Abstract
Introduction:
The prostate secretes enzymes and nutrients to promote sperm motility. Recent reports suggest that the prostate may also secrete testosterone, which is believed to be a fuel for prostate tumour growth. The aim of this study was to determine if a difference in serum testosterone levels exists between men on luteinizing hormone releasing-hormone (LHRH) agonists who have undergone radical prostatectomy, radiation or hormone therapy as primary prostate cancer treatment.
Methods:
Serum testosterone levels were evaluated in 165 consecutive prostate cancer patients using LHRH analogues for >3 months. We excluded patients receiving either radiation or chemotherapy at time of time of testosterone measurement. Patients were classified based on primary treatment: (1) radical prostatectomy; (2) radiation; or (3) primary hormone therapy. We used one-way ANOVA to compare testosterone levels. Pearson correlation was used to correlate testosterone with prostate-specific antigen (PSA) and time on LHRH agonists. Multivariable linear regression was used to predict serum testosterone levels.
Results:
The median (interquartile range) serum testosterone levels were 1.4 (1–1.9), 1.3 (1–1.625) and 1.25 (0.9–1.525) nmol/L for radical prostatectomy, radiation and primary hormone therapy groups, respectively. There was no statistically significant difference in testosterone levels between the groups (p = 0.3). No correlation was found between testosterone and PSA levels or time on LHRH (r = 0.02 and r = 0.01), respectively. Multivariable linear regression showed that none of the clinical variables were predictors of serum testosterone levels.
Conclusion:
Our study suggests that primary treatment does not affect serum testosterone levels among men using LHRH analogues.
Introduction
Prostate cancer is the most common cancer among Canadian men with 25 500 cases diagnosed in 2011.1 In the 1940s, Huggins and Hodge were the first to report the androgen dependence of prostate cancer.2 The most frequent treatment for advanced prostate cancer or recurrence following localized treatment is androgen deprivation therapy (ADT),3,4 most commonly with a luteinizing hormone releasing-hormone (LHRH) analogue.5
LHRH agonist injections act by suppressing the hypothalamic-pituitary-gonadal axis via negative feedback. They exert a nonpulsatile, constant stimulation to the anterior pituitary gland, which in turn decreases LH and testosterone production.6 Though LHRH agonists have been cited to reduce the levels of circulating serum testosterone to the point of castration, there are still considerable levels of this hormone in the serum following hormone therapy.7 There is also speculation as to the cut-off that constitutes as a castrate level of serum testosterone achieved during LHRH intervention, since hormone therapy may not produce an acceptable end value of testosterone in some patients.6 Although still debatable, a serum testosterone level of 20 ng/dL is used to describe chemical castration with LHRH agonists.6–9
Androgens, such as testosterone and dihydrotestoster-one (DHT), are present in significant levels in prostate tissue despite the castrate serum androgen levels in circulation.10–12 The realization that hormone refractory prostate cancer cells continue to be affected by androgen signalling has prompted a change in terminology; this state is now referred to as castrate-resistant prostate cancer (CRPC).13–15 The fact that prostate cancer is still affected by androgen signalling even in the CRPC phase also contributed to a rapidly growing treatment regimen for patients with CRPC such as arbiretarone (a CYP-17 inhibitor),14 MDV3100 (a potent androgen receptor antagonist) and others.15
The prostate tissue itself may act as an endocrine organ and secrete testosterone.11 As testosterone is important even in CRPC, we assessed whether serum testosterone levels differ among patients on LHRH agonists who have undergone radical prostatectomy, radiation or hormone therapy as their primary treatment. We also assessed whether there is a difference in testosterone levels between patients who use different LHRH analogues.
Methods
This study was approved by the Research Ethics Boards at University Health Network (Princess Margaret Hospital division) and the Ontario Cancer Registry in Toronto, Ontario, Canada. We examined the records of patients treated for prostate cancer in the surgical, medical and radiation oncology departments of the Princess Margaret Hospital. A total of 165 consecutive prostate cancer patients (age: 56–90 years) were identified as having obtained LHRH injections at time of data collection. Patients were excluded if they: (a) were receiving adjuvant therapies at the time of testosterone measurement and (b) had received LHRH agonists for less than three months.
The following clinical variables were collected: age, time on LHRH, primary therapy type, disease status (biochemical recurrence, locally advanced, metastatic or CRPC, serum testosterone and prostate specific antigen [PSA] values. Testosterone measurements were performed at the same laboratory (Toronto General Hospital) using the ADVIA Centaur (Siemens Healthcare Diagnostics Inc., Tarrytown, NY) testosterone assay (competitive immunoassay using direct chemiluminescent technology). Time on LHRH therapy was calculated in months from LHRH start date to the point of study enrolment. In addition, the brand of LHRH agonist (Lupron [Abbott Laboratories, Saint Laurent,QC], Zoladex [AstraZeneca Canada, Mississauga, ON], Suprefact [sanofi-aventis, Laval, QC], Eligard [sanofi-aventis, Laval, QC], Trelstar [Watson Pharma Inc., Parsippany, NJ] was recorded.
For statistical analysis, the cohort was classified into three groups based on primary treatment: (1) radical prostatectomy; (2) radiation; and (3) primary hormone therapy. Pearson correlation was used to correlate testosterone levels with serum PSA and time on LHRH agonists. Serum testosterone levels were compared between patients using the five different brands of LHRH agonists using one-way ANOVA. Testosterone levels were compared between the three groups (radical prostatectomy, radiation, primary hormone therapy) using one-way ANOVA. Multivariable linear regression analysis was used to measure the ability of the clinical variables as predictors of serum testosterone levels. Statistical analysis was performed using SPSS version 19 (SAS Institute, Cary, NC), and a two-sided p value of <0.05 was considered statistically significant.
Results
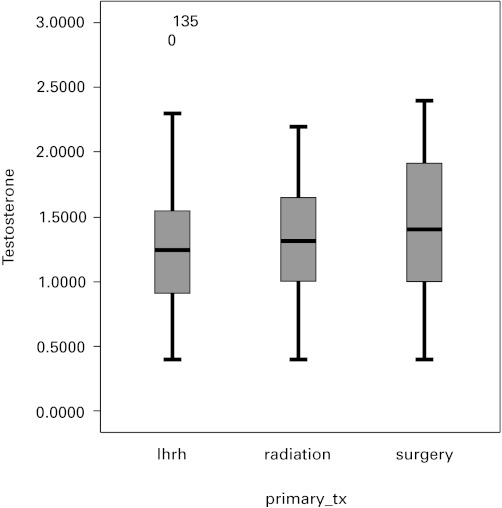
We tallied the summary characteristics and descriptive statistics of the study cohort (Table 1). Of the 165 patients in our study, 61 (37%) underwent a radical prostatectomy, 52 (31.5%) had radiation and 52 (31.5%) were on primary hormone therapy. The median (interquartile range [IQR]) of PSA values were 0.21 (0–3.49), 0.82 (0–7.38) and 1.55 (0–9.87) ug/L for radical prostatectomy, radiation and primary hormone therapy, respectively. The median (IQR) of serum testosterone levels were 1.4 (1–1.9), 1.3 (1–1.625) and 1.25 (0.9–1.525) nmol/L for radical prostatectomy, radiation and primary hormone therapy, respectively. One-way ANOVA did not reveal a statistically significant difference in serum testosterone between the three groups (p = 0.3) (Fig. 1).
Table 1.
Summary and descriptive statistics of prostate cancer patients on LHRH agonists in Ontario (n=165)
| Clinical variables | Radical prostatectomy (n=61) | Radiation (n=52) | Primary hormone therapy (n=52) |
|---|---|---|---|
| Age | |||
| Median | 72 | 77 | 78.6 |
| IQR | 66–79 | 73–81 | 70–85 |
| PSA (ug/L) at time of testosterone | |||
| Median | 0.21 | 0.82 | 1.55 |
| IQR | 0–3.49 | 0–7.38 | 0–9.87 |
| Serum testosterone (nmol/L) | |||
| Median | 1.4 | 1.3 | 1.25 |
| IQR | 1–1.9 | 1–1.625 | 0.9–1.525 |
| Time on LHRH (months) | |||
| Median | 60 | 60 | 66 |
| IQR | 36–108 | 25.75–108 | 24–108 |
| Disease status* | |||
| 1. Biochemical recurrence | 42.623 | 30.7692 | 1.9231 |
| 2. Metastases | 27.8689 | 25 | 44.2308 |
| 3. CRPC | 4.918 | 9.6154 | 5.7692 |
| 4. Locally advanced | 16.3934 | 32.6923 | 40.3846 |
| 5. Not known | 8.1967 | 1.9231 | 7.6923 |
PSA: prostate-specific antigen; IQR: interquartile range; LHRH: luteinizing hormone releasing-hormone; CRPC: castrate-resistant prostate cancer.
Values are percentages of total in each group.
Fig. 1.
Box plot displaying serum testosterone levels by primary therapy (radical prostatectomy, radiation, luteinizing hormone releasing-hormone). The median (interquartile range) serum testosterone levels were 1.4 (1–1.9), 1.3 (1–1.625) and 1.25 (0.9–1.525) nmol/L for radical prostatectomy, radiation primary hormone therapy groups, respectively. There was no statistically significant difference in serum testosterone between the groups (p = 0.3).
No association was found between PSA and time on LHRH versus testosterone levels (r = 0.02 and r = 0.01, respectively). Multivariable linear regression indicated that none of the clinical variables (PSA, age, time on LHRH, primary therapy type, disease status) were predictors of testosterone levels.
Brand of LHRH agonist was recorded for each individual (Table 2). Among the cohort, Zoladex was the most extensively used LHRH agonist (72/165) and Trelstar the least prescribed (5/165), no patient was prescribed a LHRH antagonist (Table 2).
Table 2.
Descriptive statistics of prostate cancer patients according to type of LHRH agonist (n=65)
| Brand of LHRH (median and IQR) | Age | PSA (ug/L) at time of testosterone | Serum testosterone (nmol/L) | Duration of LHRH use (months) | Disease status* |
|---|---|---|---|---|---|
| †1: 44.44% | |||||
| 2: 16.66% | |||||
| Eligard (n=18) | 72.5 (68.25–80) | 0.03 (0–1.9625) | 1.2 (0.95–1.55) | 48 (24.25–81) | 3: 0% |
| 4: 33.33% | |||||
| 5: 5.55% | |||||
| 1: 26%, 2 (34%), 3 (6%), 4 (30%), 5 (4%) | |||||
| 2: | |||||
| Lupron (n=50) | 78 (71.25–83) | 2.695 (0.51–10.01 | 1.3 (1–1.8) | 3: | |
| 4: | |||||
| 5: | |||||
| Suprefact (n=19) | 76 (71–83) | 0.25 (0–3.2) | 1.5 (1.05–1.6) | 108 (78–132) | 1 (36.84%), 2 (26.315%), 3 (0%), 4 (26.315%), 5 (10.526%) |
| Trelstar (n=19) | 67 (66–76) | 0 (0–0.06) | 1.1 (0.7–1.2) | 12 (12–24) | 1 (40%), 2 (20%), 3 (0%), 4 (20%) 5 (20%) |
| Zoladex (n=73) | 74 (67–80) | 0.3 (0–15.79) | 1.3 (0–1.8) | 48 (24–108) | 1 (18.6%), 2 (34.3%), 3 (11.4%), 4 (30%), 5 (5.7%) |
1: Biochemical recurrence; 2: metastases; 3: castrate-resistant prostate cancer; 4: locally advanced; 5: not known.
IQR: interquartile range; HRH: luteinizing hormone releasing-hormone; PSA: prostate-specific antigen.
Values are percentages of total in each group.
The median (IQR) of serum testosterone levels were 1.2 (0.95–1.55), 1.3 (1–1.8), 1.5 (1.05–1.6), 1.1 (0.7–1.2) and 1.3 (0.8–1.8) nmol/L for Eligard, Lupron, Suprefact, Trelstar and Zoladex groups, correspondingly (Table 2). The median (IQR) of PSA values were 0.03 (0–1.9625), 2.695 (0.51–10.01), 0.25 (0–3.2), 0 (0–0.06) and 0.35 (0–15.79) ug/L for Eligard, Lupron, Suprefact, Trelstar and Zoladex groups, respectively (Table 2). There was no statistically significant difference in serum testosterone levels (p = 0.3) between all five brands of LHRH agonists. In addition, Eligard, Suprefact and Trelstar were administered to a larger proportion of the cohort who had biochemical recurrence with percentages in each group of 44, 36.8 and 44, respectively (Table 2). Lupron and Zoladex were largely prescribed for cohort patients with metastatic prostate cancer (Table 2).
Discussion
The role of the prostate in androgen production has been recently demonstrated.11 Our study is the first to explore whether the primary treatment for prostate cancer (surgery, radiation or primary ADT) affects circulating levels of testosterone among men subsequently treated with ADT. We found that no difference exists in serum testosterone levels; furthermore, serum testosterone levels were not associated with duration of ADT, ADT formulation or disease status (i.e., PSA recurrence, metastasis or CRPC).
There is evidence that even hormone refractory prostate cancer cells continue to be affected by androgen signalling.12,13,16,17 Potential mechanisms accounting for this include intratumoral amplification of the androgen receptor (AR), mutations of the AR, changes in levels of AR co-factors, increased expression of enzymes involved in androgen synthesis, enhanced intracellular conversion of adrenal androgens to testosterone and DHT within the tumour micro-environment, and ligand-independent activation of the AR.14,18 Because of these processes, there is a gradual shift during prostate cancer progression from endocrine sources of androgens (i.e., from the testes and adrenal glands) to paracrine, autocrine and intracrine sources within the tumour micro-environment. Our study further demonstrates that circulating testosterone among patients treated with ADT is not dependent on primary treatment. However, measurement of testosterone levels within the tumour micro-environment may produce different results.19
The primary limitations of the study include the retrospective nature of the data and a modest population size. PSA and serum testosterone testing were performed in the same laboratory, thus eliminating measurement bias for these laboratory values. However, the serum testosterone measurements were obtained only once, and at different times during of ADT. Furthermore, we do not have details regarding the reasons for obtaining the serum testosterone measurement. However, variations in the duration of androgen suppression were not correlated to testosterone levels and were controlled for in the multivariable analysis. Finally, information on the exact mode of delivery of radiation treatment, as well as radiation dose, was unavailable and we were not able to correlate these variables to circulating testosterone levels.
Conclusion
Our study highlights several important concepts. The first is that despite increased testosterone production by prostate tissue in the castrate-resistant patient, it appears that the prostate may not be a significant source of circulating testosterone. From the preliminary evidence collected, we suggest that surgical removal of the prostate (radical prostatectomy) does not confer an added advantage in reducing serum testosterone levels. The second observation is that serum testosterone levels were not correlated with time on ADT, PSA level or specific ADT formulations.
We are the first to report that circulating levels of testosterone are independent of primary treatment among patient treated with ADT. Our study is single centre and retrospective; therefore, further studies exploring circulating and intratumoural levels of testosterone are needed to determine if primary treatment can affect the testosterone tumour micro-environment.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Prostate Cancer Canada 2011. http://www.prostatecancer.ca/Prostate-Cancer/Prostate-Cancer/Statistics (Accessed May 2, 2012).
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatises in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/S0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 3.Koo JM, Shim BS. Significance of Serum Testosterone for Prostate-Specific Antigen (PSA) Elevation and Prediction of Prostate Cancer in Patients with PSA Above 10 ng/ml. Korean J Urol. 2010;51:831–5. doi: 10.4111/kju.2010.51.12.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akaza H, Homma Y, Usami M, et al. Efficacy of primary hormone therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int. 2006;98:573–9. doi: 10.1111/j.1464-410X.2006.06349.x. [DOI] [PubMed] [Google Scholar]
- 5.Soyupek F, Soyupek S, Perk H, et al. Androgen deprivation therapy for prostate cancer: effects on hand function. Urol Oncol. 2008;26:141–6. doi: 10.1016/j.urolonc.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Gomella LG. Effective Testosterone Suppression for Prostate Cancer: Is There a Best Castration Therapy. Rev Urol. 2009;11:52–60. [PMC free article] [PubMed] [Google Scholar]
- 7.Pai HH, Pickles T, Keyes M, et al. Randomized study evaluating testosterone recovery using short-versus long-acting luteinizing hormone releasing hormone agonists. Can Urol Assoc J. 2011;5:173–9. doi: 10.5489/cuaj.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlotta A, Debruyne F. Expert opinion on optimal testosterone control in Prostate Cancer. Eur Urol Suppl. 2005;4:37–41. doi: 10.1016/j.eursup.2005.08.005. [DOI] [Google Scholar]
- 9.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–4. doi: 10.1016/S0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Geller J, Kirshner M. Acute effects of testicular and adrenal cortical blockade protein synthesis and dihydrotestosterone content of human prostate tissue. J Clin Endocrinol Metab. 1985;61:129–33. doi: 10.1210/jcem-61-1-129. [DOI] [PubMed] [Google Scholar]
- 11.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 12.Titus MA, Gregory CW, Ford OH, et al. Steroid 5©-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–71. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo G, Buonerba C, Autorino R, et al. Castration-resistant prostate cancer current and emerging treatment strategies. Drugs. 2010;70:983–1000. doi: 10.2165/10898600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher HI, Fizazi K, Saad F, et al. Effect of MDV3100, an androgen receptor signaling inhibitor (ARSI), on overall survival in patients with prostate cancer postdocetaxel: Results from the phase III AFFIRM study. J Clin Oncol. 2012;30 (suppl 5; abstr LBA1). [Google Scholar]
- 16.Labrie F. Hormonal therapy for prostate cancer. Prog Brain Res. 2010;182:321–41. doi: 10.1016/S0079-6123(10)82014-X. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mostaghel EA, Montgomery B, Nelson PS. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol Oncol. 2009;27:251–7. doi: 10.1016/j.urolonc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]