Abstract
This study investigated the in vivo properties of two heavy chain antibody fragments (VHH), ni3A and pa2H, to differentially detect vascular or parenchymal amyloid-β deposits characteristic for Alzheimer's disease and cerebral amyloid angiopathy. Blood clearance and biodistribution including brain uptake were assessed by bolus injection of radiolabeled VHH in APP/PS1 mice or wildtype littermates. In addition, in vivo specificity for Aβ was examined in more detail with fluorescently labeled VHH by circumventing the blood-brain barrier via direct application or intracarotid co-injection with mannitol. All VHH showed rapid renal clearance (10–20 min). Twenty-four hours post-injection 99mTc-pa2H resulted in a small yet significant higher cerebral uptake in the APP/PS1 animals. No difference in brain uptake were observed for 99mTc-ni3A or DTPA(111In)-pa2H, which lacked additional peptide tags to investigate further clinical applicability. In vivo specificity for Aβ was confirmed for both fluorescently labeled VHH, where pa2H remained readily detectable for 24 hours or more after injection. Furthermore, both VHH showed affinity for parenchymal and vascular deposits, this in contrast to human tissue, where ni3A specifically targeted only vascular Aβ. Despite a brain uptake that is as yet too low for in vivo imaging, this study provides evidence that VHH detect Aβ deposits in vivo, with high selectivity and favorable in vivo characteristics, making them promising tools for further development as diagnostic agents for the distinctive detection of different Aβ deposits.
Introduction
Besides neurofibrillary tangles, Alzheimer's disease (AD) is characterized by cerebral deposition of β-amyloid (Aβ) in so-called senile or diffuse plaques [1]. Similar vascular deposits of Aβ associated with cerebral amyloid angiopathy (CAA) lead to loss of vessel wall integrity increasing the risk of brain haemorrhages [2]. Present in 30% of the non-demented population over 60 years of age, CAA co-exists in 90% of the AD patients and forms an important complication in the development of immunotherapeutic strategies [3]–[5]. Although, the exact role of Aβ regarding the underlying pathogeneses remains unsolved, accumulation is believed to start 20–30 years prior to clinical onset [6], [7]. Distinctive in vivo detection of the different Aβ deposits therefore renders important knowledge regarding early diagnosis and preventive therapy development.
Currently, a gross differentiation can only be made based on the occipital predilection of CAA, while existing PET ligands, like 11C-PiB, target Aβ in its fibrillar amyloid form rather than specific vascular or parenchymal types of Aβ deposits [8].
Previously, we have selected heavy chain antibody fragments with high affinity specific for either CAA or all types of human Aβ deposits [9]. Derived from the Camelid heavy chain antibody repertoire, which completely lack light chains, their single N-terminal domain (VHH) is fully capable of antigen binding with affinities comparable with those of conventional antibodies [10], [11].
Blood-brain barrier (BBB) passage was shown to be favorable in an in vitro assay [12]; therefore, this study assessed the in vivo characteristics of two distinct Aβ targeting VHH, ni3A and pa2H, for their potential use to differentially detect AD and CAA. First, pharmacologic behaviour and biodistribution were examined after administration of radiolabeled VHH into a transgenic AD/CAA mouse model. Secondly, fluorescently labeled VHH were administered after the BBB was circumvented to evaluate their ability to specifically bind Aβ deposits in vivo.
Materials and Methods
Production of ni3A and pa2H
VHH ni3A and pa2H were selected from respectively a non-immune or an immune library created after immunisation with post-mortem brain parenchyma of a patient with Down's syndrome. VHH were subcloned and produced as previously reported including a myc- or VSV-tag for detection and a his-tag for purification [9]. Similarly, pa2H free of any additional peptide tags was commercially produced by overexpression in yeast (BAC, Leiden, the Netherlands).
Animal studies
All studies were performed using 12–16 month old transgenic mice or wildtype littermates from a colony set up using the APPswe/PS1dE9 strain (APP/PS1) (JAX), known to accumulate vascular and parenchymal Aβ deposits [13], and have been approved by the institutional Animal Ethics Committee (DEC) at the Leiden University Medical Center, permit number 09132. Besides standard genotyping, after each experiment amyloid pathology was confirmed by standard Thioflavin T staining.
Human material
Human brain tissue was obtained of AD/CAA patients or controls as confirmed by neuropathological examination in agreement with the guidelines of the ethics committee of the LUMC. Patient anonymity was strictly maintained. All tissue samples were handled in a coded fashion, according to Dutch national ethical guidelines (Code for Proper Secondary Use of Human Tissue, Dutch Federation of Medical Scientific Societies).
Murine specificity of the selected VHH
To evaluate appropriate use of the APP/PS1 mouse model, murine cryosections (10 µm) were stained according previous protocols [9], [12] with in addition a standard anti-mouse-to-mouse kit (ARK, Dako Cytomation). Final preparations were analyzed with an automated Pannoramic MIDI microscope (3DHistech).
Biodistribution and clearance
Radiolabeling
VHH were labeled according to two different protocols. First, his-tagged VHH were labeled directly with technetium-99m (99mTc) using a previously published protocol [14]. Briefly, 20 µl of VHH in PBS solution (450–500 ng/µl) was added to 8 µl of an aseptic mixture of 950 mg/l Sn(Cl)2.2H2O and 2 g/l Na4P2O7.10H2O (Technescan PYP, Covidien, Petten, the Netherlands) in saline. After addition of 4 µl of 10 mg/ml of KBH4 (crystalline, Sigma Chemical Co, St. Louis, MO) in 0.1 M NaOH, and 100 µl of Na[99mTcO4] solution (approximately 200–700 MBq/ml, Technekow, Covidien, Petten, the Netherlands) the mixture was gently stirred at room temperature for at least 30 min before use. Analysis of the labeling solution, referred to as 99mTc-VHH, yielded a radiochemical purity of >95% without detectable unreduced or free 99mTcO4 [15].
Secondly, untagged VHH were chelated for indium-111 (111In) using diethylene triamine penta-acetic acid (DTPA). Untagged pa2H was chelated in a total volume of 1.0 ml with 20-fold molecular excess of p-SCN-Bn-DTPA (Macrocyclics, Dallas, TX) at pH 8.5 in phosphate buffer for 5 hr at 37.5°C and purified by dialysis using phosphate buffered saline (PBS). 111In chloride (25 µl, 111 MBq/ml, Covidien, the Netherlands) was added to DTPA-pa2H conjugate (0.1 ml) in 0.25 M ammonium acetate buffer (0.8 ml) at pH 5.5 and incubated for 1 hr at room temperature. The reaction was quenched with 50 mM ethylene diamine tetra-acetic acid (EDTA) (50 µl) to chelate residual non-bound 111In and the radiolabeled antibody was then purified using a Sephadex™ G-25 column (PD 10; GE Healthcare) eluted with PBS. Radiochemical purity assessed by instant thin layer chromatography (ITLC) yielded a purity of >95%.
Biodistribution and brain uptake
To study the biodistribution, animals were injected intravenously with 0.2 ml radiolabeled VHH diluted with saline (5–10 MBq/ml, 10 µg/ml). At different intervals (t = 3–6–24 hrs) post-injection APP/PS1 (n = 4) and wildtype animals (n = 4) were sacrificed (Euthanasol, AST Pharma). Similar biodistribution experiments using untagged DTPA(111In)-pa2H were only performed at 24 hours post-injection for APP/PS1 (n = 6) and wildtype mice (n = 6). Blood was collected via cardiac puncture, and various organs were removed, including the brain, which was divided into the cerebrum and cerebellum. All were weighed and counted for radioactivity (Wizard2, Perkin Elmer). After decay correction, radioactivity was expressed as the percentage of the total injected dose of radioactivity per gram tissue (%ID/g). Blood/cerebrum ratios were calculated to correct for possible confounding effects accountable by residual blood. Similarly, muscle/cerebrum determined target-to-non-target ratios. Differences were regarded significant when p≤0.05 using an unpaired one or two tailed t-test. Experiments at t = 24 hrs were repeated twice using 99mTc-pa2H.
Blood clearance and analysis
Simultaneously, blood half-life was examined by collecting 5 µl tail samples at several time points between 3–90 minutes post-injection of radiolabeled VHH into transgenic or wildtype mice (n = 4–6). Combined with the cardiac blood samples corresponding half-lives were calculated using GraphPad Prism.
Similarly, 10 µl samples obtained at 10 and 90 minutes post-injection were mixed with 90 µl heparin (34 U/ml saline) and 900 µl PBS. Centrifugation for 10 minutes at 7,000 rpm separated plasma from the cell pellet. Radioactivity was measured separately to determine the blood distribution of radiolabeled VHH over time.
Specificity of radiolabeled pa2H
Aβ specificity of pa2H-his after 99mTc-radiolabeling was tested by quantitative competition autoradiography. Human and murine brain cryosections (20 µm) were blocked with 1% bovine serum albumin (BSA)/PBS at 37°C for 1 hour followed by similar application of the labeling solution, which was diluted to 1 µg/ml by 1%BSA/PBS with or without additional 1 hour pre-incubation with excess monomeric or fibrillar Aβ1–40 (rPeptide) at 37°C. Fibrils were produced using existing protocols [16].
After rinsing 3 times with PBS, radioactivity was counted for 15 minutes by a gamma camera (Toshiba GCA7100/UI). A similar region of interest was fitted for each scintigram to assess binding of 99mTc-pa2H-his with 0.1 ml of diluted labeling solution as a reference. Binding was expressed as the % of radioactivity compared to the section without any competitor. Experiments were performed in triplicate.
In vivo Aβ targeting by VHH
Fluorescent labeling
Tagged VHH were fluorescently labeled with Alexa Fluor 594 protein labeling kit (Molecular Probes, Invitrogen) according to the manufacturer's guidelines, except using only half of the recommended amount of dye. Briefly spun to remove possible aggregates, extensive dialysis removed excess free label. The labeling degree and protein concentration (200–600 ng/µl) were determined using the Nanodrop ND1000 (Isogen Life Sciences). Protein integrity was confirmed by mass spectrometry.
Immunofluorescence using VHH-Alexa594
To examine whether the fluorescent labeling affected antigen recognition, human and murine cryosections (10 µm) were rinsed with PBS, fixed in ice-cold acetone for 10 minutes before overnight incubation with VHH- Alexa594 in 1% BSA/PBS in a wet chamber. Washed 3×5 minutes with PBS, sections were mounted and analyzed using a fluorescence microscope (Leica DMR5500B).
In vivo Aβ imaging by topical application
Four APP/PS1 animals received permanent cranial windows to allow serial in vivo imaging of the brain by multiphoton microscopy. Animals were anaesthetized using 2% isoflurane gas inhalation, and the exposed skull was partly replaced by a round glass coverslip glued into place using Krazyglue® according to previous surgical protocols [17], [18]. Prior to fixation of the cranial window, a drop of 40–60 µl of VHH-Alexa594 (275–400 ng/µl) was applied directly onto the exposed brain for 30 minutes and briefly rinsed with PBS. Colocalization with the Aβ deposits was based either upon their typical green autofluorescence or by intraperitoneal injections of Methoxy-X04 one day prior surgery [16]. Animals were imaged immediately following surgery, which was typically less than 90 minutes after beginning of the procedure, and re-imaged under isoflurane anaethesia (2%) for several days to study the washout. Images were acquired with a Bio-Rad 1024 multiphoton microscope equipped with a Ti:Sapphire laser (Mai Tai, Spectra Physics) and external photodetectors (Hamamatsu Photonics). Areas were imaged to approximately 200 µm deep in 5 µm steps with a 20× objective (UMPlanFl, NA = 0.95; Olympus). Maximum intensity projections were reconstructed using ImageJ.
Specific in vivo Aβ binding after BBB disruption
A systemic approach to study the in vivo behaviour of the VHH throughout a larger area within the brain involved intracarotid infusion (60 µl/min) of 100 µl pa2H-his-Alexa594 along with 600 µl 15% mannitol selectively into the right carotic artery to disrupt the BBB [19]. At t = 2 and 24 hours post-injection., transgenic (n = 9) and wildtype animals (n = 3) were euthanized (Euthanasol, AST Pharma), and perfused with 4% paraformaldehyde (PFA). Resected brains were stored in 4% PFA with 10% sucrose for 4 hours followed by overnight fixation in 4% PFA with 30% sucrose. Next, the brains were snap frozen and sectioned completely to obtain consecutive 30-µm-thick cryosections. Besides standard Thioflavin T staining for amyloid, adjacent sections were immunostained for Aβ (6F/3D, DakoCytomation) [20] with 1∶100 goat-antimouse-Alexa488 (Invitrogen) to assess colocalization. Images obtained by a Leica DM5500B microscope were merged using Adobe Photoshop CS3.
Results
Murine specificity of the selected VHH
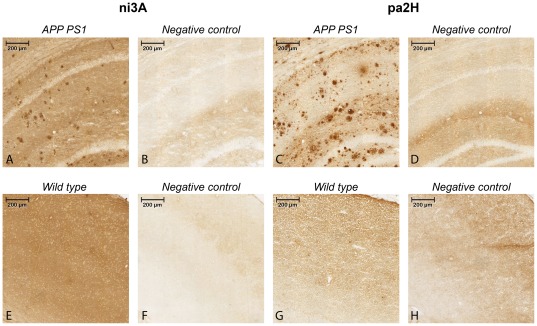
Immunostained brain sections of aged APP/PS1 and wildtype littermates using tagged VHH ni3A and pa2H were made to assess their capacity to selectively recognize different types of deposits. (Figure 1) Pa2H stained positive for all forms of Aβ depositions. In this transgenic mouse model, ni3A did not show selective affinity for vascular Aβ; both vascular and parenchymal Aβ depositions were clearly labeled. Compared to ni3A, equivalent staining protocols with pa2H resulted in higher specificity for Aβ combined with a low unspecific background binding. For neither VHH specific affinity was detected within the brain sections of wildtype animals.
Figure 1. Immunostaining on murine APP/PS1 sections using ni3A and pa2H.
The upper panels (A–D) show 10× magnifications of the resulting staining with cryosections of aged APP/PS1 mouse brain tissue including negative controls, while the lower panels (E–H) show similar staining performed with wildtype littermates.
Biodistribution and clearance
Biodistribution and brain uptake
The distribution of a bolus injection of radiolabeled tagged ni3A and pa2H over time is shown in Table 1 and 2. No significant differences in organ uptake between wildtype and transgenic animals were found, except for the brain uptake of 99mTc-pa2H after 24 hours. Although the amount was low (0.038%I.D./g), cerebral uptake was 40% higher in the transgenic animals. The cerebrum/blood ratio did not differ, indicating that this difference was not caused by different VHH concentrations within the blood pool. For the cerebellum similar results were found. Repeated experiments for this particular endpoint resulted in similar findings.
Table 1. Biodistribution of 99mTc-ni3A in mice.
| t = 3 hr | t = 6 hr | t = 24 hr | ||||
| Tissue/organ | Wildtypes | APP/PS1 | Wildtypes | APP/PS1 | Wildtypes | APP/PS1 |
| blood | 1.202±0.379 | 1.146±0.131 | 0.778±0.048 | 0.808±0.115 | 0.451±0.073 | 0.363±0.051 |
| heart | 0.525±0.129 | 0.508±0.109 | 0.347±0.049 | 0.337±0.127 | 0.252±0.041 | 0.216±0.014 |
| lungs | 0.850±0.184 | 0.819±0.208 | 0.659±0.184 | 0.743±0.301 | 0.375±0.113 | 0.291±0.055 |
| liver | 1.078±0.235 | 1.000±0.293 | 1.078±0.188 | 1.223±0.424 | 0.568±0.149 | 0.488±0.164 |
| kidneys | 15.531±2.986 | 15.192±3.075 | 10.266±1.657 | 14.294±4.337 | 9.089±6.152 | 9.901±1.158 |
| spleen | 0.590±0.257 | 0.531±0.084 | 0.792±0.144 | 0.753±0.291 | 0.397±0.056 | 0.465±0.234 |
| muscle | 0.171±0.075 | 0.120±0.069 | 0.111±0.088 | 0.086±0.022 | 0.043±0.007 | 0.048±0.008 |
| cerebrum | 0.035±0.009 | 0.035±0.007 | 0.031±0.007 | 0.035±0.008 | 0.018±0.003 | 0.019±0.001 |
| cerebellum | 0.073±0.031 | 0.063±0.009 | 0.098±0.009 | 0.096±0.014 | 0.029±0.005 | 0.026±0.002 |
| cerebrum/blood ratio | 0.030±0.003 | 0.030±0.004 | 0.040±0.010 | 0.043±0.004 | 0.040±0.001 | 0.053±0.008 * |
| cerebrum/muscle ratio | 0.242±0.142 | 0.335±0.116 | 0.407±0.270 | 0.428±0.171 | 0.422±0.029 | 0.403±0.100 |
= P<0.05 wildtype mice compared to APP/PS1 mice.
A bolus injection of 2 µg 99mTc-ni3A was administered intravenously into 12–14 month old APP/PS1 mice or their wild type littermates. At three time points after injection the animals were sacrificed and various tissues and entire organs were removed, weighed and counted for radioactivity. Values are expressed as a percentage of the injected dose per gram tissue (mean ± SD).
Table 2. Biodistribution of radiolabeled pa2H in mice.
| 99mTc-pa2H | DTPA(111In)-pa2H | |||||||
| t = 3 hr | t = 6 hr | t = 24 hr | t = 24 hr | |||||
| Tissue/organ | Wildtypes | APP/PS1 | Wildtypes | APP/PS1 | Wildtypes | APP/PS1 | Wildtypes | APP/PS1 |
| blood | 0.566±0.003 | 0.654±0.015 | 1.009±0.054 | 1.244±0.123 | 0.575±0.084 | 0.696±0.049 | 0.006±0.001 | 0.004±0.002 |
| heart | 0.273±0.121 | 0.240±0.017 | 0.623±0.101 | 0.763±0.031 | 0.367±0.059 | 0.393±0.007 | 0.017±0.084 | 0.014±0.003 |
| lungs | 0.843±0.256 | 0.537±0.010 | 0.930±0.242 | 1.088±0.035 | 0.620±0.160 | 0.622±0.031 | 0.016±0.011 | 0.014±0.003 |
| liver | 2.615±0.796 | 1.866±0.016 | 3.014±1.021 | 3.392±1.932 | 1.430±0.402 | 1.161±0.470 | 0.066±0.029 | 0.075±0.0.23 |
| kidneys | 9.243±1.787 | 6.241±0.530 | 14.306±4.105 | 15.612±1.042 | 9.824±2.810 | 8.608±0.738 | 8.859±3.623 | 7.689±2.930 |
| spleen | 1.515±0.503 | 1.319±0.060 | 6.498±1.623 | 6.258±0.208 | 3.584±1.381 | 1.747±0.100 | 0.044±0.022 | 0.048±0.006 |
| muscle | 0.356±0.379 | 0.054±0.006 | 0.174±0.022 | 0.347±0.026 | 0.102±0.023 | 0.113±0.018 | 0.059±0.020 | 0.059±0.044 |
| cerebrum | 0.014±0.003 | 0.017±0.001 | 0.033±0.005 | 0.044±0.004 | 0.027±0.004 | 0.038±0.002 * | 0.001±0.000 | 0.001±0.001 |
| cerebellum | 0.023±0.001 | 0.026±0.001 | 0.054±0.016 | 0.067±0.001 | 0.030±0.007 | 0.045±0.000 * | 0.003±0.001 | 0.002±0.001 |
| cerebrum/blood ratio | 0.025±0.005 | 0.026±0.003 | 0.033±0.004 | 0.035±0.004 | 0.047±0.003 | 0.055±0.008 | 0.013±0.011 | 0.041±0.055 |
| cerebrum/muscle ratio | 0.083±0.081 | 0.309±0.067 | 0.190±0.007 | 0.177±0.135 | 0.270±0.032 | 0.346±0.377 | 0.113±0.066 | 0.203±0.146 |
= P<0.05 wildtype mice compared to APP/PS1 mice.
A bolus injection of 2 µg radiolabeled pa2H was administered intravenously into 12–14 month old APP/PS1 mice or their wildtype littermates. At three or one time points after injection of radiolabeled pa2H respectively with or without additional peptide tags, the animals were sacrificed and various tissues and entire organs were removed, weighed and counted for radioactivity. Values are expressed as a percentage of the injected dose per gram tissue (mean ± SD).
To investigate whether these findings were not confounded by either the non-specific radiolabeling procedure or the presence of additional peptide tags, the biodistribution experiment was repeated with untagged DTPA(111In)-pa2H. (Table 2) With this labeling protocol, we no longer observed a significantly higher cerebral uptake in amyloid-bearing mice. Regardless of the tag, the majority of radiolabeled VHH was excreted via the kidneys. Cellular involvement as shown by distinctive hepatic clearance or splenal activity was low. In comparison to 99mTc-ni3A, 99mTc-pa2H showed about 3 times higher clearance via liver and spleen. Also, the clearance rate for 99mTc-pa2H was lower, independent of genotype. However, within the first 3 hours 99mTc-ni3A resulted in a higher general organ uptake, with exception of the aforementioned liver and spleen.
Blood clearance and analysis
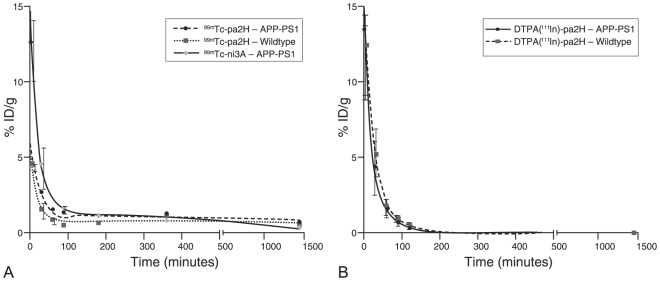
Blood clearance of the tagged 99mTc-VHH consisted of a fast and a slow component. (Figure 2A) In general, the majority of the radiolabeled VHH was cleared from the blood with a half-life of 10–20 minutes (Table 3). The actual half-life of the slow component of 99mTc- VHH could only be calculated with limited accuracy, since the half-life was longer than the blood sampling period. In line with the above biodistribution, six hours post-injection, the blood levels of 99mTc-pa2H were remarkably higher compared to earlier time points, which is characteristic for a second passage. Within the first 90 minutes about 80% of the 99mTc-VHH remained within the blood plasma, indicating that no significant cellular uptake occurred. (Table 4)
Figure 2. Blood clearance.
These graphs represent the blood half lives of tagged 99mTc-ni3A and -pa2H (A), and untagged DTPA(111In)-pa2H (B) in APP/PS1 mice and wildtype littermates. Data is shown as percentage of injected dose per gram of blood (%ID/g) over time. Based upon this plot the clearance is suggested to respectively consist of a fast and a slow phase, or only a single phase.
Table 3. Blood half lives of radiolabeled VHH.
| Fast t½ | Slow t½ | ||||||
| VHH | genotype | (min) | (95% C.I.) | % | (min) | % | (95% C.I.) |
| 99mTc-ni3A | APP/PS1 | 14.71 | (8.65–49.13) | 89.7 | 580 | 10.3 | (101.8–∞) |
| Wildtype | ND | ND | ND | ND | |||
| 99mTc-pa2H | APP/PS1 | 21.89 | (14.24–39.38) | 79.8 | 2562 | 20.2 | (975.0–∞) |
| Wildtype | 10.78 | (7.27–20.76) | 87.1 | 5861 | 12.9 | (969.3–∞) | |
| DTPA(111In)-pa2H | APP/PS1 | 19.69 | 12.63–44.60 | 100 | - | - | |
| Wildtype | 15.83 | 9.30–53.37 | 100 | - | - | ||
Half lives were determined by fitting a one or a two phase exponential decay model based on blood obtained from both tail vein and cardiac puncture at several time points after intravenous bolus injection of 2 µg radiolabeled VHH in 12–14 month old APP/PS1 mice and wildtype littermates, as depicted in Figure 2. Please note that DTPA(111In)-pa2H was produced without any additional peptide tags.
Table 4. Blood distribution of 99mTc-pa2H.
| Sample | Time p.i. | Fraction | APP/PS1 | Wildtype | ||
| (min) | (%) | sd | (%) | sd | ||
| 99mTc-pa2H | 10 | Plasma | 88,9 | 6,2 | 80,3 | 5,2 |
| Cell Pellet | 11,1 | 19,7 | ||||
| 90 | Plasma | 83,6 | 8,7 | 72,0 | 8,2 | |
| Cell Pellet | 16,4 | 28,0 | ||||
At different time point after bolus injection of 99mTc-pa2H blood collected from the tail vein of 12–14 month old APP/PS1 mice or wildtype littermates. Separated into the cell pellet and plasma, samples were counted for radioactivity. Fractions are expressed in percentage of total activity at that time point. No significant differences were calculated using a student t-test (p<0.05).
In contrast to tagged 99mTc- VHH, the blood clearance of untagged DTPA(111In)-pa2H was mono-exponential, with a similar rapid clearance within 20 minutes, but without a slow component. (Figure 2B)
Specificity of 99mTc-pa2H
After radiolabeling of the tagged pa2H it's specificity for Aβ was unaffected, as shown by scintigraphic analysis; binding of 99mTc-pa2H was higher in those sections including Aβ. (Table 5) Furthermore, binding was significantly (p<0.001) reduced when the tracer was pre-incubated with either monomeric or fibrillar Aβ.
Table 5. Quantitative autoradiography.
| Brain tissue | Binding of 99m Tc-VHH | Competion binding | |
| Monomeric Aβ | Fibrillar Aβ | ||
| ng (± sd) | ng (± sd) | ng (± sd) | |
| APP/PS1 | 98.8 (±20,7)* | 60.1 (±22.2) | 31.4 (±14.3) |
| Wildtype | 86.4 (±14.8) | 56.4 (±19.5) | 27.1 (±12.5) |
| AD human | 190.1 (±73.5)* | 81.4 (± N.D.) | 42.3 (±29.2) |
| Control human | 102.3 (±30.2) | 27.2 (± N.D.) | 49.9 (±17.4) |
Differences in radioactivity were measured after application of 1 µg 99mTc-pa2H to human and murine APP/PS1 brain sections.
Statistical difference (p<0.05) between either murine or human control versus Aβ bearing sections.
In vivo Aβ targeting by VHH
In vivo Aβ imaging by topical application
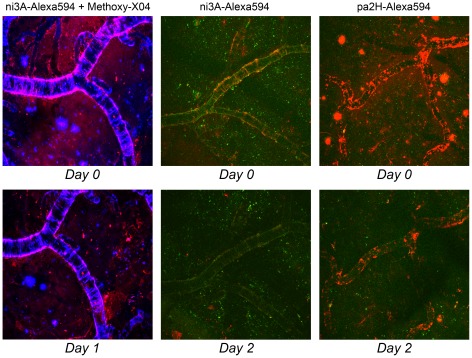
After direct application onto the exposed mouse brain, fluorescent VHH were followed up for at least 48 hours by in vivo multiphoton microscopy. (Figure 3) Specific in vivo labeling of Aβ plaques by ni3A-Alexa594 was initially confirmed by colocalization with Methoxy-X04, a known in vivo amyloid targeting fluorophore. Beside possible binding competition with the VHH, Methoxy-X04 hampered good validation due to signal cross-over into the red channel. However, colocalization based on the typical autofluorescence patterns of the different Aβ deposits resulted in similar findings. Selectivity was confirmed by lack of nonspecific background signal. Although both VHH were capable of targeting Aβ in vivo, only pa2H-Alexa594 was detectable after two days, mainly bound to vascular amyloid.
Figure 3. In vivo Aβ imaging after direct brain application.
Topical application of ni3A- or pa2H-Alexa594 (red) as visualized over time by intravital multiphoton microscopy in APP/PS1 mice clearly shows the specific in vivo labeling of different Aβ deposits. In the left, vascular and parenchymal Aβ deposits, detected by prior labeling with Methoxy-X04 (blue), colocalize with ni3A-Alexa594 (red) directly following topical application. One day later, labeling of the plaques has diminished to almost none with some residual left bound to CAA. With interpretation hampered by Methoxy-X04, middle images show a similar experiment. Colocalization with Aβ deposits based upon autofluorescence (green) gave comparable results and almost complete wash out after two days. Pa2H-Alexa594 (red), as shown in the right images, remains bound to vascular Aβ even two days after application, when the plaques remained undetected. All images are maximum intensity projections of a 3D cortical volume with a field of view 615×615 µm.
Specific in vivo Aβ binding after BBB disruption
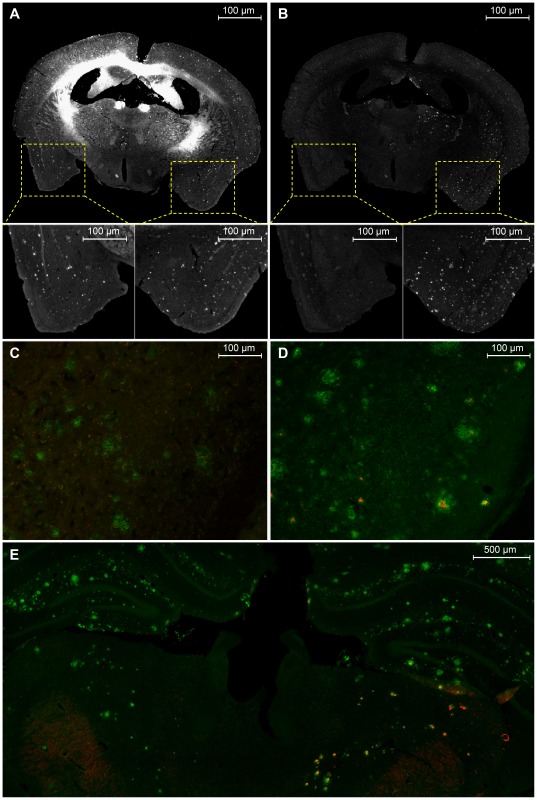
Based on the above findings, co-injections of pa2H-Alexa594 with mannitol were done in the right carotid artery to selectively open the BBB in the ipsilateral hemisphere to study the in vivo characteristics throughout the brain. Two hours post-injection, fluorescence was detected in the right hemisphere, co-localizing with Aβ. (Figure 4) Even within the deeper brain structures, no nonspecific binding was observed. Aβ related fluorescent signal remained detectable for at least 24 hours post-injection. Without BBB disruption or within wildtype littermates, no apparent Aβ labeling could be detected.
Figure 4. Specific in vivo Aβ binding after BBB disruption.
After disruption of the BBB using a co-injection of 15% mannitol with pa2H-Alexa594 into the right carotid artery of an aged APP/PS1 mouse sacrificed 2 hrs post injection, amyloid plaques are clearly depicted in both hemispheres using a Thioflavin T (ThT) staining (A), while the pa2H-Alexa594 signal is only detected in the right hemisphere (B). More careful examination shows all Alexa594 signal colocalizes with ThT in the right hemisphere, while in the left only some autofluorescense can be detected. Furthermore, immunofluorescense anti-Aβ staining of the plaques using Alexa488 within the left hemisphere (C) results only in green signal, while within the right hemisphere (D) the red signal from pa2H-Alexa594 nicely colocalizes within the plaques. Experiments performed in a similar setting but sacrificed 24 hrs post-injection, showed similar results with pa2H-Alexa594 still nicely corresponding to the green labeling of the anti-Aβ staining within the right hemisphere (E).
Immunofluorescence using VHH-Alexa594
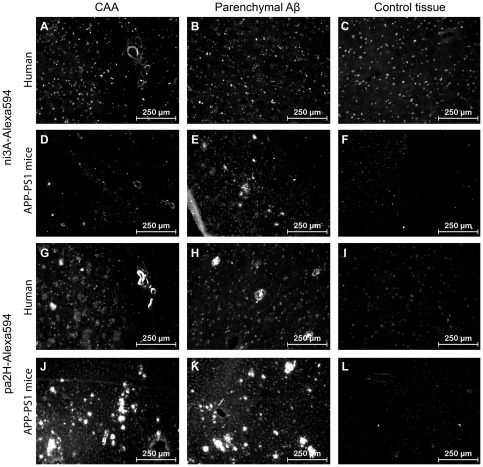
Selectivity for specific Aβ deposits was not altered after fluorescent labeling of the VHH, since on human sections, ni3A-Alexa594 selectively stained vascular Aβ (Figure 5 A–C), and pa2H-Alexa594 stained both parenchymal and vascular Aβ.(Figure 5 G–I) On murine material all Aβ deposits were stained by both fluorescent VHH.(Figure 5 D–F & J–L)
Figure 5. Immunofluorescence with VHH-Alexa594.
Shown are the results of immunofluorescence staining with ni3A- and pa2H-Alexa594 on cryosections of APP/PS1 murine and human AD/CAA brain tissue, including wildtype or healthy controls. Both VHH stain positive for CAA in all sections (A, D, G, J). Only ni3A-Alexa594 stained negative for human parenchymal Aβ (B), while pa2H stained positive for several types of parenchymal Aβ deposits (G, H, J, K) in both humans and mice. In either human of murine control tissue no such staining patterns were observed.(C, F, I, L)
Discussion
In this study, we assessed two previously described VHH for their potential to cross the blood-brain barrier and distinctively detect vascular and parenchymal Aβ deposits in vivo.
Specific detection of parenchymal and vascular amyloid in APP/PS1 mice
Both VHH stained positive for Aβ upon APP/PS1 brain sections confirming appropriate use of this transgenic model. In vivo binding to parenchymal and vascular Aβ was confirmed when the BBB was circumvented. Signal remained detectable for at least 24 hours while in vivo pa2H showed a high affinity combined with a low off-rate. However, previously shown selectivity for solely vascular Aβ in human post-mortem brain sections by ni3A was not observed within this mouse model (Figures 1,3,5). Fluorescent or radiolabeling prior in vivo application did not affect their specificity. The unique specific reactivity of ni3A for vascular amyloid deposition on human brain material is not yet completely understood [9]. Known differences in morphology and composition of human and murine Aβ deposits might help to understand ni3A's specific reactivity [21], [22]. Human plaques consist of discontinuous patches with decreased density and random fibrillar orientation within the amyloid core; murine plaques are generally built up by long organized fibrils, resulting in densely packed amyloid plaques with a relatively large core [23]. Besides morphological differences, posttranslational modifications of Aβ differ from mouse to man leading to alterations of the Aβ molecule itself [21], [24], [25]. Differences in metal ion content are known to influence the tertiary structure [26], [27]. Previous epitope mapping revealed that ni3A has no other cross reaction but to Aβ1–42 [9], which is highly abundant in parenchymal and vascular deposits in both humans and APP/PS1 mice. All together, this leads to the conclusion that the selective reactivity of ni3A must depend on the structural presentation of Aβ1–42, in which case murine parenchymal plaques probably show structural similarities to human CAA.
In vivo blood-brain barrier passage
Previous in vitro data suggested that our VHH actively migrated across the BBB in a more efficient way than FC5, a VHH specifically selected to pass the BBB [12]. However, the in vivo experiments resulted only in a small cerebral uptake of the tagged 99mTc-pa2H at 24 hours after intravenous administration, and the current brain uptake levels were insufficient to assess the uptake kinetics in vivo with for example SPECT imaging. (data not shown) Additional experiments with untagged DTPA(111In)-pa2H further confirmed the current limitations as hardly any cerebral uptake was observed with this labeling protocol. The increased brain uptake for 99mTc-pa2H compared to DTPA(111In)-pa2H may be due to the slower blood clearance for 99mTc-pa2H. The observed fast blood clearance and relatively high renal retention for the VHH in this study is in line with previous reports [28], [29], and typical for peptides and proteins smaller than the filtering threshold of the glomerular membrane (<60 kDa) [30]. However, in general, a short blood residential time effectively reduces the blood-to-brain transfer.
In vivo studies with the BBB crossing VHH FC5 demonstrated 4%ID/g brain uptake, which is much higher than our findings [31]. This discrepancy may be due to the lower dose that we used, but several other factors may also play a part. For FC5 it is known it uses receptor-mediated endocytosis via the α(2,3)-sialoglycoprotein [32]. For our VHH, in vitro active transport mechanisms are involved, but the specific receptors are as yet unknown [12]. Possibly, the in vivo BBB passage may be limited by the availability of these receptors in our mouse model.
To improve BBB penetration for the amyloid-targeting VHH, one could increase the blood circulation time by multimerization or by conjugating the VHH to an albumin-targeting moiety or VHH [33], [34]. An alternative approach would be to incorporate the VHH into a BBB-targeting nanoparticle. Recently, several nanoparticle carrier systems have been developed for brain delivery of therapeutics that would also be suitable for loading with VHH [35].
Diagnostic and therapeutic value of VHH
In general, VHH constitute many unique characteristics that make them interesting tools for either diagnostics or therapeutics. Compared to conventional monoclonal antibodies or Fab', VHH express a similar unique level of specificity and affinity, but because of their single domain, production and modification is relatively easy and cost-efficient [29].
Currently used amyloid-targeting ligands, like 11C-PiB recognize amyloid plaques rather than Aβ. In contrast, we already showed that VHH may be more specific to a certain sub-types of Aβ accumulation [9]. Further selection may allow the in vivo detection of the full range of Aβ aggregates from oligomers to dense core plaques to CAA.
Besides diagnostics, several VHH have shown their potential therapeutic value in vitro, preventing aggregation of amyloid fibrils, oligomeric forms of Aβ and polyA-binding protein nuclear 1 [36]–[39]. In the latter case, even complete clearance of existing aggregates was reported. Whether VHH evaluated in this study possess similar abilities is currently under investigation. However, within the data presented here, we observed that several Aβ plaques, as detected by their autofluorescence, could no longer be seen two days after VHH application. (Figure 3) Whereas current passive immunotherapies targeting Aβ are hampered by unwanted immunogenic side effects, repetitive administration of VHH has shown to be non-immunogenic [4], [40]. Furthermore, their selective binding to different Aβ species, like ni3A's specific binding for CAA, could shift Aβ brain efflux in the favored direction, which could be used to tailor anti-Aβ therapy to further reduce therapy-induced complications, e.g. CAA related microbleeds [4], [5], [41].
These initial in vivo studies to investigate whether Aβ specific VHH can be exploited as diagnostic tools show promising results for further development. Although capable of strong specific binding in vivo with low unspecific background binding and favorable wash-out, issues regarding higher brain uptake and clearance need to be addressed in the future.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Research was supported by: (1) the Center for Translational Molecular Medicine, LeARN, http://www.ctmm.nl; (2) Innovatiegericht OnderzoeksProgramma (IOP)Genomics, IGE05005, http://www.agentschapnl.nl/nl/programmas-regelingen/iop-genomics; (3) the Center for Medical Systems Biology, CMSB2, http://www.cmsb.nl; and (4) The Netherlands Organisation for Scientific Research (NWO) Athena, 700.58.801, http://www.nwo.nl/nwohome.nsf/pages/NWOA_6ZXCX3. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–2606. doi: 10.1161/STROKEAHA.108.536839. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller RO, Preston SD, Subash M, Carare RO. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther. 2009;1:6. doi: 10.1186/alzrt6. doi: 10.1186/alzrt6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jicha GA. Is passive immunization for Alzheimer's disease ‘alive and well’ or ‘dead and buried’? Expert Opin Biol Ther. 2009;9:481–491. doi: 10.1517/14712590902828285. doi: 10.1517/14712590902828285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg SM, Bacskai BJ, Hyman BT. Alzheimer disease's double-edged vaccine. Nat Med. 2003;9:389–390. doi: 10.1038/nm847. doi: 10.1038/nm847. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 9.Rutgers KS, van Remoortere A, van Buchem MA, Verrips CT, Greenberg SM, et al. Neurobiol Aging; 2009. Differential recognition of vascular and parenchymal beta amyloid deposition. doi: 10.1016/j.neurobiolaging.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 11.Harmsen MM, De Haard HJ. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007;77:13–22. doi: 10.1007/s00253-007-1142-2. doi: 10.1007/s00253-007-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutgers KS, Nabuurs RJ, van den Berg SA, Schenk GJ, Rotman M, et al. Neuroscience; 2011. Transmigration of beta amyloid specific heavy chain antibody fragments across the in vitro blood-brain barrier. doi: 10.1016/j.neuroscience.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 13.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, et al. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 14.Welling MM, Paulusma-Annema A, Balter HS, Pauwels EK, Nibbering PH. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27:292–301. doi: 10.1007/s002590050036. [DOI] [PubMed] [Google Scholar]
- 15.Welling MM, Korsak A, Gorska B, Oliver P, Mikolajczak R, et al. Kit with technetium-99m labelled antimicrobial peptide UBI 29–41 for specific infection detection. Journal of Labelled Compounds & Radiopharmaceuticals. 2005;48:683–691. doi: 10.1002/jlcr.961. [Google Scholar]
- 16.Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, et al. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002;61:797–805. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- 17.Skoch J, Dunn A, Hyman BT, Bacskai BJ. Development of an optical approach for noninvasive imaging of Alzheimer's disease pathology. J Biomed Opt. 2005;10:11007. doi: 10.1117/1.1846075. doi: 10.1117/1.1846075. [DOI] [PubMed] [Google Scholar]
- 18.Robbins EM, Betensky RA, Domnitz SB, Purcell SM, Garcia-Alloza M, et al. Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J Neurosci. 2006;26:365–371. doi: 10.1523/JNEUROSCI.3854-05.2006. doi: 10.1523/JNEUROSCI.3854-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadghiri YZ, Sigurdsson EM, Wisniewski T, Turnbull DH. Magnetic resonance imaging of amyloid plaques in transgenic mice. Methods Mol Biol. 2005;299:365–379. doi: 10.1385/1-59259-874-9:365. [DOI] [PubMed] [Google Scholar]
- 20.Natte R, Maat-Schieman ML, Haan J, Bornebroek M, Roos RA, et al. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol. 2001;50:765–772. doi: 10.1002/ana.10040. [DOI] [PubMed] [Google Scholar]
- 21.Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 23.van Groen T, Kiliaan AJ, Kadish I. Deposition of mouse amyloid beta in human APP/PS1 double and single AD model transgenic mice. Neurobiol Dis. 2006;23:653–662. doi: 10.1016/j.nbd.2006.05.010. doi: 10.1016/j.nbd.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Bussiere T, Bard F, Barbour R, Grajeda H, Guido T, et al. Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance. Am J Pathol. 2004;165:987–995. doi: 10.1016/s0002-9440(10)63360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson JA, Burns DK. Mouse models of Alzheimer's disease: a quest for plaques and tangles. ILAR J. 2002;43:89–99. doi: 10.1093/ilar.43.2.89. [DOI] [PubMed] [Google Scholar]
- 26.Adlard PA, Bush AI. Metals and Alzheimer's disease. J Alzheimers Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- 27.Leskovjan AC, Lanzirotti A, Miller LM. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer's disease. Neuroimage. 2009;47:1215–1220. doi: 10.1016/j.neuroimage.2009.05.063. doi: 10.1016/j.neuroimage.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gainkam LO, Huang L, Caveliers V, Keyaerts M, Hernot S, et al. Comparison of the biodistribution and tumor targeting of two 99mTc-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J Nucl Med. 2008;49:788–795. doi: 10.2967/jnumed.107.048538. doi: 10.2967/jnumed.107.048538. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Muyldermans S, Saerens D. Nanobodies(R): proficient tools in diagnostics. Expert Rev Mol Diagn. 2010;10:777–785. doi: 10.1586/erm.10.62. doi: 10.1586/erm.10.62. [DOI] [PubMed] [Google Scholar]
- 30.Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–212. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 31.Muruganandam A, Tanha J, Narang S, Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. FASEB J. 2002;16:240–242. doi: 10.1096/fj.01-0343fje. doi: 10.1096/fj.01-0343fje. [DOI] [PubMed] [Google Scholar]
- 32.Abulrob A, Sprong H, Van Bergen en HP, Stanimirovic D. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–1214. doi: 10.1111/j.1471-4159.2005.03463.x. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 33.Coppieters K, Dreier T, Silence K, de Haard H, Lauwereys M, et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006;54:1856–1866. doi: 10.1002/art.21827. doi: 10.1002/art.21827. [DOI] [PubMed] [Google Scholar]
- 34.Tijink BM, Laeremans T, Budde M, Stigter-van WM, Dreier T, et al. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Mol Cancer Ther. 2008;7:2288–2297. doi: 10.1158/1535-7163.MCT-07-2384. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- 35.Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, et al. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2011;108:18837–18842. doi: 10.1073/pnas.1111405108. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chartier A, Raz V, Sterrenburg E, Verrips CT, van der Maarel SM, et al. Prevention of oculopharyngeal muscular dystrophy by muscular expression of Llama single-chain intrabodies in vivo. Hum Mol Genet. 2009;18:1849–1859. doi: 10.1093/hmg/ddp101. doi: 10.1093/hmg/ddp101. [DOI] [PubMed] [Google Scholar]
- 37.Dumoulin M, Last AM, Desmyter A, Decanniere K, Canet D, et al. A camelid antibody fragment inhibits the formation of amyloid fibrils by human lysozyme. Nature. 2003;424:783–788. doi: 10.1038/nature01870. doi: 10.1038/nature01870. [DOI] [PubMed] [Google Scholar]
- 38.Lafaye P, Achour I, England P, Duyckaerts C, Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol Immunol. 2009;46:695–704. doi: 10.1016/j.molimm.2008.09.008. doi: 10.1016/j.molimm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Verheesen P, de Kluiver A, van Koningsbruggen S, de Brij M, De Haard HJ, et al. Prevention of oculopharyngeal muscular dystrophy-associated aggregation of nuclear polyA-binding protein with a single-domain intracellular antibody. Hum Mol Genet. 2006;15:105–111. doi: 10.1093/hmg/ddi432. doi: 10.1093/hmg/ddi432. [DOI] [PubMed] [Google Scholar]
- 40.Stijlemans B, Conrath K, Cortez-Retamozo V, van Xong H, Wyns L, et al. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J Biol Chem. 2004;279:1256–1261. doi: 10.1074/jbc.M307341200. doi: 10.1074/jbc.M307341200. [DOI] [PubMed] [Google Scholar]
- 41.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci. 2005;25:10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]