAMP levels; however, this response is not altered in the presence of a Gαs minigene, suggesting alternative G protein involvement (21). Lpar5 is expressed in spleen, heart, platelets, gastrointestinal lymphocytes, dorsal root ganglia (DRG), and the developing brain (21–24). The high expression level in DRG suggested the possible involvement of LPA5 in pain signaling. Here we report the generation of an Lpar5-deficient mouse and its evaluation in the PSNL model toward determining its contribution to neuropathic pain.
EXPERIMENTAL PROCEDURES
Lpar5 Gene Targeting
Portions of the Lpar5 genomic locus were amplified from a BAC clone obtained from Children's Hospital Oakland Research Institute using a high fidelity Pfx50 DNA polymerase (Invitrogen). Most of the coding exon (from the ATG to the BamHI site) was replaced in frame with enhanced GFP using overlap PCR. A neomycin cassette flanked by loxP sites and an introduced HindIII restriction enzyme site was inserted into the BamHI site of the coding exon. All of the amplified genomic fragments and modified fragments were assembled in pBluescript, and the entire targeting construct was sequenced at the Scripps Research Institute Center for Protein and Nucleic Acid Research.
The targeting construct was linearized and electroporated into R1 ES cells (purchased from the lab of Andras Nagy). Genomic DNA was extracted from neomycin-resistant clones, digested with HindIII, and screened for homologous recombination by Southern blotting with the indicated probe using standard techniques. Positive clones were rescreened and tested for pathogens, and two clones were injected into blastocysts at the Scripps Research Institute Mouse Genetics Core. The resultant chimeras were crossed to C57BL/6J female mice to assay for germ line transmission. Heterozygous mice were then bred together to generate null mutant animals. Genotypes of all heterozygous cross offspring were confirmed by Southern blotting and PCR genotyping with the following primers: GFP Int Rev, 5′-GTGGTGCAGATGAACTTCAGG-3′; 92GTFor, 5′-CAGAGTCTGTATTGCCACCAG-3′; and 92GT Rev, 5′-GTCCACGTTGATGAGCATCAG-3′. Wild type and mutant PCR product sizes are ∼450 and 220 base pairs, respectively.
Reverse Transcription-PCR
To confirm loss of Lpar5 gene transcripts, bone marrow, spleen, and thymus were dissected from wild type and null mutant mice. Single cell suspensions were made from all tissues, and cells were pelleted at 1400 rpm in a centrifuge. The cell pellets were resuspended in TRIzol reagent (Invitrogen), and total RNA was isolated following the manufacturer's protocol. RNA was DNase-treated, and cDNA was synthesized using the SuperScript II first strand cDNA synthesis system (Invitrogen). Reverse transcription PCR was used to amplify β-actin and Lpar5 transcripts from the cDNA with the following primer pairs: β-actin For, 5′-TGGAATCCTGTGGCATCCATGAAC-3′; β-actin Rev, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′; 92RTFor, 5′-ACTCCACGCTGGCTGTATATG-3′; and 92RTRev, 5′-GTAGCCAAAGGCCTGGTATTC-3′.
In Situ Hybridization
For in situ hybridization analysis, a probe corresponding to the Lpar5 deleted region was cloned into pBluescript and linearized with restriction enzymes on either side of the insert, and sense and antisense runoff probes were labeled with digoxigenin labeling mix (Roche Applied Science) using T7 and T3 RNA polymerases (Roche Applied Science), respectively. In situ hybridization was performed according to Braissant et al. (25) with modifications (26). Briefly, freshly frozen blocks were cut at 16–20 μm, and the sections were fixed with 4% paraformaldehyde, permeablized, dehydrated, and stored at −80 °C until use. Prehybridization was performed at 70 °C for 3 h, and the probes were incubated at 68 °C overnight. Signal was detected with anti-digoxigenin/AP Fab fragments (Roche Applied Science) at a 1:1,000 dilution overnight and visualized in nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Millipore). Double staining was then performed on the same slide using anti-NeuN (Millipore) and anti-pCREB (Cell Signaling) antibodies at dilutions of 1:200 and 1:500, respectively. The cells were double stained for both pCREB and LPA5 and quantified from at least eight sections from three different animals, presented as the means ± S.D.
Partial Sciatic Nerve Ligation
The partial sciatic nerve ligation procedure was modified from Seltzer et al. (3). Briefly, 2–4-month-old Lpar5 null mutant and wild type mice in a C57 background were deeply anesthetized using an isoflurane vaporizer with a nose cone throughout PSNL surgery. The right side of the mouse was opened, the sciatic nerve was exposed, and approximately one-half to one-third of the sciatic nerve was tightly ligated using 10-0 fine sutures. The wound was then closed, and the skin was stitched for recovery. Paw withdrawal threshold responses were monitored before and after surgery.
Behavioral Testing
Paw withdrawal threshold was performed with an automated von Frey apparatus (Ugo Basile, Italy). The mice were acclimated in plastic cages with metal mesh bottoms for an hour prior to testing in a temperature- and humidity-controlled testing room. Paw withdrawal threshold (gram) against gradually increasing mechanical stimuli (0–50 g in 20 s) was tested four separate times with at least a 1-min interval between tests. The average response was normalized to presurgery controls and presented as the means ± S.D.
Detection of Demyelination
Dorsal root was collected and fixed in 1% paraformaldehyde, 3% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4) with 5 mm CaCl2 overnight at 4 °C. The fixed dorsal root fiber was then osmicated in 1% OsO4, 0.12 m cacodylate buffer, 3.5% sucrose on ice for 2 h. The samples were then dehydrated in a graded alcohol series followed by acetone treatment and embedded in epoxy resin. Sections of 1 μm were cut and stained with 1% toluidine blue O and examined under a light microscope.
Immunohistochemistry
Spinal cord and DRG from L4–L6 regions were collected and freshly frozen in OCT compound (Sakura Finetek). Using a cryostat, 16-μm sections were cut and fixed with 4% paraformaldehyde, blocked with 3% normal goat serum and 0.1% Triton X-100 in PBS. Primary antibodies against Caα2δ1 (1:200; Sigma), PKCγ (1:500; Santa Cruz Biotechnologies), and glial fibrillary acidic protein (GFAP; 1:500; Sigma) were diluted in blocking buffer and incubated overnight at 4 °C. After washing three times in PBS, the corresponding secondary antibodies were diluted 1:1,000 in the same buffer and incubated for 1 h at room temperature. pCREB staining was performed using a rabbit anti-pCREB antibody (1:500; Cell Signaling) under the same conditions and visualized using ABC and DAB kits (Vector Labs) following standard protocols. At least four animals were used for each treatment, and six sections from each animal were analyzed. pCREB staining was quantified using ImageJ software, and the number of pCREB positive nuclei within Laminae I and II were counted, and statistical analysis was done using analysis of variance and post-hoc Tukey tests.
RESULTS
Generation of LPA5-deficient Mice
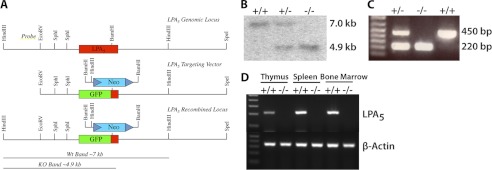
Initial gene expression studies (21) identified LPA5 in DRG, suggesting functional consequences of this receptor that might be assessed through an Lpar5 null mutant mouse that was not yet available. Gene targeting was therefore used to eliminate and replace most of the Lpar5 coding region in frame with enhanced GFP allowing for the production of Lpar5-deficient mice (Fig. 1A). Mice heterozygous for the Lpar5 knock-in allele were crossed to each other and wild type (+/+), heterozygous (+/−), and null mutant offspring (−/−) were produced, at near Mendelian ratios (data not shown), indicating that Lpar5 is dispensable for embryonic viability. Genotypes of mice generated from crosses of heterozygous mice were determined by PCR and confirmed by Southern blotting (Fig. 1, B and C).
FIGURE 1.
Targeted disruption of the Lpar5 genomic locus and generation of LPA5-deficient mice by homologous recombination. A, schematic diagram of the Lpar5 gene targeting strategy. To generate the Lpar5 targeting vector, a portion of the Lpar5 coding region was removed and replaced in frame by enhanced GFP (middle panel). ES cell clones positive for homologous recombination were identified by digestion of genomic DNA with HindIII and Southern blotting with the external probe shown in the top panel. ES cell clones with nonhomologous recombination events showed a 7-kb band, whereas clones with homologously recombined DNA produced a 4.9-kb band. B, Southern blot showing the properly recombined product for a wild type (7 kb), heterozygous, and homozygous animal (4.9 kb). C, PCR genotyping showing the Lpar5 wild type (450 bp) and mutant (220 bp) products, primers are indicated in A. D, RT-PCR of cDNA from thymus, spleen, and bone marrow from wild type and Lpar5 homozygous mutant mice shows an absence of Lpar5 mRNA in tissues from LPA5-deficient animals. β-Actin control for all tissues is shown.
Lpar5 is expressed in lymphoid tissues including the thymus and spleen (21). To confirm deletion of Lpar5, total RNA was isolated from wild type and null mutant thymus, spleen, and bone marrow and the loss of Lpar5 mRNA was assessed by RT-PCR. An absence of an Lpar5-specific RT-PCR product in null mutant tissues confirmed proper targeting of the Lpar5 genomic locus (Fig. 1D). Expression of Lpar5 in these lymphoid organs suggests that Lpar5 plays a functional role in these tissues; however, thymus and spleen lymphocyte proportions and numbers were similar in both wild type and LPA5-deficient mice (data not shown). The actual function of LPA5 in the immune system remains to be determined.
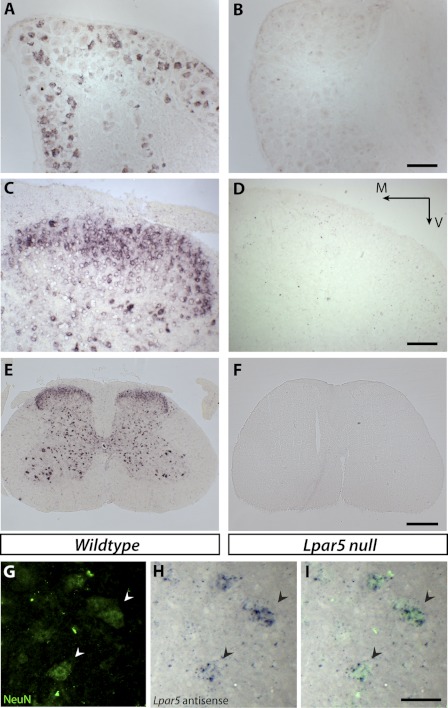
Using in situ hybridization, Lpar5 mRNA was detected in a subset of DRG neurons, and this expression pattern was completely absent from LPA5-deficient animal tissues (Fig. 2, A and B). Lpar5 was also expressed in the dorsal horn area of the lumbar spinal cord, and as expected, the specific signal is absent in tissues from Lpar5-deficient mice (Fig. 2, C and D). Immunolabeling with an antibody against the neuronal specific marker, NeuN, combined with Lpar5 in situ hybridization showed that the LPA5-expressing cells are neurons, including dorsal horn sensory neurons and central nervous system motor neurons of the spinal cord ventral horn (Fig. 2, E–I). The expression of LPA5 in both DRG and spinal dorsal horn neurons is consistent with a role for LPA5 in pain processing.
FIGURE 2.
In situ hybridization and immunolabeling of tissues from wild type and null mutant mice with Lpar5 digoxigenin-labeled antisense probes and NeuN antibody. A and B, sections of DRG from wild type (A) and Lpar5 null mice (B). C and D, sections of spinal cord dorsal horn from wild type (C) and Lpar5 null mice (D) show Lpar5 expression in the dorsal horn area. Scale bar, 100 μm. M, medial; V, ventral. E and F, low magnification images of whole spinal cord sections from wild type (E) and Lpar5 null mice (F). Note that both dorsal horn and ventral horn neurons are labeled. Scale bar, 400 μm. G–I, anti-NeuN antibody immunostaining (G) and in situ hybridization against Lpar5 (H) confirmed Lpar5 expression in double labeled neurons (I). The arrowheads indicate the same cells from G–I. Scale bar, 50 μm.
Lpar5 Null Mice Are Protected from Injury-induced Neuropathic Pain
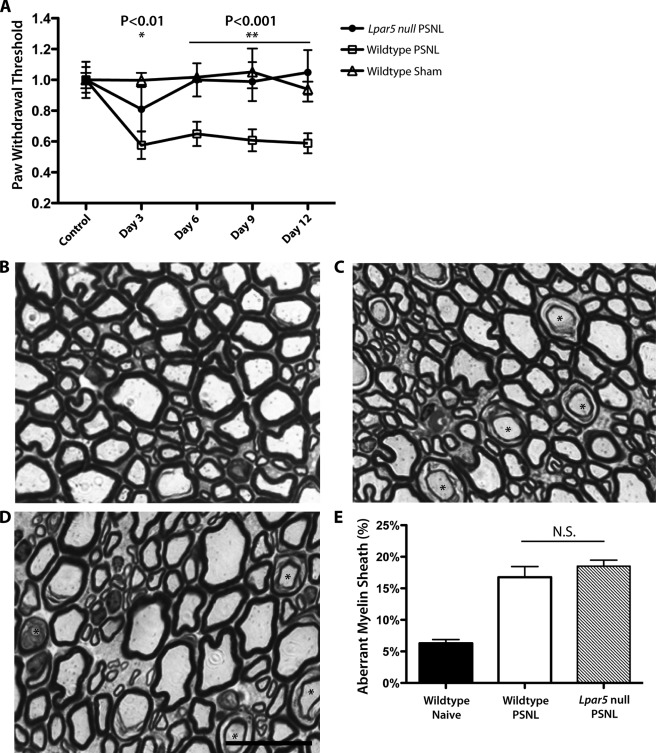
It has been shown that LPA1 is required for the initiation of injury-induced neuropathic pain (7). However, other LPA receptors have not been evaluated in this context. To examine the role of LPA5 in the development of neuropathic pain, we utilized PSNL, as described by Seltzer et al. (3) to induce neuropathic pain in Lpar5 null mice. Neuropathic pain status was assessed with an automated Von Frey apparatus to record paw withdrawal threshold against mechanical stimulus. After PSNL, wild type mice developed neuropathic pain that was evident by a decreased paw withdrawal threshold; however, the threshold of Lpar5 null mice remained at base-line levels (Fig. 3A). This phenotype was similar to that observed in Lpar1 null mice and raised the question of whether the pain response occurs via common or distinct LPA-dependent pathway(s).
FIGURE 3.
Loss of PSNL-induced neuropathic pain in Lpar5 null mice. A, paw withdrawal threshold against mechanical stimuli after PSNL or sham operation. p value compares homozygous null mutants with wild type mice with PSNL. *, p < 0.01; **, p < 0.001. B–D, representative semi-thin sections of L5 dorsal roots, 6 days after PSNL, from naïve wild type (B), PSNL wild type (C), and PSNL Lpar5 null mice (D). Scale bar, 20 μm. E, percentage of axons with aberrant myelin sheath (asterisks in C, D) in PSNL or naïve wild type versus null mutant mice. N.S., no significant difference.
Following PSNL injury, affected neurons are often demyelinated, which can lead to cross-talk between axons that can then contribute to the development of neuropathic pain (27, 28). In wild type animals subjected to PSNL, both the sciatic nerve (data not shown) and dorsal root from L4–L6 showed an increased percentage of myelin sheath aberrations resembling demyelination (7). However, in Lpar1 null mice, these myelin sheath aberrations in the dorsal root were decreased compared with control mice, which correlated with reduced pain responses (7). Because Lpar5 null mutant mice showed a similar attenuated neuropathic pain response, this led us to examine the myelination status in the dorsal root area of Lpar5 null mice after PSNL. Similar myelin sheath splitting was observed and was significantly increased in the PSNL animals (Fig. 3, B–D, asterisk). Surprisingly, at post-surgery day 6, there was no significant difference observed between heterozygous control and null mutant mice (Fig. 3E). In addition, classic characteristics of demyelination, including naked axons and macrophage infiltration, were not seen in any dorsal root sections regardless of genotype or treatment. This result indicates that in contrast to LPA1, LPA5 is not significantly involved in the demyelination process induced by nerve injury.
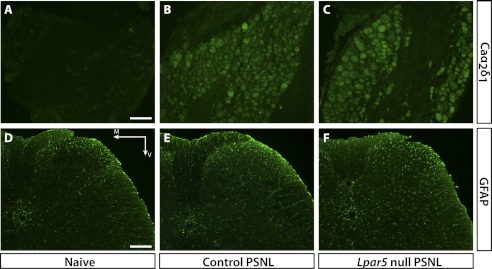
Two markers of neuropathic pain have been shown in LPA1-involved neuropathic pain development, the α2δ1 subunit of voltage-gated calcium channels (Caα2δ1), and the γ-isoform of PKCγ. Caα2δ1 has been shown to be elevated in the DRG following nerve injury and correlates with allodynia status; PKCγ elevation in the spinal cord dorsal horn has also been shown to participate in neuropathic pain development (29–31). Both markers were significantly elevated in control mice subjected to PSNL, but not in mice deficient for LPA1 (7). We compared the expression of Caα2δ1 in DRG of control versus Lpar5-deficient mice that had been subjected to PSNL. Surprisingly, Caα2δ1 (Fig. 4, A–C) expression levels were not reduced in Lpar5 null mutants, whereas PKCγ levels were not changed regardless of genotypes or treatments (data not shown).
FIGURE 4.
Immunohistochemistry showing Caα2δ1 immunoreactivity in DRG as well as GFAP immunoreactivity in the L5 spinal cord region 6 days after PSNL. A–C, Caα2δ1 is significantly up-regulated in DRG after PSNL; however, no significant difference was observed between heterozygous control and null mutant mice. Scale bar, 100 μm. D–F, GFAP was significantly increased in PSNL mice, indicating astrocyte activation; however, Lpar5 null mutant mice showed similar, if not increased, GFAP immunostaining compared with the heterozygous control. Scale bar, 200 μm. M, medial; V, ventral.
It has also been reported that astrogliosis increases after PSNL and correlates with the development of neuropathic pain (32). To assess the extent of astrogliosis, expression of GFAP, an astrocyte marker, was examined in the spinal dorsal horn prior to and after injury. Consistent with Caα2δ1 levels, GFAP is up-regulated following PSNL, and expression is not altered by the loss of LPA5 (Fig. 4, D–F), as quantified by pixel intensities and counts (data not shown).
pCREB Expression through LPA5 Is Associated with Neuropathic Pain
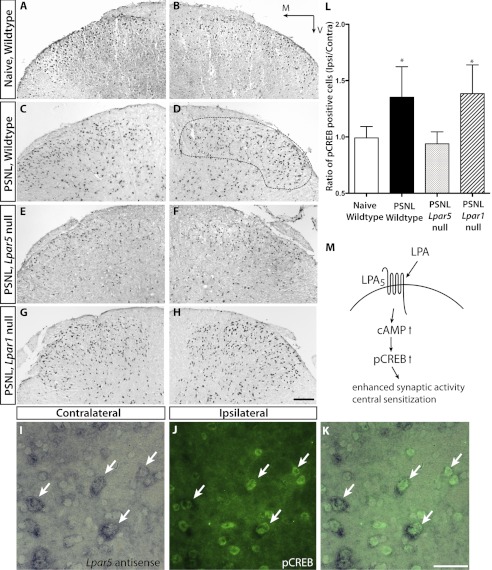
In the spinal cord dorsal horn, pCREBs are up-regulated during pain induced by two different models of neuropathic pain: chronic constriction injury and PSNL (33, 34). Furthermore, down-regulation of pCREB is associated with anti-hyperalgesic effects (35, 36). Interestingly, in contrast to LPA1, exposure of LPA5 mutants to LPA stimulation results in increased cellular cAMP levels (21, 22). This result led us to determine whether or not the loss of LPA5 can affect pCREB expression in the spinal cord dorsal horn after nerve injury. Six days after PSNL, the ipsilateral to contralateral ratio of pCREB expression in the spinal cord dorsal horn Laminae I-II was markedly reduced in Lpar5 null mice as compared with wild type controls as determined by analysis of variance and post-hoc Tukey tests (Fig. 5, A–H and L). Furthermore, double-labeling of neurons for Lpar5 mRNA and pCREB protein demonstrated co-localization of both molecules in the same cell, suggesting that LPA5 directly affects pCREB activation (Fig. 5, I–K). Among LPA5 positive cells, 95.4 ± 3.6% were also pCREB positive, whereas 83.4 ± 6.0% of pCREB positive cells were LPA5 positive. This strongly suggests that in the absence of LPA5, up-regulation of pCREB in response to PSNL is abrogated, leading to protection from neuropathic pain (Fig. 5M). Additionally, this effect was not seen in Lpar1 null mice, demonstrating that a different mechanism was involved.
FIGURE 5.
pCREB expression in the spinal dorsal horn L4–L6, 6 days after PSNL. Immunolabeling of pCREB is markedly increased in control mice after PSNL (C and D) compared with naïve mice (A and B). Attenuated expression of pCREB was observed in Lpar5 null mice that were subjected to the same nerve injury (E and F). This reduction was not observed in Lpar1 null mice (G and H). Scale bar, 100 μm. M, medial; V, ventral. I–K, co-localization of Lpar5 and pCREB is shown by Lpar5 in situ hybridization in conjunction with pCREB immunostaining. The arrows indicate the same cells from I–K. L, quantification of pCREB immunostaining, represented by ratios of labeled cells between ipsilateral and contralateral sides of the same section in Laminae I-II (dashed line in D). Statistical significance was established using analysis of variance and post-hoc Tukey tests. *, p < 0.05. M, schematic diagram of proposed LPA5 involvement in neuropathic pain development.
DISCUSSION
Prior studies showed the importance of LPA signaling through LPA1 in the initiation of neuropathic pain. Here, through the use of the first reported Lpar5-deficient mouse, a distinct LPA receptor, LPA5, was identified as a new influence on neuropathic pain as assessed by PSNL. The mechanism through which LPA5 produces protection appears to be distinct from LPA1 (7), demonstrating that different receptors for the same endogenous ligand can affect pain development through nonidentical molecular and cellular pathways.
LPA5 gene expression in DRG and spinal cord dorsal horn neurons, as revealed by in situ hybridization, suggests a possible role in pain. Interestingly, normal pain sensation is not altered in LPA5-deficient mice, whereas the development of nerve injury-induced neuropathic pain is abolished. This protective effect is not seen in heterozygous mice that show wild type PSNL susceptibility, indicating that a single allele of Lpar5 is sufficient for the development of neuropathic pain. These results also suggest that LPA5 is activated during injury, but not under basal conditions, which is consistent with the increased levels of LPA detected in spinal cord dorsal horn and dorsal roots following nerve injury (37).
In the initial report describing the involvement of LPA1 in neuropathic pain development, a significant reduction of demyelination in the dorsal root area of Lpar1-deficient mice was proposed to be the protective mechanism (7). Subsequent studies suggested that LPA-LPA1 signaling was responsible for dorsal root demyelination (38). In contrast, no difference in nerve injury-induced demyelination between wild type and Lpar5-deficient mice was detected here, despite maintained LPA1 expression, indicating that LPA5 loss does not prevent demyelination, despite protecting against the development of neuropathic pain. This observation provides the first evidence that, despite sharing the same endogenous ligand and partially overlapping expression patterns, LPA1 and LPA5 participate in neuropathic pain development through distinct pathways.
In DRG, Caα2δ1 expression increases with nerve injury-induced neuropathic pain and is thought to increase the excitability of DRG neurons (29, 30). In Lpar1 null mice, the activation of Caα2δ1 is greatly reduced (7). Combined with the reduction of demyelination, these phenomena suggest that the loss of LPA1 signaling prevents abnormal pain signaling transmission from the periphery into the central nervous system. By contrast, Caα2δ1 still shows increased expression in Lpar5 null mice when challenged with nerve injury, indicating relatively normal pain transmission through the DRG to the spinal cord dorsal horn and implicating a distinct locus of action.
Astrocytes are known to be activated during neuropathic pain as manifested by astrogliosis and are thought to contribute to the development of neuropathic pain (39). Upon activation, astrocytes can release cytokines/chemokines including IL-1β, TNFα, IL-6, and monocyte chemotactic protein 1, which can promote the neuropathic pain phenotype (40). These molecules are also important for maintaining the synaptic connectivities required for proper neuronal signaling by interacting with neurons through the release of neurotransmitters such as glutamate (41). Astrogliosis in the L4–L6 spinal dorsal horn was identified by GFAP immunostaining, which demonstrated that astrocytes are indeed activated in all injured animals. Nevertheless, the severity of astrogliosis was equivalent between Lpar5 null versus wild type animals subject to PSNL. These data indicate that the spinal cord dorsal horn continues to receive normal pain signals despite the loss of LPA5 and the accompanying abrogation of the pain phenotype.
In wild type animals exposed to PSNL, LPA5 could contribute to increased pCREB expression through at least two different pathways. Increased levels of LPA following nerve injury provide a high concentration of ligand, which activates LPA5 in dorsal horn neurons and causes increased production of cAMP and pCREB; up-regulation of these molecules then leads to increased sensitivity of dorsal horn neurons to pain stimuli. In addition, the increased LPA concentration may also activate a subgroup of LPA5 expressing DRG neurons that could stimulate neurons in the dorsal horn, thus leading to increased pCREB expression within the spinal cord. Additional experiments are required to elucidate the detailed mechanism; however, the abrogated increase in pCREB and the observed failure to develop neuropathic pain in Lpar5 null mice was behaviorally unambiguous and also distinct from cellular end points observed with the loss of LPA1. We speculate that the loss of LPA5 activity reduces central neuronal signaling required to induce neuropathic pain, which has both mechanistic implications and therapeutic implications for pharmaceutical properties of desirable agents.
Cellular cAMP levels have been shown to increase in response to LPA signaling through LPA5. This response is the opposite of that observed with LPA1-Gαi activation that reduces cAMP (21, 22), representing a possible basis for the differences observed between Lpar5 and Lpar1 null mutants. Increased cAMP can lead to increased CREB phosphorylation and hyperalgesia via central sensitization (42, 43). Decreased pCREB expression is also associated with decreased pain in model systems (35, 36). In the dorsal horn of naïve mice, a low level of pCREB expression was observed; however, this was strongly increased in animals challenged with nerve injury (33, 44), in concert with previous reports. Importantly and consistent with a loss of cAMP production, PSNL-induced pCREB expression was markedly reduced in Lpar5 null mice. Reduced pCREB expression may in part account for the protective effects of LPA5 loss, because increased pCREB expression has been strongly associated with neuropathic pain after nerve injury (35, 36). Furthermore, this specific reduction in pCREB expression was not observed in LPA1-deficient mice (Fig. 5, G and H), thus demonstrating that each receptor subtype has distinct effects that separately contribute to neuropathic pain.
The mechanistic differences observed between LPA1- and LPA5-deficient animals also raise the question of whether LPA controls neuropathic pain development through one or more than one pathway. LPA1-deficient mice challenged with PSNL are protected from neuropathic pain, showing reduced demyelination and calcium channel up-regulation yet continue to show pCREB up-regulation. By comparison, LPA5-deficient mice were also protected from neuropathic pain yet continued to show demyelination and calcium channel up-regulation, responses, while instead exhibiting central reductions in pCREB. Taken together, the observed protection against pain produced by receptor loss, despite key molecular differences, indicates that LPA1 and LPA5 act through mechanistically distinct parallel pathways to regulate neuropathic pain development.
Differential activation of LPA receptors by alternative LPA species such as 1-acyl and 1-alkyl LPA may also provide additional insights into how these pathways function. Consistent with this view, LPA1–3 have been reported to respond to acyl-LPA with at least 10-fold improvement over 1-alkyl LPA, whereas LPA5 seem to have a preference of 1-alkyl LPA (45, 46). This mechanism could provide a way to differentially activate LPA receptor subtypes and the identified parallel pathways that influence neuropathic pain development. Moreover, it would not be surprising if additional LPA receptor subtypes contribute to neuropathic pain, based on analyses of LPA3-deficient animals in distinct pain models (47).
In conclusion, LPA5 appears to contribute to neuropathic pain through central pCREB activation. The protective effects observed in Lpar5-deficient animals following PSNL are mechanistically distinct from those mediated through LPA1, in part reflecting the opposing actions of these different LPA receptors on cAMP levels and the production of pCREB. These data support LPA signaling in the development of neuropathic pain and suggest that inhibition of LPA1, LPA5, and perhaps other receptor subtypes via pharmacological antagonism could provide a basis for novel pain therapeutics.
Acknowledgment
We would like to thank Grace Kennedy for technical assistance with histology and in situ hybridization.
This work was supported, in whole or in part, by National Institutes of Health Grants MH051699 and NS048478 (to J. C.). This work was also supported by an Amira predoctoral fellowship (to M. L.).
- PSNL
- partial sciatic nerve ligation
- LPA
- lysophosphatidic acid
- DRG
- dorsal root ganglion
- GFAP
- glial fibrillary acidic protein
- pCREB
- phosphorylated cAMP response element-binding protein.
REFERENCES
- 1. Bouhassira D., Lantéri-Minet M., Attal N., Laurent B., Touboul C. (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136, 380–387 [DOI] [PubMed] [Google Scholar]
- 2. Torrance N., Smith B. H., Bennett M. I., Lee A. J. (2006) The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain 7, 281–289 [DOI] [PubMed] [Google Scholar]
- 3. Seltzer Z., Dubner R., Shir Y. (1990) A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43, 205–218 [DOI] [PubMed] [Google Scholar]
- 4. Gao X., Kim H. K., Chung J. M., Chung K. (2005) Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain 116, 62–72 [DOI] [PubMed] [Google Scholar]
- 5. Miletic G., Pankratz M. T., Miletic V. (2002) Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany thermal hyperalgesia following chronic constriction injury in rats. Pain 99, 493–500 [DOI] [PubMed] [Google Scholar]
- 6. Sorkin L. S., Yaksh T. L. (2009) Behavioral models of pain states evoked by physical injury to the peripheral nerve. Neurotherapeutics 6, 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inoue M., Rashid M. H., Fujita R., Contos J. J., Chun J., Ueda H. (2004) Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10, 712–718 [DOI] [PubMed] [Google Scholar]
- 8. Karliner J. S. (2004) Mechanisms of cardioprotection by lysophospholipids. J. Cell Biochem. 92, 1095–1103 [DOI] [PubMed] [Google Scholar]
- 9. Swaney J. S., Chapman C., Correa L. D., Stebbins K. J., Bundey R. A., Prodanovich P. C., Fagan P., Baccei C. S., Santini A. M., Hutchinson J. H., Seiders T. J., Parr T. A., Prasit P., Evans J. F., Lorrain D. S. (2010) A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br. J. Pharmacol. 160, 1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panupinthu N., Lee H. Y., Mills G. B. (2010) Lysophosphatidic acid production and action. Critical new players in breast cancer initiation and progression. Br. J. Cancer 102, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye X., Hama K., Contos J. J., Anliker B., Inoue A., Skinner M. K., Suzuki H., Amano T., Kennedy G., Arai H., Aoki J., Chun J. (2005) LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yung Y. C., Mutoh T., Lin M. E., Noguchi K., Rivera R. R., Choi J. W., Kingsbury M. A., Chun J. (2011) Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl Med. 3, 99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin M. E., Herr D. R., Chun J. (2010) Lysophosphatidic acid (LPA) receptors. Signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 91, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noguchi K., Herr D., Mutoh T., Chun J. (2009) Lysophosphatidic acid (LPA) and its receptors. Curr. Opin Pharmacol. 9, 15–23 [DOI] [PubMed] [Google Scholar]
- 15. Contos J. J., Fukushima N., Weiner J. A., Kaushal D., Chun J. (2000) Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. U.S.A. 97, 13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Contos J. J., Ishii I., Fukushima N., Kingsbury M. A., Ye X., Kawamura S., Brown J. H., Chun J. (2002) Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg2/Edg4) lysophosphatidic acid receptor knockout mice. Signaling deficits without obvious phenotypic abnormality attributable to lpa2. Mol. Cell Biol. 22, 6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumida H., Noguchi K., Kihara Y., Abe M., Yanagida K., Hamano F., Sato S., Tamaki K., Morishita Y., Kano M. R., Iwata C., Miyazono K., Sakimura K., Shimizu T., Ishii S. (2010) LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 116, 5060–5070 [DOI] [PubMed] [Google Scholar]
- 18. Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C. W., Mutoh T., Lin M. E., Teo S. T., Park K. E., Mosley A. N., Chun J. (2010) LPA receptors. Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50, 157–186 [DOI] [PubMed] [Google Scholar]
- 19. Cohen A., Sagron R., Somech E., Segal-Hayoun Y., Zilberberg N. (2009) Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K(2P)2.1 channel. Mol. Cell Neurosci. 40, 382–389 [DOI] [PubMed] [Google Scholar]
- 20. Xie W., Matsumoto M., Chun J., Ueda H. (2008) Involvement of LPA1 receptor signaling in the reorganization of spinal input through Aβ-fibers in mice with partial sciatic nerve injury. Mol. Pain 4, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee C. W., Rivera R., Gardell S., Dubin A. E., Chun J. (2006) GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 281, 23589–23597 [DOI] [PubMed] [Google Scholar]
- 22. Kotarsky K., Boketoft A., Bristulf J., Nilsson N. E., Norberg A., Hansson S., Owman C., Sillard R., Leeb-Lundberg L. M., Olde B. (2006) Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 318, 619–628 [DOI] [PubMed] [Google Scholar]
- 23. Amisten S., Braun O. O., Bengtsson A., Erlinge D. (2008) Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb. Res. 122, 47–57 [DOI] [PubMed] [Google Scholar]
- 24. Ohuchi H., Hamada A., Matsuda H., Takagi A., Tanaka M., Aoki J., Arai H., Noji S. (2008) Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev. Dyn. 237, 3280–3294 [DOI] [PubMed] [Google Scholar]
- 25. Braissant O., Wahli W. (1998) A simplified in situ hybridization protocol using non-radioactively labeled probes to detect abundant and rare mRNAs on tissue sections. Biochemica 1, 10–16 [Google Scholar]
- 26. Plenz G., Weissen B., Steffen I. (2003) Detection of mRNAs on cryosections of the cardiovascular system using DIG-labeled RNA probes. Biochemica 1, 19–21 [Google Scholar]
- 27. Smith K. J., McDonald W. I. (1982) Spontaneous and evoked electrical discharges from a central demyelinating lesion. J. Neurol. Sci. 55, 39–47 [DOI] [PubMed] [Google Scholar]
- 28. Rasminsky M. (1978) Ectopic generation of impulses and cross-talk in spinal nerve roots of “dystrophic” mice. Ann. Neurol. 3, 351–357 [DOI] [PubMed] [Google Scholar]
- 29. Luo Z. D., Calcutt N. A., Higuera E. S., Valder C. R., Song Y. H., Svensson C. I., Myers R. R. (2002) Injury type-specific calcium channel α2 δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 303, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 30. Luo Z. D., Chaplan S. R., Higuera E. S., Sorkin L. S., Stauderman K. A., Williams M. E., Yaksh T. L. (2001) Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 21, 1868–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malmberg A. B., Chen C., Tonegawa S., Basbaum A. I. (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science 278, 279–283 [DOI] [PubMed] [Google Scholar]
- 32. Coyle D. E. (1998) Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia 23, 75–83 [PubMed] [Google Scholar]
- 33. Ma W., Quirion R. (2001) Increased phosphorylation of cyclic AMP response element-binding protein (CREB) in the superficial dorsal horn neurons following partial sciatic nerve ligation. Pain 93, 295–301 [DOI] [PubMed] [Google Scholar]
- 34. Song X. S., Cao J. L., Xu Y. B., He J. H., Zhang L. C., Zeng Y. M. (2005) Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats. Acta Pharmacol. Sin. 26, 789–798 [DOI] [PubMed] [Google Scholar]
- 35. Liou J. T., Liu F. C., Hsin S. T., Yang C. Y., Lui P. W. (2007) Inhibition of the cyclic adenosine monophosphate pathway attenuates neuropathic pain and reduces phosphorylation of cyclic adenosine monophosphate response element-binding in the spinal cord after partial sciatic nerve ligation in rats. Anesth. Analg. 105, 1830–1837 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y., Cheng X., Xu J., Liu Z., Wan Y., Ma D. (2011) Anti-hyperalgesic effect of CaMKII inhibitor is associated with downregulation of phosphorylated CREB in rat spinal cord. J. Anesth. 25, 87–92 [DOI] [PubMed] [Google Scholar]
- 37. Ma L., Uchida H., Nagai J., Inoue M., Aoki J., Ueda H. (2010) Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 333, 540–546 [DOI] [PubMed] [Google Scholar]
- 38. Nagai J., Uchida H., Matsushita Y., Yano R., Ueda M., Niwa M., Aoki J., Chun J., Ueda H. (2010) Autotaxin and lysophosphatidic acid 1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol. Pain 6, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gao Y. J., Ji R. R. (2010) Targeting astrocyte signaling for chronic pain. Neurotherapeutics 7, 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milligan E. D., Watkins L. R. (2009) Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 10, 23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bezzi P., Domercq M., Vesce S., Volterra A. (2001) Neuron-astrocyte cross-talk during synaptic transmission. Physiological and neuropathological implications. in Progress in Brain Research (Castellano Lopez M. N.-S. B., ed) pp. 255–265, Elsevier Science Publishing Co., Inc., New York: [DOI] [PubMed] [Google Scholar]
- 42. Hoeger-Bement M. K., Sluka K. A. (2003) Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J. Neurosci. 23, 5437–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kawasaki Y., Kohno T., Zhuang Z. Y., Brenner G. J., Wang H., Van Der Meer C., Befort K., Woolf C. J., Ji R. R. (2004) Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J. Neurosci. 24, 8310–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ji R. R., Rupp F. (1997) Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia. Relationship to c-fos induction. J. Neurosci. 17, 1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams J. R., Khandoga A. L., Goyal P., Fells J. I., Perygin D. H., Siess W., Parrill A. L., Tigyi G., Fujiwara Y. (2009) Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J. Biol. Chem. 284, 17304–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujiwara Y., Sardar V., Tokumura A., Baker D., Murakami-Murofushi K., Parrill A., Tigyi G. (2005) Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J. Biol. Chem. 280, 35038–35050 [DOI] [PubMed] [Google Scholar]
- 47. Ma L., Uchida H., Nagai J., Inoue M., Chun J., Aoki J., Ueda H. (2009) Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid. An initiator of nerve injury-induced neuropathic pain. Mol. Pain 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]