Abstract
Although ventral parietal cortex (VPC) activations can be found in a variety of cognitive domains, these activations have been typically attributed to cognitive operations specific to each domain. In this article, we propose a hypothesis that can account for VPC activations across all the cognitive domains reviewed. We first review VPC activations in the domains of perceptual and motor reorienting, episodic memory retrieval, language and number processing, theory of mind, and episodic memory encoding. Then, we consider the localization of VPC activations across domains, and conclude that they are largely overlapping with some differences around the edges. Finally, we assess how well four different hypotheses of VPC function can explain findings in various domains, and conclude that a bottom-up attention hypothesis provides the most complete and parsimonious account.
Introduction
Recently, the cognitive functions of ventral parietal cortex (VPC), which is comprised of the supramarginal gyrus (SMG) and the angular gyrus (AG), have become the focus of intense research interest and lively theoretical debate. The research interest was stimulated by the ubiquity of VPC activations in functional neuroimaging studies in a variety of cognitive domains, including perceptual and motor reorienting, episodic memory retrieval, language and number processing, and social cognition. Indicative of this interest, a recent symposium at a major cognitive neuroscience conference was devoted to the functions of a single VPC subregion.1 The theoretical debate reflects the difficulty of explaining how VPC can play a role in very dissimilar tasks, from reorienting to simple visual targets and actions, to remembering personal events, to understanding language and performing mental calculations, and to attributing beliefs to other people. Cognitive neuroscientists have generated very different hypotheses about the functions of VPC depending on the specific tasks they investigated, and it is uncertain if a single global function could explain VPC contributions to so many different tasks.
When cognitive neuroscientists try to explain why a broad brain region is recruited by different cognitive tasks, they usually adopt one of two theoretical positions. One position, which may be called the fractionation view, is that the different tasks engage distinct subregions within the broad area, which each subregion mediating very different cognitive processes. According to this view, the idea that the different tasks engage the same region is only an illusion, which is dispelled by careful demarcation of the subregions engaged by each task. An alternative position, which can be called the overarching view, is that although functional subdivisions within the broad brain region do in fact exist, the differences are more graded because each subregion mediates a different aspect of the global cognitive function supported by the broad region. The debate between fractionation and overarching views has occurred for several brain regions, with the lateral prefrontal cortex being a clear example. While some authors have emphasized differences in function between various lateral prefrontal subregions (e.g., ventrolateral vs. dorsolateral; 2, 3 anterior vs. posterior ventrolateral4, 5), others have proposed all lateral prefrontal subregions contribute to different aspects of an executive control function.6, 7 For other brain regions, most researchers support an overarching view. For example, most cognitive neuroscientist today believe that ventral occipito-temporal regions play a general role in visual processing, while at the same time acknowledge that within this broad area, different subregions are specialized in processing faces, scenes, faces, etc.8 Put another way, most overarching views focus on the general function of the region,9 whereas the fractionation view focuses on differences in the nature of representations.10
Currently, a debate between fractionation and overarching views involves theories about VPC function. Whereas some researchers have emphasized sharp functional differences between anterior (SMG) and posterior (AG) VPC, 11, 12 others have proposed a global function for all VPC subregions.13 In the current article, we review functional neuroimaging evidence regarding VPC activations in various cognitive domains. After reviewing this evidence, we consider the issue of localization across VPC subregions, and finally turn to different overarching accounts of VPC function.
Functional neuroimaging of VPC function
This section reviews functional neuroimaging evidence of VPC activations in various cognitive domains (for a brief summary on lesion evidence regarding VPC function, see Box 1). We focus on five cognitive domains: (1) perceptual and motor reorienting, (2) episodic memory retrieval, (3) language and number processing, (4) theory of mind, and (5) episodic memory encoding. Although VPC activations are also found in other domains, these domains were chosen because they are areas where VPC is assumed to be one of the core regions mediating the function and specific hypotheses regarding VPC function have been advanced. Given the complexity of these domains, we focus only on the most typical paradigms and contrasts yielding VPC activations within each domain (see Table 1) and mention only a few representative studies for each of them. Our goal is to show a broad pattern across domains (the ‘big picture’) rather than to explain the complexity of activation patterns within each domain. In other words, we focus on the forest rather than the trees.
Box 1. Effects of VPC lesions.
Reports of cognitive disturbances following VPC lesions date to the turn of the 20th century.111 Among the symptoms that have been noted in the past century are disorders of attention, action (apraxia), of language and reading, of mathematical calculations, of body schema, of spatial orientation and construction, of short-term or working memory, and of intentionality and theory of mind.112–115 The most prominent attention disorder associated with VPC lesions is hemi-spatial neglect, which is characterized by defective detection of events and impaired exploratory activities in the contralesional part of space. The frame of reference of neglect can be extra-personal or peri-personal, within egocentric or object-based frame of reference.116–118 The source of the neglect is attributed, in part, to an inability to disengage attention automatically from the intact region and direct it to the contralesional side.103, 104, 119 However, other evidence suggest that neglect is typically accompanied by other spatial and non-spatial attentional deficits that affect both sides of space.113 An even more debilitating attentional disorder caused by bilateral parietal lesions is Balint-Holmes’ Syndrome which includes an inability to perceive and report more than one object at a time, and even to bind the features of an object together also 112 . Ideomotor apraxia, an impairment in imitating gestures, pantomiming tool use and making meaningful gestures to command, can occur after left, inferior parietal lesions, especially if the gestures require a series of sequential movements.34 Gerstmann’s syndrome120 is yet another visuomotor syndrome consisting of a tetrad of symptoms: finger agnosia (the inability to recognize, name, and select individual fingers of both hands), left-right disorientation, agraphia (impaired writing), and acalculia (impaired mathematical calculation and symbol recoding and manipulation121). Although this syndrome is typically associated with left AG damage,122 the heterogeneous deficits may not reflect the function of this region and could be caused by the damage of white matter fibers passing through AG.123, 124
VPC lesions have been also associated with reading,125, 126 working memory,112, 127 and reasoning disorders.128 Among reading disorders, left AG damage has been associated with some forms of developmental dyslexia.129–131 Although attention directly plays a part in some types of acalculia132 and dyslexia,133 there is reason to believe that it does so indirectly in many other forms of these disorders via short short-term or working-memory (WM), both of which are intimately tied to attention (see below). WM deficits have been reported following AG lesions, with verbal and spatial short-term memory being impaired following damage to the left and right side, respectively.127, 134, 135 Although these deficits were initially attributed to impaired phonological or visuospatial WM buffers,115, 136 more recently they have been linked to impaired attention whose operation is needed to activate and sustain the long-term memory representations that constitute the contents of WM.137–139 Finally, VPC lesions have been associated with deficits in reasoning about one’s own and others’ mental states, such as thoughts, intentions, and beliefs, or Theory of Mind (ToM).82, 91, 114 Although reading and reasoning involve some mechanisms that are different, the deficits following VPC lesions may share an impairment in WM which arises from deficient attention-mediated processing140 that, as we noted, is necessary to activate and sustain the contents of WM.
If it were not difficult enough to find a common explanation to account for the variety of functions associated with VPC lesions, one has to overcome the discouraging evidence that each of the elements of the syndromes that are described tend not to cohere with one another. Thus, different aspects of Gerstmann’s and Balint’s syndrome are as likely to occur independently of one another123, 141, 142 as together.123, 124, 143 The same applies to the different manifestations of hemi-neglect,117 and to different aspects of ToM.82, 91 As noted above, the heterogeneity of symptoms may reflect the disconnection of regions outside VPC rather than VPC functions per se.123, 124 Nonetheless, a deficit in bottom-up attention could contribute to several of these disorders, as well as to memory difficulties following VPC damage, as reviewed in the main text.
Table 1. Domains of VPC activations. Strengths and weaknesses of VPC hypotheses.
Cognitive domains showing frequent VPC activations and hypotheses that could account for these activations
| Cognitive Domain Paradigm |
Typical Contrasts |
Semantic Retrieval H. | WM Maintenance H. | Multimodal Integration H. | Bottom-Up Attention H. |
|---|---|---|---|---|---|
| Perceptual/Motor Reorienting | |||||
| Posner task | Invalid > valid targets | − | − | − | + |
| Oddball task | Deviant > standard | − | − | − | + |
| Episodic Memory Retrieval | |||||
| Recollection | Recollection> familiarity | + | + | ++ | + |
| Recognition confidence | High > Low confidence “old” | + | + | ++ | + |
| High > Low confidence “new” | − | − | − | + | |
| Language/Number Processing | |||||
| Word comprehension | Word > nonword/pseudowords | ++ | + | − | + |
| Sentence comprehension | Anomalous > standard | − + | + | − | + |
| Mental calculation | Exact > approximate answer | + | + | − | + |
| Known answer vs. calculation | + | − | − | + | |
| Theory of mind (ToM) | |||||
| TOM stories | false belief story > false photo story | + | + | − | + |
| Episodic Memory Encoding | |||||
| Subsequent memory | Subsequent forgotten > remembered | − | − | − | + |
Notes: H.: hypothesis
Perceptual and motor reorienting
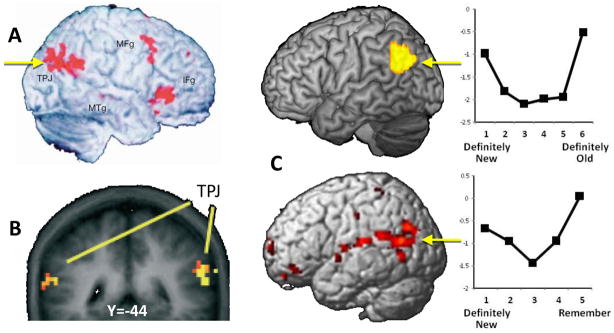
Functional neuroimaging studies that linked VPC to perceptual reorienting have used mainly two tasks, Posner and oddball. Although VPC activations during these tasks are usually bilateral and occur in both SMG and AG, most studies highlight the right temporo-parietal junction or TPJ (for VPC anatomy see Box 2). In a typical Posner task, a central cue indicates whether a target stimulus is likely to occur on the left or the right of the screen, and the target occurs in the expected (valid) location in the majority of the trials. Whereas dorsal parietal cortex (DPC) activity is greater during the cue period, VPC activity is greater during the target period.14 Moreover, target-related VPC activity tends to be greater for invalid than valid trials (e.g., Figure 1-A),15–18 consistent with a reorienting of attention when expectations are violated.19, 20 In a typical oddball paradigm, subjects process a long series of identical stimuli (“standards”) intermixed with a few (e.g., 10%) deviant stimuli (“oddballs”), which attract reflexive attention.21–28 Compared to standards, oddball stimuli elicit VPC activity bilaterally (e.g., Figure 1-B). This occurs only when the oddballs are somehow relevant to the main task, indicating that the effect is not merely due to novelty or saliency. 29, 30 In sum, functional neuroimaging studies of Posner and oddball tasks have strongly linked VPC to reorienting attention to locations or stimuli that were not part of the focus of attention but are nonetheless related to the task at hand.
Box 2. VPC anatomy and connectivity.
As illustrated by Figure I, VPC (also known as inferior parietal lobule—IPL) is a region ventral to the intra-parietal sulcus (IPS) and anterior to post-central gyrus. VPC is comprised of the supramarginal gyrus (SMG) and the angular gyrus (AG). The imprecise term temporo-parietal junction (TPJ) usually refers to an SMG area around the end of the Sylvian fissure but it has been applied to activations in AG and in the superior temporal gyrus. AG largely corresponds to Brodmann Area (BA) 39, and SMG, to BA 40. Cytoarchitectonically (see Figure II), AG can subdivided into anterior area PGa and posterior area PGp, whereas SMG can be subdivided into at least five different areas.144, 145
VPC has direct anatomical connections with most frontal and temporal lobe regions (see Figure III).146 Functional connectivity differs for various VPC subregions. For example, a recent resting-state fMRI study found that PGa is more strongly connected with basal ganglia and ventrolateral prefrontal cortex, whereas PGp is more strongly connected with the hippocampus and posterior cingulate (see Figure IV).147
Figure I. VPC is comprised of SMG (BA 40) and AG (BA 39). Figure II. AG can be subdivided into areas PGa and PG, and SMG into areas PFop, PFt, PF, PFm, and PFcm (from ref. 145). Figure III. VPC is connected to the frontal lobes via superior longitudinal (SFL), fronto-occipital (FOF), and arcuate (AC) fasciculi and to the temporal lobes via middle (MdlF) and inferior (ILF) longitudinal fasciculi (from ref. 146). Figure IV. PGa is more strongly connected with regions such as the ventrolateral prefrontal cortex whereas PGp is more strongly connected with the hippocampus and other regions (from ref. 147)
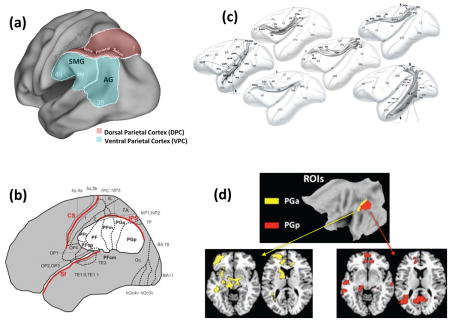
Figure 1. Perceptual reorienting and Episodic Retrieval.
Examples of VPC activations during perceptual reorienting and episodic retrieval. A. Invalid>valid trials in a Posner task (from ref. 15) B. Relevant > irrelevant distractors in an oddball task (from ref 30). C. Confidence during recognition memory (top panel from ref. 44, bottom panel from ref.45)
VPC, particularly the left supramarginal gyrus, is also activated by a motor version of the Posner task, in which a cue indicates which finger must be used to respond to a subsequent target. 31 Transcranial magnetic stimulation (TMS) of this region interferes with participants’ ability to redirect the motor action when the target is invalid.32 This effect may be interpreted as a deficit in disengaging motor attention.33 This idea fits with evidence that errors in ideomotor apraxia following VPC lesions34 tend to be greater for tasks that require a sequence of movements,35, 36 and hence involve rapid reorienting of motor attention. Impairments in tool use may also reflect a deficit in redirecting attention from a typical reach to a tool-appropriate reach.33 Consistent with the role of VPC in reorienting motor attention, a study found that VPC stimulation in awake patients undergoing surgery led to a sense of having acted though no action was detected.37
Episodic memory retrieval
Episodic memory retrieval tasks, such as old/new recognition memory tasks, consistently activate VPC.38 These activations are usually bilateral but are more frequent in left VPC, consistent with the verbal nature of the stimuli typically employed in these studies. The activations tend to increase as a function of recollection and confidence.13, 39–41 The involvement of VPC in recollection (rich, vivid memories) has been demonstrated with both subjective measures, such as the Remember-Know procedure, and objective measures, such as source memory.13, 39–42 Interestingly, compared to low-confidence responses, VPC shows greater activity not only for high-confidence “old” responses but also for high-confidence “new” responses.13, 43–45 Thus, when VPC activity is plotted as a function of perceived oldness (from “definitely new” to “definitely old” responses) it shows a clear U-function (see Figure 1-C). This U-function suggest that VPC activity tracks the relevancy of recognition responses or cues rather than memory recovery per se. Consistent with this idea, recent evidence suggest that VPC tracks the violation of expectations during retrieval.46, 47 Whereas VPC activity is greater when memory recovery is high (recollection, high confidence, etc), dorsal parietal cortex (DPC) activity is greater when recovery is low (familiarity, low confidence, etc). Based on this and other dissociations, we have proposed an Attention to Memory (AtoM) model13, 40, 48 whereby VPC and DPC mediate bottom-up and top-down attention, respectively, during episodic retrieval (see Box 3).
Box 3. The AtoM model.
The Attention-to-Memory (AtoM) model was formulated to explain the contribution of DPC and VPC to episodic memory retrieval.13, 39–41 The AtoM model makes an explicit distinction between the mnemonic roles of DPC and VPC based on the differential roles these regions play in attention. According to a prominent model,103 DPC mediates endogenous attention, which enables selection of stimuli based on internal goals, whereas the VPC mediates exogenous attention, which enables detection of relevant stimuli when attention is not directly focused on them. According to the AtoM hypothesis, DPC and VPC serve analogous attention roles in memory retrieval: DPC mediates the allocation of attention to memory retrieval operations (top-down AtoM), whereas VPC mediates the bottom-up capture of attention by salient memory contents (bottom-up AtoM). Consistently, processing in DPC is prominent when memory retrieval loads heavily on top-down attention (e.g., strategic retrieval operations), whereas processing in VPC is prominent when the recovered memory is salient and therefore captures attention bottom-up (e.g., recollection). 13, 39–42 Moreover, PPC patients may be unable to retrieve and re-experience relevant memory contents spontaneously (bottom-up), in the face of a generally preserved ability to access memory contents if adequately probed (top-down).105, 106, 108, 148
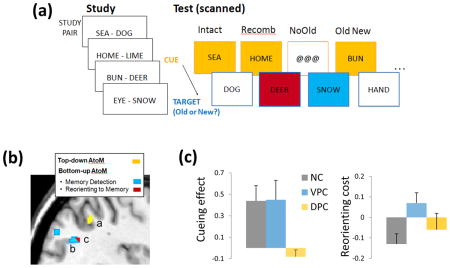
Using fMRI, Ciaramelli and collaborators47 dissociated the functional profile of DPC and VPC in episodic memory within a single, “Posner-like”, recognition memory paradigm. Participants studied word pairs and then detected studied (target) words among new words. In some conditions, a studied word cued the upcoming target word, facilitating recognition performance (Figure I). Left DPC (a) was engaged when participants searched for\anticipated memory targets upon presentation of memory cues, whereas left VPC mediated target detection on noncued (b) and invalidly cued trials (c) (Figure II). These results mirror closely those obtained in the perceptual domain.14 These findings were confirmed in a small sample of patients with lesions limited to VPC and DPC. DPC patients did not show a normal advantage in the validly cued compared to the non-cued condition, whereas patients with VPC lesions had problems detecting memory probes that violated mnemonic expectations (Figure III).
Figure I. Example trials of the “Posner-like” recognition memory paradigm. Figure II. Left DPC (a) is associated with top-down attention to memory during cued recognition decisions; left VPC is associated with (bottom-up) detection of non-cued (b) and invalidly cued (c) memory targets. Figure III. Damage to DPC causes a reduction of the Cueing effect (Acc_Intact – Acc_No Old\Acc_NoOld); Damage to VPC causes an increase in the Reorienting cost after invalid cues (RT_Recombined – RT_NoOld \ RT_NoOld) (from ref 47).
Language and number processing
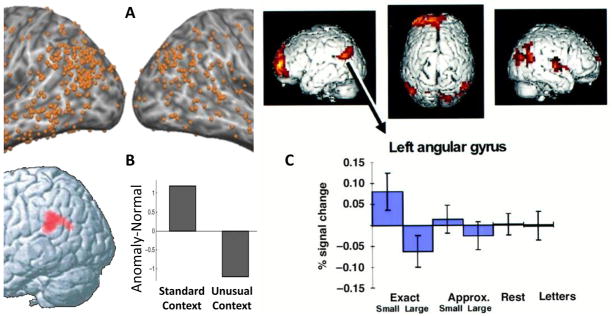
In language studies, VPC activations are frequently found during words and sentence comprehension. Regarding word comprehension, a recent metaanalysis49 identified VPC as one of the regions most strongly associated with semantic processing of spoken and written words, including contrasts such as words vs. nonwords,50–52 familiar vs. unfamiliar people names,53, 54 semantically related vs. unrelated words,55, 56 and semantic vs. non-semantic tasks.57, 58 Although researchers in this domain usually emphasize left AG, the results of the metaanalysis (see Figure 2-A) clearly shows that VPC activations are bilateral and occur in both AG and SMG. Regarding sentence comprehension, VPC is also activated while reading or hearing sentences compared to lists of random words or pseudowords.59–61 Several studies found that VPC activity was greater for sentences with semantic violations than for normal sentences.62–64 This effect may reflect the demands of retrieving semantic knowledge or the demands of processing discourse coherence. To investigate these two explanations, one study64 compared normal vs. anomalous sentences (e.g., With lights you can see more/less at night.) that were preceded either by a standard context or by an unusual context that explained the anomaly (e.g, Lamp posts block the night sky. This is sad for astronomers.). As illustrated by Figure 2-B, VPC showed greater activity for anomalous sentences in the standard context, but for normal sentences in the unusual context, suggesting that VPC activity tracks discourse incoherence rather than knowledge retrieval per se.
Figure 2. Language and number processing.
Examples of VPC activations during language and numerical processing. A. Metaanalysis of semantic processing during word comprehension (from ref. 49). B. VPC activity increases with discourse incoherence (from ref. 64). D. VPC activity is greater for exact than approximate number calculation (from ref. 67).
In the related domain of number processing, VPC activations also tend to be bilateral (e.g., Figure 2-C) but again researchers emphasize the role of left AG. In general, VPC activations are found in conditions that require the retrieval of numerical facts.65 For example, VPC activity is greater for exact (e.g., 4+5→ select correct: 9 or 7) than approximate (e.g., 4+5→ select closer: 8 or 3) calculations.66, 67 Also, VPC activity is greater for problems whose answer is stored in memory, including multiplying single digits,68, 69 adding single digits up to a total of 10,67 and comparing small numbers.70 Moreover, VPC shows greater activity when participants solve previously trained than untrained multiplications problems (e.g., 14×7=98)71 or when they report using retrieval rather calculation strategies.72 All these findings are consistent with numerical fact retrieval.
Theory of mind
Theory of mind (ToM) refers to the ability to think about mental states in oneself and other people, including thoughts and beliefs. Functional neuroimaging studies have investigated ToM using a variety of stimuli, including stories,73–77 cartoons,78–80 and animations.74, 81 VPC activations are very frequent during TOM tasks, particularly when using stories.82 ToM stories usually involve “false beliefs” 73–77 as in the classic Sally-Anne story: Sally put her ball in a basket and left the room. While Sally was away, Anne moved the ball from the basket to a box. When Sally came back, did she look for her ball in the basket or in the box? Answering this question correctly requires inferring Sally’s mental state, which is the false belief that the ball is in place where she left it. As a control condition, several studies 75–77, 83 have used “false photograph” stories such as the following: A photograph was taken of an apple hanging on a tree branch. While the photograph was being developed, strong wind blew the apple to the ground. Did the developed photograph show the apple on the branch or on the ground? The false belief vs. false photo contrast, which has been called a “ToM localizer”, typically yields activations in bilateral VPC regions, including both AG and SMG. However, these activations are often labeled TPJ and the right hemisphere is emphasized.84, 85
Episodic Memory Encoding
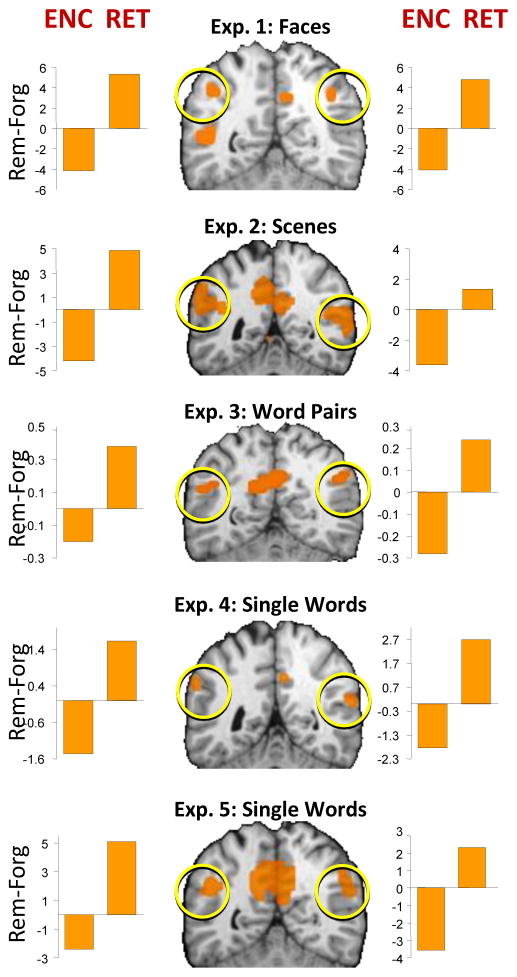
Whereas in all the domains above, VPC activations are generally associated with successful performance, in the episodic encoding domain they are associated with failed performance. In contrast with medial temporal lobe and ventrolateral prefrontal cortex, which typically show greater activity for subsequently remembered than forgotten items86 (subsequent memory effect—SME), VPC usually shows greater activity for subsequently forgotten than remembered items87, 88(reverse SME). Interestingly, the VPC regions showing reverse SME effects during encoding overlap the ones showing memory success effects during retrieval. As illustrated by Figure 3, across five different experiments involving a variety of stimuli, 89 same VPC regions associated with memory failure during encoding (subsequently forgotten > remembered) were associated with memory success during retrieval (remembered > forgotten). This “encoding-retrieval flip” finding markedly constraints possible accounts of VPC function because it implies that VPC mediates a cognitive process that is (1) beneficial for perceptual/motor reorienting, episodic memory retrieval, language/number processing, and TOM, but (2) detrimental for episodic memory encoding. As discussed later, few cognitive processes can fulfill both requirements.
Figure 3. Encoding-Retrieval Flip.
Contrasts between retrieval and encoding in the same participants show that VPC is associated with retrieval success (remembered > forgotten) but with encoding failure (forgotten > remembered) (From ref. 89)
Localization of VPC activations across cognitive domains
As noted before, VPC activations in all the cognitive domains reviewed have been found in both left and right hemispheres and in both anterior and posterior VPC subregions. Despite this fact, most domains emphasize one hemisphere and one VPC subregion. The focus on a single hemisphere probably originated in the brain damage literature, which often underscored trends lesion lateralization (e.g., neglect is more frequent after right than left parietal damage). The focus on a single VPC subregion is partly due to the popularity of certain anatomical labels, such as TPJ and AG. For example, perceptual reorienting studies tend to label most VPC activations “TPJ”, even if they occur in posterior VPC (e.g., Figure 1-A), whereas language/number processing studies tend to label most VPC activations “AG”, even if they extend into SMG (e.g., Figure 2-B). We are not suggesting that the spatial distribution of VPC activations is absolutely identical across all cognitive domains. The spatial distributions of VPC activations in different domains are neither perfectly segregated nor perfectly overlapping; they are largely overlapping with some differences around the edges. The next section provides two examples of this overlap-with-differences pattern, and the following section considers possible explanations.
Examples of the overlap-with-differences pattern
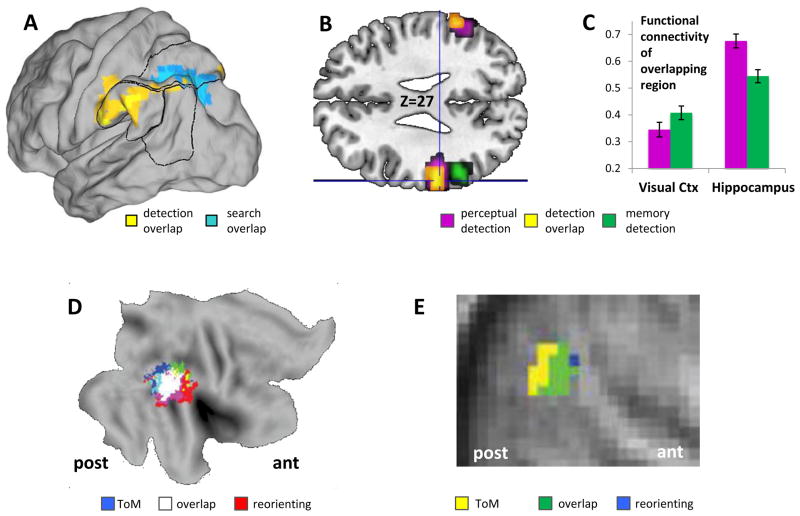
One example of overlaps with differences around the edges is between the domains of perceptual reorienting and episodic retrieval. A metaanalysis of left VPC activations in these two domains concluded that activation peaks were more anterior (SMG) for perceptual reorienting but more posterior (AG) for episodic retrieval.11 To investigate this idea, a recent fMRI study compared VPC activations in these two domains within-participants.90 In the perceptual reorienting task, participants searched a stream of consonants on the screen and pressed a key when they detected a vowel (an oddball task), whereas in a episodic retrieval task, they searched their memory for previously studied word-chains and pressed a key when they detected the last word of a chain. Consistent with the AtoM model (see Box 3), conjunction analyses showed that in both tasks the search phase activated DPC whereas the detection phase activated VPC (Figure 4-A). The overlap in VPC was found within SMG (yellow region in Figure 4-B), but, consistent with the aforementioned metaanalysis,11 VPC activations in the episodic retrieval task extended posteriorly towards AG (green region in Figure 4-B). As discussed later, functional connectivity of the overlapping (yellow) region varied according to the task (Figure 4-C). In sum, the results of this study suggest that perceptual reorienting and episodic retrieval engage overlapping VPC regions with some differences around the edges.
Figure 4.
VPC overlaps across functions. A. VPC overlap for perceptual and memory detection (from ref. 90). B. In the same study, memory detection activity extends more posteriorly than memory detection. C. Functional connectivity of the overlapping left VPC region (in yellow) with visual cortex was stronger during perception than memory whereas functional connectivity with the hippocampus was stronger during memory than perception. D. Metaanalysis showing overlap in right VPC activity for perceptual reorienting and TOM (from ref. 91). E. Overlap between reorienting and TOM within participants (from ref. 77)
VPC overlaps across functions
Another example of overlaps with differences around the edges involves the domains of perceptual reorienting and ToM. Even though VPC activations in these domains are typically bilateral and involve both SMG and AG, both domains tend to emphasize the role of right TPJ. To investigate the distribution of activations in this region, Decety and Lamm91 conducted a metaanalysis including perceptual reorienting and ToM studies. Activations in these two domains largely overlapped within SMG (in white in Figure 4-D) but from there reorienting activity extended anteriorly and ToM activity, posteriorly. A similar pattern was found by Mitchell 77 when he compared a ToM task (false belief vs. false photo stories) to a Posner task (invalid > valid trials) within participants: there was a large overlap (in green in Figure 4-E) and from there reorienting and ToM activity extended anteriorly and posteriorly, respectively. In another study comparing these two domains, the differences were more along a ventral-dorsal axis. 92 In sum, when VPC activations across domains are compared across studies11, 91 or within-participants77, 90 the results consistently show large areas of overlap with some differences around the edges.
Explaining the overlap-with-differences pattern
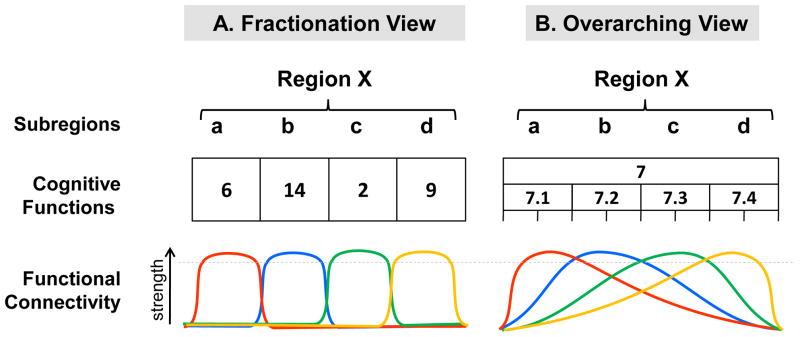
How shall one interpret the finding that VPC activations for different cognitive functions largely overlap but there are some differences around the edges? As mentioned in the Introduction, there are two different ways of conceptualizing the spatial distribution of cognitive functions across the subregions of a large area such as VPC. According to a fractionation view (Figure 5-A), the broad region does not have a global function; instead, each of its subregions mediates a distinct—sometimes very different—function (subregion ‘a’ mediates process 6; subregion ‘b’ mediates process 14, etc). Likewise, each subregion is assumed to be connected with a different network (e.g., subregion ‘a’ is connected to a ‘red’ network; subregion ‘b’, to a ‘blue’ network, and so forth). According to an overarching view (Figure 5-B), in contrast, the broad region has a global function (e.g., function 7) and its various subregions mediate different aspects of the same function (e.g., subregion ‘a’ mediates process 7.1 and subregion ‘b’, process 7.2, etc). These various processes are dissociable by experimental manipulations and may appear as very different at a concrete level. However, they can still be described as consistent with a global function for the entire region, described at more abstract levels. As suggested in the introduction, whereas the subregions may differ at the representation level, they all contribute to different aspects of the same function. Representational processes may be partly determined by variations in functional connectivity, but these variations are assumed to be graded rather than sharp, perhaps reflecting intermixed populations of neurons and their projections.
Figure 5. Fractionated vs. overarching views of functional organization.
Fractionated vs. overaching views of functional organization across hypothetical subregions 1–4 of hypothetical Region X.
In general, available functional neuroimaging evidence about VPC function fits better with the overarching than the fractionation view. The fractionation view cannot easily account for substantial overlaps in VPC across different very cognitive domains, such as attention, episodic retrieval, and TOM (see Figure 4). In contrast, the overarching view can explain these overlaps on the assumption that different cognitive domains engage different aspects of the same broad VPC function. One way of conceptualizing these different aspects is that that they reflect the application of a similar cognitive operation to different kinds of information. For example, the overlap between perception and memory detection depicted in Figure 4-A can be explained by assuming that VPC mediates a detection process that can be applied to perceptual information as well as to memory information. Consistent with this idea, the overlapping VPC region showed stronger connectivity with visual cortex during a perception task but stronger connectivity with the hippocampus during a memory task (see Figure 4-C). The overarching view can also explain differences around the edges under the assumption that the strength of VPC connectivity with different brain regions differs gradually across VPC subregions (see Figure 5-B). For example, if one assumes that connectivity with the hippocampus is stronger for posterior VPC regions (AG), then the overarching view predicts that VPC activations extend more posteriorly during a memory than a perception task (Figure 4-B), even if the VPC processes engaged by these tasks are very similar. The assumption that the localization of VPC activation vary according to functional connectivity can also explain why memory tasks using words, which interact with a left-lateralized language network, tend to elicit stronger activations in left VPC, whereas perceptual reorienting tasks using spatial information, which interact with a right-lateralized spatial processing network, tend to elicit stronger activations in right VPC. Even if left and right VPC mediate similar cognitive operations, hemispheric asymmetries can be expected depending on other brain regions engaged by the task.
In sum, the overarching view fits very well with evidence of overlaps with differences around the edges. This evidence suggests that VPC has a global function but different subregions apply this process to different types of information and goals, which varies according to functional connectivity. A similar idea has been previously proposed for frontal lobe subregions, which could mediate a general executive control function by applies it to different posterior regions depending on the task. 6, 9, 93, 94 The open question is what global VPC function could explain the involvement of this region in perceptual/motor reorienting, episodic memory retrieval, language/number processing, and ToM tasks, as well as its association with encoding memory failure. This is the topic of the next section.
Hypotheses about a global VPC function
The sections below discuss four hypotheses that were originally proposed to account for the contribution of a specific VPC subregion to a particular cognitive domain. Here we assess whether these hypotheses could be expanded to account for VPC contributions to all the domains reviewed, namely perceptual/motor reorienting, episodic retrieval, ToM, language/number processing, and episodic encoding (see Table 1).
Semantic retrieval hypothesis
According to Binder and collaborators,49 left AG activations during language comprehension reflect the recovery of semantic knowledge. Can this hypothesis account for VPC activations in other cognitive domains? The easiest extension of the semantic retrieval hypothesis is to the number processing domain, where left AG activations have been already attributed to the retrieval of particular kind of semantic knowledge, namely numerical facts.65 The semantic retrieval hypothesis could explain some motor reorienting findings, such as deficits in processing meaningful actions in ideomotor apraxia. The semantic retrieval hypothesis could also account for VPC activations during episodic memory retrieval under the assumption that episodic retrieval requires the retrieval of concepts. As noted by Binder and collaborators “to recall, for example, that ‘I played tennis last weekend’ logically entails retrieval of the concepts ‘tennis’, ‘play’ and ‘weekend’“ (p. 2781). Finally, the semantic retrieval hypothesis can account for the involvement of VPC during ToM tasks such false belief stories because these stories tend to require additional semantic processing in order integrate different perspectives.
On the other hand, the semantic retrieval hypothesis does not fare as well in accounting for VPC activations in other domains (see minuses in Table 1). First, perceptual and motor reorienting studies involve the detection of meaningless sensory stimuli or the execution of meaningless movements, and hence, they require very little semantic processing. Second, “definitely new” recognition memory trials (see Figure 1-C) do not seem to involve additional semantic retrieval. Third, as illustrated by Figure 2-A, there is evidence that VPC activations during sentence comprehension track discourse incoherence rather than semantic retrieval per se.64 Finally, given that semantic processing generally enhances encoding,95 the semantic retrieval hypothesis cannot easily explain why VPC activity is associated with encoding failure rather than success.
Working memory maintenance hypothesis
Within the episodic retrieval domain, VPC activations have been attributed to the maintenance of information within working memory (WM).38 This hypothesis can explain why VPC activity during episodic retrieval is greater for recollection-based and confident “old” recognition trials given that trials involve greater information recovery and hence a heavier WM load. Can the WM hypothesis account for VPC activations in other cognitive domains? “In the motor reorienting domain, this hypothesis could account for neuropsychological evidence of apraxia in VPC patients, which could arise from deficits in maintaining and manipulating information in WM.96 The WM hypothesis could account for language and number processing findings by assuming that recovering semantic information (e.g., words >nonwords) increases WM load. However, semantic retrieval may also lead to chunking which reduces WM load. Showing some advantage over the semantic retrieval hypothesis, the WM maintenance hypothesis could account for the role of VPC in addressing text incoherence (Figure 2-B), which requires holding and comparing alternative interpretations within WM. For similar reasons, the WM maintenance hypothesis provides a good account for ToM findings, which require simultaneous consideration of alternative viewpoints.
However, the WM maintenance hypothesis has several weaknesses (see minuses in Table 1). First, perceptual and motor reorienting effects entail very little or no change in WM load. In the perceptual reorienting domain, it could be argued that invalid>valid and deviant > standard contrasts involve WM updating but it is difficult to distinguish between WM updating and bottom-up attention, which is the focus of a different hypothesis. Second, high-confidence “new” recognition responses are unlikely to involve much memory recovery and WM load. Third, when solving math problems, mental calculation should load WM more, not less, than retrieving the answer from memory. 72 Finally, the WM maintenance hypothesis cannot easily explain encoding failure findings, unless one argues that VPC activity reflects the maintenance of irrelevant information within WM.
Multimodal integration hypothesis
Episodic recollection is characterized by the recovery of multiple types of information, such sensory, conceptual, and emotional aspects of the same event, and the role of VPC has been attributed to the integration of these multimodal features. There are two versions of this multimodal integration hypothesis. According episodic buffer view,41 left AG activity reflects the maintenance of integrated multimodal information by the episodic buffer in Baddeley’s WM model.97 According to the cortical binding of relational activity (CoBRA) view,98 left AG is a convergence zone that binds episodic features stored in disparate neocortical regions to promote consolidation of memories in neocortex. These two views have different assumptions and make different predictions but we consider them here only in terms of their shared assumption regarding multimodal integration. In this regard, the main strength of both views is accounting for the involvement of VPC in episodic recollection, which—by definition—involves greater multimodal integration than familiarity. For the same reason, the multimodal integration hypothesis can explain very well why VPC activity increases with the amount of information recollected 41, 99–101 and with confidence in “old” responses (see Figure 1-C). It can also be stretched to account the application of actions in tool use or to visuo-spatial targets.
Conversely, the multimodal integration hypothesis has difficulty accommodating phenomena that do not involve multimodal integration. First, within the episodic retrieval domain, this hypothesis cannot easily explain strong VPC activity for high-confidence “new” responses, given that multimodal information recovery is minimal or null. One possible counterargument 98 is that rejecting nonstudied items involves remembering studied items but there is evidence that this “recall-to-reject strategy” is unlikely during item recognition.102 Second, perceptual and motor reorienting effects entail very little multimodal integration because most paradigms involve the same kind of information (e.g., spatial, finger movements). The same can be said about language and number processing findings. Third, although TOM stories require considering multiple points of view, these different viewpoints are all conceptual and do not necessarily involve different types of information. Finally, like semantic retrieval and WM maintenance hypotheses, the multimodal integration hypothesis cannot explain why VPC activity is associated with encoding failure. The episodic buffer view predicts that maintaining integrated multimodal information in WM should be beneficial for encoding. According to CoBRA, the contribution of VPC to binding of multimodal information occurs after encoding, and hence, VPC is not expected to be associated with encoding success.98 However, CoBRA cannot explain why VPC is associated with encoding failure.
Bottom-up attention hypothesis
According to Corbetta and Shulman’s dual-attention model,103 a dorsal frontoparietal system that includes DPC mediates the selection of perceptual stimuli based on internal goals or expectations (endogenous, or goal-driven attention), whereas a ventral frontoparietal system that includes VPC enables the detection of salient and behaviorally relevant stimuli in the environment, especially when they were previously unattended (exogenous, or stimulus-driven attention). This exogenous attention account of VPC explains very well evidence of VPC activations during perceptual and motor reorienting tasks but it cannot accommodate VPC activations during tasks without obvious environmental targets, including episodic retrieval, ToM, and language/number processing tasks.
However, this situation changes dramatically if one modifies this hypothesis so that it includes not only attention captured by environmental stimuli but also attention captured by internal (memory-based) information.13, 40, 48, 104 According to this bottom-up attention (BUA) hypothesis, VPC activity reflects the capture of bottom-up attention by information entering WM either from senses or from long-term memory. A clear example of the memory-trigged BUA is the startle response we may display when we suddenly remember that we forgot an important appointment. Most of the time, however, bottom-up capture of attention by incoming memories is more subtle and consists of a brief awareness that new episodic or semantic information entered WM. Unlike the exogenous attention hypothesis, the BUA hypothesis can accommodate not only the findings in the perceptual and motor reorienting domain but all reviewed findings in the domains of episodic retrieval, language/number processing, and episodic encoding.
Episodic retrieval
The BUA hypothesis can explains VPC activations during episodic retrieval.13, 40, 48 Given that recollected episodic details capture BUA, this hypothesis can easily explain why VPC activity is greater for recollection than for familiarity,41 increases with the amount of information recollected,41, 99–101 and is stronger for high- than low-confidence “old” responses.43–45 Importantly, the BUA hypothesis is the only one of the four hypotheses considered that can explain why VPC activity is stronger for high- than low-confidence “new” responses.44, 45 BUA is captured not only by rich memories but also by salient retrieval cues, such as items that appear very novel in a recognition memory task. According to the BUA hypothesis, what drives VPC activity is the extent to which memories or retrieval cues capture attention, and in a recognition memory test both “definitely old” and “definitely new” items are the most salient relevant items. Thus, the BUA hypothesis provides a parsimonious account for the U-function in Figure 1-C.
Although the current review is focused on functional neuroimaging evidence, it is worth noting that the BUA hypothesis can also explain subtle memory deficits in patients with VPC lesions. The BUA hypothesis predicts that VPC lesions should not impair memory per se but only the extent to which these memories capture BUA. Thus, VPC patients should have a deficit in spontaneously reporting memories (BUA) but they should be able to report them when guided by the memory task (top-down attention). This is exactly what the few studies available have shown. In one study, VPC patients spontaneously reported fewer details in their autobiographical memories but they were able to provide the missing details when prompted by the experimenter.105 Similarly, patients with VPC lesions subjectively rated their memories as impoverished but were able to recall source memory information when specifically questioned.106 This is analogous to the neglect syndrome, which does not affect perception per se but bottom-up attention to percepts. Accordingly, memory deficits in VPC patients may be described as memory neglect.13, 48 Simons and collaborators noted that VPC lesions reduce confidence in episodic memories and impair the subjective experience of recollection.107, 108 The BUA hypothesis can account for these effects and additionally explain many other findings outside the episodic retrieval domain.
Language and number processing
The BUA hypothesis can account for VPC activations during language and number processing in a way that is similar to that of episodic retrieval: when information from long-term memory enters WM, it captures BUA. For example, evidence of greater VPC activity for words than nonwords,50–52 for familiar than unfamiliar names of people,53, 54 and for semantically related than unrelated words, 55, 56 is explained by the idea that retrieved semantic knowledge captures BUA. VPC activity is likely to greater for semantic than nonsemantic tasks57, 58 if responses to the former are more immediate and hence effective in capturing BUA. The BUA hypothesis can also accounts for mental calculation findings: VPC show greater activity for problems with known than unknown answers 67–70 and for problems with exact than approximate answers66, 67 because a known, exact answer “pops” into mind capturing BUA. For the same reason, VPC activity is greater for retrieval than calculation strategies 72 and for trained than untrained problems.71
Turning to sentences, the BUA hypothesis can explain greater VPC activity for sentences than random words 59–61 because access to the sentence meaning is likely to captures BUA more strongly. On the other hand, anomalous sentences activate VPC more than normal sentences62–64 because BUA is captured by the violation of expectations (reorienting). The BUA hypothesis can also explain why this effect is reversed when the sentences are preceded by an unusual context explaining the anomalous sentence64(see Figure 2-B): in the unusual context, the normal sentence is the one that violates expectations and captures BUA more strongly. Alternatively, comprehending inconsistent sentences may require more attentional switches between the different units of the phrase.
ToM
As previously proposed by other authors,77, 91, 104 BUA could account for VPC activity during ToM tasks. Like anomalous sentences62–64 and incoherent texts,64 false belief stories requires processing information detected outside the main focus of attention. Consistent with the BUA account, ToM and perceptual reorienting tasks recruit overlapping VPC regions across different studies 91 and within participants.77 Activations in neighboring regions92 are also consistent with the BUA hypothesis because BUA is only one of several factors accounting for localization within parietal cortex. Other factors include the nature of the stimuli (e.g., verbal vs. nonverbal, spatial vs. nonspatial) and the nature of the task, and hence, contrasts between dissimilar stimuli are likely to yield different locations even if the same process is involved.
Encoding-retrieval flip
The BUA hypothesis is the only one of the four hypotheses considered that can explain why VPC activity is associated with success during retrieval but with failure during encoding (see Figure 3). During retrieval, successful performance requires disengaging attention from the retrieval cue and reorienting it towards a recovered memory. In typical encoding conditions, in contrast, to-be-encoded items are in the focus of attention and no reorienting of attention is required. In these conditions, attention reorienting usually reflects distraction by unrelated stimuli or thoughts, and hence, VPC activity tends be associated with failure to encode target items. However, the BUA hypothesis predicts that if one measures subsequent memory for the items that captured BUA, then VPC activity should predict encoding success rather than failure.48
Summary
Among the hypotheses assessed, the BUA hypothesis provides a more complete account of VPC activations across cognitive domains (see Table 1). The semantic retrieval and WM buffer hypotheses provide generally good accounts of episodic retrieval, language/number processing, and ToM findings but they cannot easily explain perceptual/motor reorienting and episodic encoding findings. The multimodal integration hypothesis appears is limited mainly to recollection and high-confidence “old” findings. In contrast, the BUA hypothesis can explain not only internal/conceptual findings in memory, language, and ToM domains but also the external/perceptual finding in the perceptual/motor reorienting domain.
Caveats about the BUA hypothesis
Although the BUA hypothesis provides an excellent account of VPC activations across cognitive domains (see Table 1), it is important to consider several potential issues for this hypothesis.
First, a potential issue for any theory postulating a global VPC function is the fact that this region consists of several subregions with different cytoarchitectonic structure and connectivity (see Box 2). This is not a problem for the BUA hypothesis, which acknowledges that the different VPC subregions mediate different domains. The BUA hypothesis does not propose the domains mediated by different VPC subregions are identical; it only assumes that these different domains can be conceptualized as different aspects of BUA. They could mediate different BUA processes or the same BUA process applied to different types of input (e.g., mnemonic input from the medial temporal lobes or visual input from occipital cortex). Differences in cytoarchitectonic structure and connectivity among VPC subregions do not always imply sharp differences in cognitive operations. If this were the case, then various ventral occipito-temporal subregions, which differ in both cytoarchitectonic structure and connectivity, could not be said to mediate different aspects of visual information processing, yet serve an overarching common visual function.
Second, a potential problem for the BUA hypothesis would be evidence of fMRI dissociations between anterior and posterior VPC subregions. As reviewed before, however, meta-analyses of fMRI data11, 91 do not show sharp dissociations between these subregions; they show overlaps with differences around the edges. Overarching views like BUA hypothesis can easily account for differences around the edges. A recent fMRI study found a dissociation between anterior and posterior VPC regions for visual vs. memory search, respectively.12 This finding could be accommodated by an overarching view, though it must be noted that in that particular study the paradigm focused on top-down attention rather than to bottom-up attention, and hence the results are not directly related to the BUA hypothesis.
Third, it has been argued that AG and SMG mediate different functions because the AG is more likely than SMG to show deactivations compared to the resting baseline and to form part of the default mode network.104 We believe that knowing whether a region is activated or deactivated compared to the resting baseline would be informative about the function only if one could specify the cognitive processes active during rest, but this is very difficult or impossible. If one assumes that rest involves retrieval from episodic memory, then AG could be more involved in episodic retrieval than SMG is. This idea is consistent with the results of meta-analyses11, as well as with evidence that VPC activations extend more posteriorly for BUA to memory than for BUA perception (Figure 4-B). As noted before, this finding is not inconsistent with the BUA hypothesis because the exact localization of BUA-related activations within VPC may reflect the informational input (e.g., medial temporal lobe vs. visual cortex).
Finally, one weakness of the BUA hypothesis is that direct evidence that VPC activations reflect BUA is strong for only some of the domains reviewed. At present, the link between VPC activity and BUA is very strong in the perceptual/motor reorienting domain14 and moderately strong in the episodic retrieval domain.13, 40 In the other domains, the BUA hypothesis provides a convincing account of many reported VPC activations but this hypothesis has not been directly tested. In the language and number processing domains, we have argued that when information from long-term memory enters WM (e.g., words meaning, math facts), it captures BUA. However, strong evidence would require directly manipulating BUA during language and math tasks, which, to our knowledge, has not been done. Likewise, we proposed that the involvement of BUA in ToM reflects the reorientation of attention during false belief stores. Although this hypothesis is consistent with the overlap of ToM and perceptual reorienting activations across studies 91 and within participants.77 (see Figure 4-D and 4-E), direct evidence for this hypothesis is missing. Similarly, although BUA can account for the encoding-retrieval flip by assuming that BUA is captured by irrelevant information during encoding but by recovered memories during retrieval, direct evidence for this hypothesis is not available.
In sum, whereas the main strength of the BUA hypothesis is that it can parsimoniously explain findings in many different cognitive domains, the breadth of this hypothesis is also its main weakness. As noted by Walsh, who proposed an overarching theory of parietal cortex in terms of magnitude processing,109 attentional theories are often too non-specific and malleable. We believe that these potential weaknesses can be mitigated by making the predictions that follow from it specific, as he have attempted in this review; moreover, whatever weakness may remain is offset by the broad explanatory power of this hypothesis and its potential for integrating evidence from many cognitive domains.
Conclusions
At a more general level, the BUA hypothesis emphasizes the role that attention plays across all domains. Though such domain-general roles typically are assigned to prefrontal cortex, the close relationship of regions of posterior parietal cortex with prefrontal cortex suggests that they may share in such broadly applied functions. Closely linked to the prefrontal cortex, and lodged between the ventral perceptual stream and the dorsal action stream, the VPC is ideally situated to its function of capturing and sustaining activated representations in the service of thought, planning, and action. Specialization within the VPC does not reflect different functions, but rather the engagement of the same function, BUA, with respect to information from different domains whose input and output pathways are located, in a graded manner, in different regions of the VPC. For example, we noted that WM, which has been identified with functions of the prefrontal cortex, can itself be considered an outgrowth of the interaction of VPC-mediated attention with perceptual and long-term memory representations in different regions of neocortex. It also seems reasonable that a process such as BUA would be applied to all domains, since cognitive flexibility demands a mechanism that allows unexpected, but relevant information, to capture attention. Without such a mechanism, once a goal-directed process is initiated, it could never be interrupted, no matter how important such unexpected information would be. As with all models, we expect that as new evidence emerges, details of the model will change, but we hope that its general principles will prove more resilient and continue to guide research on the role of attention in perception, cognition and action. The fact that all these different processes recruit similar VPC regions cannot be coincidental. As pointed out by Cajal “All natural arrangements, however capricious they may seem, have a function” (110 cited by 109)
Box 4. Questions for future research.
Do cognitive deficits following VPC damage reflect the function of this region or the damage of white matter fibers passing through this region?
In studying patients with lesions, the focus is usually on the most salient deficit. Would VPC lesions typically produce an ensemble of deficits associated with the functions identified in this article?
Can transcranial magnetic stimulation (TMS) reproduce the variety of symptoms associated with VPC damage as they did in motor reorienting?
How do spatial and nonspatial aspects of VPC function relate to each other? Is it possible to manipulate the relative contribution of left and right VPC to Posner and oddball tasks by varying the nature of the stimuli (spatial vs. nonspatial, meaningful vs. meaningless)?
If the VPC activations for tasks in different domains overlap, would these tasks interfere with each other when conducted concurrently? What determines which function would capture attention?
How is multimodal integration in episodic memory98 related to binding of features in perception?149 Are the same VPC regions implicated?
Is unintentional recovery of internally generated information associated with VPC activation as much as capture by external stimuli?
What is the time course of the interaction between VPC and the regions from which it received input and directs output, such as the prefrontal cortex? Can these time courses predict the onset of conscious awareness?
Acknowledgments
This work was supported by NIA grants AG19731 and AG34580 to RC and NSERC grant A8347 to MM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roberto Cabeza, Center for Cognitive Neuroscience, Duke University.
Elisa Ciaramelli, Department of Psychology, University of Bologna.
Morris Moscovitch, Department of Psychology, University of Toronto.
References
- 1.Rosenberg-Lee M, Menon V. Symposium: New Perspectives on the Cognitive Functions of the Angular Gyrus. 2011 Meeting of the Cognitive Neuroscience Society; MIT Press; 2011. [Google Scholar]
- 2.Petrides M. Functional organization of the human frontal cortex for mnemonic processing: Evidence from neuroimaging studies. Annals of the New York Academy of Sciences. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Annals of the New York Academy of Sciences. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 4.Poldrack RA, et al. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 5.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 8.Grill-Spector K, Malach R. The human visual cortex. Annual Review of Neuroscience. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- 9.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscovitch M, Umiltà C. Modularity and neuropsychology: Implications for the organization of attention and memory in normal and brain-damaged people. In: Schwartz ME, editor. Modular processes in dementia. MIT Press; 1990. [Google Scholar]
- 11.Hutchinson JB, et al. Posterior parietal cortex and episodic retrieval: Convergent and divergent effects of attention and memory. Learning & Memory. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sestieri C, et al. Attention to Memory and the Environment: Functional Specialization and Dynamic Competition in Human Posterior Parietal Cortex. Journal of Neuroscience. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabeza R, et al. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbetta M, et al. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 15.Arrington CM, et al. Neural mechanisms of visual attention: Object-based selection of a region in space. Journal of Cognitive Neuroscience. 2000;12:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- 16.Kincade JM, et al. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer AR, et al. The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage. 2006;30:938–949. doi: 10.1016/j.neuroimage.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 18.Vossel S, et al. What is “Odd” in Posner’s Location-cueing Paradigm? Neural Responses to Unexpected Location and Feature Changes Compared. Journal of Cognitive Neuroscience. 2009;21:30–41. doi: 10.1162/jocn.2009.21003. [DOI] [PubMed] [Google Scholar]
- 19.Doricchi F, et al. Neural Correlates of the Spatial and Expectancy Components of Endogenous and Stimulus-Driven Orienting of Attention in the Posner Task. Cerebral Cortex. 2010;20:1574–1585. doi: 10.1093/cercor/bhp215. [DOI] [PubMed] [Google Scholar]
- 20.Shulman GL, et al. Interaction of Stimulus-Driven Reorienting and Expectation in Ventral and Dorsal Frontoparietal and Basal Ganglia-Cortical Networks. Journal of Neuroscience. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy G, et al. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. Journal of Neurophysiology. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 22.Linden DE, et al. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cerebral Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- 23.Clark VP, et al. Responses to rare visual target and distractor stimuli using event-related fMRI. Journal of Neurophysiology. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- 24.Marois R, et al. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 25.Braver TS, et al. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 26.Kiehl KA, et al. Neural sources involved in auditory target detection and novelty processing: An event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- 27.Stevens MC, et al. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. Neuroimage. 2005;26:782–792. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bledowski C, et al. Attentional systems in target and distractor processing: a combined ERP and fMRI study. Neuroimage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Downar J, et al. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- 30.Serences JT, et al. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 31.Rushworth MFS, et al. The attentional role of the left parietal cortex: The distinct lateralization and localization of motor attention in the human brain. Journal of Cognitive Neuroscience. 2001;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- 32.Rushworth MFS, et al. Complementary localization and lateralization of orienting and motor attention. Nature Neuroscience. 2001;4:656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- 33.Rushworth MFS, et al. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 34.De Renzi E. Methods of limb apraxia examination and their bearing on the interpretationof the disorder. In: Roy EA, editor. Neuropsychological studies of apraxia and related disorders. Elsevier; 1985. pp. 45–64. [Google Scholar]
- 35.Kimura D, Archibal Y. Motor Functions of Left Hemisphere. Brain. 1974;97:337–350. doi: 10.1093/brain/97.1.337. [DOI] [PubMed] [Google Scholar]
- 36.Haaland KY, et al. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- 37.Desmurget M, et al. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 38.Wagner A, et al. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Skinner EL, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Ciaramelli E, et al. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spaniol J, et al. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Cabeza R. Trusting our memories: Dissociating the neural correlates of confidence in veridical vs. illusory memories. Journal of Neuroscience. 2007;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daselaar SM, et al. Triple Dissociation in the Medial Temporal Lobes: Recollection, Familiarity, and Novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- 45.Yonelinas AP, et al. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor AR, et al. The Inferior Parietal Lobule and Recognition Memory: Expectancy Violation or Successful Retrieval? Journal of Neuroscience. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciaramelli E, et al. Top-Down and Bottom-Up Attention to Memory Are Dissociated in Posterior Parietal Cortex: Neuroimaging and Neuropsychological Evidence. Journal of Neuroscience. 2010;30:4943–4956. doi: 10.1523/JNEUROSCI.1209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabeza R. Role of posterior parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binder JR, et al. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binder JR, et al. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- 51.Binder JR, et al. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Fiebach CJ, et al. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- 53.Woodard JL, et al. Temporally graded activation of neocortical regions in response to memories of different ages. J Cogn Neurosci. 2007;19:1113–1124. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiura M, et al. Cortical mechanisms of person representation: recognition of famous and personally familiar names. Neuroimage. 2006;31:853–860. doi: 10.1016/j.neuroimage.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Prince SE, et al. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychological Science. 2007 doi: 10.1111/j.1467-9280.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 56.Luo J, Niki K. Role of medial temporal lobe in extensive retrieval of task-related knowledge. Hippocampus. 2002;12:487–494. doi: 10.1002/hipo.10027. [DOI] [PubMed] [Google Scholar]
- 57.Daselaar SM, et al. Medial temporal lobe activity during semantic classification using a flexible fMRI design. Behav Brain Res. 2002;136:399–404. doi: 10.1016/s0166-4328(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 58.Scott SK, et al. Going beyond the information given: a neural system supporting semantic interpretation. Neuroimage. 2003;19:870–876. doi: 10.1016/s1053-8119(03)00083-1. [DOI] [PubMed] [Google Scholar]
- 59.Homae F, et al. From perception to sentence comprehension: The convergence auditory and visual information of language in the left inferior frontal cortex. Neuroimage. 2002;16:883–900. doi: 10.1006/nimg.2002.1138. [DOI] [PubMed] [Google Scholar]
- 60.Humphries C, et al. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Human Brain Mapping. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humphries C, et al. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newman SD, et al. Differential effects of syntactic and semantic processing on the subregions of Broca’s area. Cognitive Brain Research. 2003;16:297–307. doi: 10.1016/s0926-6410(02)00285-9. [DOI] [PubMed] [Google Scholar]
- 63.Ni W, et al. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- 64.Menenti L, et al. When Elephants Fly: Differential Sensitivity of Right and Left Inferior Frontal Gyri to Discourse and World Knowledge. Journal of Cognitive Neuroscience. 2009;21:2358–2368. doi: 10.1162/jocn.2008.21163. [DOI] [PubMed] [Google Scholar]
- 65.Dehaene S, et al. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- 66.Dehaene S, et al. Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 67.Stanescu-Cosson R, et al. Understanding dissociations in dyscalculia - A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain. 2000;123:2240–2255. doi: 10.1093/brain/123.11.2240. [DOI] [PubMed] [Google Scholar]
- 68.Chochon F, et al. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of Cognitive Neuroscience. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- 69.Gruber O, et al. Dissociating neural correlates of cognitive components in mental calculation. Cerebral Cortex. 2001;11:350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- 70.Ansari D, et al. Linking visual attention and number processing in the brain: The role of the temporo-parietal junction in small and large symbolic and nonsymbolic number comparison. Journal of Cognitive Neuroscience. 2007;19:1845–1853. doi: 10.1162/jocn.2007.19.11.1845. [DOI] [PubMed] [Google Scholar]
- 71.Grabner RH, et al. Fact learning in complex arithmetic and figural-spatial tasks: the role of the angular gyrus and its relation to mathematical competence. Hum Brain Mapp. 2009;30:2936–2952. doi: 10.1002/hbm.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grabner RH, et al. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47:604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 73.Fletcher PC, et al. Other Minds in the Brain - a Functional Imaging Study of Theory of Mind in Story Comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 74.Gobbini MI, et al. Two takes on the social brain: A comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 75.Saxe R, Powell LJ. It’s the thought that counts: Specific brain regions for one component of theory of mind. Psychological Science. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- 76.Saxe R, Kanwisher N. People thinking about thinking people - The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18:262–271. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- 78.Gallagher HL, et al. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi C, et al. Children’s and adults’ neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia. 2007;45:1522–1532. doi: 10.1016/j.neuropsychologia.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommer M, et al. Neural correlates of true and false belief reasoning. Neuroimage. 2007;35:1378–1384. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 81.Castelli F, et al. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 82.Carrington SJ, Bailey AJ. Are There Theory of Mind Regions in the Brain? A Review of the Neuroimaging Literature. Human Brain Mapping. 2009;30:2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. Neuroimage. 2008;40:1912–1920. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 84.Saxe R, Wexler A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 85.Saxe R. The right temporo-parietal junction: a specific brain region for thinking about thoughts. In: Leslie A, German T, editors. Handbook of Theory of Mind. 2010. [Google Scholar]
- 86.Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- 87.Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 88.Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daselaar SM, et al. Posterior Midline and Ventral Parietal Activity is Associated with Retrieval Success and Encoding Failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabeza R, et al. Overlapping Parietal Activity in Memory and Perception: Evidence for the Attention to Memory (AtoM) Model. Journal of Cognitive Neuroscience. 2011;11:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- 92.Scholz J, et al. Distinct Regions of Right Temporo-Parietal Junction Are Selective for Theory of Mind and Exogenous Attention. PLoS ONE. 2009;4:7. doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimamura AP. Memory and frontal lobe function. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; 1995. pp. 803–814. [Google Scholar]
- 94.Moscovitch M, Umilta C. Modularity and neuropsychology: Modules and central processes in attention and memory. In: Schwartz MF, editor. Modular deficits in Alzheimer’s Disease. MIT Press; Bradford: 1990. pp. 1–59. [Google Scholar]
- 95.Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- 96.Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: a parametric functional magnetic resonance imaging study. J Neurosci. 2010;30:3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Sciences. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 98.Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- 99.Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vilberg KL, Rugg MD. Functional Significance of Retrieval-Related Activity in Lateral Parietal Cortex: Evidence From fMRI and ERPs. Human Brain Mapping. 2009;30:1490–1501. doi: 10.1002/hbm.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vilberg KL, Rugg MD. An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. Neuroimage. 2009;45:562–571. doi: 10.1016/j.neuroimage.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rotello CM, Heit E. Two-process models of recognition memory: Evidence for recall-to-reject? Journal of Memory and Language. 1999;40:432–453. [Google Scholar]
- 103.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 104.Corbetta M, et al. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berryhill ME, et al. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davidson PSR, et al. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simons JS, et al. Is the pareital lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 108.Simons JS, et al. Dissociation Between Memory Accuracy and Memory Confidence Following Bilateral Parietal Lesions. Cerebral Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends in Cognitive Sciences. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Cajal SR. Advice for a young investigator. MIT Press; 1896/1999. [Google Scholar]
- 111.Critchley M. The parietal lobes. Edward Arnold; 1953. [Google Scholar]
- 112.Vallar G. Spatial neglect, Balint-Homes’ and Gerstmann’s syndrome, and other spatial disorders. CNS Spectr. 2007;12:527–536. doi: 10.1017/s1092852900021271. [DOI] [PubMed] [Google Scholar]
- 113.Husain M, Nachev P. Space and the parietal cortex. Trends in Cognitive Sciences. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Shallice T, Cooper RP. The organization of mind. Oxford University Press; 2011. [Google Scholar]
- 116.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buxbaum LJ, et al. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62:749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- 118.Heilman KM, et al. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 4. Oxford University Press; 2003. pp. 296–346. [Google Scholar]
- 119.Gillebert CR, et al. Lesion evidence for the critical role of the intraparietal sulcus in spatial attention. Brain. 2011;134:1694–1709. doi: 10.1093/brain/awr085. [DOI] [PubMed] [Google Scholar]
- 120.Gerstmann J. Syndrome of finger agnosia, disorientation for right and left, agraphia and acalculia. Arch Neurol Psychiatry. 1940;44:398–407. [Google Scholar]
- 121.Dehaene S, et al. Arithmetic and the brain. Curr Opin Neurobiol. 2004;14:218–224. doi: 10.1016/j.conb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 122.Strub RL, Geschwind N. Localization in Gerstmann syndrome. In: Kertesz A, editor. Localization in Neuropsychology. Academic Press; 1983. [Google Scholar]
- 123.Benton AL. The Fiction of the “Gerstmann Syndrome”. J Neurol Neurosurg Psychiatry. 1961;24:176–181. doi: 10.1136/jnnp.24.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Resenberg-Lee M, Menon V. New perspectives on the cognitive functions of the angular gyrus. 2011 Meeting of the Cognitive Neuroscience Society; MIT Press; 2011. [Google Scholar]
- 125.Dehaene S. Reading in the brain. Viking; 2009. [Google Scholar]
- 126.Geschwind N. Disconnexion syndromes in animal and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 127.De Renzi E, Nichelli PF. Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex. 1975;11:341–354. doi: 10.1016/s0010-9452(75)80026-8. [DOI] [PubMed] [Google Scholar]
- 128.Mason RA, Just MA. Differentiable cortical networks for inferences concerning people’s intentions versus physical causality. Hum Brain Mapp. 2010;32:313–329. doi: 10.1002/hbm.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galaburda AM, et al. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- 130.Shaywitz BA, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- 131.Suresh PA, Sebastian S. Developmental Gerstmann’s syndrome: a distinct clinical entity of learning disabilities. Pediatr Neurol. 2000;22:267–278. doi: 10.1016/s0887-8994(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 132.Dehaene S. Varieties of numerical abilities. Cognition. 1992;44:1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- 133.Behrmann M, et al. Perceptual and conceptual mechanisms in neglect dyslexia. Two contrasting case studies. Brain. 1990;113 (Pt 4):1163–1183. doi: 10.1093/brain/113.4.1163. [DOI] [PubMed] [Google Scholar]
- 134.Markowitsch HJ, et al. Short-term memory deficit after focal parietal damage. J Clin Exp Neuropsychol. 1999;21:784–797. doi: 10.1076/jcen.21.6.784.853. [DOI] [PubMed] [Google Scholar]
- 135.Danckert J, Ferber S. Revisiting unilateral neglect. Neuropsychologia. 2006;44:987–1006. doi: 10.1016/j.neuropsychologia.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 137.Cowan N. An embedded-processes model of working memory. In: Miyake A, Shah P, editors. Models of working memory: mechanisms of active maintenance and executive control. Cambridge University Press; 1999. pp. 62–101. [Google Scholar]
- 138.Chein JM, et al. Using neuroimaging to evaluate models of working memory and their implications for language processing. J Neuroling. 2003;16:315–339. [Google Scholar]
- 139.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McKinnon MC, Moscovitch M. Domain-general contributions to social reasoning: theory of mind and deontic reasoning re-explored. Cognition. 2007;102:179–218. doi: 10.1016/j.cognition.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 141.Martory MD, et al. Pure global acalculia following a left subangular lesion. Neurocase. 2003;9:319–328. doi: 10.1076/neur.9.4.319.15549. [DOI] [PubMed] [Google Scholar]
- 142.Roux FE, et al. Writing, calculating, and finger recognition in the region of the angular gyrus: a cortical stimulation study of Gerstmann syndrome. J Neurosurg. 2003;99:716–727. doi: 10.3171/jns.2003.99.4.0716. [DOI] [PubMed] [Google Scholar]
- 143.Mayer E, et al. A pure case of Gerstmann syndrome with a subangular lesion. Brain. 1999;122 (Pt 6):1107–1120. doi: 10.1093/brain/122.6.1107. [DOI] [PubMed] [Google Scholar]
- 144.Caspers S, et al. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 145.Caspers S, et al. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- 146.Schmahmann JD, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 147.Uddin LQ, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciaramelli E, et al. Mental Space Travel: Damage to Posterior Parietal Cortex Prevents Egocentric Navigation and Reexperiencing of Remote Spatial Memories. Journal of Experimental Psychology-Learning Memory and Cognition. 2010;36:619–634. doi: 10.1037/a0019181. [DOI] [PubMed] [Google Scholar]
- 149.Treisman A. Feature binding, attention and object perception. Philos Trans R Soc Lond B Biol Sci. 1998;353:1295–1306. doi: 10.1098/rstb.1998.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]