Abstract
Background
Sugarcane is one of the most important crops in Brazil, mainly because of its use in biofuel production. Recent studies have sought to determine the role of sugarcane endophytic microbial diversity in microorganism-plant interactions, and their biotechnological potential. Epicoccum nigrum is an important sugarcane endophytic fungus that has been associated with the biological control of phytopathogens, and the production of secondary metabolites. In spite of several studies carried out to define the better conditions to use E. nigrum in different crops, little is known about the establishment of an endophytic interaction, and its potential effects on plant physiology.
Methodology/Principal Findings
We report an approach based on inoculation followed by re-isolation, molecular monitoring, microscopic analysis, plant growth responses to fungal colonization, and antimicrobial activity tests to study the basic aspects of the E. nigrum endophytic interaction with sugarcane, and the effects of colonization on plant physiology. The results indicate that E. nigrum was capable of increasing the root system biomass and producing compounds that inhibit the in vitro growth of sugarcane pathogens Fusarium verticillioides, Colletotrichum falcatum, Ceratocystis paradoxa, and Xanthomomas albilineans. In addition, E. nigrum preferentially colonizes the sugarcane surface and, occasionally, the endophytic environment.
Conclusions/Significance
Our work demonstrates that E. nigrum has great potential for sugarcane crop application because it is capable of increasing the root system biomass and controlling pathogens. The study of the basic aspects of the interaction of E. nigrum with sugarcane demonstrated the facultative endophytism of E. nigrum and its preference for the phylloplane environment, which should be considered in future studies of biocontrol using this species. In addition, this work contributes to the knowledge of the interaction of this ubiquitous endophyte with the host plant, and also to a better use of microbial endophytes in agriculture.
Introduction
Endophytic microorganisms, primarily bacteria and fungi, inhabit, for at least one period of their life cycle, the interior of the host plant without inducing disease symptoms or producing external structures [1]. The bioprotective effects of these organisms, such as growth promotion and tolerance to herbivory and abiotic stress, are well-known for some temperate climate plants (Poaceae) [2], [3]. The diversity of endophytic fungi is greater in tropical regions. Plants in these regions are considered to be true reservoirs of fungal diversity [1], [4], [5], but the endophyte-plant interaction under these conditions is not yet fully understood [6]. In fact, the plant interior is now recognized as a prolific environment for the discovery of fungi with new biological activities [7], [8], [9], especially biocontrol capabilities [10], [11], [12]. Although endophytes have potential use in agriculture, the incomplete understanding of the biology of the endophyte-plant interaction presents impedes their wider use [2], [13].
Microorganisms that naturally associate with sugarcane, especially atmospheric nitrogen fixing bacteria and plant growth-promoting bacteria, have contributed to more productive agriculture with decreased environmental impact [14], [15]. The investigation of sugarcane endophytic bacterial communities and their related soil microbial populations has resulted in a greater comprehension of the bacterial population dynamics in the tropical agricultural environment [16] and the potential for the biological control of sugarcane pathogens [17], [18]. However, there have been few studies on the fungal communities associated with Saccharum officinarum [19], [20]. Our group has previously assessed the endophytic fungal communities associated with sugarcane [21], [22], [23] and their biotechnological potential [24], [25], [26], [27]. Notably, the ascomycete E. nigrum has been frequently isolated as an endophyte of sugarcane plants [21], [22], [23], [24].
E. nigrum Link (syn. E. purpurascens Ehrenb. ex Schlecht.) is a widespread mitosporic ascomycete (Dothideomycetes) that colonizes different types of substrates and is associated with plant primary decomposition [28]. Similar to other ubiquitous fungi, E. nigrum can display an endophytic lifestyle [29] in a variety of plants that are not taxonomically related [21], [30], [31], [32], [33], which suggests the development of adaptations to overcome the different types of plant defenses. E. nigrum is especially known for its biocontrol activity against pathogens, such as Sclerotinia sclerotiorum in sunflower [34], Pythium in cotton [35], phytoplasma in apple trees [36] and Monilinia spp. in peaches and nectarines [37], [38], [39], [40]. In spite of its biotechnological potential, little is known about the interaction of E. nigrum with plants [36], [41], and there have been no studies of the endophytic interaction of E. nigrum with tropical plants. Some studies of the E. nigrum endophytic interaction have been performed; for example, primers for the variable ITS1 and ITS2 regions were developed to detect endophytic E. nigrum in grapevines with and without phytoplasma symptoms [32]. In another study, the inoculation of an endophytic E. nigrum strain from an apple tree in the model plant Catharanthus roseus triggered defense responses against “Candidatus Phytoplasma mali” and reduced symptom severity [36]. These findings illustrate the potential for the use of endophytic E. nigrum in different host plants and warrant a further investigation of the physiological and molecular aspects of the interaction.
The aim of this work was to gain insight into the interaction of this common sugarcane endophyte with the host plant and to assess the antagonistic capacity of E. nigrum against S. officinarum plant pathogens. The growth responses of the sugarcane plants to colonization by E. nigrum were also investigated, and aspects of the lifestyle of this fungus are discussed.
Results and Discussion
E. nigrum Establishes a Facultative Endophytic Interaction, Preferentially Colonizes the Phylloplane, and Causes Transient Changes in the Sugarcane-associated Fungal Community
The basic aspects of the interaction of the common sugarcane endophyte E. nigrum with the host plant were investigated by inoculation on the leaves and roots of plants grown in a greenhouse and later re-isolation in a time-course experiment. In addition to monitoring E. nigrum colonization by isolation from disinfected and non-disinfected sugarcane organs over time, this approach permitted the comparison of the E. nigrum isolation frequency with the total isolation frequency of plant-associated fungi. Importantly, the Random Amplified Polymorphic DNA (RAPD) profiles of all of the re-isolated E. nigrum were the same as those of the original P16 strain (Figure S1).
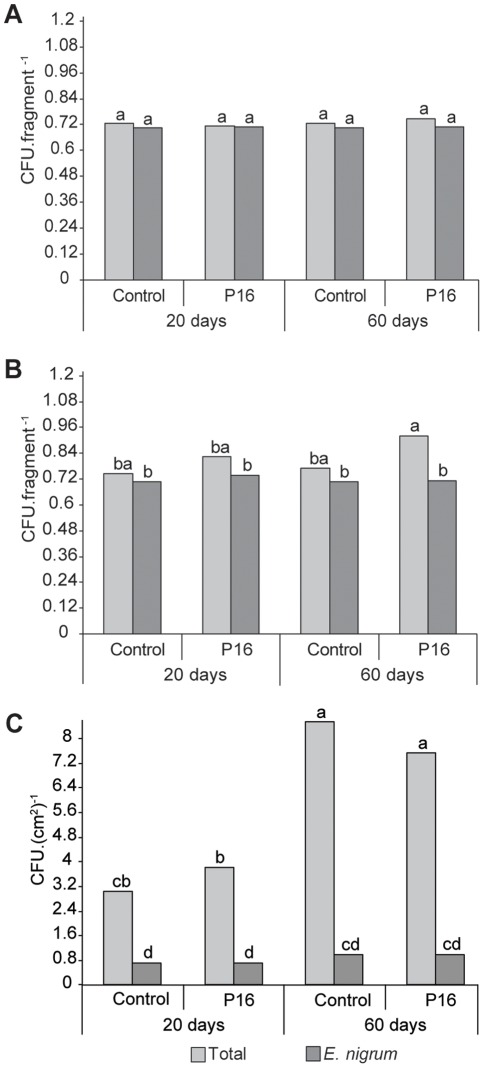
The isolation analysis of the disinfected sugarcane leaves and sheaths did not reveal significant differences in the number of E. nigrum CFUs recovered from these organs during the two sampling periods, as observed from the analysis of variance (Figure 1a and Figure 1b). E. nigrum was not isolated as an endophyte from the leaves and sheaths of the control plants in any sample period. In studying the effect of the inoculation with E. nigrum mycelia fragments on the endophytic fungal isolation frequency in these organs, the analysis also did not reveal differences in the number of CFUs recovered from the leaves over time (Figure 1a), but the number of CFUs recovered from the sheaths increased as the plant aged (Figure 1b). Most of the colonies obtained from the inoculated plants after disinfection were E. nigrum, which demonstrates the capacity of this fungus to endophytically colonize not only senescent leaves but also newly opened leaves on the sugarcane plants (not shown). These results also confirm previous reports on the sugarcane endophytic behavior of E. nigrum [21], [22] and indicate that E. nigrum is capable of disseminating to other tissues after the inoculation on leaves with mycelial fragments.
Figure 1. E. nigrum and sugarcane-associated fungi re-isolation from the phylloplane of sugarcane grown in greenhouse.
E. nigrum and sugarcane-associated fungi were re-isolated 20 and 60 days after inoculation of the P16 endophytic strain on leaves of sugarcane plants. The E. nigrum isolation frequency was compared with the total isolation frequency of sugarcane-associated fungi. Isolation frequency of the endophytic fungi from leaves (a) and sheaths (b) is shown in CFU per leaf/sheath fragment. Isolation frequency of epiphytic fungi (c) is shown in CFU per cm2 and includes abaxial and adaxial surfaces of the leaf fragments. All the data were transformed with √ x + 0.5 and submitted to analysis of variance followed by Tukey’s test. Means followed by the same letter indicate that they were not statistically different (Tukey’s test, P>5%). Control indicates the non-inoculated plants, while P16 indicates plants inoculated with the E. nigrum P16 strain.
A different scenario was observed for the epiphytic environment. The number of E. nigrum CFU recovered from the leaf surface increased as the plant aged, as observed from the analysis of variance (Figure 1c). E. nigrum was recovered from the leaf surface of the control plants 60 days after inoculation (Figure 1c), which suggests that the inoculated plants served as a potential inoculum source in the greenhouse environment, as confirmed by RAPD analysis (not shown). This finding is difficult to explain because the leaves of the inoculated plants did not present symptoms and were not found in the leaf litter, where the fungi could release conidia. We observed an increase in the epiphytic fungal isolation frequency in the leaves of plants 20 days after inoculation with E. nigrum, but this effect did not persist as the plant aged (Figure 1c), which reflects the establishment of an equilibrium in the cultivable epiphytic fungal community or transient changes in the epiphytic fungal community as a result of the E. nigrum colonization. Moreover, an increase was also detected in the epiphytic fungal isolation frequency related to plant senescence (Figure 1c), which has been reported as a common phenomenon in studies on fungal diversity on plants [42].
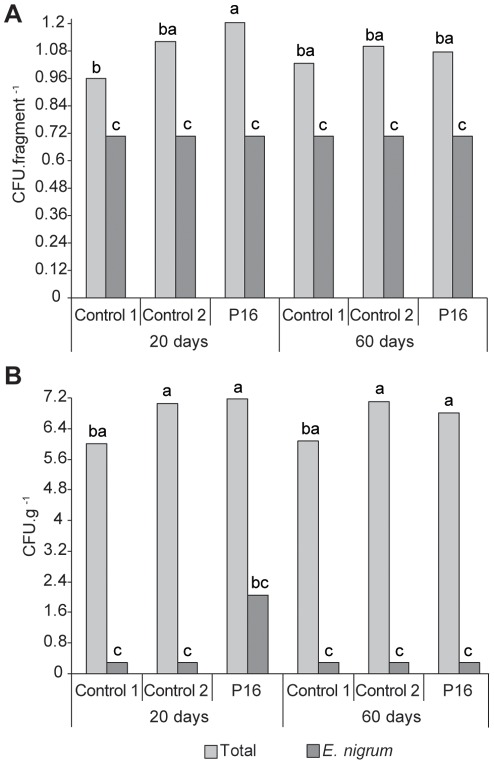
E. nigrum was not recovered as an endophyte from inoculated sugarcane plants after root superficial disinfection (Figure 2a), which indicates that E. nigrum did not colonize the endophytic root environment under the analyzed conditions. However, 20 days after inoculation, we observed a significant increase in the total isolation frequency for fungi inside the sugarcane roots (Figure 2a), but this frequency declined 60 days after inoculation. E. nigrum was recovered from the rhizosphere only during the first isolation period (Figure 2b). These differences may have been caused by the establishment of a balance in the fungal population as the plants aged; however, the presence of E. nigrum may also have brought about transient alterations in the root endophytic fungal community, as observed for the leaf epiphytic environment. Importantly, the observed shift in the plant-associated fungal isolation frequency after E. nigrum inoculation may be the results of a combination of many factors, such as the type of inoculum and substrate [43], interspecific competition, the type of exudate released, and the chemical compounds on the leaf surface [10], [44].
Figure 2. E. nigrum and sugarcane-associated fungi re-isolation from the root environment of sugarcane grown in greenhouse.
E. nigrum and sugarcane-associated fungi were re-isolated 20 and 60 days after inoculation of the P16 endophytic strain in the roots of sugarcane plants. The E. nigrum isolation frequency was compared with the total isolation frequency of sugarcane-associated fungi. Control 1 indicates non-inoculated roots, while Control 2 indicates roots inoculated only with sterilized wheat seeds. Isolation frequency of the endophytic fungi from roots (a) is shown in CFU per root fragment. Isolation frequency of the rhizosphere fungi (b) is shown in CFU per gram of substrate. The data were transformed with √× + 0.5 (a) and Log (× + 2) (b) and submitted to analysis of variance followed by Tukey’s test. Means followed by the same letter indicate that they were not statistically different (Tukey’s test, P>5%).
Altogether, these results demonstrate that E. nigrum established a facultative endophytic interaction with sugarcane, preferentially in the phylloplane environment. This finding is in agreement with studies that have reported the isolation of this fungus mainly from sugarcane leaves [21], [22]. E. nigrum is considered to be a ubiquitous species in the epiphytic [28] and endophytic [29] environments and features characteristics such as melanized conidia, which increase UV tolerance and have been related to the capacity to colonize the phylloplane [37]. E. nigrum persistence in the phylloplane may be related to the different physiological conditions present in the plant tissues, but studies on other fungi have shown that there may be a preference for the tissue and conditions present in the studied plant organ [43]. These results demonstrate the importance of this approach for studying the interaction between endophytes and the microbial communities associated with the plant.
E. nigrum Colonizes Sugarcane Leaves Through Natural Openings and Uses the Epidermis as a Preferential Niche
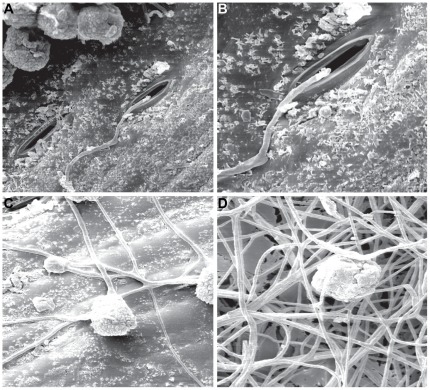
The germination of E. nigrum conidia on sugarcane leaf fragments was investigated by scanning electronic microscopy (SEM), which showed that conidia germination occurred at 12–16 hours after inoculation and that the hyphae penetrated the leaf tissue through the stomata (Figure 3). No changes associated with the direct penetration of the surface, such as the development of structures similar to the appressorium or changes on the leaf surface to which the conidia and hyphae were attached, were observed. Random hyphal ramification was observed 40 hours after surface colonization, and the hypha seems to firmly adhere to the cuticle (Figure 3). After 64 hours, the leaf surface was completely covered with E. nigrum hyphae (Figure 3).
Figure 3. E. nigrum conidia germination on sugarcane leaf fragments analyzed by scanning electronic microscopy.
Scanning electronic microscopy analysis of the conidia germination of the E. nigrum P16 endophytic strain on sugarcane leaf fragments. (a–b) After 12 hours of incubation in wet chamber it was possible to visualize the conidia germination and hyphae next to the stomata (1000X and 2000X, respectively). (c) After 40 hours of incubation it was possible to visualize hyphal ramification and random surface colonization (1000X). (d) After 64 hours, the leaf surface was completely covered with E. nigrum hyphae (1000X).
The absence of direct penetration and changes on the leaf surface was also observed in the leaf disks of bean plants colonized by E. nigrum conidia [41], which suggests that this fungi is not able to induce disease symptoms in bean plants, as we showed in sugarcane. However, other studies are needed to determine whether the internal parenchyma is colonized or whether the fungus is restricted to the sugarcane epidermal intercellular spaces. In a recent assessment of the capacity of several E. nigrum endophytic isolates obtained from healthy S. officinarum leaves to secrete hydrolytic enzymes, high lipase production was observed, including for the P16 strain used in the present study [21]. Therefore, the colonization pattern observed by microscopy and the high lipase secretion suggest that the sugarcane epidermis is a preferential niche for this fungus, as was shown by the previous re-isolation analyses.
Among the endophytic fungi, there are many epiphyte species that belong to ubiquitous genera that can live inside the host plant [29]. It has been suggested that endophytic communities contain epiphyte species that show facultative leaf penetration, such as Alternaria, Cladosporium and Epicoccum [30]. However, studies have not been performed to test these hypotheses, and the analyses performed demonstrated that facultative endophytism is part of the E. nigrum life strategy. Furthermore, the occurrence of this fungus inside various, taxonomically unrelated plants [21], [30], [31], [32], [36], [45], [46], [47] suggests the development of adaptations to overcome different types of plant defenses, which is characteristic of a generalist lifestyle.
E. nigrum Colonizes Sugarcane Asymptomatically and Increases Root System Biomass
Conidia of the E. nigrum P16 strain were inoculated in axenic sugarcane plants to investigate the possible effects of this fungus on plant survival in the in vitro rooting phase. At 72 hours of incubation, a mycelial film around the roots was observed (not shown). The general characteristics of the colonized plants were not altered compared with the control, and plant senescence was not postponed, which demonstrates the non-pathogenic character of the P16 strain.
Because E. nigrum P16 was not able to induce disease symptoms in in vitro propagated sugarcane plantlets, we investigated the effect of this fungus on the greenhouse acclimatization of sugarcane plants. This analysis was performed because the acclimatization process can be a stress period for plants [48] and is an opportune time to introduce protective microbial inoculants [49]. We observed no difference in the survival of inoculated plants after the acclimatization period compared with the control, which confirms that E. nigrum sugarcane colonization in this period is also asymptomatic. The absence of pathogenicity in Epicoccum endophytic isolates was demonstrated previously when strains of this fungus were re-introduced in in vitro propagated pejibaye plants to promote plantlet growth [46] and when pathogenicity tests were carried out in Quercus spp. plants for the use of E. nigrum in Diplodia corticola control [45]. More recently, an endophytic apple tree E. nigrum isolate was inoculated in C. roseus plants to control phytoplasma dissemination, and no disease symptoms were detected in pathogenicity tests with C. roseus plants inoculated with the E. nigrum isolate [36].
We further analyzed the effect of E. nigrum colonization of axenic sugarcane roots on plant growth after the acclimatization process by analyzing the accumulation of root and canopy fresh and dry matter. After 60 days of growth in a greenhouse, the dry matter accumulation in the roots of the colonized plants was greater than that in the control plants as demonstrated by the analysis of variance (Table 1), which suggests that this fungus increased the rooting capacity of the plant at this post-acclimatization phase. Although inoculation of the P16 strain tended to decrease the canopy biomass, significant differences were not found for the total dry matter of the inoculated plants compared with the control plants (Table 1). Root colonization by E. nigrum may have changed the carbon and dry matter distribution among the different parts of the plant because an increase in the root:canopy dry matter ratio was observed (Table 1), which indicates greater biomass allocation to the roots than to the canopy in the presence of E. nigrum. Importantly, before the acclimatization process, E. nigrum could not be recovered from the substrate (not shown), which indicates that the sugarcane root growth resulted from colonization by E. nigrum. However, we cannot discard other factors that could explain this effect, such as the alterations of the microbial community composition in the root environment, because in our previous experiments E. nigrum inoculation induced changes in the total isolation frequency of endophytic root fungi.
Table 1. Effect of the colonization of the E. nigrum P16 strain on the accumulation of root and canopy fresh and dry matter of the SP70-1143 sugarcane variety, after 60 days of growth in a greenhouse.
| Treatment | Fresh weight (g)(1) | ||||
| Roots | Aerialparts | Total | Root/aerial parts (2) | ||
| SP70-1143 (control) | 12.123a | 14.118a | 26.241a | 0.86578b | |
| SP70-1143 (P16) | 11.663a | 10.664b | 22.327b | 1.09208a | |
| Treatment | Dry weight (g) (1) | ||||
| Roots | Aerial parts | Total | Root/ aerial parts (2) | ||
| SP70-1143 (control) | 1.206ba | 4.076a | 5.282a | 0.30269b | |
| SP70-1143 (P16) | 1.527a | 3.039b | 4.566a | 0.50920a | |
Data were submitted to analysis of variance followed by Tukey’s test. Means followed by the same letter indicate that they were not statistically different (Tukey’s test, P>5%, means from 10 replicates).
Root:canopy dry matter ratio.
Although E. nigrum P16 induced a reduction of the canopy biomass, the increase in root systems could increase the adaptation of the inoculated plants in field conditions. Also, as disease symptoms were not observed in vitro, the presence of this fungus may increase plant fitness under specific conditions, such as in the presence of pathogens and/or pests, as has been reported for other interactions involving E. nigrum [36], [37], [50]. In fact, the results obtained in the present study indicate that physiological alterations could occur in the host plant as a result of E. nigrum colonization. Physiological and structural alterations, such as callose accumulation, have been observed in C. roseus plants inoculated with an endophytic apple tree E. nigrum strain to control phytoplasma symptoms [36], which indicates that physiological changes and bioprotective effects also could occur in non-host plants as a result of the E. nigrum colonization. Furthermore, the ability to produce plant growth-regulator-like molecules has been suggested to underlie the growth response to inoculation by endophytic fungi [1]. Plant hormone production of an E. nigrum endophytic strain has been observed in the culture medium [51]. Therefore, if these compounds are produced during the interaction with the host plant, they could be involved in root growth such as that observed in the present study.
Indeed, little is known about the costs and benefits of the association of endophytes with tropical plants [6], [10], [52], [53], despite the remarkably common occurrence of this interaction. Interestingly, endophytes have been demonstrated to induce an alert state in plants that is characterized by an increased capacity to express basic defense responses following biotic and abiotic challenges [13], [54]. In fact, even for the fairly well-studied association between Clavicipitaceae fungi and temperate climate grasses (Poaceae), some beneficial effects, such as the growth response of the plants to the presence of endophytes, are variable and depend on the host genotype, nutrient availability and environmental stresses [13]. Although further analyses are needed to assess the reproducibility of the effect of E. nigrum P16 on sugarcane root growth by addressing different conidia concentrations, sugarcane varieties, types of stress, and plot development strategies, colonization by endophytic fungi may increase the cost to the plant, as demonstrated by the physiological alterations resulting from asymptomatic E. nigrum colonization.
Sugarcane Endophytic E. nigrum Inhibits Several Plant Pathogens
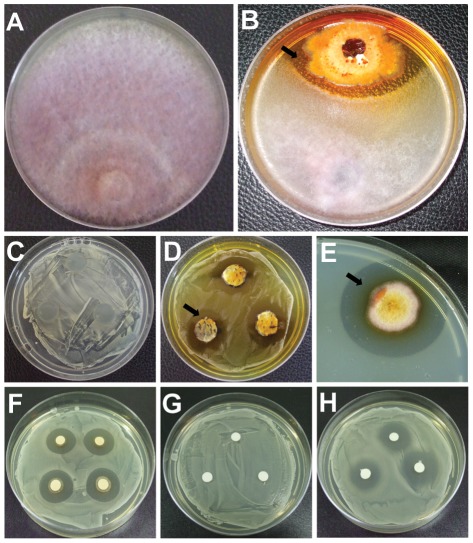
Endophytic fungi are known to protect plants against several biotic stresses, in part via production of secondary metabolites with biological activities. Therefore, we investigated the antagonistic potential of the sugarcane endophytic E. nigrum P16 strain against different phytopathogens. E. nigrum reduced C. paradoxa and F. verticillioides radial growth by more than 50% (Table 2), and an inhibition zone formed among the colonies that was stable even after 20 days of culture (Figure 4a–b), which indicates that diffusible compounds were released in the culture medium by the antagonistic endophyte. The inhibitory activity against X. albilineans was observed by the agar block method (Figure 4c–d), which yielded a 22.6 mm (±0.1 mm) inhibition zone, and by the method of diffusion in semi-solid agar (Figure 4e), which yielded a 9.0 mm (±0.1 mm) inhibition zone. These findings demonstrate that bioactive compounds were produced in the initial and the more advanced stages of E. nigrum growth on solid media.
Table 2. Antagonism test of the sugarcane endophytic E. nigrum P16 strain against the sugarcane phytopathogens C. paradoxa and F. verticillioides.
| Treatment | C. paradoxa | F. verticillioides | |||||
| Radialgrowth | Growthreduction | Inhibitionzone | Radialgrowth | Growthreduction | Inhibition zone | ||
| Control | 75 mma | – | – | 75 mma | – | – | |
| P16 | 31 mmb | 58.6% | 4.5 (±0.1 mm) | 34.5 mmb | 54% | 4 (±0.1 mm) | |
Data were submitted to analysis of variance followed by Tukey’s test. Means followed by the same letter indicate that they were not statistically different (Tukey’s test, P>5%, means from 3 replicates).
Figure 4. In vitro antagonism of the E. nigrum P16 endophytic strain against sugarcane phytopathogens.
Antagonism test between E. nigrum P16 and F. verticillioides (a–b). An inhibition zone (dark arrow) formed among the colonies can be observed (b), in comparison with the control plate with only F. verticillioides (a). The inhibitory activity against X. albilineans was observed by the agar block method (c–d) and by the method of diffusion in semi-solid agar (e). The E. nigrum P16 ethyl acetate extract also inhibited X. albilineans growth (f–h). DMSO was used as control treatment (g). Spectinomycin (50 mg.mL−1) was used as positive control (h).
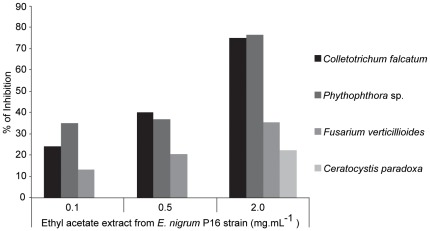
Because we observed that diffusible compounds were produced during E. nigrum growth in solid medium, we also investigated if the organic extracts of the supernatant (ethyl acetate) and the mycelium (methanol) of the E. nigrum culture had inhibitory activity. The extract obtained from the mycelium did not exhibit antimicrobial activity under the assessment conditions, possibly because the compounds were not stored in the mycelia or their production was low and their presence could not be detected by the methods used. However, the ethyl acetate extract inhibited X. albilineans growth (Figure 4f–h) and produced a 15.5 mm (±0.1 mm) inhibition zone; the inhibition zone produced by the antibiotic spectinomycin, which was used as a positive control, was 19.0 mm (±0.1 mm). The E. nigrum extract also significantly reduced C. falcatum, F. verticillioides, C. paradoxa and Phythophthora sp. growth at concentrations ranging from 0.1 to 2.0 mg.mL−1. C. falcatum and Phythophthora sp. were more sensitive to the extract, with colony diameter reductions of 75% and 76.47%, respectively, in the presence of 2.0 mg.mL−1 of the extract (Figure 5).
Figure 5. Antimicrobial activity of the ethyl acetate extract from E. nigrum P16 endophytic strain.
Antifungal and anti-oomycete activity of the ethyl acetate extract from E. nigrum P16 strain. The percentage of growth inhibition of the pathogens is showed in the y axis. The means of three replicates for each extract concentration analysed were used to calculate the percent reduction in pathogen mycelial growth by the equation [1– (mean colony diameter of the control/mean colony diameter of the treatment)×100].
The antagonistic activity of E. nigrum has already been demonstrated against different fungal and oomycete phytopathogens [34], [35], [39], [45], [47], [55], [56], [57], [58], [59], [60], [61], [62], [63]. Some antimicrobial compounds produced by E. nigrum have been characterized, such as epicorazines A–B [64], epirodines A–B [65], flavipin [66], epicoccines A–D [67], epipiridones and epicocarines [68]. In particular, flavipin and epicorazines A–B have been associated with E. nigrum biocontrol activity against Monilinia spp., Pythium ssp. and Phythophthora ssp. [55], [69], [70]. In addition, flavipin also inhibits plant pathogenic bacteria such as Corynebacterium michiganense, Erwinia carotovora var. atroseptica, Pseudomonas phaseolicola, P. putida, P. syringae and X. phaseoli [69], which illustrates the broad spectrum of action of this metabolite. Therefore, the common sugarcane endophyte E. nigrum [21], [22], [23] may act as a natural antagonist for several sugarcane pathogens if it produces these compounds during the interaction with the host plant.
Concluding Remarks
A study of the basic aspects of the interaction of E. nigrum with sugarcane demonstrated the facultative endophytism of E. nigrum and its preference for the phylloplane environment, which should be considered in future studies of biocontrol using this species. Furthermore, an increase in the root system biomass was observed in plants inoculated with E. nigrum, which demonstrates the need for greater investigation of the physiological alterations and molecular mechanisms involved in the symbiosis. Endophytic fungi have received increasing interest as a promising source of potential control agents against plant pathogens [10], [11]. Although endophytes have potential uses in agriculture, the incomplete understanding of the biology of the endophyte-plant interaction impedes their widespread use [2], [13]. For example, with the exception of the Clavicipitaceae endophytes, little is known about the function of the secondary metabolites in the endophyte-plant interaction. The present study demonstrates that the sugarcane leaf endophyte E. nigrum inhibited the in vitro growth of different microorganisms, which indicates that this endophyte could be a natural antagonist for plant pathogens in sugarcane tissues. These findings suggest that the plant interior is a prolific environment for discovering E. nigrum strains that may produce new metabolites and opens the possibility of characterizing different isolates of this fungus to select more promising strains. In this context, a new natural product, epicolactone, was recently isolated from the ethyl acetate extract of the E. nigrum P16 strain analyzed in the present work [71].
The compounds produced by E. nigrum that are responsible for inhibiting plant pathogens must be characterized. Although E. nigrum has a fairly diverse secondary metabolism, there are no genetic studies on the biosynthesis of bioactive compounds. Our results should encourage future studies to evaluate secondary metabolite synthesis by E. nigrum and define the best conditions for using this fungus in sugarcane culture. Such studies are underway in our laboratory and have been facilitated by the optimization of genetic transformation protocols [24] and the complete genomic sequencing of the E. nigrum P16 strain, which was recently approved by our group (FAPESP Grant 10/08286-2 - BioProject Accession PRJNA7784).
Materials and Methods
Strains, Growth Conditions, and Conidia Production
The E. nigrum P16 strain was isolated from surface-disinfected healthy sugarcane leaves [21] and maintained in Potato Dextrose Agar (PDA) (Difco). To obtain conidia, E. nigrum was inoculated on sterile sugarcane leaf fragments on Petri dishes containing agar-agar (1.5% p/v). After incubation for 25 days at 28°C with a 16-hour light period, a conidia suspension was prepared (1×106 conidia.mL−1). Fusarium verticillioides, Ceratocystis paradoxa, Colletotrichum falcatum (sugarcane pathogens), and Phythophthora sp. (Citrus sp. pathogen) were maintained in PDA. Xanthomonas albilineans (sugarcane pathogen) was maintained in Nutrient Agar (NA) (Difco). The pathogens were obtained from the collection of microbial strains in the Laboratory of Microbial Genetics, Department of Genetics, ESALQ/USP, Piracicaba, São Paulo, Brazil.
E. nigrum Inoculation in Sugarcane Seedlings
Thirty-day-old sugarcane plants (SP80-1842, conventional variety) were grown on trays with PlantMax commercial substrate (Eucatex, Brazil) and kindly supplied by the Sugarcane Technology Center (Centro de Tecnologia Canavieira S.A.). To inoculate the roots, 20 g wheat seed was placed in flasks, moistened with 10 mL distilled water and sterilized in an autoclave three consecutive times. Two E. nigrum mycelia disks were then inoculated over the seeds and incubated at 28°C for 15 days. The 1-kg pots were filled with PlantMax commercial plant substrate, and the seedlings were transferred to these pots with 20 g of the previously prepared inoculum so that the seeds colonized by the fungus were in contact with the roots. A randomized block design was used with the three treatments (SP80-1842 - not inoculated, SP80-1842+ wheat seeds, SP80-1842+ E. nigrum P16 strain) with three replicates. This experimental design was considered for the analysis of variance (ANOVA). The inoculum was prepared by transferring three mycelia disks to flasks containing 200 mL potato broth culture medium (12 flasks). After growth for 15 days at 28°C, the mycelia were filtered, and the mass was estimated. The mycelia were then mixed with PBS buffer (8 g NaCl, 0.2 g KCl, 1.4 g Na2HPO4, 0.24 g KH2PO4, 1.000 mL distilled water, pH 7.4) at a proportion of 70 g.L−1, homogenized and inoculated on the sugarcane leaves. The plants were kept under highly humid conditions in a greenhouse for 48 hours (wet chamber made with transparent plastic bags). Application of the PBS buffer was used as the control. A randomized block design was used with two treatments (SP80-1842 not inoculated, SP80-1842+ E. nigrum P16 strain) and three replicates, in which each plant was considered a replicate. This experimental design was considered for the analysis of variance (ANOVA).
Re-isolation of E. nigrum from Sugarcane Plants
E. nigrum and sugarcane-associated fungi were re-isolated 20 and 60 days after inoculation. For the re-isolation, the endophytic fungal community from the leaves, sheath, and roots and the phylloplane epiphytic and rhizosphere fungal communities were isolated. The leaf and sheath endophytes were isolated after superficial disinfection (70% ethanol for 60 seconds, 3% sodium hypochlorite (v/v) for 90 seconds, 70% ethanol for 60 seconds and rinsed twice with sterilized water). Seven leaf fragments (0.5 cm2) were transferred to Petri dishes containing PDA supplemented with tetracycline (50 µg mL−1).
For the isolation of root endophytes, the roots were washed in running water, and a 2-g sample was disinfected superficially (70% ethanol for 60 seconds, 3% sodium hypochlorite (v/v) for 180 seconds, 70% ethanol for 60 seconds and rinsed twice with sterilized water). To assess the disinfection efficiency, a 100-µL aliquot of the water used in the last wash was sown on the PDA culture medium. The plates were incubated at 28°C for 5–15 days, and the number of colonies was converted to colony forming units (CFUs) per fragment. The data were transformed with √×+0.5 and submitted to analysis of variance and the Tukey test at the level of 5% significance using SAS software (Copyright (c) 1989–1996 by SAS Institute Inc., Cary, NC, USA).
To isolate the fungi from the phylloplane surfaces, ten leaf fragments (5.0×1.5 cm2) were transferred to flasks containing glass spheres (0.2 cm diameter) and 50 mL PBS buffer. After shaking for 2 hours (200 rpm) at 28°C, 100 µL aliquots were sown on PDA supplemented with tetracycline (50 µg mL−1) and incubated at 28°C for 7 days. The number of fungal colonies was converted to CFUs per square centimeter based on the upper and lower surfaces of the leaf fragments used. These data were transformed with √×+0.5 and submitted to analysis of variance and the Tukey test at 5% significance using SAS software (Copyright (c) 1989–1996 by SAS Institute Inc., Cary, NC, USA).
To isolate the fungi from the rhizosphere, the excess extract was removed, the roots were shaken vigorously to collect the substrate adhering to them, and 5-g samples were transferred to Erlenmeyer flasks containing glass spheres and 50 mL PBS buffer. After incubation for one hour at 28°C under agitation (200 rpm), the dilutions were sown on PDA supplemented with tetracycline (50 µg mL−1) and incubated at 28°C for 5 days. The number of CFUs per gram of extract was transformed with log (×+2) and submitted to analysis of variance and the Tukey test at 5% significance using SAS software (Copyright (c) 1989–1996 by SAS Institute Inc., Cary, NC, USA).
Genetic Identity of E. nigrum Re-isolates
The colonies obtained in the isolation that were morphologically similar to E. nigrum were compared with the original P16 strain using RAPD markers [72]. For the comparison, three mycelia disks from monoconidial colonies of the sugarcane-recuperated isolates were transferred to flasks containing 50 mL potato broth. After 7 days of growth at 28°C, the mycelia were collected by filtration, and the genomic DNA was extracted with the Wizard Genomic DNA Purification Kit (Promega, USA) and used as a template in PCR reactions.
The OP-X12 (5′ TCGCCAGCCA 3′), OP-X17 (5′ GACACGGACC 3′) and OP-X19 (5′ TGGCAAGGCA 3′) oligonucleotides (Operon Technologies, USA) were used in the RAPD reactions and prepared in duplicate to a final volume of 25 µL with 0.25 mM dNTPs, 3.0 mM MgCl2, 1× buffer (50 mM KCl and 20 mM Tris-HCl, pH 8.4), 0.5 U.µL−1 Taq DNA polymerase (Fermentas Life Sciences, Brazil), 0.4 µM each primer/starter and 5 ng genomic DNA. The amplification was performed in a PTC - 200 thermocycler (MJ Research) with an initial denaturation at 94°C for four minutes followed by 40 amplification cycles. Each cycle consisted of one minute at 92°C, one minute at 35°C, two minutes at 72°C and a final extension of five minutes at 72°C. The PCR products were separated in a 1.5% agarose gel, stained with ethidium bromide and photodocumented under ultraviolet light.
Colonization of Sugarcane Leaf Fragments
To investigate the conidia germination of the P16 strain on sugarcane leaf fragments, four–month-old plant leaves of the SP80-1842 variety grown at the Sugarcane Technology Center (Centro de Tecnologia Canavieira S.A.) were superficially disinfected (70% ethanol for 60 seconds, 3% sodium hypochlorite (v/v) for 90 seconds, 70% ethanol for 60 seconds and rinsed twice with sterilized water) and transferred to a wet chamber (Petri dishes containing filter paper moistened with sterilized distilled water). Aliquots of 10 µL of a conidia suspension (1×105 conidia.mL−1) were inoculated on the abaxial surface of the leaf fragments. The plates were incubated at 28°C with a 16-hour photoperiod, and 5-mm leaf fragments were collected at regular intervals (0 h, 6 h, 12 h, 18 h, 24 h, 30 h, 36 h, 42 h, 48 h and 72 h) and fixed in Karnovsky solution (2.5% glutaraldehyde, 2.5% formaldehyde in 0.05 M sodium cacodylate, pH 7.2, 0.001 M CaCl2). The samples were fixed with osmium tetroxide (1% OsO4 in 0.1 M cacodylate buffer), dehydrated in ethanol solutions with increasing concentrations (30, 50, 70, 90 and 100%) and then dried to the critical point and metallized. The analysis was performed with a Zeiss DSM 940 A scanning electronic microscope at the Research Support Nucleus for Electronic Microscopy (NAP/MEPA, ESALQ/USP, Piracicaba, SP).
Greenhouse Evaluation of E. nigrum on Sugarcane Growth
Axenic plants of the SP70-1143 variety at the rooting stage were donated by the Sugarcane Technology Center (Centro de Tecnologia Canavieira S.A.). First, the general aspect of the plants was assessed visually. The conidia of the P16 strain were inoculated in flasks containing 20 mL Murashige and Skoog (MS) culture medium [73], and the concentration was adjusted to 1×105 conidia.mL−1. The plants were transferred to the flasks, and the roots were immersed in the culture medium and incubated at 28° with a 16-hour photoperiod. The plants were inspected visually at 24-, 48-, 72- and 96-hour intervals (three plants for each interval) or until the non-inoculated plants entered a state of senescence.
Subsequently, 50-mL Falcon type tubes were prepared containing 7 mL of MS culture medium [73] inoculated with 1×105 conidia.mL−1. Micropropagated plantlets with homogeneous characteristics (same canopy size and similar root number in the in vitro culture) were individualized and aseptically transferred to the tubes containing conidia. The plants were incubated at 28°C with a 16-hour photoperiod for three days and were then acclimatized in a greenhouse. For acclimatization, plastic trays with 200-mL wells were filled with commercial PlantMax substrate. The plantlets were transplanted to the substrate and maintained in a wet chamber under greenhouse conditions for 10 days.
After this period, the wet chamber was removed, and the plants were watered every two days with 50 mL water. After a 60-day growth period under greenhouse conditions, the plants were collected and assessed for fresh and dry matter root and canopy accumulation. E. nigrum was also re-isolated and analyzed by RAPD, as reported previously. To measure the dry matter, the plants were incubated in a chamber at 80°C for 24 hours and then at 65°C until a constant weight was reached. The analysis of variance was carried out with 10 plants from each treatment (SP70-1143 not inoculated; SP70-1143+ E. nigrum P16 strain) in a complete randomized design using SAS software (Copyright (c) 1989–1996 by SAS Institute Inc., Cary, NC, USA); the Tukey test was applied at the level of 5% significance.
Antagonism Against Plant Pathogenic Microorganisms
E. nigrum and the plant pathogens were cultivated for 7 days at 28°C in PDA. E. nigrum mycelia disks (6 mm in diameter) were transferred to PDA medium 48 hours before inoculation with pathogens. The pathogens were inoculated 5 cm away from the E. nigrum P16 colony. As a control, the pathogen was inoculated without the Epicoccum colonies. After five days of incubation at 28°C, the inhibition zone and the percentage of growth inhibition of the pathogen were calculated in relation to the control. The tests were performed in triplicate.
X. albilineans inhibition was evaluated by the agar block method [74]. E. nigrum was cultured for 15 days in 15 mL PDA at 28°C. The bacterial culture was prepared in 10 mL nutrient broth (3 g meat extract, 5 g peptone, 1000 mL distilled water, pH 6.8) and incubated with agitation (100 rpm) for 24 hours at 30°C. An aliquot (50 µL) of this culture was sown on 15 mL NA (Difco), and E. nigrum disks (8 mm diameter) were then transferred to the dishes containing the bacteria. The inhibition halo was measured after 24 hours of growth at 30°C. The control consisted of inoculating the PDA disks, and the bioassay was performed in triplicate.
Activity against X. albilineans was also investigated using a previously reported method [75]. E. nigrum fragments were inoculated in 10 mL PDA, and the dishes were incubated for 72 hours at 28°C in the dark. X. albilineans was cultured as described previously, and one aliquot of the culture (1%, v/v) was transferred to semisolid NA (0.5% agar; 45°C–50°C). Then, 7 mL of this culture medium was poured over the E. nigrum cultures, and the dishes were stored for 8 hours at 4°C before incubation at 30°C for 24 hours. The tests were performed in triplicate, and the inhibition zone was then measured.
Antimicrobial Activity of the Organic Extract of E. nigrum Cultures
To produce the bioactive compounds in liquid culture medium, 3 mycelia disks (8-mm diameter) from E. nigrum cultures grown on PDA for 7 days at 28°C were inoculated in flasks containing 200 mL potato broth supplemented with 2% yeast extract (200 g boiled potato, 20 g glucose, 20 g yeast extract, 1000 mL distilled water, pH 6.8). The flasks were incubated at 28°C without light in static culture for 45 days. The mycelia and the fermentative culture medium were separated by filtering under vacuum, and the filtrate was submitted to three consecutive extraction steps with ethyl acetate. Ethyl acetate and the filtrate were mixed at a ratio of 1∶3 (v/v), and after agitation, the aqueous and organic phases were separated in a separation funnel.
The mycelia were immersed in flasks containing 100 mL dichloromethane and left at room temperature for 48 hours. The organic solvent was collected by filtration, and the mycelia were again immersed in 100 mL dichloromethane and methanol (1∶1), left to rest for 48 hours and then immersed in methanol alone for an additional 48 hours. The solvents were concentrated in a rotating evaporator under reduced pressure.
Aliquots of 20 µL of the organic extracts (100 mg.mL−1 in dimethylsulfoxide) were inoculated on 6-mm-diameter filter paper disks placed on the surface of Petri dishes containing culture medium inoculated with X. albilineans, as described previously. The experiment was performed in triplicate, and the control consisted of the inoculation of 20 µL dimethylsulfoxide and/or spectinomycin (Sigma-Aldrich) (50 mg.mL−1). The dishes were incubated at 30°C for 24 hours. The inhibition halo diameters were measured perpendicularly with a ruler. The ethyl acetate extract was also assessed against F. verticillioides, C. paradoxa and C. falcatum; the mycelial fragments of the pathogen were inoculated on Petri dishes (60×15 mm) containing 5 mL PDA, and increasing concentrations of the extract (0.1, 0.5, 2.0 mg.mL−1), and dimethylsulfoxide (control) alone were added. The colony diameters were measured after 4 days of incubation at 28°C. The experiment was performed in triplicate in a complete randomized design. The analysis of variance followed by Tukey’s test was conducted using SAS software (Copyright (c) 1989–1996 by SAS Institute Inc., Cary, NC, USA). The Tukey’s test was applied at the level of 5% significance. The percent reduction in pathogen mycelial growth was also calculated using the following equation: [1– (mean colony diameter of the control/mean colony diameter of the treatment) x 100].
Supporting Information
RAPD profile generated with primer OPX12 (a), OPX17 (b), and OPX19 (c) of the original E. nigrum P16 strain (left) and six endophytic re-isolates obtained from sugarcane leaves variety SP80-1842, 20 days after inoculation in greenhouse. Amplification products were separated in 1.4% agarose gels and stained with ethidium bromide. (M) DNA ladder 1 Kb (Fermentas Life Sciences, Brazil).
(DOC)
Acknowledgments
We are grateful to Dra. Sabrina M. Chábregas (Centro de Tecnologia Canavieira S.A., Piracicaba, São Paulo, Brazil) for sugarcane plants; Dr. Elliot W. Kitajima (“Luiz de Queiroz” College of Agriculture, University of São Paulo, Piracicaba, São Paulo, Brazil) for microscopy facilities; Dra. Joelma Marcon and Dra. Priscila B. Rosseto (Department of Genetics, “Luiz de Queiroz” College of Agriculture, University of São Paulo, Piracicaba, São Paulo, Brazil) for assistance with isolation techniques; Dr. Fernando L. Melo (University of Brasília, Distrito Federal, Brazil) for the invaluable suggestions on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by FAPESP (State of São Paulo Research Foundation) (Grants 02/14143-3 and 10/08286-2). The authors also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a doctoral fellowship to LCLF (Graduate Program in Plant Genetics and Breeding, “Luiz de Queiroz” College of Agriculture, University of São Paulo). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Azevedo JL, Araújo WL. Diversity and applications of endophytic fungi isolated from tropical plants. In: Ganguli BN, Deshmukh SK, editors. Fungi: multifaceted microbes. Boca Raton: CRC Press; 2007. pp. 189–207. [Google Scholar]
- 2.Kuldau G, Bacon C. Clavicipitaceous endophytes: their ability to enhance resistance of grasses to multiple stresses. Biological Control. 2008;46:57–71. [Google Scholar]
- 3.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology. 2004;55:315–340. doi: 10.1146/annurev.arplant.55.031903.141735. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell M. The Fungi: 1, 2, 3 … 5.1 million species? American Journal of Botany. 2011;98:426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 5.Suryanarayanan TS. Diversity of fungal endophytes in tropical trees. In: Pirttilä AM, Frank AC, editors. Endophytes of Forest Trees: Biology and Applications, Forestry Sciences. Springer Netherlands. 2011;80:67–80. [Google Scholar]
- 6.Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biology Reviews. 2007;21:51–66. [Google Scholar]
- 7.Aly AH, Debbab A, Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Applied Microbiology and Biotechnology. 2011;90:1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- 8.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suryanarayanan TS, Thirunavukkarasu N, Govindarajulu MB, Sasse F, Jansen R, et al. Fungal endophytes and bioprospecting. Fungal Biology Reviews. 2009;23:9–19. [Google Scholar]
- 10.Arnold AE, Mejia LC, Kyllo D, Rojas EI, Maynard Z, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15649–15654. doi: 10.1073/pnas.2533483100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backman PA, Sikora RA. Endophytes: An emerging tool for biological control. Biological Control. 2008;46:1–3. [Google Scholar]
- 12.Ownley BH, Gwinn KD, Vega FE. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. Biocontrol. 2010;55:113–128. [Google Scholar]
- 13.Singh LP, Gill SS, Tuteja N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signaling and Behavior. 2011;6:175–191. doi: 10.4161/psb.6.2.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldani JI, Reis VM, Baldani VLD, Dobereiner J. A brief story of nitrogen fixation in sugarcane - reasons for success in Brazil. Functional Plant Biology. 2002;29:417–423. doi: 10.1071/PP01083. [DOI] [PubMed] [Google Scholar]
- 15.Boddey RM, Urquiaga S, Alves BJR, Reis V. Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant and Soil. 2003;252:139–149. [Google Scholar]
- 16.Dini-Andreote F, Andreote FD, Costa R, Taketani RG, van Elsas JD, et al. Bacterial soil community in a Brazilian sugarcane field. Plant and Soil. 2010;336:337–349. [Google Scholar]
- 17.Luvizotto DM, Marcon J, Andreote FD, Dini-Andreote F, Neves AAC, et al. Genetic diversity and plant-growth related features of Burkholderia spp. from sugarcane roots. World Journal of Microbiology & Biotechnology. 2010;26:1829–1836. [Google Scholar]
- 18.Mendes R, Pizzirani-Kleiner AA, Araújo WL, Raaijmakers JM. Diversity of cultivated endophytic bacteria from sugarcane: genetic and biochemical characterization of Burkholderia cepacia complex isolates. Applied and Environmental Microbiology. 2007;73:7259–7267. doi: 10.1128/AEM.01222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azeredo LA, Gomes EA, Mendonça-Hagler LC, Hagler AN. Yeast communities associated with sugarcane in Campos, Rio de Janeiro, Brazil. International Microbiology. 1998;1:205–208. [PubMed] [Google Scholar]
- 20.Nasim G, Ali A, Munawar A, Bajwa R. Seasonal dynamics of AM fungi in sugarcane (Saccharum officinarum L. Cv. Spf-213) in relation to red rot (Colletotrichum falcatum) disease from Punjab, Pakistan. Pakistan Journal of Botany. 2008;40:2587–2600. [Google Scholar]
- 21.Fávaro LCL, Melo FL, Aguilar-Vildoso CI, Araújo WL. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS One. 2011;6:e14828. doi: 10.1371/journal.pone.0014828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart RM, Romão AS, Pizzirani-Kleiner AA, Azevedo JL, Araújo WL. Culturable endophytic filamentous fungi from leaves of transgenic imidazolinone-tolerant sugarcane and its non-transgenic isolines. Archives of Microbiology. 2010;192:307–313. doi: 10.1007/s00203-010-0557-9. [DOI] [PubMed] [Google Scholar]
- 23.Romão AS, Araújo WL. Efeito do cultivo de cana-de-açúcar geneticamente modificada sobre a comunidade fúngica associada. In: Costa-Maia L, Malosso E, Yano-Melo AM, editors. Micologia: avanços no conhecimento. 5° Congresso Brasileiro de Micologia, Recife: Editora Universitária da UFPE; 2007. pp. 150–159. [Google Scholar]
- 24.Fávaro LCL, Araújo WL. Resumos do 5° Congresso Brasileiro de Micologia, Recife: Editora Universitária da UFPE. p; 2007. Transformação do fungo endofítico de cana-de-açúcar Epicoccum nigrum com o gene da proteína verde fluorescente (GFP) mediada por Agrobacterium tumefaciens.199 [Google Scholar]
- 25.Pallu APS, Fávaro LCL, Rodrigues MBC, Ferreira A, Araújo WL, et al. Agrobacterium-mediated transformation of the endophytic fungus Penicillium pinophilum associated with sugarcane. Abstracts of the III International Conference on Environmental, Industrial and Applied Microbiology, Lisboa. 2009. pp. 130–139.
- 26.Romão AS. PhD Thesis. “Luiz de Queiroz” College of Agriculture, University of São Paulo; 2010. Analysis of sugarcane-associated fungal community and study of the interaction Trichoderma virens - host plant.268 [Google Scholar]
- 27.Rojas JD, Sette LD, Araújo WL, Lopes MS, da Silva LF, et al. The diversity of polyketide synthase genes from sugarcane-derived fungi. Microbial Ecology. 2012;63:565–577. doi: 10.1007/s00248-011-9938-0. [DOI] [PubMed] [Google Scholar]
- 28.Andrews JH, Harris RF. The ecology and biogeography of microorganisms on plant surfaces. Annual Review of Phytopathology. 2000;38:145–180. doi: 10.1146/annurev.phyto.38.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Schulz B, Boyle C. The endophytic continuum. Mycological Research. 2005;109:661–686. doi: 10.1017/s095375620500273x. [DOI] [PubMed] [Google Scholar]
- 30.Fisher PJ, Petrini O. Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L). New Phytologist. 1992;120:137–143. [Google Scholar]
- 31.Larran S, Perello A, Simon MR, Moreno V. The endophytic fungi from wheat (Triticum aestivum L.). World Journal of Microbiology & Biotechnology. 2007;23:565–572. [Google Scholar]
- 32.Martini M, Musetti R, Grisan S, Polizzotto R, Borselli S, et al. DNA-dependent detection of the grapevine fungal endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Disease. 2009;93:993–998. doi: 10.1094/PDIS-93-10-0993. [DOI] [PubMed] [Google Scholar]
- 33.Camatti-Sartori V, da Silva-Ribeiro RT, Valdebenito-Sanhueza RM, Pagnocca FC, Echeverrigaray S, et al. Endophytic yeasts and filamentous fungi associated with southern Brazilian apple (Malus domestica) orchards subjected to conventional, integrated or organic cultivation. Journal of Basic Microbiology. 2005;45:397–402. doi: 10.1002/jobm.200410547. [DOI] [PubMed] [Google Scholar]
- 34.Pieckenstain FL, Bazzalo ME, Roberts AMI, Ugalde RA. Epicoccum purpurascens for biocontrol of Sclerotinia head rot of sunflower. Mycological Research. 2001;105:77–84. [Google Scholar]
- 35.Hashem M, Ali E. Epicoccum nigrum as biocontrol agent of Pythium damping-off and root-rot of cotton seedlings. Archives of Phytopathology and Plant Protection. 2004;37:283–297. [Google Scholar]
- 36.Musetti R, Grisan S, Polizzotto R, Martini M, Paduano C, et al. Interactions between ‘Candidatus Phytoplasma mali’ and the apple endophyte Epicoccum nigrum in Catharanthus roseus plants. Journal of Applied Microbiology. 2011;110:746–756. doi: 10.1111/j.1365-2672.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 37.De Cal A, Larena I, Linan M, Torres R, Lamarca N, et al. Population dynamics of Epicoccum nigrum, a biocontrol agent against brown rot in stone fruit. Journal of Applied Microbiology. 2009;106:592–605. doi: 10.1111/j.1365-2672.2008.04030.x. [DOI] [PubMed] [Google Scholar]
- 38.Larena I, Melgarejo P. Development of a new strategy for monitoring Epicoccum nigrum 282, a biological control agent used against brown rot caused by Monilinia spp. in peaches. Postharvest Biology and Technology. 2009;54:63–71. [Google Scholar]
- 39.Larena I, Torres R, De Cal A, Linan M, Melgarejo P, et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biological Control. 2005;32:305–310. [Google Scholar]
- 40.Mari M, Torres R, Casalini L, Lamarca N, Mandrin JF, et al. Control of post-harvest brown rot on nectarine by Epicoccum nigrum and physico-chemical treatments. Journal of the Science of Food and Agriculture. 2007;87:1271–1277. [Google Scholar]
- 41.Zhou T, Reeleder RD. Colonization of bean flowers by Epicoccum purpurascens. Phytopathology. 1991;81:774–778. [Google Scholar]
- 42.Frohlich J, Hyde KD, Petrini O. Endophytic fungi associated with palms. Mycological Research. 2000;104:1202–1212. [Google Scholar]
- 43.Akello J, Dubois T, Gold CS, Coyne D, Nakavuma J, et al. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.). Journal of Invertebrate Pathology. 2007;96:34–42. doi: 10.1016/j.jip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Saunders M, Kohn LM. Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytologist. 2009;182:229–238. doi: 10.1111/j.1469-8137.2008.02746.x. [DOI] [PubMed] [Google Scholar]
- 45.Campanile G, Ruscelli A, Luisi N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. European Journal of Plant Pathology. 2007;117:237–246. [Google Scholar]
- 46.Almeida CV, Yara R, Almeida M. Endophytic fungi in shoot tip of the pejibaye cultivated in vivo and in vitro. Pesquisa Agropecuária Brasileira. 2005;40:467–470. [Google Scholar]
- 47.Lahlali R, Hijri M. Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiology Letters. 2010;311:152–159. doi: 10.1111/j.1574-6968.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- 48.Grattapaglia D, Machado MA. Micropropagação. In: Torres AC, Caldas LS, editors. Técnicas e Aplicações da Cultura de Tecidos de Plantas. Brasília: ABCTP/EMBRAPA CNPH; 1990. pp. 99–170. [Google Scholar]
- 49.Quecine MC, Lacava PT, Silva MCP, Araújo WL, Pizzirani-Kleiner AA. Resumos do 54°Congresso Brasileiro de Genética, Salvador: Sociedade Brasileira de Genética. p; 2008. Expressing heterologous gene cry by endophytic bacterium Pantoea agglomerans and its potential on biocontrol of Diatraea saccharalis.133 [Google Scholar]
- 50.Newcombe G, Shipunov A, Eigenbrode S, Raghavendra A, Ding H, et al. Endophytes influence protection and growth of an invasive plant. Communicative and Integrative Biology. 2009;2:29–31. doi: 10.4161/cib.2.1.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowan DD, Latch GCM. Utilization of endophyte-infected perennial ryegrasses for increased insect resistance. In: Bacon CW, White JFJ, editors. Biotechnology of endophytic fungi of grasses. Boca Raton: CRC Press; 1994. pp. 169–183. [Google Scholar]
- 52.Pinto LSRC, Azevedo JL, Pereira JO, Vieira MLC, Labate CA. Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytologist. 2000;147:609–615. doi: 10.1046/j.1469-8137.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 53.Arnold AE, Engelbrecht BMJ. Fungal endophytes nearly double minimum leaf conductance in seedlings of a neotropical tree species. Journal of Tropical Ecology. 2007;23:369. [Google Scholar]
- 54.Van Wees SCM, Van der Ent S, Pieterse CMJ. Plant immune responses triggered by beneficial microbes. Current Opinion in Plant Biology. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Brown AE, Finlay R, Ward JS. Antifungal compounds produced by Epicoccum purpurascens against soil-borne plant pathogenic fungi. Soil Biology & Biochemistry. 1987;19:657–664. [Google Scholar]
- 56.Kortekamp A. Epicoccum nigrum Link: A biological control agent of Plasmopara viticola (Berk. et Curt.) Berl. et De Toni? Vitis. 1997;36:215–216. [Google Scholar]
- 57.Mielnichuk N, Lopez SE. Interaction between Epicoccum purpurascens and xylophagous basidiomycetes on wood blocks. Forest Pathology. 2007;37:236–242. [Google Scholar]
- 58.Zhou T, Reeleder RD, Sparace SA. Interactions between Sclerotinia sclerotiorum and Epicoccum purpurascens. Canadian Journal of Botany. 1991;69:2503–2510. [Google Scholar]
- 59.Elmer PAG, Alcock EA, Perry F. Epicoccum nigrum as a biological control and as a source of antimicrobial metabolites. Proceedings of the 13° Biennial Conference of Australasian Plant Pathology Society, Cairns, p. 2001. 339
- 60.Fowler SR, Jaspers MV, Walter M, Stewart A. Suppression of overwintering Botrytis cinerea inoculum on grape rachii using antagonistic fungi. New Zealand Plant Protection. 1999;52:141–147. [Google Scholar]
- 61.Chand T, Logan C. Antagonists and parasites of Rhizoctonia solani and their efficacy in reducing stem canker of potato under controlled conditions. Transactions of the British Mycological Society. 1984;83:107–112. [Google Scholar]
- 62.Royse DJ, Ries SM. The influence of fungi isolated from peach twigs on the pathogenicity of Cytospora cincta. Phytopathology. 1978;68:603–607. [Google Scholar]
- 63.Webber JF, Hedger JN. Comparison of interactions between Ceratocystis ulmi and elm bark saprobes in vitro and in vivo. Transactions of the British Mycological Society. 1986;86:93–101. [Google Scholar]
- 64.Baute MA, Deffieux G, Baute R, Neveu A. New antibiotics from the fungus Epicoccum nigrum. I. Fermentation, isolation and antibacterial properties. Journal of Antibiotics. 1978;31:1099–1101. doi: 10.7164/antibiotics.31.1099. [DOI] [PubMed] [Google Scholar]
- 65.Ikawa M, Mcgrattan CJ, Burge WR, Iannitelli RC, Uebel JJ, et al. Epirodin, a polyene antibiotic from mold Epicoccum nigrum. Journal of Antibiotics. 1978;31:159–160. doi: 10.7164/antibiotics.31.159. [DOI] [PubMed] [Google Scholar]
- 66.Bamford PC, Norris GLF, Ward G. Flavipin production by Epicoccum spp. Transactions of the British Mycological Society. 1961;44:354–356. [Google Scholar]
- 67.Zhang Y, Liu S, Che Y, Liu X. Epicoccins A-D, epipolythiodioxopiperazines from a Cordyceps-colonizing isolate of Epicoccum nigrum. Journal of Natural Products. 2007;70:1522–1525. doi: 10.1021/np070239u. [DOI] [PubMed] [Google Scholar]
- 68.Wangun HVK, Hertweck C. Epicoccarines A, B and epipyridone: tetramic acids and pyridone alkaloids from an Epicoccum sp. associated with the tree fungus Pholiota squarrosa. Organic & Biomolecular Chemistry. 2007;5:1702–1705. doi: 10.1039/b702378b. [DOI] [PubMed] [Google Scholar]
- 69.Madrigal C, Tadeo JL, Melgarejo P. Relationship between flavipin production by Epicoccum nigrum and antagonism against Monilinia laxa. Mycological Research. 1991;95:1375–1381. [Google Scholar]
- 70.Madrigal C, Melgarejo P. Morphological effects of Epiccocum nigrum and its antibiotic flavipin on Monilinia laxa. Canadian Journal of Botany. 1995;73:425–431. [Google Scholar]
- 71.Araújo FDS, Fávaro LCL, Oliveira FL, Araújo WL, Aparício R, et al. Epicolactone: a new natural product isolated from the fungus Epicoccum nigrum. Abstracts of the ZiNG Natural Products Conference, Lanzarote, 2012. 2012.
- 72.Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- 74.Ichikawa T, Date M, Ishikura T, Ozaki A. Improvement of kasugamycin-producing strain by agar piece method and prototroph method. Folia Microbiologica. 1971;16:218–224. doi: 10.1007/BF02884210. [DOI] [PubMed] [Google Scholar]
- 75.Spelhaug SR, Harlander SK. Inhibition of foodborne bacterial pathogens by bacteriocins from Lactococcus lactis and Pediococcus pentosaceous. Journal of Food Protection. 1989;52:856–862. doi: 10.4315/0362-028X-52.12.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RAPD profile generated with primer OPX12 (a), OPX17 (b), and OPX19 (c) of the original E. nigrum P16 strain (left) and six endophytic re-isolates obtained from sugarcane leaves variety SP80-1842, 20 days after inoculation in greenhouse. Amplification products were separated in 1.4% agarose gels and stained with ethidium bromide. (M) DNA ladder 1 Kb (Fermentas Life Sciences, Brazil).
(DOC)