Background: Fenretinide, an in-trial chemotherapeutic, improves insulin sensitivity in mice and humans.
Results: Fenretinide reduces Des1 expression and prevents ceramide accumulation, while protecting against lipid-induced insulin resistance.
Conclusion: Fenretinide decreases ceramide biosynthesis, and increases levels of dihydroceramides, thus preserving insulin responsiveness.
Significance: These data suggest that Des1 may be a viable therapeutic target for normalizing glucose homeostasis.
Keywords: Ceramide, Diabetes, Insulin Resistance, Resveratrol, Sphingolipid, Dihydroceramide, Fenretinide
Abstract
Fenretinide is a synthetic retinoid that is being tested in clinical trials for the treatment of breast cancer and insulin resistance, but its mechanism of action has been elusive. Recent in vitro data indicate that fenretinide inhibits dihydroceramide desaturase, an enzyme involved in the biosynthesis of lipotoxic ceramides that antagonize insulin action. Because of this finding, we assessed whether fenretinide could improve insulin sensitivity and glucose homeostasis in vitro and in vivo by controlling ceramide production. The effect of fenretinide on insulin action and the cellular lipidome was assessed in a number of lipid-challenged models including cultured myotubes and isolated muscles strips incubated with exogenous fatty acids and mice fed a high-fat diet. Insulin action was evaluated in the various models by measuring glucose uptake or disposal and the activation of Akt/PKB, a serine/threonine kinase that is obligate for insulin-stimulated anabolism. The effects of fenretinide on cellular lipid levels were assessed by LC-MS/MS. Fenretinide negated lipid-induced insulin resistance in each of the model systems assayed. Simultaneously, the drug depleted cells of ceramide, while promoting the accumulation of the precursor dihydroceramide, a substrate for the reaction catalyzed by Des1. These data suggest that fenretinide improves insulin sensitivity, at least in part, by inhibiting Des1 and suggest that therapeutics targeting this enzyme may be a viable therapeutic means for normalizing glucose homeostasis in the overweight and diabetic.
Introduction
The rapid and unabated climb of type 2 diabetes has prompted the search for novel therapies, and recent efforts have placed a renewed appreciation on the utility of drugs intended to treat other diseases. This focus on teaching old drugs new tricks (1, 2) has led to a paradigm shift from the maxim “one drug, one target” to “one drug, many targets.” Fenretinide is a prime example of an increasingly promiscuous drug. Originally slated as an anticancer therapy (3, 4), fenretinide has recently been shown to have insulin-sensitizing and antidiabetic effects in mice and overweight humans (5–7). Clinical trials are currently underway investigating the utility of this compound in the control of insulin resistance.2 Although initial studies cited retinol-binding protein 4 (RBP4)3 as the relevant target of fenretinide that explains its insulin-sensitizing effects (6), more recent evidence suggested that fenretinide might have additional actions relevant to metabolic disease (5). Interestingly, fenretinide was recently shown to block the synthesis of ceramide, a mediator of lipid- and inflammation-induced insulin resistance (9, 10), by inhibiting dihydroceramide desaturase (Des1), which catalyzes the final step in de novo ceramide synthesis (5, 11–13).
De novo ceramide production involves a biosynthetic pathway initiated by the enzyme serine palmitoyltransferase, which condenses serine and palmitoyl-CoA to produce 3-ketosphinganine. Sequential reactions catalyzed by 3-ketosphinganine reductase, (dihydro)ceramide synthase (CerS), and Des1 enzymes lead to ceramide formation. Inhibitors of serine palmitoyltransferase (i.e. myriocin and cycloserine) and CerS (fumonisin B1) have potent insulin-sensitizing effects both in vitro and in vivo (9, 10). The final reaction in this pathway is catalyzed by Des1, which introduces a characteristic double bond in the fatty acyl chain that forms the sphingosine backbone of ceramide.
Inhibition of Des1 allows a quantifiable accumulation of dihydroceramide, which can be incorporated into other sphingolipids, while preventing ceramide accumulation. Using a cell culture model of insulin resistance, we herein demonstrate that fenretinide blocks lipid-induced insulin resistance by preventing ceramide accumulation, while simultaneously inducing dihydroceramide accrual. After confirming the role of Des1 using cell culture models, we found that a single injection of a therapeutic dose of fenretinide in mice was sufficient to promote dihydroceramide accumulation. Moreover, prolonged fenretinide treatment of diet-induced obese mice improved diet-induced insulin resistance and hepatic steatosis, while selectively lowering ceramide/dihydroceramide ratios. These observations identify a new mechanism of action for fenretinide that contributes to its insulin-sensitizing properties. Moreover, the studies suggest that therapeutics targeting ceramide synthesis through the inhibition of Des1 may be efficacious in the treatment of insulin resistance.
EXPERIMENTAL PROCEDURES
Animals
Male mice (C57Bl/6) were from The Jackson Laboratory (Bar Harbor, ME). Male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). Mice were separated at 6 weeks to continue eating standard chow or high-fat diet (D12492; Research Diets, Inc., New Brunswick, NJ). Studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at the Duke-National University of Singapore Graduate Medical School.
Cell Culture
Cells were maintained in DMEM + 10% fetal bovine serum (Invitrogen). For differentiation into myotubes, C2C12 myoblasts were grown to confluency, and the medium was replaced with DMEM + 10% horse serum (Invitrogen). Myotubes were used for experiments on day 4 of differentiation. For lipid treatment, palmitic acid (Sigma P5585), oleic acid (Sigma O1008), or N-acetyl-d-sphingosine (C2-ceramide; Sigma A7191) was dissolved in ethanol and diluted to the desired concentration in DMEM. Palmitate and oleate solutions were then conjugated to 2% (w/v) BSA (Sigma A9576). Fenretinide (H7779) and resveratrol (R5010) were from Sigma. Des1 siRNA oligonucleotide was added to Opti-MEM and Lipofectamine (Invitrogen) and left to incubate for 15 min before placing on confluent cells overnight. Cells were treated for 48 h following transfection.
Isolated Muscles
Palmitate was dissolved in ethanol (200 mm), and ethanol or free fatty acids were conjugated to bovine serum albumin (BSA) by diluting 1:25 in Krebs-Henseleit buffer supplemented with 20% BSA and heating (55 °C for 30 min, with occasional vortexing). The final incubation medium was prepared by diluting the conjugated BSA solution 1:8 in freshly oxygenated Krebs-Henseleit buffer. Male Sprague-Dawley rats (150–200 grams) were deeply anesthetized with sodium pentobarbital (110 mg/kg, intraperitoneal), and soleus muscles were isolated, laterally bisected, and transferred to 25-ml Erlenmeyer flasks containing 2 ml of Krebs-Henseleit buffer supplemented with 2.5% BSA, 8 mm glucose, and 1 mm HEPES (pH 7.2). Muscles were maintained in a shaking water bath at 29 °C while being continuously gassed with 95% O2, 5% CO2. Following this incubation, muscles were rapidly frozen in liquid nitrogen or stimulated with insulin (300 microunits) for 60 min, and the incorporation of 2-[3H]DOG was assessed during the final 20 min of the incubation using methods described previously (14). Free fatty acids were present throughout the 2-DOG uptake assay. Insufficient tissue remained for lipid analysis.
Single-dose Injections
C57Bl/6 mice received intraperitoneal injections of fenretinide (10 mg/kg) 12 h before sacrifice. Fenretinide was dissolved in dimethyl sulfoxide (DMSO) and resuspended in warmed PBS (15).
High-fat Diet and Fenretinide Intervention
Male mice (C57Bl6) were fed a standard chow or a high-fat diet (HFD)(D12492; Research Diets, Inc., New Brunswick, NJ) from 5 to 17 weeks, at which point half of the HFD-fed mice began receiving fenretinide in drinking water for 4 weeks. Fenretinide (FEN; H7779, Sigma) was dissolved in 100% ethanol and diluted in water to 10 μg/ml. Control treatment water received an equal amount of ethanol (0.5%). FEN water was prepared in low-light conditions and administered in light-protective bottles. Water was replaced every 1–2 days, and no precipitation of FEN was noted at any time. Animal weights were recorded at the beginning and end of the treatment period. Following a 4-week FEN treatment, mice underwent intraperitoneal glucose (G7021, Sigma) and insulin (Actrapid, Novo Nordisk) tolerance tests. For both tests, mice were fasted for 6 h and received an injection of either glucose (1 g/kg of body weight) or insulin (0.75 units/kg of body weight). Blood glucose was determined at the times indicated by the Bayer Contour® glucose meter, and insulin was measured with the rat/mouse insulin ELISA kit (EZRMI-13K; Millipore; Billerica, MA). The insulin resistance index was assessed by using fasting blood glucose and insulin levels to compute the homeostatic model assessment of insulin resistance (HOMA-IR), where a higher number represents greater insulin resistance.
Lipid Analysis
For isolation of lipids, pellets were resuspended in 900 μl of ice-cold chloroform/methanol (1:2) and incubated for 15 min on ice and then briefly vortexed. For cell lipid analysis, a portion of the cell suspension (prior to pelleting) was used for protein measurement (Thermo Scientific). Separation of aqueous and organic phases required the addition of 400 μl of ice-cold water and 300 μl of ice-cold chloroform. The organic phase was collected into a fresh vial, and lipids were dried under a gentle nitrogen stream. An Agilent high performance liquid chromatography (HPLC) system coupled with an Applied Biosystems triple quadrupole/ion trap mass spectrometer (3200 QTRAP) was used for quantification of individual phospholipids. Multiple reaction monitoring transitions were set up for quantitative analysis of various polar lipids (16, 17). Levels of individual lipids were quantified using spiked internal standards, including dimyristoyl phosphatidylcholine (28:0-PC), C17-ceramide, C8-glucosylceramide, and C12-sphingomyelin, which were obtained from Avanti Polar Lipids (Alabaster, AL). Neutral lipids were analyzed using a sensitivity HPLC/electrospray mass ionization/multiple reaction monitoring method, modified from a previous method (18). TAG was calculated as relative contents to the spiked d5-TAG 48:0 internal standard (CDN Isotopes), whereas DAG was quantified using 4ME 16:0 Diether DG (Avanti) as an internal standard. Lipids of interest were compared with cellular phosphate levels to ensure equal and accurate comparison between treatments.
Histological Analysis of Liver
Frozen livers were embedded in OCT compound (Tissue-Tek) and sectioned at 10 μm in a cryostat. Sections were stained with Oil red O (Sigma) dissolved in 70% isopropyl alcohol.
Quantitative Real-time PCR
Total RNA was extracted and purified from tissues using TRIzol (Invitrogen) according to the manufacturer's recommendations. cDNA was synthesized from mRNA by reverse transcription-PCR using a commercial cDNA synthesis kit with oligo(dT) primers (iScript select cDNA synthesis; Bio-Rad). Quantitative real-time PCR was performed by following the instructions accompanying the Qiagen QuantiFast SYBR Green PCR kit and using a Qiagen QIAcube. A sample containing no cDNA was used as a nontemplate control to verify the absence of primer dimers. β-Actin reactions were performed side by side with every sample analyzed. Changes in the mRNA level of each gene for each treatment were normalized to that of the β-actin control mRNA according to Pfaffl (19).
Protein Analysis
Tissue extracts were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted using methods described previously (20). Protein detection was performed using the Odyssey infrared imaging system (LI-COR; Lincoln NE) according to the manufacturer's instructions. Primary antibodies were from Cell Signaling, and secondary antibodies were from LI-COR.
Statistics
Data are presented as the mean ± S.E. Data were compared by two-tailed Student's t test or two-way analysis of variance with Tukey's post hoc analysis (GraphPad Prism; La Jolla, CA). Significance was set at p < 0.05.
RESULTS
Fenretinide Inhibits Ceramide Formation and Protects Insulin Signaling in Cultured Myotubes and Isolated Muscle Strips
Prolonged incubation of cells and tissues with palmitate is known to induce endogenous ceramide synthesis, which potently inhibits insulin-stimulated signaling and glucose uptake. As described above, inhibitors of the first and third enzymatic steps (i.e. serine palmitoyltransferase and ceramide synthase, respectively) prevent lipid-induced insulin resistance in vitro and in vivo (10, 21). Of the various interventions we (9) and others (22, 23) have used to inhibit ceramide synthesis to restore insulin signaling in high-lipid conditions, relatively little has been done to elucidate the efficacy of Des1 inhibition on these same outcomes.
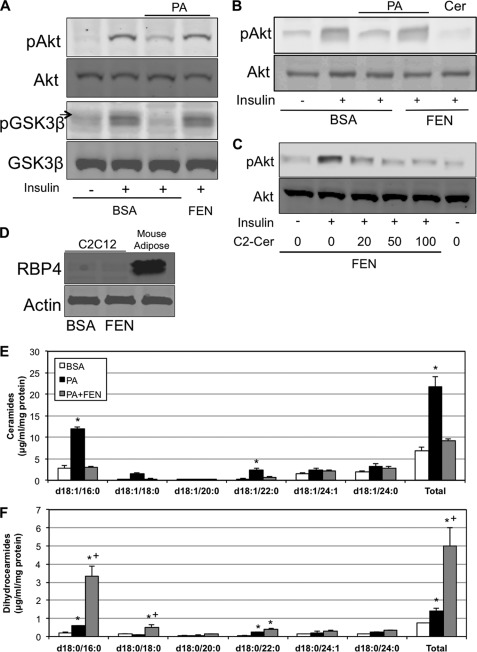
FEN was shown previously to improve insulin resistance in diet-induced obese mice (6). The proposed mechanism was that it promotes the urinary excretion of RBP4, an adipose-derived secretagogue implicated in insulin resistance. However, subsequent studies revealed that the compound could combat hepatic steatosis and obesity in RBP4-null mice, suggesting the existence of other targets that mediated its antidiabetic actions (5). We were intrigued by recent observations using cell culture systems revealing that fenretinide inhibits Des1 (13, 24), which prompted us to speculate that the regulation of ceramide levels may be an essential component of the antidiabetic actions of the drug. We tested this hypothesis initially in cultured myotubes treated with exogenous palmitate conjugated to BSA. We had previously shown that in this system, palmitate inhibited insulin activation of Akt/PKB by inducing sphingolipid formation. Indeed, inhibition of ceramide biosynthesis using inhibitors of serine palmitoyltransferase or ceramide synthase negated this ceramide effect (10). Herein we demonstrate that fenretinide negates the antagonistic effects of palmitate on insulin action (Fig. 1A), whereas the readdition of ceramide to the culture bypasses the protective effect of FEN (Fig. 1B). Indeed, even at relatively low doses (20 μm), the addition of C2-ceramide overrode the protective effect of FEN on insulin signaling (Fig. 1C). The effect of fenretinide in this system was not via RBP4 as the protein was not detected in the cell-autonomous myotube culture (Fig. 1D). In support of its role as a Des1 inhibitor, FEN completely prevented the palmitate-induced increase in ceramides (Fig. 1E), while eliciting a roughly 5-fold increase in dihydroceramides (Fig. 1F).
FIGURE 1.
Ceramide inhibition with fenretinide protects insulin signaling. A, murine C2C12 myotubes were treated with 0.75 mm BSA-conjugated PA for 16 h in the presence or absence of FEN (5 μm) followed by 10 min of insulin treatment (100 nm). pAkt, phosphorylated Akt; pGSK3β, phospho-glycogen synthase kinase 3β; GSK3β, glycogen synthase kinase 3β. B and C, the readdition of C2-ceramide (100 μm) bypassed the protection offered by FEN (B), an effect seen with as little as 20 μm ceramide (C). D, the improvement in insulin signaling with FEN does not require RBP4 as C2C12 cells appear to lack the protein. E and F, although PA induces ceramide and dihydroceramide accumulation, FEN prevents ceramide synthesis (E), whereas accumulating dihydroceramides (F). *, p < 0.05 for treatment versus BSA. +, p < 0.05 for PA+FEN versus PA (n = 3–6).
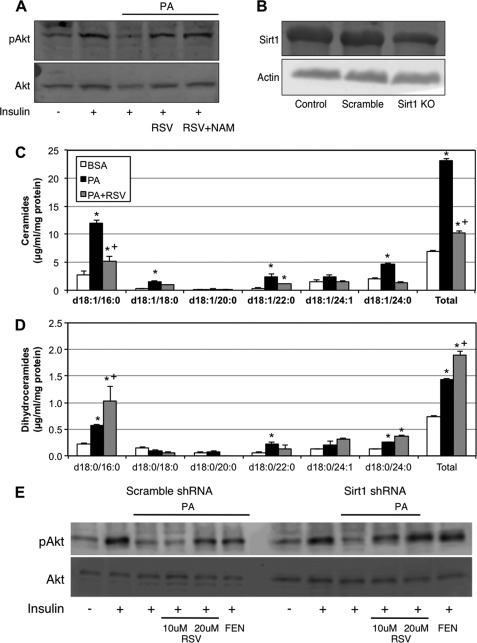
With recent evidence suggesting that resveratrol (RSV), a well studied component of red wine that also has insulin-sensitizing properties, shared structural similarity with fenretinide, which also rendered it a direct Des1 inhibitor (25), we performed parallel experiments with RSV. The results were comparable; RSV restored insulin signaling (Fig. 2A) and inhibited palmitate (PA)-induced ceramide accumulation (Fig. 2C), whereas preferentially inducing dihydroceramides (Fig. 2D). These effects appear to be independent of the RSV target sirt1 as neither treatment with nicotinamide (Fig. 2A) nor reducing sirt1 using shRNA (Fig. 2B) had an effect on insulin signaling (Fig. 2E).
FIGURE 2.
Resveratrol inhibits Des1 and protects insulin signaling. A, similar to fenretinide treatment, the addition of 20 μm RSV improved insulin signaling (100 nm, 10 min) in C2C12 myotubes exposed to 0.75 mm PA for 16 h. pAkt, phosphorylated Akt; Sirt1 KO, Sirt1 knock-out. C and D, RSV significantly reduced ceramides and increased dihydroceramides when added to PA-containing medium in comparison with PA alone. A, B, and E, to confirm that these effects occurred independently of Sirt1, similar to Sirt1 inhibition with nicotinamide (NAM) (A), Sirt1 ablation with shRNA (B) had no effect on RSV-mediated improvements in insulin signaling (E). *, p < 0.05 for treatment versus BSA. +, p < 0.05 for PA+RSV versus PA (n = 3–6).
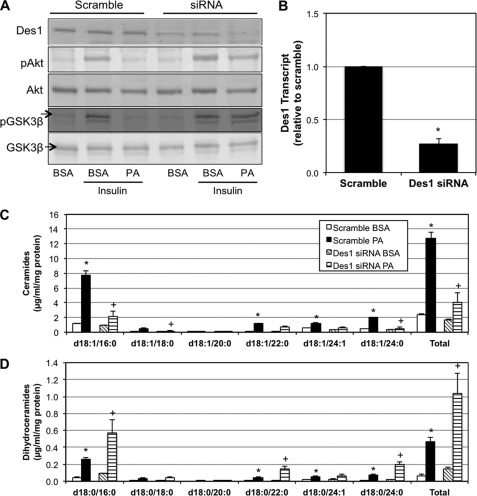
Having established the efficacy of a purported pharmacological inhibitor of Des1 in preventing lipid-induced insulin resistance, we sought to eliminate any concern over the possibility of the insulin-sensitizing effects of FEN being the result of other off-target effects. To validate the efficacy of Des1 inhibition on modifying sphingolipid profile and protecting insulin signaling, Des1 siRNA was used to lower myotube Des1 levels (Fig. 3A). Of the two Des1 isoforms in mammals, Des1 displays specificity for synthesis of ceramide (via Δ4-desaturase), whereas Des2 (via Δ4-desaturase/C4-hydroxylase) produces both ceramides and phytoceramides. Additionally, the two isoforms exhibit diverse tissue distribution, with Des2 being highly enriched in epithelial cells (gut, kidneys, skin) (24). Accordingly, Des1 was explored in this system. As opposed to scrambled siRNA, siRNA directed against Des1 prevented the antagonistic effects of palmitate on insulin-stimulated Akt/PKB phosphorylation (Fig. 3, A and B). The treatment additionally prevented the phosphorylation of glycogen synthase kinase 3β (GSK3β), an anabolic enzyme that is an Akt/PKB substrate. As predicted, Des1 knockdown prevented the significant increase in ceramides in response to PA seen in cells treated with a scrambled siRNA construct (Fig. 3C). Moreover, Des1 knockdown increased dihydroceramide levels in both the absence and the presence of PA (Fig. 3D). The prominent glycerolipids TAG and DAG were not affected by Des1 knockdown (data not shown).
FIGURE 3.
Ablation of Des1 inhibits ceramide accumulation and protects insulin signaling. A, Akt Ser-473 phosphorylation (pAkt) was determined in C2C12 myotubes 48 h after transfection with Des1 siRNA or control. Cells were treated for 16 h with 0.75 mm PA followed by insulin stimulation (100 nm; 10 min). Des1 knockdown protected insulin signaling. pGSK3β, phospho-glycogen synthase kinase 3β; GSK3β, glycogen synthase kinase 3β. A and B, Des1 knockdown was confirmed by Western blot (A) and quantitative PCR (B). To confirm the quality of Des1 knockdown, levels of ceramides and dihydroceramides were determined and found to vary significantly between treatments. C and D, Des1 knockdown robustly inhibited ceramide accumulation (C) and induced a significant increase in dihydroceramides in response to 0.75 mm PA (D) when compared with control conditions. *, p < 0.05 for treatment versus BSA. +, p < 0.05 for dihydroceramides versus ceramides in BSA with Des1 siRNA (n = 4–5).
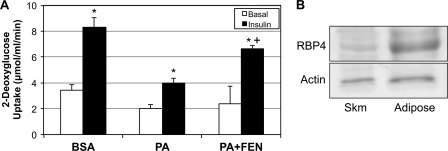
These data strongly indicate that FEN negates the palmitate-induced impairment of insulin signaling in cultured myotubes by inhibiting ceramide formation. Also, the increase in dihydroceramide is highly suggestive that Des1 is the likely site of action. To further explore the role of FEN on insulin action, we measured glucose uptake in isolated rat soleus muscle strips. We have used this system previously, where we showed that palmitate induced insulin resistance via a ceramide-dependent mechanism (10). Moreover, using this model, we demonstrated that muscles haploinsufficient for Des1 are protected from palmitate-induced insulin resistance (9). As predicted, the reduction in insulin-stimulated glucose uptake caused by palmitate was improved by the inclusion of FEN in the medium (Fig. 4A). As in the cultured myotubes, we found virtually no RBP4 in the rodent muscle (Fig. 4B), confirming that the drug has an alternative mechanism of action. These data strongly indicate that fenretinide can improve insulin sensitivity in a cell- and tissue-autonomous manner.
FIGURE 4.
Fenretinide prevents lipid-induced reductions in insulin-stimulated glucose uptake. Following excision, rat soleus was incubated in gassed medium for 6 h with 1 mm PA in the presence or absence of FEN (10 μm) followed by 1 h of insulin incubation (300 microunits). A, 2-[3H]DOG uptake was found to be significantly improved with the addition of FEN to PA when compared with PA alone. B, to confirm that the effect occurs independently of RBP4, we measured RBP4 protein in whole muscle and found extremely low levels. *, p < 0.05 for insulin stimulation versus basal. +, p < 0.05 for PA+FEN versus PA alone (n = 5). Skm, skeletal muscle.
Fenretinide Improves Glucose Homeostasis and Alters Sphingolipid Levels in Vivo
To determine whether FEN is able to target the sphingolipid synthesis pathway in vivo, we measured lipids from soleus muscle and liver of mice 12 h after the delivery of the drug by intraperitoneal injection. The single injection of the drug only slightly reduced muscle ceramide levels, but robustly increased dihydroceramide levels (Fig. 5, A and B). A similar, although less robust, effect was observed in the liver (Fig. 5, C and D). However, although FEN had a more pronounced effect on shorter acyl chain dihydroceramide species (e.g. C16) in muscle, it appeared to affect a longer chain species more specifically in the liver (C24). This is consistent with the recent observation that the dominant ceramide synthase in the liver (CerS2) makes predominantly longer ceramides. Altogether, these data again indicate that FEN modulates sphingolipid levels and are highly suggestive that it inhibits Des1 in vivo.
FIGURE 5.
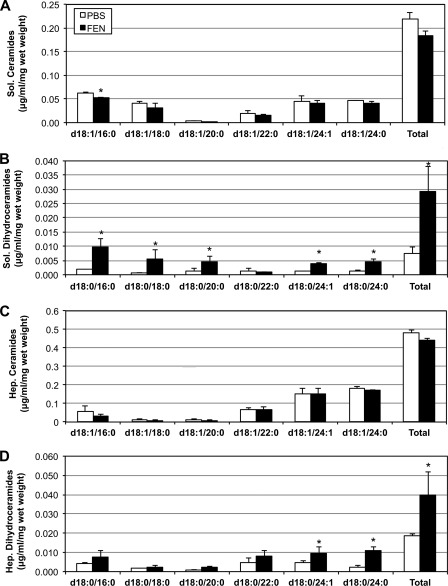
Acute fenretinide injection selectively inhibits ceramide accumulation. HFD-fed male C57Bl/6 mice received 10 mg/kg of FEN via intraperitoneal injection 12 h prior to sacrifice. A and C, ceramides tended to decrease in both the soleus (Sol.) and the liver (Hep.) (A and C), but did not reach significance. B and D, however, FEN injection induced a roughly 6-fold increase in dihydroceramides in the soleus (B) and a 2-fold increase in the liver (D). *, p < 0.05 for treatment versus PBS injection (n = 5).
With evidence supporting an acute effect of fenretinide in the regulation of Des1, we sought next to determine the effect of chronic FEN on lipid profiles and metabolic parameters in an in vivo model of obesity and insulin resistance. Five-week-old mice (C57Bl/6) were placed on an HFD for 16 weeks, with a subset receiving FEN for the final 4 weeks. FEN treatment improved glucose tolerance and insulin sensitivity as determined by both glucose and insulin tolerance tests (Fig. 6, A and B). The improvement in insulin sensitivity in HFD-fed mice receiving FEN was confirmed by computing the HOMA-IR from fasting blood glucose and insulin levels (Fig. 6C). The magnitude of effects observed is comparable with those reported previously (5). However, in contrast to earlier studies (5), we did not see a significant discrepancy in weight between mice on HFD versus those receiving an HFD plus FEN (Fig. 6D).
FIGURE 6.
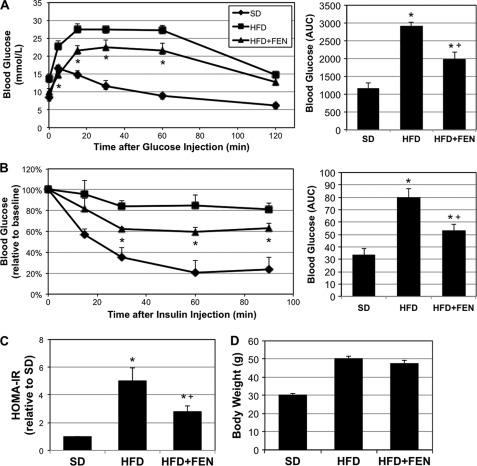
Chronic fenretinide treatment improves glucose tolerance and insulin sensitivity in diet-induced obese mice. Male mice (C57Bl/6) were made obese by HFD for 12 weeks prior to 4-week FEN treatment administered in drinking water (10 μg/ml). Following the treatment period, intraperitoneal glucose (1g/kg of body weight) and insulin (0.75 units/kg of body weight) tolerance tests were conducted. A and B, FEN treatment resulted in improved glucose (A) and insulin (B) tolerance when compared with HFD alone (area under curve (AUC) also shown). *, p < 0.05 for HFD+FEN versus HFD. Fasting insulin and glucose were used to determine the HOMA-IR. C, HFD-fed mice had a significantly elevated HOMA-IR value when compared with animals fed SD, and this was reduced in the HFD+FEN group. D, body weight increased on HFD, but was unaffected by FEN. *, p < 0.05 for treatment versus SD. +, p < 0.05 for HFD+FEN versus HFD (n = 6).
Mice fed an HFD demonstrated significantly elevated muscle and hepatic ceramides when compared with animals on a standard diet (SD) (Fig. 7, A and C). Treatment with FEN (HFD+FEN) completely normalized ceramide levels such that by the end of the treatment period, tissue ceramide levels were not different from the SD controls (Fig. 7, A and C). Dihydroceramides in both tissues increased in a stepwise manner with HFD and HFD+FEN (Fig. 7, B and D). Note that when the FEN was given over the prolonged, 4-week period, it was included in the drinking water, rather than being given by an acute intraperitoneal injection. Thus, the differences in ceramides and dihydroceramides likely reflect the pharmacodynamics of drug action.
FIGURE 7.
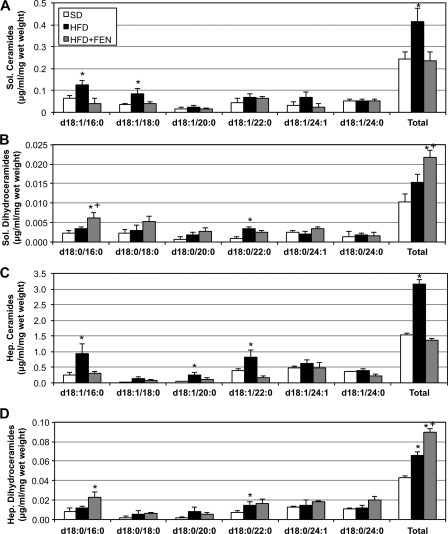
Fenretinide reduces muscle and liver ceramides and increases dihydroceramides. Lipids from soleus (Sol.) and liver (Hep.) were extracted from tissues of mice receiving SD, HFD, or HFD with fenretinide (HFD+FEN) after 4 weeks of treatment. A–D, in both soleus (A and B) and liver (C and D), the HFD-induced increase in ceramides was prevented with FEN treatment (A and C). Moreover, FEN increased dihydroceramides in both tissues (B and D). *, p < 0.05 for treatment versus SD. +, p < 0.05 for HFD+FEN versus HFD (n = 6).
Previous studies have observed that ceramides strongly correlate with the degree of hepatic steatosis (26) and that treatment of diet-induced obese rodents with various sphingolipid inhibitors prevents and reverses hepatic steatosis (22, 27–30). We report similar findings with FEN. Although FEN did not affect hepatic DAG levels (Fig. 8A), the drug significantly reduced hepatic TAG levels (Fig. 8B). Oil red O staining of liver sections further confirmed a reversal of steatosis with FEN supplementation (Fig. 8C).
FIGURE 8.
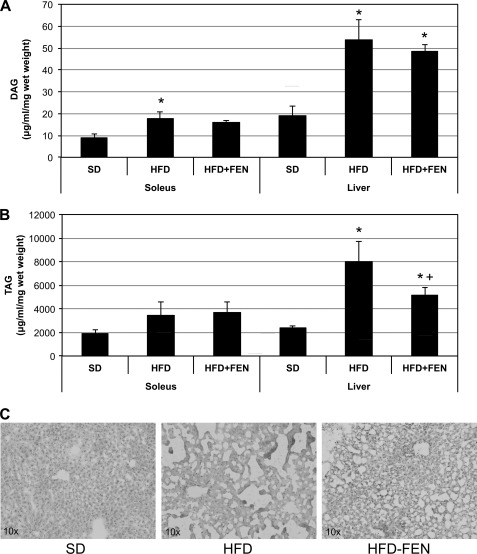
Fenretinide reduces soleus and liver neutral lipid content. A, DAG increased significantly with HFD versus SD, and FEN had no effect. B and C, in contrast, the increased TAG content in liver with HFD was significantly inhibited with FEN, suggesting a reversal of hepatic steatosis with FEN. *, p < 0.05 for treatment versus SD. +, p < 0.05 for HFD+FEN versus HFD (n = 6).
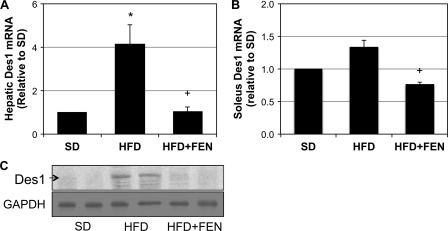
Fenretinide Regulates Des1 Expression
We were surprised to observe that high-fat feeding markedly increased Des1 transcript and protein levels in the liver (Fig. 9A). Even more surprising, treatment with fenretinide, which inhibits Des1 activity in cell culture (31), reduced hepatic Des1 expression in vivo (Fig. 9C). A smaller effect was observed in muscle, where the Des1 mRNA did not show a statistically significant increase (p = 0.063). However, as in the liver, FEN treatment significantly reduced Des1 mRNA (Fig. 9B). These data suggest that prolonged inhibition of Des1 activity may lead to a compensatory down-regulation in protein expression levels.
FIGURE 9.
Fenretinide inhibits high-fat diet-induced increase in Des1 transcription. Liver and soleus were extracted from mice fed SD, HFD, or HFD with fenretinide (HFD+FEN) for 4 weeks. A–C, hepatic Des1 mRNA and protein levels were markedly elevated with HFD (A and C), but less so in soleus (B). However, inclusion of FEN to HFD resulted in a significant reduction in Des1 transcript in soleus when compared with HFD alone. *, p < 0.05 for treatment versus SD. +, p < 0.05 for HFD+FEN versus HFD (n = 6).
Monounsaturated Oleate Prevents Des1 Expression
We have previously shown that ceramide synthesis occurs only in response to saturated, not unsaturated, fatty acids (9, 32). A number of studies have shown that the monounsaturated fatty acid oleate (OA) protects cells from insulin resistance and ceramide synthesis. This was previously attributed to an increased shunting of lipids into the glycerolipid pathway as many of the enzymes involved in that process show preference for that fatty acid (33). We confirm earlier observations that OA protects myotubes from the deleterious effects of PA on insulin signaling (supplemental Fig. 1A). In exploring the mechanism of this protection, we found that OA completely prevented the PA induction of ceramide (supplemental Fig. 1C). Interestingly, although OA prevented the increase in ceramide caused by palmitate, these treatment conditions were associated with a robust induction of dihydroceramides (supplemental Fig. 1D). We analyzed Des1 transcript levels and found that OA prevented the transcription of Des1 mRNA caused by PA (supplemental Fig. 1B). Rather than increasing amounts of DAG or TAG, OA, when given in concert with PA, decreased DAG levels (supplemental Fig.1F) without affecting TAG (supplemental Fig. 1E). These findings corroborate other recent work by Hu et al. (34) and suggest that some of the protective effects of OA may relate to its ability to specifically modulate Des1 expression.
DISCUSSION
Because of its ability to slow tumor growth and its favorable toxicological profile, fenretinide has been studied extensively as a chemotherapeutic for slowing cancer progression. Interestingly, studies in both rodents and humans reveal that the compound has insulin-sensitizing properties, suggesting an alternative use for this drug (5–7). Because of these studies, clinical trials evaluating the utility of the drug for treating insulin resistance have been initiated.
The data presented herein indicate that the insulin-sensitizing effect of fenretinide in liver and muscle results from its ability to, in a cell-autonomous fashion, inhibit the formation of ceramides. Evidence supporting this includes the following. First, the addition of C2-ceramide in place of palmitate bypasses the site of fenretinide action (Fig. 1); second, knockdown of Des1 by siRNA recapitulates the effects of fenretinide in vitro (Fig. 3); and third, fenretinide improves glucose homeostasis, while selectively lowering the ceramide/dihydroceramide ratio in vivo (Figs. 5–7). When considered in concert with the recent observation that FEN inhibits Des1 activity in vitro (31), the data are suggestive of a role for fenretinide in the regulation of Des1 activity in vivo.
The earliest report of the efficacy of fenretinide in controlling glucose homeostasis came from the Barbara Kahn laboratory (6), which demonstrated that the compound was efficacious in high-fat-fed mice. The work derived from studies on RBP4, an adipose-derived secretagogue implicated in insulin resistance. Knock-out mice lacking RBP4 placed on a high-fat diet have increased insulin sensitivity when compared with wild-type littermates. Fenretinide displaces retinol from RBP4, leading to the excretion of the latter through the kidneys. However, the subsequent observation that fenretinide had antiobesity and antisteatotic actions in RBP4-null mice suggests that the compound has additional targets relevant to metabolic disease (5). Herein we demonstrate that the insulin-sensitizing effects of fenretinide in cultured myotubes and isolated muscles are independent of RBP4, which is not present in these systems. It is noteworthy that although Preitner et al. (5) observed a lack of obesity in the mice fed HFD with fenretinide, we observed no significant weight difference; HFD mice fed fenretinide gained weight comparable with HFD mice receiving no fenretinide (Fig. 6D). We suspect that the difference lies in the treatment periods. Although Preitner et al. (5) began fenretinide treatment at the onset of HFD intervention, our fenretinide treatment was intentionally much more acute and introduced later in life, after obesity was already achieved.
A quick review of the literature is likely to create confusion about the effect of fenretinide on ceramide metabolism. Early studies erroneously suggested that fenretinide induced a general elevation of ceramides (36–38). Indeed, this increase was thought to trigger the stress responses that halt the growth and promote death of transformed cells. However, the methods used to measure sphingolipids in these early studies could not distinguish ceramides from dihydroceramides. When conducting a lipidomic profile of fenretinide-treated cells using mass spectroscopy, the Merrill group (13) found that fenretinide actually inhibits ceramide synthesis, resulting in the accumulation of its immediate precursor, dihydroceramide (12). This finding is corroborated in the studies here. Importantly, fenretinide exerts similar effects on ceramide metabolism in vivo in both acute and chronic conditions. We observed an increase in dihydroceramides in the liver and soleus muscle of mice following an acute single injection of fenretinide. The observed differences in ceramide were minor (∼20–30%) following this acute dosing regimen. Due to the fact that a typical cell has substantially more ceramides than dihydroceramides, a slight change in ceramide inhibition at the level of Des1 translates into a comparably large change in dihydroceramides. Nonetheless, this finding shows that a single dose of fenretinide is capable of promoting dihydroceramide accumulation, and greater effects would possibly be noticed with the alteration of the drug dose and injection site. When exploring the chronic effects of fenretinide, we determined that 4 weeks of fenretinide treatment is sufficient to significantly modify tissue lipid profiles and improve insulin sensitivity in diet-induced obese mice. Specifically, diet-induced obese mice receiving fenretinide had reduced ceramide levels and elevated dihydroceramides, which was associated with a reduced HOMA-IR value and improved glucose and insulin tolerance. Interestingly, fenretinide also significantly reduced hepatic TAG, suggesting a reversal in hepatic steatosis (35).
Fenretinide (N-(4-hydroxyphenyl)retinamide) is synthesized from the modification of the carboxyl end of retinoic acid with an N-4-hydroxyphenyl group. Until very recently, the precise mechanisms and full extent of the antagonizing effect of fenretinide on Des1 activity have not been completely elucidated. Work in the Merrill laboratory (13) points to a critical role for the molecular structure of fenretinide. In particular, their work implicates the phenol group found on fenretinide, which may disrupt the electron transport necessary for the desaturation of the dihydroceramide backbone to form ceramide (8), a proposition supported by the similar effects of the polyphenol resveratrol in Des1 inhibition (25). Additionally, fenretinide has a structure roughly similar to dihydroceramide. Rahmaniyan et al. (31) found that this structural similarity allows fenretinide to directly bind Des1, displaying a competitive and then irreversible dose-dependent inhibition as early as 2 h.
In conclusion, these experiments reveal that the modulation of ceramide synthesis likely accounts for some of the effects of fenretinide, which is being explored as a therapeutic means to improve insulin sensitivity in the obese. Because it selectively alters ratios of the substrates versus products of the Des1 reaction, it suggests that Des1 is a probable target in vivo. The peripheral studies here support the idea that Des1 may be a viable therapeutic target for improving insulin resistance. The enzyme is induced by factors associated with obesity, and silencing the enzyme pharmacologically or genetically has a potent insulin-sensitizing effect. Studies evaluating whether it is capable of inducing ceramides and lowering dihydroceramides in the human subjects receiving the drug are warranted.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK081456-01 (to S. A. S.). This work was also supported by the Singapore Ministry of Education Academic Research Fund (Grant MOE2009-T2-2-016) (to S. A. S.); the National Medical Research Council (Grant IRG09may004) and the Singapore National Research Foundation (CRP Award 2007-04)(to S. A. S.); the Duke-National University of Singapore (Duke-NUS) Signature Research Program funded by the Agency for Science, Technology and Research, Singapore, and the Ministry of Health, Singapore (to S. A. S.); and a Brigham Young University (BYU) Mentoring Environment grant (to B. T. B.).

This article contains supplemental Fig. 1.
Veterans Research Medical Foundation, unpublished data.
- RBP4
- retinol-binding protein 4
- Des1
- dihydroceramide desaturase 1
- CerS
- (dihydro)ceramide synthase
- FEN
- fenretinide
- HFD
- high-fat diet
- SD
- standard diet
- HOMA-IR
- homeostatic model assessment of insulin resistance
- DAG
- diacylglycerol
- TAG
- triacylglycerol
- RSV
- resveratrol
- PA
- palmitate
- OA
- oleate.
REFERENCES
- 1. Antonoff M. B., D'Cunha J. (2010) Teaching an old drug new tricks: metformin as a targeted therapy for lung cancer. Semin. Thorac. Cardiovasc. Surg. 22, 195–196 [DOI] [PubMed] [Google Scholar]
- 2. Heemskerk J., Tobin A. J., Bain L. J. (2002) Teaching old drugs new tricks: Meeting of the Neurodegeneration Drug Screening Consortium, 7–8 April 2002, Washington, DC, USA. Trends Neurosci. 25, 494–496 [DOI] [PubMed] [Google Scholar]
- 3. Bonanni B., Lazzeroni M. (2009) Retinoids and breast cancer prevention. Recent Results Cancer Res. 181, 77–82 [DOI] [PubMed] [Google Scholar]
- 4. Wang H., Charles A. G., Frankel A. J., Cabot M. C. (2003) Increasing intracellular ceramide: an approach that enhances the cytotoxic response in prostate cancer cells. Urology 61, 1047–1052 [DOI] [PubMed] [Google Scholar]
- 5. Preitner F., Mody N., Graham T. E., Peroni O. D., Kahn B. B. (2009) Long-term fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Serum retinol-binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 7. Johansson H., Gandini S., Guerrieri-Gonzaga A., Iodice S., Ruscica M., Bonanni B., Gulisano M., Magni P., Formelli F., Decensi A. (2008) Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 68, 9512–9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng W. (2006) Fenretinide increases dihydroceramide and dihydrosphingolipids due to inhibition of dihydroceramide desaturase. Ph.D. thesis, Georgia Institute of Technology, Atlanta, GA [Google Scholar]
- 9. Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., Pagliassotti M. J., Scherer P. E., Summers S. A. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121, 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holland W. L., Brozinick J. T., Wang L. P., Hawkins E. D., Sargent K. M., Liu Y., Narra K., Hoehn K. L., Knotts T. A., Siesky A., Nelson D. H., Karathanasis S. K., Fontenot G. K., Birnbaum M. J., Summers S. A. (2007) Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 [DOI] [PubMed] [Google Scholar]
- 11. Munoz-Olaya J. M., Matabosch X., Bedia C., Egido-Gabás M., Casas J., Llebaria A., Delgado A., Fabriàs G. (2008) Synthesis and biological activity of a novel inhibitor of dihydroceramide desaturase. ChemMedChem. 3, 946–953 [DOI] [PubMed] [Google Scholar]
- 12. Valsecchi M., Aureli M., Mauri L., Illuzzi G., Chigorno V., Prinetti A., Sonnino S. (2010) Sphingolipidomics of A2780 human ovarian carcinoma cells treated with synthetic retinoids. J. Lipid Res. 51, 1832–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H., Maurer B. J., Liu Y. Y., Wang E., Allegood J. C., Kelly S., Symolon H., Liu Y., Merrill A. H., Jr., Gouazé-Andersson V., Yu J. Y., Giuliano A. E., Cabot M. C. (2008) N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol. Cancer Ther 7, 2967–2976 [DOI] [PubMed] [Google Scholar]
- 14. Brozinick J. T., Jr., Birnbaum M. J. (1998) Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J. Biol. Chem. 273, 14679–14682 [DOI] [PubMed] [Google Scholar]
- 15. Vratilova J., Frgala T., Maurer B. J., Patrick Reynolds C. (2004) Liquid chromatography method for quantifying N-(4-hydroxyphenyl)retinamide and N-(4-methoxyphenyl)retinamide in tissues. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 808, 125–130 [DOI] [PubMed] [Google Scholar]
- 16. Fei W., Shui G., Gaeta B., Du X., Kuerschner L., Li P., Brown A. J., Wenk M. R., Parton R. G., Yang H. (2008) Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan R., Uchil P. D., Jin J., Shui G., Ott D. E., Mothes W., Wenk M. R. (2008) Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J. Virol. 82, 11228–11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shui G., Guan X. L., Low C. P., Chua G. H., Goh J. S., Yang H., Wenk M. R. (2010) Toward one step analysis of cellular lipidomes using liquid chromatography coupled with mass spectrometry: application to Saccharomyces cerevisiae and Schizosaccharomyces pombe lipidomics. Mol. Biosyst. 6, 1008–1017 [DOI] [PubMed] [Google Scholar]
- 19. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. (2003) A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278, 10297–10303 [DOI] [PubMed] [Google Scholar]
- 21. Chavez J. A., Holland W. L., Bär J., Sandhoff K., Summers S. A. (2005) Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 280, 20148–20153 [DOI] [PubMed] [Google Scholar]
- 22. Yang G., Badeanlou L., Bielawski J., Roberts A. J., Hannun Y. A., Samad F. (2009) Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297, E211–E224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmitz-Peiffer C., Craig D. L., Biden T. J. (1999) Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 274, 24202–24210 [DOI] [PubMed] [Google Scholar]
- 24. Zheng W., Kollmeyer J., Symolon H., Momin A., Munter E., Wang E., Kelly S., Allegood J. C., Liu Y., Peng Q., Ramaraju H., Sullards M. C., Cabot M., Merrill A. H., Jr. (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism, and roles in membrane structure, dynamics, signaling, and autophagy. Biochim. Biophys. Acta 1758, 1864–1884 [DOI] [PubMed] [Google Scholar]
- 25. Signorelli P., Munoz-Olaya J. M., Gagliostro V., Casas J., Ghidoni R., Fabriàs G. (2009) Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 282, 238–243 [DOI] [PubMed] [Google Scholar]
- 26. Yetukuri L., Katajamaa M., Medina-Gomez G., Seppänen-Laakso T., Vidal-Puig A., Oresic M. (2007) Bioinformatics strategies for lipidomics analysis: characterization of obesity-related hepatic steatosis. BMC Syst. Biol. 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bijl N., Scheij S., Houten S., Boot R. G., Groen A. K., Aerts J. M. (2008) The glucosylceramide synthase inhibitor N-(5-adamantane-1-yl-methoxy-pentyl)-deoxynojirimycin induces sterol regulatory element-binding protein-regulated gene expression and cholesterol synthesis in HepG2 cells. J. Pharmacol. Exp. Ther. 326, 849–855 [DOI] [PubMed] [Google Scholar]
- 28. Aerts J. M., Ottenhoff R., Powlson A. S., Grefhorst A., van Eijk M., Dubbelhuis P. F., Aten J., Kuipers F., Serlie M. J., Wennekes T., Sethi J. K., O'Rahilly S., Overkleeft H. S. (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56, 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao H., Przybylska M., Wu I. H., Zhang J., Maniatis P., Pacheco J., Piepenhagen P., Copeland D., Arbeeny C., Shayman J. A., Aerts J. M., Jiang C., Cheng S. H., Yew N. S. (2009) Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology 50, 85–93 [DOI] [PubMed] [Google Scholar]
- 30. Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., Merrill A. H., Jr., Nikolova-Karakashian M. (2009) Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 284, 8359–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rahmaniyan M., Curley R. W., Jr., Obeid L. M., Hannun Y. A., Kraveka J. M. (2011) Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 286, 24754–24764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chavez J. A., Summers S. A. (2003) Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 419, 101–109 [DOI] [PubMed] [Google Scholar]
- 33. Henique C., Mansouri A., Fumey G., Lenoir V., Girard J., Bouillaud F., Prip-Buus C., Cohen I. (2010) Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J. Biol. Chem. 285, 36818–36827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu W., Ross J., Geng T., Brice S. E., Cowart L. A. (2011) Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. J. Biol. Chem. 286, 16596–16605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bikman B. T., Summers S. A. (2011) Sphingolipids and hepatic steatosis. Adv. Exp. Med. Biol. 721, 87–97 [DOI] [PubMed] [Google Scholar]
- 36. Wang H., Maurer B. J., Reynolds C. P., Cabot M. C. (2001) N-(4-Hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 61, 5102–5105 [PubMed] [Google Scholar]
- 37. Li X., Ling W., Pennisi A., Khan S., Yaccoby S. (2009) Fenretinide inhibits myeloma cell growth, osteoclastogenesis, and osteoclast viability. Cancer Lett. 284, 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saeed Z., Guilbault C., De Sanctis J. B., Henri J., Marion D., St-Arnaud R., Radzioch D. (2008) Fenretinide prevents the development of osteoporosis in Cftr-KO mice. J. Cyst. Fibros. 7, 222–230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.