Abstract
Postsynaptic densities (PSDs) are responsible for organizing receptors and signaling proteins that regulate excitatory transmission in the mammalian brain. To better understand the assembly and 3D organization of this synaptic structure, we employed electron cryotomography to visualize general and fine structural detail of PSDs isolated from P2, P14, P21 and adult forebrain in the absence of fixative and stains. The PSDs at P2 are a loose mesh of filamentous and globular proteins and during development additional protein complexes are recruited onto the mesh. Quantitative analysis reveals that while the surface area of PSDs is relatively constant, the thickness and protein occupancy of the PSD volume increase dramatically between P14 and adult. One striking morphological feature is the appearance of lipid raft-like structures, first evident in PSDs from 14 day old animals. These detergent resistant membranes stain for GM1 ganglioside and their terminations can be clearly seen embedded in protein “bowls” within the PSD complex. In total, these results lead to the conclusion that the PSD is assembled by the gradual recruitment and stabilization of proteins within an initial mesh that systematically adds complexity to the structure.
Keywords: Postsynaptic Density, PSD, tomography, cryo, synaptic development
1.0
The postsynaptic density (PSD) is one of the most complex protein structures in the mammalian nervous system, where it lines the cytoplasmic face of postsynaptic membranes (Gray, 1959, Cotman and Taylor, 1972, Cotman et al., 1974, Blomberg et al., 1977, Cohen et al., 1977, Cohen and Siekevitz, 1978, Matus and Taff-Jones, 1978). As a testament to its complexity, proteomic analyses have estimated the structure contains between 100 and 500 unique proteins (Collins et al., 2006, Dosemeci et al., 2007), and it was calculated to have an average mass of 1.1 GDa (Chen et al., 2005). The function of the PSD is many-fold. Proteins embedded within the complex span the cleft to stabilize the synapse as a functional unit through interactions with proteins embedded in the presynaptic membrane. On the postsynaptic side, the PSD clusters neurotransmitter receptors and organizes the intracellular network of signaling molecules within the postsynaptic compartment. Morphologically, PSDs have been described as a disc shaped organelle that is flat or slightly concave when viewed in plastic-embedded thin sections (Gray, 1959; Cohen et al, 1978). Their dimensions range from 0.2–1.2 μm in diameter with an approximate thickness of 60 nm, and they appear to remain mostly intact after biochemical isolation using Triton X-100 (Cotman and Taylor, 1972; Cotman et al., 1974; Blomberg et al., 1977; Cohen et al., 1977). This isolation process has proven critical to the study of PSD structure, as there are significant limitations to gaining high-resolution images of the complex inside the crowded milieu of neuronal tissue.
The PSD’s proteomic complexity is further convoluted by a dynamic developmental and activity-dependent regulation of its structure and composition (Harris et al., 1992, Ehlers, 2003, Petralia et al., 2005, Blanpied et al., 2008, Swulius et al., 2010). As a consequence, each PSD is unique based on age and the activation history of individual synapses. The PSD’s role in organizing synaptic function, coupled with its dynamic nature, makes understanding the structural arrangement of its protein components and how it allows for dynamic rearrangement, critical to gaining a complete picture of how the synapse is built and how it functions.
Electron tomography excels in producing three-dimensional reconstructions of unique biological specimens at macromolecular resolution (4–6 nm). It has been used to study platinum shadowed (Petersen et al., 2003), negatively stained (Swulius et al., 2010) and plastic-embedded, thin sectioned PSDs (Chen et al., 2008, Chen et al., 2011). While many structural features were revealed, these preparation methods lead to limited resolution of fine morphology and can make interpretation of the underlying structure ambiguous. Electron cryotomography (ECT), however, circumvents the need for fixation and stains and allows direct visualization of protein density within the PSDs in a frozen-hydrated state. In the present study, we employed ECT to produce 3D structures of PSDs isolated at postnatal days P2, P14, P21 and P60, and by doing so, provide a clearer image of PSD fine morphology and how it changes during brain development. The use of cryo-preservation further allows a direct quantitative assessment of the protein occupancy within the volume of developing PSDs. Taken together, the alterations in morphology and protein occupancy support a developmental model that includes an initial mesh-like core structure that is “filled in” with other modular components, which leads to an increase in the ratio of protein to total PSD volume during PSD maturation. This mode of assembly would provide a mechanism for tailoring PSD structure and function to suit the requirements of individual synapses as synaptic physiological needs change.
2.0 Materials and Methods
2.1 Isolation of Postsynaptic Densities
Subcellular fractions enriched in PSDs were obtained from rat forebrains as previously described (Swulius et al., 2010). The same protocol was used to isolate PSDs from rats at P2, P14 and P21. For the P2 isolation, volumes were scaled down with respect to brain weight to compensate for the smaller amount of starting tissue. The number of rats used for each isolation was described previously (Swulius et al., 2010). For the P14 time point, the forebrains from 10 pups were used.
2.2 Cryo-preservation for EM
20 μl aliquots of frozen PSDs (in 5 mM HEPES, pH 7.4 with 20% glycerol) were thawed on ice and diluted 1:10 with 5 mM HEPES, pH 7.4. PSDs were pelleted in a 4°C microfuge for 15 min at 14,000 RPM. PSDs were re-suspended in 20 μl of 5 mM HEPES, pH 7.4 containing BSA-coated 10 nm colloidal gold as fiducial markers. 4 μl of sample was then applied to a freshly glow-discharged Quantifoil (R 2/2) EM grid and plunge frozen in liquid ethane using a Vitrobot (FEI). Vitrobot settings were 100% humidity at room temperature (~22°C) and a 5 second blot time. Grids were then stored in liquid nitrogen until use.
To make the BSA coated colloidal gold, 100 μl of 10 nm colloidal gold (Sigma) was mixed with 400 μl 5% BSA in H2O. After a 5 min incubation, the gold was pelleted for 15 min at 14,000 RPM in a microfuge. The supernatant was removed and the gold was suspended in 800 μl of 5 mM HEPES, pH 7.4 (one eighth the gold’s starting concentration). BSA coating the gold prevented non-specific binding to the PSDs.
2.3 Electron Microscopy and Image Processing
PSDs were first identified in the EM based on their size (0.2–1.2 μm in diameter) and morphology compared to cryo-preserved PSDs identified by immunolabeling with antibodies against markers known to be PSD enriched across all these developmental time points (Swulius et al., 2010). All tomographic tilt-series were collected using a Polara F30 (FEI) TEM equipped with a field emission gun running at 300 kV. Tilt-series were collected using FEI’s Batchtomo software and were shot from ± 60° at 2° tilt increments using an ~15 μm defocus. Images were captured using a Tietz 4k × 4k CCD using 2x binning. The combination of camera setting and magnification produced a final pixel size of 1.17 nm/pixel. The total dose for each complete tilt-series was ~150 e−/Å2. IMOD (http://bio3d.colorado.edu/imod/) was used to align the tilt-series and calculate tomographic reconstructions.
Amira (v 5.3.3; Visage Imaging) was used to calculate the protein-to-volume ratios of individual PSDs. To accomplish this, all of the voxels within the volume of individual PSDs, including aqueous channels within the complex, were enclosed by tracing each PSD’s outer edge in every tenth 2D slice in the XY plane, interpolating the boundary across these segmented regions and then closing the volume. The total number of voxels within this PSD volume was then calculated. Mean pixel intensities for protein and solvent were then calculated by statistical analysis of five 20×20×20 voxel cubes from within the proteinaceous regions of the PSD or from areas of solvent surrounding the structure or in well resolved areas within it, respectively. A threshold was then set halfway between the mean solvent and mean protein voxel intensities and Amira was used to segment and categorize voxels as either protein or solvent. With the number of voxels belonging to each group and the total PSD volume known, the protein to PSD volume ratio was calculated.
2.4 Labeling of PSD-associated Membranes
Isolated PSDs from the different aged rats were placed on formvar/carbon coated 400 mesh copper EM grids (Ted Pella) immediately following glow discharging. Samples were blocked by floating the grid, sample side down, on a 40 μl drop of blocking buffer (5% BSA in PBS, pH 7.4) for 10 min. The grid was then moved to a 30 μl drop of a 1:5000 dilution of biotinylated cholera toxin B subunit (Sigma) in blocking buffer for 30 min. Next, the grids were washed 3 times for 5 min each in blocking buffer, followed by a 30 min incubation on a 30 μl drop of a 1:100 dilution of streptavidin-conjugated Qdot 605 (Invitrogen). The grids were then washed once for 5 min in blocking buffer and twice for 5 minutes in nanopure water and finally negative stained with a 1:1 dilution of nano-van (Nanoprobes) in H2O. Qdot labeled PSDs were imaged using a JEOL 1400 electron microscope equipped with an Orius SC1000 4K × 2.4K CCD (Gatan). The specific quantum dot (Qdot 605) was chosen for its size and density making it easy to visualize in negatively stained specimens.
2.5 Immuno-gold labeling of PSDs for cryo-preservation
P2 or P60 PSDs were permitted to adhere to glow-discharged Quantifoil grids (R2/2) for 2 min at room temperature and then were processed for immunolabeling using a similar procedure to that described for cholera toxin labeling (section 2.4) with the following modifications, as described previously (Swulius et al., 2010). After blocking, the grids were incubated in antibodies either to βCaMKII (1:100; Invitrogen, monoclonal antibody 13-9800) or α-actinin (1:20; Sigma-Aldrich; mouse monoclonal antibody A5044), diluted in blocking buffer, for 30 min at RT. After washing, the grids were then incubated in a 1:5 dilution of either 12 nm gold labeled anti-mouse IgG (for the βCaMKII antibody) or 12 nm gold labeled anti-mouse IgM (for the α-actinin antibody), both from Jackson Labs, for 30 min at RT. The grids were then washed 2X with blocking buffer, 2X with PBS without BSA, cryo-preserved, and imaged as described above (section 2.3).
3.0 Results
3.1 Mature PSDs are composed of a mesh-like lattice and regions of densely packed protein
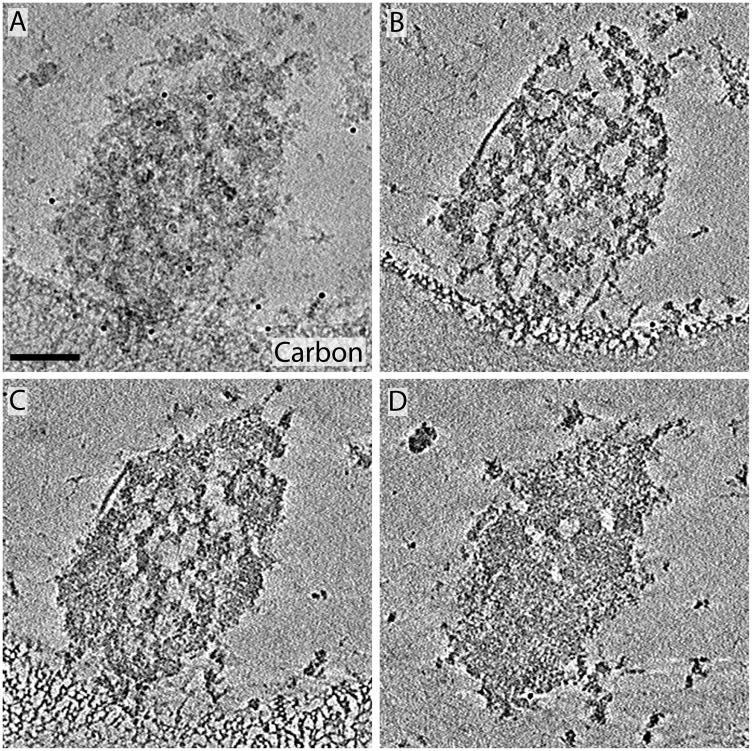
Two higher-order structural features were visualized in all cryo-tomograms of P60 PSDs, a mesh-like lattice (the PSD-mesh) and regions composed of densely packed protein (the dense material; see Figure 1). While the PSD-mesh was typically visible throughout the entire Z-dimension, the dense material tended to accumulate toward one face of the PSD in a laminar distribution (Figure 1B–D). These two features are representative of what was seen across 19 cryo-tomographic reconstructions from P60 PSDs. Movie S1 shows a series of slices through the Z-dimension of the PSD in Figure 1, providing a sense of the three-dimensional distribution of protein in the complex. The PSD in Figure 1 has a fairly regular outer boundary, but this is not uniformly the case. PSD borders were often irregular such as those shown in Figures 2 and 4.
Figure 1.
Figure 2.
Figure 4.
3.2 Changes in morphology and protein occupancy of PSDs isolated throughout development
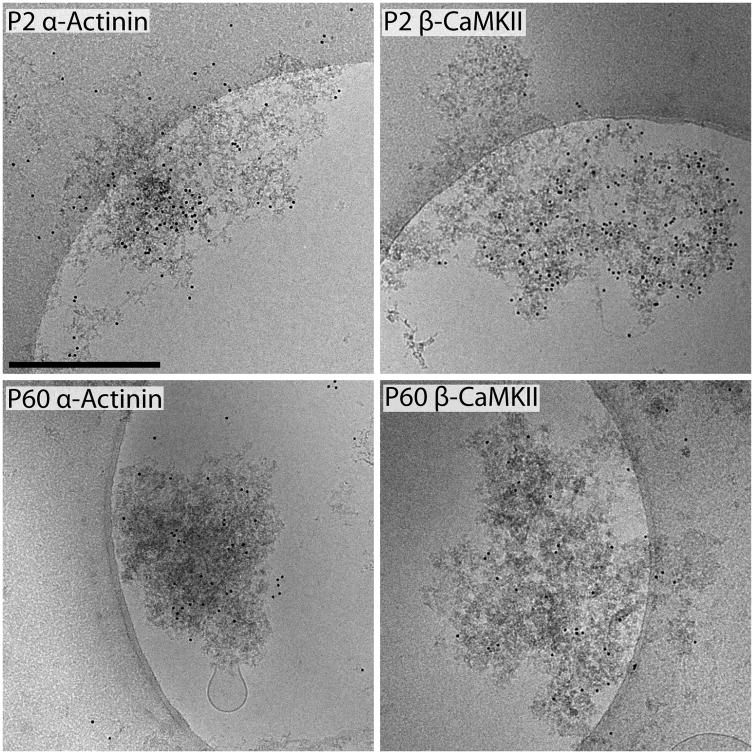
Cryo-tomographic reconstructions of PSDs at P2, P14, P21 and P60 revealed a developmental increase in identifiable structures (Figure 2) with increased age. Starting at P2, PSDs are composed entirely of PSD-mesh material (Movie 2 shows a 3D reconstruction of a P2 PSD). In the nine P2 PSDs reconstructed, there was no dense material (as identified in mature PSDs) detectable. By immuno-gold labeling PSDs from P2 (and P60) with antibodies to βCaMKII and α-actinin, two proteins previously identified as developmentally early PSD components (Swulius et al., 2010), we confirmed that these morphologically distinct mesh-like structures at P2 were PSDs (Figure 3). The majority of labeling is found over the PSDs and minimal labeling was found when primary antibodies were omitted (data not shown, but see Swulius et al., 2010). From this immunolabeling data, we conclude that the structures isolated from P2 forebrain are immature PSDs.
Figure 3.
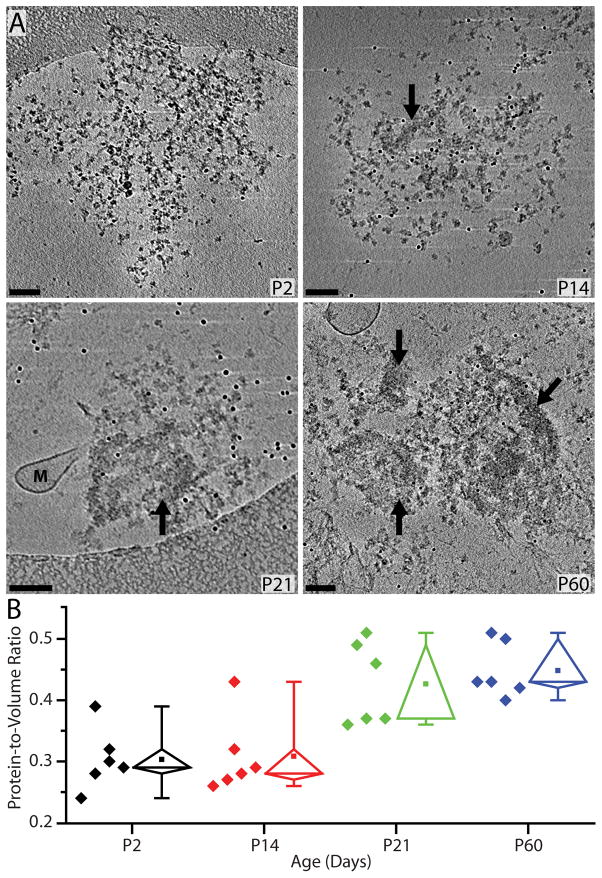
If the transition from a PSD-mesh to a mature PSD were a continuum then one would expect to see the gradual formation of dense material during development. In support of this continuum idea, the reconstructions from thirteen P14 PSDs showed evidence of the early stages of dense material formation (Figure 2 and 4A) overlaying the PSD-mesh. In general, the dense material seen at P14 was visible in small patches and was not as prominent as that seen by P21 and P60 (Figure 2 and 4A). Cryotomograms of nine P21, PSDs were largely in distinguishable from those isolated at P60, and were observed to have both a PSD-mesh and dense material.
To quantitatively examine the increase in the amount of protein within each PSD (protein occupancy) the protein-to-volume ratio was calculated for PSDs from each developmental time point (see details in section 2.3). This analysis is possible because information in the tomograms of cryopreserved specimens represents true protein density in its fully hydrated state. Results from the occupancy analysis of six PSDs at each developmental time point are illustrated in the whisker plot of Figure 4B. From P2 to P14, there was no significant increase in the protein-to-volume ratio despite the morphological appearance of occasional areas of densely packed protein in P14 PSDs. There was, however, an approximately 50% increase in the protein-to-volume ratio by P21 with only a minor additional increase by P60 (Figure 4B and Table 1).
Table 1.
Mean (± SEM) surface area, thickness and protein-to-volume ratios for PSDs isolated through development.
| P2 | P14 | P21 | P60 | |
|---|---|---|---|---|
| Surface Area (μm2) | 0.47 ± 0.09 | 0.30 ± 0.06 | 0.43 ± 0.06 | 0.44 ± 0.04 |
| Thickness (nm) | 74 ± 10 | 64 ± 6 | 107 ± 12 | 155 ± 13 |
| Protein:Volume | 0.30 ± 0.02 | 0.31 ± 0.03 | 0.43 ± 0.03 | 0.45 ± 0.02 |
Additional measurements of both total surface area and thickness of PSDs at all four developmental time points are also presented in Table 1. Except for a slight decrease in surface area at P14 the value remained relatively constant for PSDs from all four ages. In contrast, the thickness showed a continuous increase from P14 to P60 consistent with the idea that additional protein is being layered onto the PSD as development progresses. The thickness of cryo-preserved p60 PSDs is also significantly greater than that described previously (Gray, 1959, Cotman et al., 1974, Cohen and Siekevitz, 1978). Perhaps fixation or other post-sample processing leads to collapse or shrinkage in the thickness of PSDs reported in these earlier studies.
3.3 Fine morphology of cryo-preserved PSDs
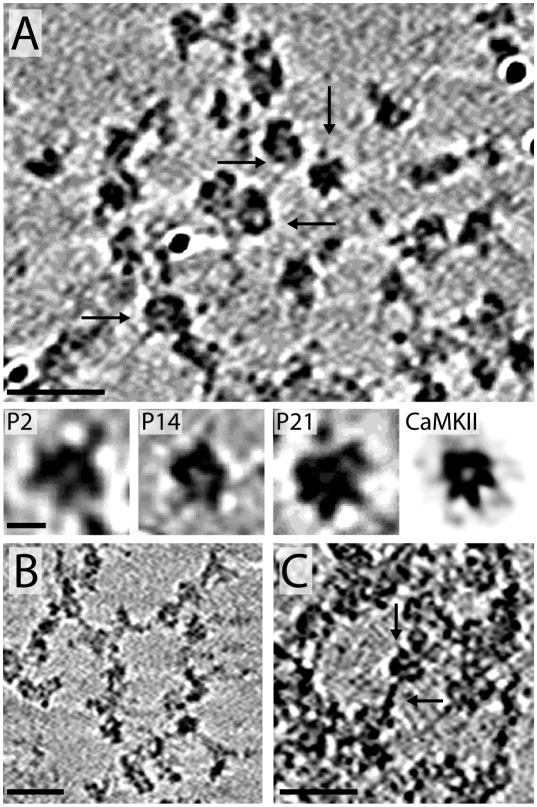
Upon closer inspection, the PSD-mesh can be seen as a complex mixture of globular and filamentous proteins (Figure 5). Almost every PSD examined (regardless of developmental stage) contained networks of globular proteins that are similar in size (20–25 nm across) and shape, suggesting they could be the same molecule or complex of molecules (Figure 5A). They are typically most prevalent early in development and become less obvious as their appearance is obscured by the denser structure of the mature PSD. It is sometimes the case that the globular network appears more visible toward the edges of the PSD circumference.
Figure 5.
Without direct immunochemical or higher resolution morphological evidence it is difficult to identify the composition of these globular modules. There are a variety of receptors, ion channels, enzymes and other protein complexes (e.g., proteasomes) associated with the PSD that are all candidates (Sheng and Hoogenraad, 2007). Based on the size and morphology it is possible that some of these globular molecules are CaMKII. CaMKII is the one of the most abundant proteins in PSDs (Dosemeci et al., 2007) and is present in PSDs as early as E19 (Swulius et al., 2010). Depending on the angle and cross-section, the globular complexes in the PSDs sometimes show small protrusions that resemble the gear shaped core of the CaMKII holoenzyme (lower panels of Figure 5A, Kolodziej et al., 2000). The multisubunit (dodecameric) nature of CaMKII provides ample possibilities for the enzyme to play a scaffolding role to organize molecules into local signaling modules within the PSD.
Early in development (P2) when the PSD-mesh is the least obscured by accumulated protein components, strands of connected proteins (8–14) nm across (Figure 5B) are abundant. While this mesh is likely made of more than two protein components, its morphology is similar to the protein network seen when the PSD proteins Shank and Homer are combined in vitro (Hayashi et al., 2009), suggesting that these two proteins, perhaps along with CaMKII, may be major components of the PSD-mesh. In addition, α-actinin is a significant component of P2 PSDs (Swulius et al., 2010) and its rod shaped structure (5 nm across 34 nm long) would be consistent with this filamentous material. In addition to the strands of connected proteins there are short filamentous proteins seen in PSDs (~5 nm across and 15–20 nm long) as illustrated in Figure 5C. The association with globular components suggests that proteins of this size could be adaptor proteins such as PSD-95 or one of the other MAGUKs known to link proteins together within the PSD.
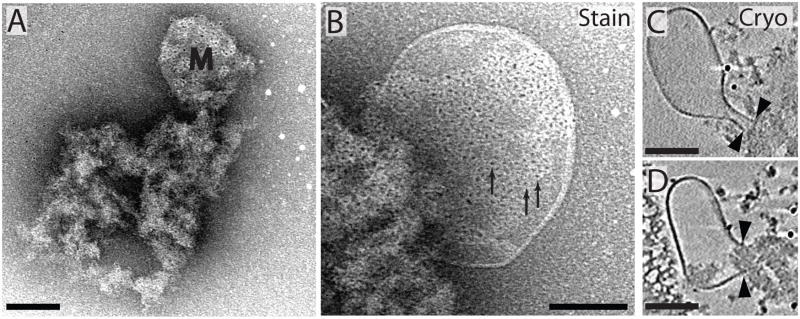
3.4 Detergent-resistant membranes enriched in the raft-associated lipid GM1 are directly connected to PSD dense material
Detergent resistant membranes in PSD fractions have been described previously (Petersen et al., 2003, Swulius et al., 2010), but whether they could be considered integral components of the PSD, or were bound superficially via specific or non-specific interactions could not be discerned without ECT. Figures 6A–B show examples of membrane blebs protruding from a p60 PSD in negative stain. In the tomographic slices of cryopreserved specimens (Fig. 6C–D), the membrane blebs clearly have discontinuous ends that meet and interact directly with the protein of the PSD’s dense material, suggesting they are not random membrane material that binds to the PSD during isolation. Interestingly, the appearance of the dense protein material at P14 coincided with the occasional appearance of detergent-resistant membranes (Figure 3), suggesting that proteins critical for interactions with this membrane component are recruited to the PSD in a developmentally regulated fashion.
Figure 6.
Lipid rafts were previously identified as being associated with PSDs (Suzuki et al., 2001, Petersen et al., 2003, Suzuki et al., 2008) so it was possible these membrane blebs were lipid rafts. To examine this possibility, we used a biotinylated cholera toxin (which binds the raft-associated lipid GM1) and streptavidin-conjugated Q-Dots to explore the lipid composition of these membranes. As evident in Figure 6A and B, membrane blebs showed heavy cholera toxin labeling along their surface, and labeling was not evident if cholera toxin was eliminated from the labeling reactions (data not shown). A much lower labeling density of Q-dots was seen randomly scattered within the PSD, suggesting that there is some GM1 (or some other cholera toxin binding molecule) associated randomly at low levels in the PSD structure. The cholera toxin labeled PSDs in Figure 6 were isolated at P60, but experiments performed on PSDs isolated at P14 and P21 produced similar labeling of the membrane blebs (data not shown).
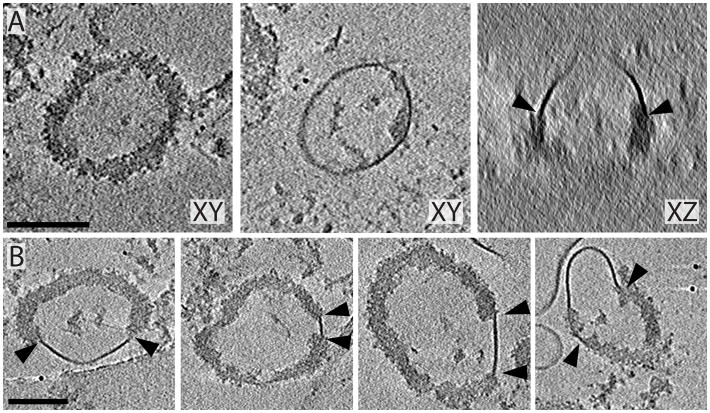
In many mature PSDs, dense material formed bowl shaped structures (50–150 nm in diameter at their opening) that were often in contact with the detergent-resistant membranes. Slices through these bowls appear as rings of dense material in the tomograms. Figure 7A shows such a ring in a 20-nm thick slice and illustrates how the bowl is capped with membrane that is attached all the way around the perimeter of its open end. When viewed from the side (Figure 7A, right panel) the continuity of the membrane with the dense material can be clearly seen. The membrane sometimes formed a closed cap around the perimeter of the bowl’s opening as seen in Figure 7A, but was also seen attached to smaller regions of the bowl’s dense material as in Figure 7B. While these bowls/rings of densely packed protein were reported previously in negatively stained PSDs (Swulius et al., 2010), their connection with detergent-resistant membrane was not evident. In fact, in cryo-preserved PDSs, 65% of the bowl structures observed (16 of 25) were directly attached to lipid, implying that these two structures are functionally connected.
Figure 7.
4.0 Discussion
Here we combined a developmental approach with state-of-the-art ECT, to capture different states of the PSD during its assembly. The morphological details preserved in vitrified PSDs is remarkable compared to those obtained through negative stain or platinum shadowing (Petersen et al., 2003, DeGiorgis et al., 2006, Swulius et al., 2010). One reason is that the contrast in cryo-preserved specimens is generated directly by the sample density instead of a chemical stain or heavy metal coat. From these results, we can propose a model for PSD assembly based on gross morphological features. Early in development (P2) PSDs are composed entirely of PSD-mesh that serves as a scaffold of relatively constant surface area. Interspersed regions of densely packed protein then appear gradually (generally in a new layer) through development (P14–P60) that leads to thickening of the PSD. Interestingly, while the surface area of the PSD reported here is typical of that described previously (Harris et al., 1992, Petersen et al., 2003, Swulius et al., 2010), the thickness of the PSD isolated from mature rat brain (P60) is significantly greater (~150 nm) than that reported previously (Gray, 1959, Cohen et al., 1977, Cohen and Siekevitz, 1978). We hypothesize that this is due to capturing the PSDs in a vitrified layer of aqueous buffer that avoids distortions (collapse) induced by other methodologies. The transition between early lattice-dominated PSDs and later multi-layered, more mature PSDs was evident in P14 PSDs and appeared mostly complete by P21. This coupled with an increase in protein occupancy within PSDs supports a model that PSDs are assembled by recruitment of individual or small assemblies of proteins as development proceeds. By employing time-lapse fluorescence microscopy of PSD molecules in living neurons, Bresler et al. (2004) reached a similar conclusion. Our results do no rule out the possibility that the earliest foundation of the PSD (the PSD-mesh or some precursor molecules) is recruited to the synapse in a pre-assembled form as part of a mobile transport packet as reported for NMDARs and PSD-95 (Prange and Murphy, 2001, Washbourne et al., 2002)
The idea of an underlying mesh as a central component of PSDs is not new. Petersen et al (Petersen et al., 2003), made similar observations on platinum shadowed adult PSDs. Intriguingly, a mesh-like arrangement similar to what we report was also described by in vitro reconstitution experiments using purified Shank/Homer proteins (Hayashi et al., 2009), suggesting these two molecules are potential candidates for core PSD-mesh proteins. From immuno-gold labeling experiments (Swulius et al., 2010), α-actinin is another reasonable candidate for a component of this mesh considering the relatively high labeling at both E19 and P2 and for its ability to bind to other integral PSD components such as αCaMKII and NMDA receptors (Walikonis et al., 2001, Robison et al., 2005, Merrill et al., 2007) as well as the cytoskeletal protein actin (Blanchard et al., 1989). Studies have shown that little PSD-95 is present early in development (Petralia et al., 2005, Swulius et al., 2010), so it cannot be a major component of the initial PSD-mesh. Other MAGUK family proteins, like PSD-93, SAP-102 and chapsyn-110, are present early in development (Petralia et al., 2005, Sheng and Hoogenraad, 2007), and given their rod shape (~15 nm long and 5 nm in diameter) they are most likely important molecules in PSD-mesh formation and development.
It is also possible that the PSD-mesh is, in part, made of cytoskeletal proteins such as actin and tubulin, as both of these proteins are well documented components of PSD fractions (Walikonis et al., 2000, Li et al., 2004, Peng et al., 2004, Yoshimura et al., 2004, Dosemeci et al., 2007). However, there were no obvious structures consistent with actin filaments or microtubules in the PSDs observed here. One possibility is that they do not form typical filaments or microtubules like they do in the cytoplasm and only make short connections between various proteins within the PSD or perhaps they play a non-filamentous role. That being said, filamentous actin is well documented within post-synaptic spines and is often seen running in bundles from the PSD into the cytoplasm (Rostaing et al., 2006). The fact that such filaments are not seen in our preparations, suggests that the PSD isolation process does not fully preserve certain aspects of the post-synaptic compartment.
Another potential component of the early assembly of the mesh is CaMKII. CaMKII is the most abundant protein present in PSDs (Collins et al., 2006, Dosemeci et al., 2007) and is present in PSDs as early as E19 increasing in amount into adulthood (Swulius et al., 2010). The level of detail provided by ECT already reveals globular PSD components that resemble the 20 nm diameter CaMKII molecules and similar structures were also identified as CaMKII in tomographic reconstructions of platinum shadowed PSDs (Petersen et al., 2003). Future studies combining high resolution immuno-gold labeling and ECT will establish the identity, distribution and co-localization of individual proteins that produce the functional PSD complex.
Another developmentally regulated structure evident in the PSDs was the appearance of detergent-resistant membranes beginning around P14. Membrane patches surrounded by a rim of particles were also identified in platinum shadowed PSDs (Petersen et al., 2003); however, they appeared flattened into the matrix of the PSDs. In ECT, the membranes could often be seen ballooning out from the PSD, sometimes for significant distances, and their intimate contact with protein particles within the PSD could be well delineated. Synaptic lipid rafts are well documented (Suzuki et al., 2001, Renner et al., 2009, Delint-Ramirez et al., 2010) and electron micrographs of negatively stained synaptic raft fractions show membrane structures (Suzuki et al., 2001) virtually identical to those seen in our PSD fractions. In addition, PSD and lipid raft fractions isolated from the same synaptic plasma membrane share many of the same components (Suzuki et al., 2008, Delint-Ramirez et al., 2010). The PSD is known to stay firmly attached to the plasma membrane during isolation. It seems plausible that the tight integration of these lipid blebs into the PSD is, in part, responsible for maintaining their close association with the overlying synaptic membrane. The abundance of AMPA receptors found in dendritic raft fractions (Suzuki et al., 2001), suggests that these rafts play a role in sequestering or recycling AMPA receptors at the postsynaptic site. Further experiments are needed to determine the function of these PSD associated rafts, but their presence carries significant biological importance due to the role of rafts in regulating various cell signaling pathways (Simons and Toomre, 2000). It is worth noting that in our previous study (Swulius et al., 2010), we did not detect immuno-gold labeling of NMDA or AMPA receptors within these PSD associated membrane structures while significant staining was detectable through other areas of the PSD. This could be because AMPA and NMDA receptors were stripped away from the membrane components during the PSD isolation process, or it could represent a real composition difference between rafts that are firmly anchored to the PSD and synaptic rafts present in the overlying plasma membrane. Regardless, determining the functional role that these unique lipid-containing structures might have in regulating PSD and synaptic structure and function is certainly warranted.
Supplementary Material
ECT provides novel information about the morphology and protein disposition within unfixed PSDs.
PSDs are assembled on a mesh-like core that is “filled in” with protein components during development.
The thickness and protein occupancy of PSDs increases significantly while their surface area does not.
PSDs help determine the microenvironment of the lipids in the overlying postsynaptic membrane.
Acknowledgments
This work was supported by a grant from the NIH/NINDS R01NS026086. MNW also acknowledges an endowment from the William Wheless III Professorship. MTS acknowledges support from NIH Training Grant T32NS07467 during a portion of this work. MMF was supported by NIH Training Grant 5T32GM008280. The Polara electron microscope was supported, in part, through the Structural Biology Imaging Center at UTHSC-Houston.
Footnotes
The authors state that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanchard A, Ohanian V, Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989;10:280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Kerr JM, Ehlers MD. Structural plasticity with preserved topology in the postsynaptic protein network. Proc Natl Acad Sci U S A. 2008;105:12587–12592. doi: 10.1073/pnas.0711669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg F, Cohen RS, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977;74:204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE. Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci. 2004;24:1507–1520. doi: 10.1523/JNEUROSCI.3819-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci U S A. 2005;102:11551–11556. doi: 10.1073/pnas.0505359102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RS, Blomberg F, Berzins K, Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977;74:181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RS, Siekevitz P. Form of the postsynaptic density. A serial section study. J Cell Biol. 1978;78:36–46. doi: 10.1083/jcb.78.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, Choudhary JS, Grant SG. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Banker G, Churchill L, Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974;63:441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972;55:696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgis JA, Galbraith JA, Dosemeci A, Chen X, Reese TS. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biol. 2006;35:239–250. doi: 10.1007/s11068-007-9017-0. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Fernandez E, Bayes A, Kicsi E, Komiyama NH, Grant SG. In vivo composition of NMDA receptor signaling complexes differs between membrane subdomains and is modulated by PSD-95 and PSD-93. J Neurosci. 2010;30:8162–8170. doi: 10.1523/JNEUROSCI.1792-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Mol Cell Proteomics. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- Li KW, Hornshaw MP, Van Der Schors RC, Watson R, Tate S, Casetta B, Jimenez CR, Gouwenberg Y, Gundelfinger ED, Smalla KH, Smit AB. Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology. J Biol Chem. 2004;279:987–1002. doi: 10.1074/jbc.M303116200. [DOI] [PubMed] [Google Scholar]
- Matus AI, Taff-Jones DH. Morphology and molecular composition of isolated postsynaptic junctional structures. Proc R Soc Lond B Biol Sci. 1978;203:135–151. doi: 10.1098/rspb.1978.0097. [DOI] [PubMed] [Google Scholar]
- Merrill MA, Malik Z, Akyol Z, Bartos JA, Leonard AS, Hudmon A, Shea MA, Hell JW. Displacement of alpha-actinin from the NMDA receptor NR1 C0 domain By Ca2+/calmodulin promotes CaMKII binding. Biochemistry. 2007;46:8485–8497. doi: 10.1021/bi0623025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Modular transport of postsynaptic density-95 clusters and association with stable spine precursors during early development of cortical neurons. J Neurosci. 2001;21:9325–9333. doi: 10.1523/JNEUROSCI.21-23-09325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M, Choquet D, Triller A. Control of the postsynaptic membrane viscosity. J Neurosci. 2009;29:2926–2937. doi: 10.1523/JNEUROSCI.4445-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, MacMillan LB, Carmody LC, Bartlett RK, Colbran RJ. Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J Biol Chem. 2005;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- Rostaing P, Real E, Siksou L, Lechaire JP, Boudier T, Boeckers TM, Gertler F, Gundelfinger ED, Triller A, Marty S. Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur J Neuorsci. 2006;24(12):3463–74. doi: 10.1111/j.1460-9568.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Du F, Tian QB, Zhang J, Endo S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J Neurochem. 2008;104:596–610. doi: 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ito J, Takagi H, Saitoh F, Nawa H, Shimizu H. Biochemical evidence for localization of AMPA-type glutamate receptor subunits in the dendritic raft. Brain Res Mol Brain Res. 2001;89:20–28. doi: 10.1016/s0169-328x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Swulius MT, Kubota Y, Forest A, Waxham MN. Structure and composition of the postsynaptic density during development. J Comp Neurol. 2010;518:4243–4260. doi: 10.1002/cne.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Jensen ON, Mann M, Provance DW, Jr, Mercer JA, Kennedy MB. Identification of proteins in the postsynaptic density fraction by mass spectrometry. J Neurosci. 2000;20:4069–4080. doi: 10.1523/JNEUROSCI.20-11-04069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Yamauchi Y, Shinkawa T, Taoka M, Donai H, Takahashi N, Isobe T, Yamauchi T. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. J Neurochem. 2004;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.