Background: The structure and function of myelin P0-related 36-kDa protein are totally unknown.
Results: A novel isoform of P0, L-MPZ, contains an extra C terminus derived from 3′-UTR of P0 mRNA and is expressed in peripheral myelin.
Conclusion: L-MPZ is produced by stop codon readthrough and probably related with peripheral myelinogenesis.
Significance: Analyses of L-MPZ are crucial for understanding readthrough mechanism in mammals and myelinogenesis.
Keywords: Cell Adhesion, Membrane Proteins, Myelin, Neurobiology, Neurochemistry, Neuroscience, Schwann Cells, Translation, Neuropathy, Stop Codon Readthrough
Abstract
Myelin protein zero (P0 or MPZ) is a major myelin protein (∼30 kDa) expressed in the peripheral nervous system (PNS) in terrestrial vertebrates. Several groups have detected a P0-related 36-kDa (or 35-kDa) protein that is expressed in the PNS as an antigen for the serum IgG of patients with neuropathy. The molecular structure and function of this 36-kDa protein are, however, still unknown. We hypothesized that the 36-kDa protein may be derived from P0 mRNA by stop codon readthrough. We found a highly conserved region after the regular stop codon in predicted sequences from the 3′-UTR of P0 in higher animals. MS of the 36-kDa protein revealed that both P0 peptides and peptides deduced from the P0 3′-UTR sequence were found among the tryptic fragments. In transfected cells and in an in vitro transcription/translation system, the 36-kDa molecule was also produced from the identical mRNA that produced P0. We designated this 36-kDa molecule as large myelin protein zero (L-MPZ), a novel isoform of P0 that contains an additional domain at the C terminus. In the PNS, L-MPZ was localized in compact myelin. In transfected cells, just like P0, L-MPZ was localized at cell-cell adhesion sites in the plasma membrane. These results suggest that L-MPZ produced by the stop codon readthrough mechanism is potentially involved in myelination. Since this is the first finding of stop codon readthrough in a common mammalian protein, detailed analysis of L-MPZ expression will help to understand the mechanism of stop codon readthrough in mammals.
Introduction
Peripheral myelin protein zero (known as P02 or MPZ) is a type I transmembrane adhesion molecule, which belongs to the immunoglobulin (Ig) superfamily and constitutes >50% of the total peripheral nervous system (PNS) myelin protein in vertebrates (1). The P0 gene encodes a protein of 248 amino acids (aa) including a 29-aa signal sequence. Mature P0 protein (219 aa) is N-glycosylated and has an apparent molecular weight of ∼30 kDa. Homophilic interactions between the extracellular Ig domain lead to tight adhesion between each layer in PNS myelin (2). The highly basic intracellular domain of P0 is required for compaction of the cytoplasmic side of myelin and is also involved in extracellular adhesion (3, 4). The P0 gene consists of six exons, and protein sequences are evolutionarily highly conserved from fish to humans (1). In particular, sequences from mouse, rat, bovine, and human have >90% identity. Thus, P0 is important for the function of the PNS, and a large number of P0 mutations cause hereditary motor sensory neuropathies (5).
Several groups have suggested that a 36-kDa (or 35-kDa) autoantigen that reacted with sera from patients with neuropathies may be involved in various neuropathies. This molecule has an identical N-terminal aa sequence and partial internal sequence as P0 (6–9), but the size of this protein is somewhat larger than P0. Although there are three truncated, spliced variants of P0 that are related to the omission or partial skipping of exon 3 (10), no other mRNA transcripts have been reported. Ishida et al. (8) screened a human sciatic nerve (ScN) cDNA expression library using a serum sample obtained from a patient and found that three positive clones included small cDNA fragments matching part of the 3′-UTR of P0. The molecular structure and function of this protein are, however, totally unknown.
Translation termination is a crucial step that controls expression during protein synthesis. During this process, well-regulated stop codon readthrough is a mechanism that is thought to expand the coding potential of a limited genome in viruses (11), yeasts (for review, 12), and Drosophila (13–15). It is believed that this mechanism occurs in higher animals and that it can have substantial effects on the function of the encoded protein and on the phenotype of the cell. Rabbit β-globin is, however, the only reported example of the utilization of this mechanism in a higher organism (16, 17).
Here we show that the neuropathy-associated 36-kDa protein is a novel form of P0 that is produced by translational stop codon readthrough. We designated this protein as large myelin protein zero (L-MPZ).
EXPERIMENTAL PROCEDURES
Serum Samples
Serum that was positive for antibodies against the 36-kDa protein was obtained from a 54-year-old female patient with chronic inflammatory demyelinating polyneuropathy (CIDP) at Gifu University Hospital and was stored in aliquots at −80 °C until use. Normal serum was obtained from a 62-year-old female volunteer and was stored in aliquots at −80 °C until use. This study was approved by the Tokyo University of Pharmacy and Life Sciences Review Committee and the Gifu University Review Committee. The patient and a volunteer provided written informed consent prior to participation in the study.
Animals
Wistar rats that were 8 weeks old and postnatal day (P)1, P3, P5, and P7 Wistar rats were purchased from Japan SLC (Hamamatsu, Japan). All experiments were conducted in accordance with the guidelines on the care and use of animals of the Tokyo University of Pharmacy and Life Sciences Animal Use Committee.
Preparation of Brain and ScN Homogenates
A homogenate was prepared from 10 8-week-old male Wistar rats. All procedures were carried out on ice or at 4 °C. Homogenates were obtained as described (18) with slight modification. Briefly, ScNs were dissected and snap frozen in liquid nitrogen. The frozen tissues were then ground into powder using a mortar and homogenized with a homogenizer (Heidolph, Schwabach, Germany) in nine volumes (w/v) of 0.32 m sucrose containing 5 mm Tris-HCl, pH 7.5; 2 mm EGTA; 0.75 μm aprotinin; 1 μm leupeptin; 1 μm pepstatin A; and 0.4 mm PMSF (Homogenization Buffer). To remove chromosomal DNA, cell debris, and fibers, the homogenates were centrifuged at 500 × g for 10 min, and the supernatants were collected and stored as a whole homogenate fraction at −80 °C. The protein concentration was determined using a bicinchoninic acid assay (Pierce Biotechnology).
Isolation of Membrane, Cytosol, and Myelin Fractions from ScN Homogenates
Whole ScN homogenate was collected and centrifuged for 30 min at 200,000 × g. The supernatant collected at this stage contained the cytosolic fraction. The precipitate, which was the membrane fraction, was then re-homogenized in Homogenization Buffer. These fractions were stored at −80 °C. The myelin fraction was purified as described (18).
Western Blot Analysis
Western blot analysis using SDS-PAGE and two-dimensional gel electrophoresis (2DE) with a cationic detergent, cetyltrimethylammonium bromide (CTAB), was performed as described (18). This 2DE method using cationic detergent is appropriate for the analysis of highly hydrophobic membrane proteins, especially those in myelin (18, 19). CTAB-2DE is advantageous for the solubilization of hydrophobic proteins. The primary antibodies used in Western blotting are described below.
Peptide Absorption of Immunoreactivity
Immunoreactivity of the serum IgG in Western blotting was blocked by peptide absorption. The absorption peptides were synthesized by GenScript (Piscataway, NJ). Appropriate concentrations of the serum and antibody were incubated overnight at 4 °C with each of the synthetic peptides (20 μg/ml) in a solution containing 0.15 m NaCl and 20 mm Tris-HCl, pH 7.5 (TBS). This mixture was diluted 1:9 (v/v) in 0.3% skim milk/0.1% Tween-20/TBS and was used as the primary antibody solution for Western blotting.
Deglycosylation
Peptide:N-glycosidase F (PNGase F, EC 3.5.1.52) digestion of the peptide was performed as described (20) with slight modification. Briefly, the samples were boiled for 5 min in 0.5% SDS and 100 mm 2-mercaptoethanol and cooled to room temperature. Triton X-100 (10%) was added to a final concentration of 2.5%, and the sample was diluted to 1 μg/ml protein by adding 0.1 m phosphate buffer (PB), pH 6.8, and 25 mm EDTA. The mixture was incubated with 0.25 units of PNGase F (Roche)/30 μg protein for 15 h at 30 °C. The deglycosylated samples were analyzed by Western blotting.
MS Analysis
PNS myelin proteins were separated with CTAB-2DE and visualized with fluorescent staining (18, 21). The excised 36-kDa spot was analyzed by the Pasarow Mass Spectrometry Laboratory at the University of California at Los Angeles. Tryptic peptide fractions from HPLC chromatography were loaded into nano-electrospray emitters (Proxeon; Thermo, San Jose, CA) for immediate analysis using a nano-electrospray source-equipped mass spectrometer (LTQ-FT Ultra; Thermo). Identification of tryptic peptides from P0 and predicted L-MPZ-specific sequences was achieved by analyzing MS/MS spectra obtained using an MS/MS ion search of the Mascot Server (Matrix Science, Boston, MA).
Plasmid Constructs
Human P0 cDNA in the pUC19 vector (22) was a gift from Dr. Kiyoshi Hayasaka (Yamagata University, Yamagata, Japan). The EcoRI restriction fragment of full-length P0 was cloned into the EcoRI site of the pcDNA3.0 vector (Invitrogen, Carlsbad, CA), which contains a CMV promoter for expression in mammalian cell culture (hP0/pcDNA3). Mutants of deletion (del- L-MPZ/pcDNA3) or replacements [TAG → GCG, Ala (MutA- L-MPZ/pcDNA3); TAG → TAC, Tyr (MutY- L-MPZ/pcDNA3)] of P0 stop codon, mutant of 3′UTR-deletion just after P0 stop codon (P0–3′del/pcDNA3), and mutant of frameshift mutation by deletion of single base pair immediately after P0 stop codon (P0_only/pcDNA3) were generated from hP0/pcDNA3 using the PrimeSTAR Mutagenesis Basal kit (Takara Bio, Otsu, Japan) (Fig. 9B).
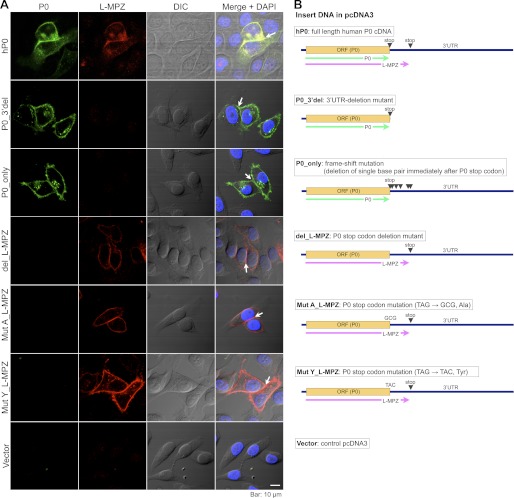
FIGURE 9.
Localization of L-MPZ and P0 in HeLa cells transfected with human P0 cDNA and several mutants. A, double immunostaining was performed using anti-P0 (first column, green) and anti-L-MPZ (second column, red) antibodies. DIC, differential interference contrast image (third column). Merge of first to third column images with DAPI-nuclear stain (blue) (forth column). L-MPZ-positive signals were colocalized with P0 signals (first row). Specific mutants expressing P0 (P0_3′del and P0_pnly) or L-MPZ-like molecules (del_, MutA_, MutY_L-MPZ) demonstrated the individual plasma membrane distribution of P0 or L-MPZ-like molecules. Just like P0, L-MPZ-positive signals were accumulated at cell-cell adhesion sites between two L-MPZ-like mutant-expressing cells (white arrows). Scale bar: 10 μm. B, illustrations of the constructions of insert DNAs in pcDNA3 expression vectors.
Cell Culture and Electroporation
Monolayers of HeLa and NIH/3T3 cells were maintained in DMEM (GIBCO-BRL/Invitrogen) supplemented with 10% FBS in 5% CO2 at 37 °C. Transfection of pcDNA3.0 (empty vector) or hP0/pcDNA3 was performed with an electroporator (MicroPorator, DigitalBio; now provided as Neon by Invitrogen) according to the manufacturer's instructions (Hela cell, 0.7 μg plasmid DNA in 10 μl solution, 1020 V, 35 ms, two pulses; NIH3T3, 15 μg plasmid DNA in 100 μl solution, 1350 V, 20 ms, two pulses).
Preparation of Transfected Cell Cultures
Transfected NIH/3T3 cells (3 × 106 cells) were plated on a 100-mm culture dish and cultured in 10% FBS/DMEM. Twenty-four hours after plating, the cells were washed with phosphate-buffered saline (PBS) and Homogenization Buffer containing 1 mm DTT. The cells were harvested with a cell scraper and lysed using a syringe with sequential passes through 21-G and 27-G needles. The supernatant was collected by centrifuging at 500 × g for 10 min and then used for Western blot analysis. Transfected HeLa cells (5 × 105 cells) were plated onto 18-mm coverslips coated with poly-l-lysine and cultured in 10% FBS/MEM-α containing GlutaMax (GIBCO-BRL/Invitrogen). The cells were used for immunostaining 24 h after plating onto the coverslips.
In Vitro Translation
In vitro translation of P0 and/or L-MPZ from hP0/pcDNA3 was performed with the TNT Quick Coupled Transcription/Translation System (Promega, Madison, WI) according to the manufacturer's instructions. l-[35S]methionine (EasyTag PerkinElmer, Waltham, MA) was used for labeling of synthesized proteins. The radiolabeled proteins were analyzed by SDS-PAGE followed by autoradiography on BioMax MR films (Kodak, Rochester, NY). For quantification, the intensity of each band was measured by ImageGauge v4.23 (Fujifilm, Tokyo, Japan), and the relative ratio of P0 and L-MPZ with or without G418 was calculated. Values were obtained from four samples. Statistical analysis was performed using a two-way ANOVA (PRISM 5; GraphPad Software, La Jolla, CA) followed by Bonferroni multiple comparison test.
Immunostaining
Transfected HeLa cells plated onto coverslips were fixed with 4% paraformaldehyde (PFA) at room temperature for 10 min and permeabilized for 5 min at room temperature in 0.1% Triton X-100 in PBS. The coverslips were preincubated for 1 h in 10% normal donkey serum (NDS)/PBS and then incubated overnight at 4 °C with primary antibodies diluted in NDS/PBS. After rinsing, the cells were incubated with Alexa Fluor 488- or 594-conjugated secondary antibodies for 1 h at room temperature. The labeled coverslips were then rinsed and mounted onto glass slides with Vectashield containing DAPI (Vector Laboratories, Burlingame, CA). Images were captured with confocal microscopy (Olympus, Tokyo, Japan).
Frozen sections of the ScN were prepared by transcardial perfusion of 8-week-old Wistar rats with 4% PFA in 0.1 m PB, pH 7.4. The 10-μm-thick frozen sections were permeabilized and blocked for 1 h in TBS containing 0.3% Triton X-100 and 10% NDS (NDS/T-TBS). The sections were then incubated overnight at 4 °C with primary antibodies diluted in NDS/T-TBS, thoroughly rinsed in TBS, and incubated with fluorescently labeled secondary antibodies for 1 h at room temperature. Finally, the labeled frozen sections were rinsed and mounted in Vectashield containing DAPI. The images were captured with confocal microscopy.
Antibodies
The following antibodies were used for immunostaining: goat polyclonal anti-P0 antibody produced against the peptide sequence (DHSRSTKAVSEK) of the C-terminal region of human P0 (1:500; Abnova, Taipei, Taiwan), rat monoclonal anti-myelin basic protein antibody (anti-MBP; 1:200; Chemicon/Millipore, Billerica, MA), mouse monoclonal anti-neurofilament (anti-NF; 1:5; Nichirei, Tokyo, Japan), and rabbit polyclonal anti-L-MPZ (1:4000) antibody, which was produced by immunization with a keyhole limpet hemocyanin-conjugated 21-aa peptide (C-LRPAVKSPSRTSLKNALKNM; synthesized by GenScript) of the human L-MPZ-specific region. The secondary antibodies used for immunostaining were Alexa Fluor 488- and 594-conjugated species-specific antibodies (1:2000; Molecular Probes/Invitrogen). For Western blotting, the following antibodies were used: the serum obtained from the patient with CIDP (1:200), goat polyclonal anti-P0 (1:4000), rabbit polyclonal anti-L-MPZ (1:40,000), mouse monoclonal anti-β-catenin (1:2,000; BD Biosciences, San Jose, CA), mouse monoclonal anti-actin (1:4,000; Sigma-Aldrich), rabbit polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000, Imgenex, San Diego, CA), mouse monoclonal anti-paxillin (1:5,000; BD Biosciences), and anti-MBP (1:2000) antibodies. The secondary antibodies used for Western blotting were horseradish peroxidase-conjugated anti-mouse, anti-rabbit, anti-rat, anti-goat (1:10000; Jackson ImmunoResearch Laboratories, West Grove, PA), and anti-human IgG (1:5000; American Qualex, San Clemente, CA).
RESULTS
Characterization of the 36-kDa Protein Recognized by Serum IgG from a Patient with Neuropathy
In the course of investigating neuropathy-associated autoantigens recognized by sera from patients with neuropathy, we found that some sera detected a 36-kDa molecule in rat ScN homogenate. To characterize this 36-kDa protein, Western blot analysis was performed using fractions from rat brain and ScN. The 36-kDa protein was detected only in the whole homogenate fraction from rat ScN and not in brain (Fig. 1A). This 36-kDa protein was detected in the β-catenin (adherence junction marker)-enriched ScN membrane fraction (Fig. 1B), especially in the MBP molecules (myelin marker)-enriched myelin fraction (Fig. 1C). To investigate N-linked glycosylation, Western blotting with PNGase F-treated ScN whole homogenate was performed. A deglycosylation assay with PNGase F revealed that both P0 and the 36-kDa protein bands were similarly shifted downward, suggesting that the 36-kDa protein had nearly the same sugar chain content as P0 (Fig. 1D). Thus, the serum from the patient recognized the peptide portion of this PNS myelin protein. These properties corresponded to those of the previously reported 36-kDa (or 35-kDa) protein antigen (6–9). Therefore, this 36-kDa protein is likely to be a common autoantigen in PNS myelin.
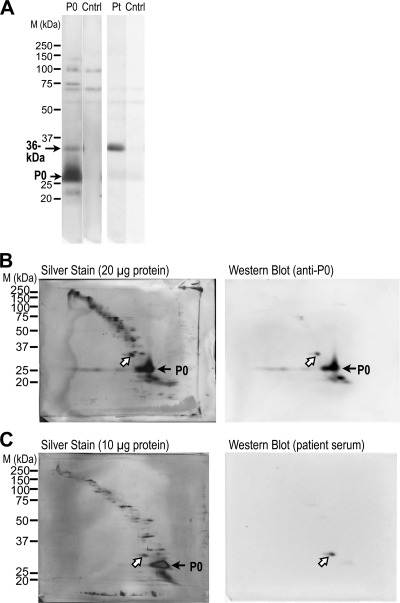
FIGURE 1.
Characterization of the 36-kDa protein detected by the serum IgG of a patient with CIDP. A, Western blotting of rat brain and ScN whole homogenates (10 μg protein). The 36-kDa band was detected in the ScN but not in the brain homogenate. B and C, Western blotting of the ScN fractions using the patient serum (Pt). The 36-kDa protein was detected in the membrane fraction (Mem) but not in the cytosolic fraction (Cyt; B) and was enriched in the myelin fraction (Myln; C). While β-actin and GAPDH (cytosolic and membrane-associated proteins) were detected both cytosolic and membrane fractions, β-catenin (adherence junction marker) was extremely enriched in the membrane fraction (B). In the myelin fraction, MBP molecules (myelin marker) were enriched but not β-catenin, GAPDH, nor paxillin (focal contact marker) (C). D, after deglycosylation of N-linked sugar chains with PNGase F treatment of ScN whole homogenate, the relative molecular weight of the 36-kDa protein was shifted down in the same manner as P0. WH, whole homogenates; Nrml, negative control using normal human serum; M, marker.
To investigate the relationship between this 36-kDa protein and P0, we tested whether anti-P0 antibody recognized the 36-kDa band using commercially available anti-P0 antibody, which recognizes the C-terminal region of human P0. A 36-kDa band was detected by both anti-P0 and the patient serum (Fig. 2A). To investigate whether these 36-kDa molecules were identical, we performed 2DE-Western blotting using CTAB, which is suitable for analyzing highly hydrophobic membrane protein spots (18). Proteins from rat ScN homogenates were visualized with silver staining of CTAB-2DE membranes (left panels of Fig. 2, B and C). The same 36-kDa spots were recognized by both the patient serum and anti-P0 antibody (white arrows in Fig. 2, B and C). This suggests that the 36-kDa molecule has an antigen in common with the P0 C-terminal region.
FIGURE 2.
Detection of the 36-kDa protein with anti-P0 antibody. A, Western blotting of ScN whole homogenates (10 μg protein). A band of the same size as the 36-kDa protein was recognized by anti-P0 (P0) antibody and by serum obtained from a patient with CIDP (Pt). B and C, 2DE Western blotting of rat ScN whole homogenate using CTAB. Spots with identical positions (white arrows) were detected using anti-P0 (B, right) antibody and patient serum (C, right). The corresponding spots (white arrows) are shown on the colloidal silver-stained membranes (B, C, left panels). Anti-P0 antibody also recognized smaller bands than the P0 band, which were probably degradation products (A and B, right). Cntrl, negative control without primary antibody; M, marker.
Evolutionarily Conserved AA Sequences Derived from the 3′-UTR of P0 mRNA
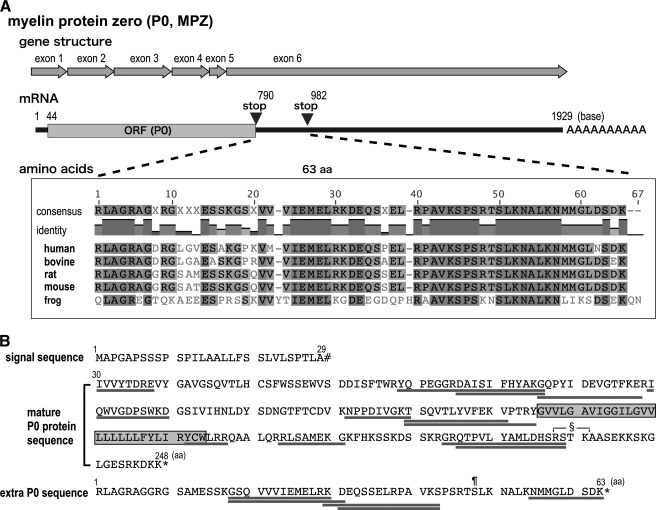
The N-terminal sequence of the 36-kDa (or 35-kDa) band detected by the sera of patients with neuropathy is identical to that of P0, and 36-kDa (or 35-kDa) bands are found in human, bovine, rat, and mouse PNS (6–9). In addition, Ishida et al. (8) showed that only small cDNA fragments containing a partial P0 3′-UTR have been obtained by expression cloning of the human ScN cDNA library using the serum from a patient with neuropathy. These data strongly suggest that this protein is a novel isoform of P0 and contains extra aa derived from the 3′-UTR of P0 mRNA. Because exon 6 encodes part of the P0 C terminus, the stop codon, and the entire 3′-UTR (Fig. 3A), we carefully examined the sequence of the 3′-UTR of P0 mRNA. After a typical stop codon (UAG), there were 189 nucleotides before the next in-frame stop codon (UGA) appeared. Evolutionary comparisons of P0 3′-UTR cDNAs revealed that the extra 63 predicted aa immediately after the regular stop codon were well conserved from frog (Xenopus laevis) to human (Fig. 3A). After the second stop codon, no additional conserved regions were found in the 3′-UTR. Interestingly, no such sequence homology was found in zebrafish P0 cDNA (data not shown). In addition, no such conserved aa sequence immediately after the regular stop codon was seen in other common myelin protein genes, including those encoding myelin proteolipid protein (PLP), myelin-associated glycoprotein, MBP, and peripheral myelin protein 22 (data not shown).
FIGURE 3.
The 36-kDa protein has evolutionarily conserved sequences derived from the 3′-UTR of P0 mRNA. A, sequence alignment of the deduced amino acid sequence of the region between the first and second stop codons in the 3′-UTR of P0 cDNA of human, bovine, rat, mouse, and frog (X. laevis). According to the degree of identity, amino acids are colored from light to dark gray. The sequence of this region is highly conserved. B, summary of the identified tryptic peptides from the 36-kDa spot detected by CTAB-2DE following MS analysis (underlined sequences). Twelve peptides were identified in the entire P0 protein sequence (aa 30–248). Five peptides were identified in the predicted sequence from the 3′-UTR of the P0 mRNA. The shaded box indicates the transmembrane region of P0. §, the protein kinase C (PKC)-mediated phosphorylation site in mature P0; ¶, the predicted PKC-mediated phosphorylation site in the 36-kDa-specific region; #, signal peptide cleavage site; *, stop codon.
To investigate whether the 36-kDa protein actually contains the extra 63 aa, tryptic peptides from the 36-kDa protein spot on CTAB-2DE gels were analyzed by MS. Twelve tryptic peptides from the 36-kDa protein corresponded to the predicted tryptic peptides from P0, and five additional peptides corresponded to the predicted sequence in the P0 3′-UTR (Fig. 3B). These peptides covered almost the entire P0 molecule including the N terminus. These data support the possibility that the 36-kDa protein is derived from P0 mRNA.
P0 has a phosphorylation site (RSTK) that is regulated by protein kinase C (PKC)-α in the cytoplasmic C-terminal region (Fig. 3B); this PKC-mediated phosphorylation is necessary for P0-mediated adhesion (4). To look for a possible predicted phosphorylation site in the extra 63 aa, we analyzed this sequence with the NetPhos 2.0 Server. One putative PKC-mediated phosphorylation site (RTSLK, Fig. 3B) was found with a high score.
Elimination of Immunoreactivity for the 36-kDa Band by the Specific Peptide Derived from the Predicted Sequence in the 3′-UTR of P0 mRNA
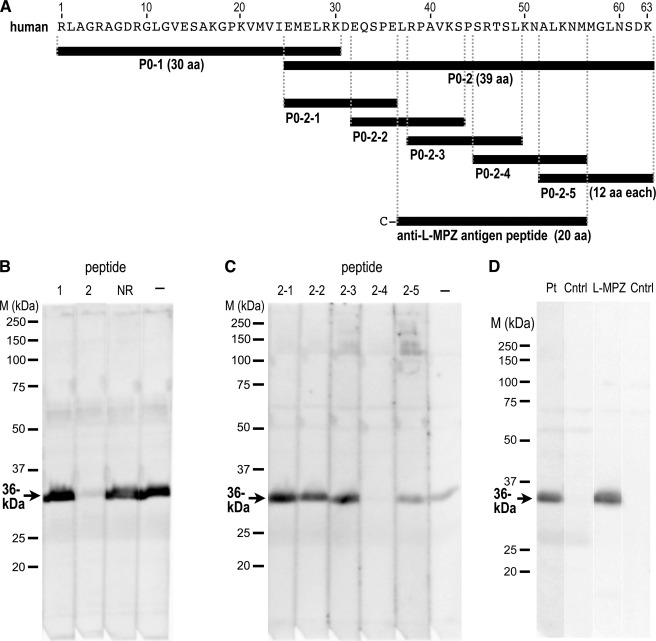
To determine whether the extra 63 aa of the 36-kDa protein contain antigenic sites, we performed peptide absorption using synthetic peptides derived from the predicted sequence in the 3′-UTR of human P0 mRNA. First, two peptides (Fig. 4A; P0–1 and P0–2) that matched the predicted extra sequence were synthesized and used for absorption. The immunoreactivity for the 36-kDa band in the patient serum was completely absorbed by the P0–2 peptide but not at all by the P0–1 peptide (Fig. 4B). To further investigate the epitope, five different 12-mer peptides (Fig. 4A; P0–2-1 to -5) that totally covered the P0–2 peptide sequence were synthesized and used for absorption. On the Western blot, the peptide P0–2-4 clearly absorbed the immunoreactivity from the patient serum (Fig. 4C), indicating that the 36-kDa protein has the extra sequence derived from the 3′-UTR of P0 mRNA, and the epitope recognized by the serum is located within the P0–2-4 sequence (SRTSLKNALKNM). Thus, because the 36-kDa protein is encoded by the P0 gene, we designated this protein as a large form of myelin P0 and called it L-MPZ. A rabbit polyclonal anti-L-MPZ was produced against a 20-aa peptide (Fig. 4A; anti-L-MPZ antigen peptide) that includes the P0–2-4 sequence. This rabbit anti-L-MPZ antibody recognized the same 36-kDa band as the band detected by the patient serum (Fig. 4D). The high antigenicity of this specific region of L-MPZ was demonstrated by the extremely high titer of this antibody in an ELISA with the peptide antigen (>1/512,000; ∼2.0 ng/ml) and Western blotting with the ScN homogenate (>1/40,000; ∼0.05 μg/ml). The immunoreactivity of anti-L-MPZ was also absorbed by both the antigen peptide and the P0–2-4 peptide (data not shown), which indicates that the epitope for the rabbit antibody and the patient IgG were in the same 12-aa region.
FIGURE 4.
Elimination of immunoreactivity for the 36-kDa band by specific peptides. A, black bars depict the synthesized peptides with the additional 63-aa sequence: two peptides derived from the 63-aa sequence (P0–1 and P0–2), five partially overlapping peptides derived from P0–2 (P0–2-1 to -5), and a peptide used to produce the specific antibody in rabbit. A cysteine residue was added to the N terminus of the latter peptide for conjugation to keyhole limpet hemocyanin. B and C, peptide absorption tests of the 36-kDa protein with immunoblots using serum IgG from a patient with CIDP. The immunoreactivity was completely blocked by the P0–2 peptide but not by P0–1 or a control non-related 21-aa peptide (NR; B). Only P0–2-4 absorbed the immunoreactivity of the 36-kDa band; P0–2-1, -2, -3, and -5 did not (C). The positive control (−) consisted of a sample containing no peptide. D, Western blotting using rabbit anti-L-MPZ produced by immunization with the synthetic peptide shown in A. The same 36-kDa band was recognized by the patient serum (Pt) and anti-L-MPZ (L-MPZ). The negative control (Cntrl) in each case had no primary antibody. Western blots of ScN whole homogenate had 10 μg protein/lane (B and C) or 5 μg protein/lane (D). M, marker.
Production of Both L-MPZ and P0 from Human P0 cDNA
The P0 gene is relatively small (7 kb) and consists of six exons (1). Exon 6 contains both stop codons (Fig. 3A). Therefore, we considered it unlikely that L-MPZ is translated from a splice variant. To test whether L-MPZ was produced from P0 mRNA, we analyzed NIH/3T3 cells transfected with a P0 expression vector containing full-length human P0 cDNA. After electroporation, the human P0 protein was transiently expressed in the NIH/3T3 cells (Fig. 5A). Both glycosylated and deglycosylated (by PNGase F) L-MPZ bands were detected in lysates from transfected NIH/3T3 cells with Western blotting using anti- L-MPZ (Fig. 5B).
FIGURE 5.
Production of both L-MPZ and P0 from transfected human P0 cDNA. A, Western blots using anti-P0 antibody or a negative control without primary antibody (Cntrl) of lysates (10 μg) from NIH/3T3 cells transfected with human P0 (hP0) or a vector control (Vec). While nonspecific band (arrowhead) was seen in each sample, P0 was detected only in cells transfected with human P0. B, glycosylated and deglycosylated (by PNGase F) L-MPZ bands were detected in lysates from human P0-transfected NIH/3T3 cells. Cntrl, negative control without primary antibody. GAPDH, loading control. M, marker.
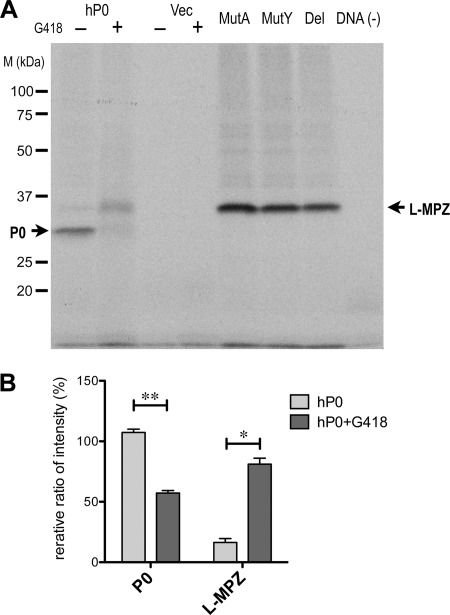
Aminoglycosides such as G418 are reported to induce stop codon readthrough in Escherichia coli, yeast, and human cultured cells (23, 24). To confirm the stop codon readthrough of P0 mRNA, we performed in vitro transcription/translation in the presence of G418 using a mammalian in vitro transcription/translation system. For effective stop codon readthrough in in vitro translation, 5 μg/ml G418 is sufficient (25). Synthesized [35S]methionine-labeled proteins were detected by autoradiography of SDS-PAGE gel (Fig. 6A). Strong band of unglycosylated P0 with the leading peptide (predicted MW: ∼27.5 kDa) was detected in the sample from the in vitro transcription/translation of human P0 cDNA without G418. Unglycosylated L-MPZ with the leading peptide (predicted MW: ∼34.4 kDa) was also detected at low levels in the fraction not treated with G418. A dramatic increase of L-MPZ was detected in the sample with human P0 cDNA in the presence of G418. The relative ratio of P0 and L-MPZ were calculated from the intensity of each band with or without G418 treatment (without G418, P0 107.3 ± 2.8, L-MPZ 16.3 ± 3.3; with G418, P0 57.1 ± 2.2, L-MPZ 81.1 ± 4.9) (%) (Fig. 6B). Next, to demonstrate P0 stop codon readthrough producing L-MPZ, three types of readthrough model mutants as L-MPZ, deletion or replacement (Ala or Tyr) of P0 stop codon in hP0/pcDNA3, were used in in vitro translation. All three types of vectors produced the bands corresponding to unglycosylated L-MPZ with the leading peptide (Fig. 6A). These results indicate that both P0 and L-MPZ were derived from P0 mRNA, possibly via stop codon readthrough. Thus, L-MPZ is probably produced in P0-expressing Schwann cells in vivo by the same mechanism.
FIGURE 6.
Synthesis of L-MPZ by in vitro transcription/translation using human P0 cDNA and G418. A, autoradiography image of the in vitro transcription/translation reaction mixture (2 μl/lane; 100–200 μg endogenous protein) on SDS-PAGE gel (10.5%) using [35S]methionine-labeling. P0 was detected in samples with the human P0 vector (hP0/pcDNA3; hP0) without G418. Increased translation of L-MPZ was detected in the hP0 sample with G418. All of three types of readthrough model mutant molecules as L-MPZ, deletion or replacement (Ala or Tyr) of P0 stop codon in hP0/pcDNA3 were detected (Del, MutA, MutY). B, quantitative analysis of the relative ratio of P0 and L-MPZ bands intensity with or without G418. Significantly, P0 was decreased in half (**: < 0.0001) and L-MPZ was increased 5 times (*: < 0.001) by G418 treatment. Statistical analysis was performed using a two-way ANOVA (PRISM 5) followed by Bonferroni multiple comparison test of 4 samples. Ratio standard was intensity of one of P0 band in hP0 without G418. Vec, transcription/translation mixture using the control vector (pcDNA3); DNA(−), transcription/translation mixture without template DNA.
Molecular Structure of L-MPZ
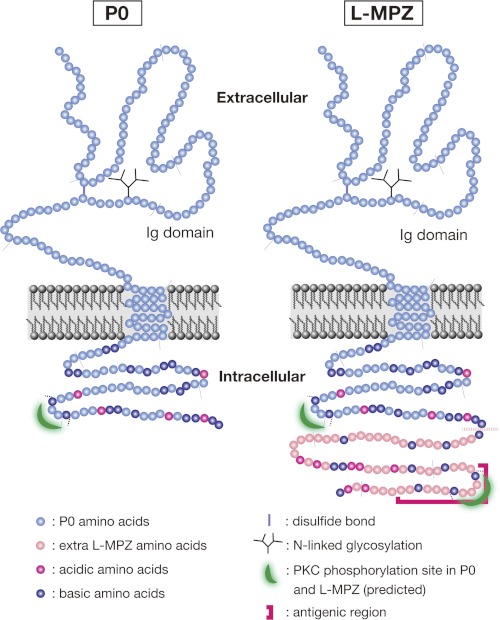
The predicted structure of L-MPZ, based on data from Western blotting, MS analysis, and the transfection study, is depicted in Fig. 7. According to the results from the deglycosylation assay and MS analysis, most of the structure of L-MPZ seems to be identical to that of P0; the N-terminal extracellular region contains a disulfide bond, an Ig-like domain, and N-linked glycosylation. There is a transmembrane region and a C-terminal cytoplasmic region that contains a putative PKC-dependent phosphorylation site. We found that L-MPZ has an extra 63 aa at the C terminus of P0, which are located in the cytoplasm. This additional region is highly basic and may contain an extra PKC-dependent phosphorylation site, which is in the antigenic site. No other known motifs or domains were found in the L-MPZ-specific extra region.
FIGURE 7.
Schematic illustration of the structure of P0 and L-MPZ. Most of the L-MPZ structure is identical to that of P0. The cytoplasmic domain of P0 is highly basic (pI 11) and includes one PKC target motif. The L-MPZ-specific portion extends from the P0 C terminus, is also highly basic (pI 10), and contains one additional predicted PKC phosphorylation site in the antigenic region. Faint gray lines are drawn every 20 aa.
Developmental Expression and Localization of L-MPZ in the PNS
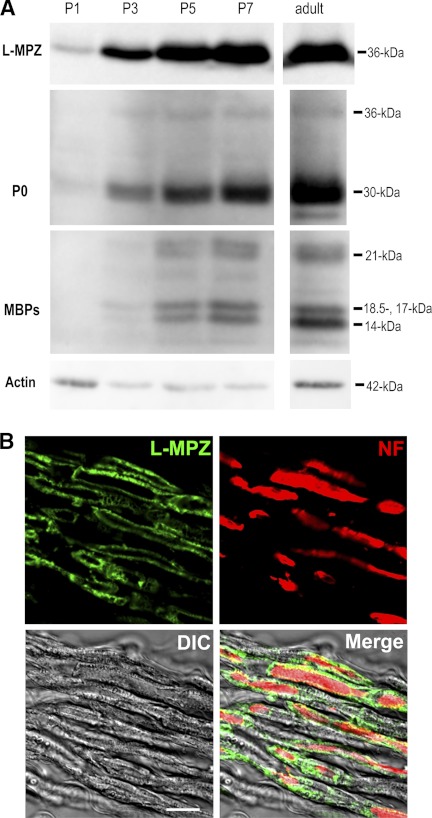
L-MPZ was enriched in the adult ScN myelin fraction and was translated from P0 mRNA. Because the expression of P0 protein is dramatically increased during early postnatal development, we next investigated the change in L-MPZ expression by stop codon readthrough during ScN development. L-MPZ and PNS myelin proteins (P0 and MBP-splice variants) were detected with Western blotting. In myelin fractions obtained during the various developmental stages, L-MPZ was detected from P1, and expression increased with age, similar to the major PNS myelin proteins (Fig. 8A). Because myelinogenesis is active around P7 in the rat PNS, up-regulation of L-MPZ in 5–7-day-old rat ScN myelin may be involved in the maturation of myelinated fibers.
FIGURE 8.
Production and localization of L-MPZ in the rat ScN. A, Western blot analysis of rat ScN whole homogenates (10 μg protein/lane) from P1, 3, 5, and 7 and adult rats. The primary antibodies were specific for L-MPZ, P0, and MBP-splice variants (MBPs; 14-, 17-, 18.5-, 21-kDa). Expression of L-MPZ began to increase at P3, in parallel with the expression patterns of the major PNS myelin proteins (P0 and MBP). Comparative control, β-actin (actin). B, distribution of L-MPZ in the adult rat ScN. Double immunostaining of longitudinal ScN sections was performed using anti-L-MPZ (green) and anti-NF (red) antibodies. L-MPZ-positive signals were detected specifically in myelin that ensheathed NF-positive axons. Scale bar: 10 μm.
To investigate the localization of L-MPZ in the PNS, double immunostaining of adult rat longitudinal ScN sections was performed using anti- L-MPZ (green) and anti-NF (red; Fig. 8B) antibodies. L-MPZ -positive signals were detected specifically in the myelin, which ensheathed NF-positive axons. Thus, L-MPZ is localized in the compact myelin of the PNS.
Cellular Localization of L-MPZ and P0 in Transfected HeLa Cells
During myelination, P0 is targeted to the plasma membrane, and homophilic binding of extracellular Ig-like domains mediates intermembrane adhesion to form compact myelin (2). In the HeLa cell culture system, forcibly expressed P0 protein is localized to the plasma membrane, especially at sites of close apposition between two P0-expressing cells (26). To investigate the cellular localization of L-MPZ and P0, we performed double immunostaining of full-length P0 cDNA-transfected HeLa cells using anti-L-MPZ and anti-P0 antibodies. L-MPZ and P0 immunoreactivities were colocalized (Fig. 9A, first row). While anti-L-MPZ can detect only L-MPZ, anti-P0 antibody detects mainly P0 but also slightly detects L-MPZ in Western blot (Fig. 2A). Therefore, to confirm the individual distribution of P0 and L-MPZ in transfected cells, five types of mutant (deletion or frameshift mutants to express only P0, deletion or replacement (Ala or Tyr) of P0 stop codon to express only L-MPZ-like molecules) were generated from full-length human P0 cDNA (Fig. 9B). The productions of these mutant molecules were confirmed by Western blotting using anti-P0 or anti- L-MPZ antibodies (data not shown). In immunocytofluorescence, both individual P0 and L-MPZ-like molecules were detected in plasma membrane (Fig. 9A, second-6th rows). Especially, just like P0, L-MPZ-positive signals were accumulated at sites of cell-cell adhesion between two L-MPZ-like mutant-expressing cells (Fig. 9A, 4th-6th rows). These results indicate that L-MPZ may also contribute to cell adhesion.
DISCUSSION
In this study, we show that the highly antigenic 36-kDa neuropathy-associated protein, designated L-MPZ, is a novel isoform of P0 that contains an additional 63 aa at the C terminus of P0. This protein is probably produced by translational readthrough of the regular P0 stop codon, because L-MPZ was produced by the identical P0 mRNA in conjunction with P0 in transfected mammalian cells and because P0 peptides and peptides with the deduced aa sequence in the 3′-UTR of P0 are both found with MS analysis of tryptic L-MPZ fragments. Stop codon readthrough was also shown by the great increase in L-MPZ synthesis with mammalian in vitro transcription/translation system in the presence of G418. P0 stop codon readthrough model mutants those only produced L-MPZ-like proteins were also synthesized by mammalian in vitro transcription/translation system. Like P0, L-MPZ was expressed during myelinogenesis, localized in compact myelin of the PNS, and accumulated at cell-cell adhesion sites in transfected cells, suggesting that L-MPZ is involved in the formation and/or maintenance of compact myelin, similar to P0. Because the L-MPZ-specific region contains an additional putative PKC phosphorylation site and is highly antigenic, L-MPZ may also have some unique roles in normal and pathological myelin conditions.
There was a high degree of homology at the aa level among the L-MPZ-specific coding regions in P0 mRNA from mammals and X. laevis (>80% identity in mammals and ∼48% identity in X. laevis), but such homologies were not seen in zebrafish or shark P0 mRNA (data not shown). These findings suggest that evolutionarily, P0 may have acquired the L-MPZ-specific region by stop codon readthrough after the evolutionary progression from amphibians. Regarding the CNS-specific myelin PLP and its alternatively spliced isoform DM20, it is believed that ancestral DM20 isoforms appeared in cartilaginous fish and evolved into PLP by the acquisition of the PLP-specific 35-aa region during the evolutionary progression from lobe-finned fish to amphibians (27). Phylogenetically, ancestral P0 is found in both the CNS and PNS from cartilaginous fish to some amphibians, and P0 became a PNS-specific protein after the evolution of amphibians (27, 28). Therefore, during the same course of evolution of PLP, acquisition of the L-MPZ-specific region may have been required for PNS-specific distribution and function in PNS myelin.
The main function of P0 is the formation and stabilization of the multilamellar membrane structure of compact myelin. Homophilic binding of extracellular Ig domains between face-to-face molecules is required for compaction. Our data suggest that L-MPZ may have the same extracellular structure (Fig. 7) that is involved in homophilic and/or L-MPZ/P0 heterophilic adhesion. PKC-mediated phosphorylation of specific residues (RSTK) in the cytoplasmic domain of P0 (Figs. 3B and 7) is necessary for P0-mediated adhesion, and alterations in this process can cause demyelinating neuropathy in humans (4). Because L-MPZ contains an additional predicted putative PKC phosphorylation site (RTSLK) in the cytoplasmic L-MPZ-specific region extending from the P0 C terminus (Fig. 3B and Fig. 7), phosphorylation of the L-MPZ-specific region may influence P0-mediated adhesion during myelin formation.
More than 120 mutations in the P0 gene have been identified in patients with hereditary neuropathies. These mutations and polymorphisms have been found in both the extracellular and intracellular regions (5). If L-MPZ has a similar structure as P0 and is involved in myelination, mutations in specific regions of L-MPZ may also modulate the adhesion and compaction of PNS myelin and cause cryptogenic neuropathies. Careful and large-scale screening of mutations or polymorphisms in the L-MPZ -specific region of the P0 gene may be important in understanding the spectrum of inherited neuropathies. The mechanism of anti-L-MPZ antibody production and its pathological role in neuropathies are still unclear. Since the epitope for the serum IgG of neuropathy patient is in the L-MPZ-specific intracellular domain and highly antigenic, myelin debris generated by PNS demyelination may induce the production of anti-L-MPZ IgG in patients with neuropathies.
Stop codon readthrough is a process that increases the diversity of genomic information. This mechanism is well documented with respect to viruses (11), yeast (for review, 12), and Drosophila (13–15). Recently, enhancing stop codon readthrough artificially with aminoglycosides has been examined for the treatment of many inherited diseases caused by genes containing premature termination codons generated by mutations or abnormal splicing, such as cystic fibrosis and Duchenne muscular dystrophy (for review, 29). In contrast, so far, stop codon readthrough has not been reported under normal conditions in humans and other mammals. P0-related 36-kDa protein believed to be L-MPZ has been detected under normal conditions in human, bovine, rat, and mouse PNS (6–9). Because the aa sequence of the specific region of L-MPZ is highly conserved from frogs to humans (Fig. 3A), it is not simply due to an accidental misreading of the stop codon. Therefore, stop codon readthrough may be essential for modulating the function of regular-sized proteins in mammals. One possible process of stop codon readthrough at L-MPZ regular stop codon is natural nonsense suppression by natural suppressor tRNA. A valiety of naturally occurring suppressor tRNAs were found in higher eukaryotes including mammals (for review, 30). Viruses use these suppressor tRNAs for the translational readthrough permitting the differential production of more than one polypeptide from a single gene in host eukaryotes. One of eukaryotic natural suppressor tRNA for UAG (amber) stop codon is tRNATyr. If L-MPZ was produced in natural suppressor tRNA system, L-MPZ might be synthesized by such suppressor tRNATyr, like one of mutant molecules produced by MutY_L-MPZ (Figs. 6A and 9A). In addition, nonsense suppression using suppressor tRNA is enhanced by G418 in mammalian culture cells (31). Because L-MPZ synthesis with in vitro transcription/translation was dramatically increased by G418, L-MPZ may be produced by the suppression of the regular P0 stop codon. On the other hand, because G418 enhanced the production of L-MPZ in vitro, it will be necessary to consider the effects of aminoglycosides and other readthrough enhancer reagents on the normal readthrough of proteins in vivo.
Further analysis focusing on L-MPZ is now in progress to improve diagnosis and develop better treatment for peripheral neuropathies, as well as to determine its functional role in normal and pathological conditions of PNS myelin. Further, because this is the first report describing stop codon readthrough in a common mammalian protein, detailed analysis of L-MPZ expression may yield greater understanding of the mechanism of stop codon readthrough in mammals.
Acknowledgments
We thank the patient for participating in this study and Dr. Kiyoshi Hayasaka for providing a human P0 cDNA clone. We also acknowledge the help of our laboratory members, especially Y. Naito, A. Nagata, T. Kikukawa, and R. Yamazaki.
This research was supported by a Japanese Health and Labor Sciences Research Grant for Research on Psychiatry and Neurological Diseases and Mental Health (H20-015), and the Promotion and Mutual Aid Corporation for Private Schools of Japan.
- P0 or MPZ
- myelin protein zero
- L-MPZ
- large myelin protein zero
- PNS
- peripheral nervous system
- aa
- amino acid
- ScN
- sciatic nerve
- CIDP
- chronic inflammatory demyelinating polyneuropathy
- P
- postnatal day
- 2DE
- two-dimensional gel electrophoresis
- CTAB
- cetyltrimethylammonium bromide
- PNGase
- Peptide:N-glycosidase
- NDS
- normal donkey serum
- MBP
- myelin basic protein
- PLP
- myelin proteolipid protein.
REFERENCES
- 1. Kirschner D., Laurence W., Feltri M. (2004) Myelin Biology and Disorders, pp. 523–545, Elsevier Academic Press, San Diego, CA [Google Scholar]
- 2. Filbin M. T., Walsh F. S., Trapp B. D., Pizzey J. A., Tennekoon G. I. (1990) Role of myelin P0 protein as a homophilic adhesion molecule. Nature 344, 871–872 [DOI] [PubMed] [Google Scholar]
- 3. Wong M. H., Filbin M. T. (1994) The cytoplasmic domain of the myelin P0 protein influences the adhesive interactions of its extracellular domain. J. Cell Biol. 126, 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu W., Shy M., Kamholz J., Elferink L., Xu G., Lilien J., Balsamo J. (2001) Mutations in the cytoplasmic domain of P0 reveal a role for PKC-mediated phosphorylation in adhesion and myelination. J. Cell Biol. 155, 439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shy M. E. (2006) Peripheral neuropathies caused by mutations in the myelin protein zero. J. Neurol. Sci. 242, 55–66 [DOI] [PubMed] [Google Scholar]
- 6. Nobile-Orazio E., Manfredini E., Sgarzi M., Spagnol G., Allaria S., Quadroni M., Scarlato G. (1994) Serum IgG antibodies to a 35-kDa P0-related glycoprotein in motor neuron disease. J. Neuroimmunol. 53, 143–151 [DOI] [PubMed] [Google Scholar]
- 7. Meléndez-Vásquez C. V., Gregson N. A. (1998) Characterization and partial purification of a novel 36 kDa peripheral myelin protein recognized by the sera of patients with neurological disorders. J. Neuroimmunol. 91, 10–18 [DOI] [PubMed] [Google Scholar]
- 8. Ishida K., Takeuchi H., Takahashi R., Yoshimura K., Yamada M., Mizusawa H. (2001) A possible novel isoform of peripheral myelin P0 protein: a target antigen recognized by an autoantibody in a patient with malignant lymphoma and peripheral neuropathy. J. Neurol. Sci. 188, 43–49 [DOI] [PubMed] [Google Scholar]
- 9. Favereaux A., Lagueny A., Vital A., Schmitter J. M., Chaignepain S., Ferrer X., Labatut-Cazabat I., Vital C., Petry K. G. (2003) Serum IgG antibodies to P0 dimer and 35 kDa P0-related protein in neuropathy associated with monoclonal gammopathy. J. Neurol. Neurosurg. Psychiatry. 74, 1262–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Besançon R., Prost A. L., Konecny L., Latour P., Petiot P., Boutrand L., Kopp N., Mularoni A., Chamba G., Vandenberghe A. (1999) Alternative exon 3 splicing of the human major protein zero gene in white blood cells and peripheral nerve tissue. FEBS Lett. 457, 339–342 [DOI] [PubMed] [Google Scholar]
- 11. Valle R. P., Drugeon G., Devignes-Morch M. D., Legocki A. B., Haenni A. L. (1992) Codon context effect in virus translational readthrough. A study in vitro of the determinants of TMV and Mo-MuLV amber suppression. FEBS Lett. 306, 133–139 [DOI] [PubMed] [Google Scholar]
- 12. von der Haar T., Tuite M. F. (2007) Regulated translational bypass of stop codons in yeast. Trends Microbiol. 15, 78–86 [DOI] [PubMed] [Google Scholar]
- 13. Steneberg P., Englund C., Kronhamn J., Weaver T. A., Samakovlis C. (1998) Translational readthrough in the hdc mRNA generates a novel branching inhibitor in the drosophila trachea. Genes Dev. 12, 956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steneberg P., Samakovlis C. (2001) A novel stop codon readthrough mechanism produces functional Headcase protein in Drosophila trachea. EMBO Rep. 2, 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stark A., Lin M. F., Kheradpour P., Pedersen J. S., Parts L., Carlson J. W., Crosby M. A., Rasmussen M. D., Roy S., Deoras A. N., Ruby J. G., Brennecke J., Harvard FlyBase curators, Berkeley Drosophila Genome Project, Hodges E., Hinrichs A. S., Caspi A., Paten B., Park S. W., Han M. V., Maeder M. L., Polansky B. J., Robson B. E., Aerts S., van Helden J., Hassan B., Gilbert D. G., Eastman D. A., Rice M., Weir M., Hahn M. W., Park Y., Dewey C. N., Pachter L., Kent W. J., Haussler D., Lai E. C., Bartel D. P., Hannon G. J., Kaufman T. C., Eisen M. B., Clark A. G., Smith D., Celniker S. E., Gelbart W. M., Kellis M. (2007) Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature 450, 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatfield D., Thorgeirsson S. S., Copeland T. D., Oroszlan S., Bustin M. (1988) Immunopurification of the suppressor tRNA dependent rabbit beta-globin readthrough protein. Biochemistry 27, 1179–1183 [DOI] [PubMed] [Google Scholar]
- 17. Chittum H. S., Lane W. S., Carlson B. A., Roller P. P., Lung F. D., Lee B. J., Hatfield D. L. (1998) Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry 37, 10866–10870 [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi Y., Miyagi Y., Baba H. (2008) Two-dimensional electrophoresis with cationic detergents, a powerful tool for the proteomic analysis of myelin proteins. Part 1: technical aspects of electrophoresis. J. Neurosci. Res. 86, 755–765 [DOI] [PubMed] [Google Scholar]
- 19. Werner H. B., Kuhlmann K., Shen S., Uecker M., Schardt A., Dimova K., Orfaniotou F., Dhaunchak A., Brinkmann B. G., Möbius W., Guarente L., Casaccia-Bonnefil P., Jahn O., Nave K. A. (2007) Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J. Neurosci. 27, 7717–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freeze H. H., Kranz C. (2010) Curr. Protoc. Protein Sci., John Wiley & Sons, New York, NY [Google Scholar]
- 21. Yamaguchi Y., Miyagi Y., Baba H. (2008) Two-dimensional electrophoresis with cationic detergents: a powerful tool for the proteomic analysis of myelin proteins. Part 2: analytical aspects. J. Neurosci. Res. 86, 766–775 [DOI] [PubMed] [Google Scholar]
- 22. Hayasaka K., Nanao K., Tahara M., Sato W., Takada G., Miura M., Uyemura K. (1991) Isolation and sequence determination of cDNA encoding the major structural protein of human peripheral myelin. Biochem. Biophys. Res. Commun. 180, 515–518 [DOI] [PubMed] [Google Scholar]
- 23. Burke J. F., Mogg A. E. (1985) Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 13, 6265–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin R., Mogg A. E., Heywood L. A., Nitschke L., Burke J. F. (1989) Aminoglycoside suppression at UAG, UAA, and UGA codons in Escherichia coli and human tissue culture cells. Mol. Gen. Genet. 217, 411–418 [DOI] [PubMed] [Google Scholar]
- 25. Manuvakhova M., Keeling K., Bedwell D. M. (2000) Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA 6, 1044–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doyle J. P., Stempak J. G., Cowin P., Colman D. R., D'Urso D. (1995) Protein zero, a nervous system adhesion molecule, triggers epithelial reversion in host carcinoma cells. J. Cell Biol. 131, 465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida M., Colman D. R. (1996) Parallel evolution and coexpression of the proteolipid proteins and protein zero in vertebrate myelin. Neuron 16, 1115–1126 [DOI] [PubMed] [Google Scholar]
- 28. Rotenstein L., Herath K., Gould R. M., de Bellard M. E. (2008) Characterization of the shark myelin Po protein. Brain Behav. Evol. 72, 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linde L., Kerem B. (2008) Introducing sense into nonsense in treatments of human genetic diseases. Trends Genet. 24, 552–563 [DOI] [PubMed] [Google Scholar]
- 30. Beier H., Grimm M. (2001) Misreading of termination codons in eukaryotes by natural nonsense suppressor tRNAs. Nucleic Acids Res. 29, 4767–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillips-Jones M. K., Hill L. S., Atkinson J., Martin R. (1995) Context effects on misreading and suppression at UAG codons in human cells. Mol. Cell Biol. 15, 6593–6600 [DOI] [PMC free article] [PubMed] [Google Scholar]