Abstract
In order to identify the combination of antibody-mediated mechanisms of neutralization that result from vaccination with anthrax vaccine adsorbed (AVA), we isolated antibody secreting cells from a single donor seven days after booster vaccination with AVA and generated nine fully human monoclonal antibodies (hmAb) with high specificity for protective antigen (PA). Two of the antibodies were able to neutralize lethal toxin in vitro at low concentrations (IC50: p6C01, 0.12 µg/ml and p6F01, 0.45 µg/ml). Passive transfer of either of these hmAbs to A/J mice prior to challenge with lethal toxin conferred 80–90% protection. We demonstrate that hmAb p6C01 is neutralizing by preventing furin cleavage of PA in a dose-dependent manner, but the mechanism of p6F01 is unclear. Three additional antibodies were found to bind to domain 3 of PA and prevent oligomerization, although they did not confer significant protection in vivo and showed a significant prozone-like effect in vitro. These fully human antibodies provide insight into the neutralizing response to AVA for future subunit vaccine and passive immunotherapeutic cocktail design.
Keywords: anthrax, Anthrax Vaccine Adsorbed, human monoclonal antibodies, passive immunotherapeutics, protective antigen
1Introduction
Bacillus anthracis has been used in the recent past as a form of biological terrorism and continues to be a significant health concern. Anthrax spores are long-lived and the mortality rate of inhalation anthrax is 45–90% even with aggressive antimicrobial treatment [1]. This is due to both rapid bacterial growth because of a poly-γ-D-glutamic acid capsule which plays important roles in the progression of the disease [2], and the effects of a tripartite secreted toxin. The toxin includes protective antigen (PA), lethal factor (LF), and edema factor (EF). PA is an 83 kDa protein which, after binding to its cell surface receptor, is cleaved by furin-like proteases to generate 63 kDa (PA63) and 20 kDa (PA20) fragments. PA63 then oligomerizes allowing EF and/or LF to bind and be internalized into the cell [3]. The PA structure has been well characterized and consists of four domains [3, 4]. When PA combines with LF, Lethal toxin (LT) forms and acts as a Zn2+-dependent protease, cleaving mitogen-activated protein kinase kinase family members as well as other intracellular substrates [5]. When PA combines with EF, Edema toxin forms which protects B. anthracis from phagocytosis by acting as a calmodulin-dependent adenylate cyclase [5].
Anthrax Vaccine Adsorbed (AVA) is the only currently licensed vaccine against B. anthracis in the United States. The vaccine is a cell-free filtrate of an attenuated strain with PA as the major component and EF and LF as minor components, as mortality from human anthrax infection is thought to be primarily toxinogenic and high toxin concentrations can lead to death even when antibiotic treatment has sterilized the blood [6]. The vaccination schedule is onerous, requiring five injections over 18 months and yearly boosters to maintain protection because anti-PA titers fall off rapidly after vaccination [7]. The vaccine most likely provides protection by inducing the production of neutralizing PA-specific antibodies. However, as measured by an in vitro assay, the overall effectiveness with regard to neutralizing antibodies is poor, with as many as 54% of vaccinees who have completed their first series not producing neutralizing antibodies detectable in the serum by one methodology [8]. Furthermore, engineered strains with resistance to ciprofloxacin remain viable terrorist threats, thus novel passive immunotherapeutics must be developed to reduce the threat of anthrax mortality [9].
Monoclonal antibodies specific to toxin components represent a promising post-exposure treatment for anthrax, particularly if given in combination with antibiotics and/or immunization [9]. The direct administration of neutralizing antibodies immediately increases serum antibody titers, protects against spore challenge in non-human primate and rabbit models, and does not interfere with the later generation of an endogenous adaptive response [10, 11]. Also, anthrax spores can have delayed germination that may initiate infection after the cessation of antibiotic treatment further highlighting the need for long-lived immunotherapeutics and efficacious active immunization [6].
Because of this neutralizing potential, many anti-PA, EF, and LF monoclonal antibodies have been developed from murine sources [12–16]. Neutralizing mouse antibodies have been humanized and have been shown to protect from spore challenge in a rabbit model [17, 18]. Several antibodies have also been characterized from SCID mice with a transplanted human immune system [19]. Fully human or chimpanzee antibodies have been limited to phage display products (with non-physiological heavy-light chain pairing) but neutralizing antibodies have been developed and characterized against PA [20, 21] and LF [10, 22]. A recent study examined a panel of human monoclonal epitopes from a Fab library, but mechanisms of protection and specific domain binding was not explored [23]. One fully human monoclonal antibody, raxibacumab, has recently been FDA approved [24] and several others have clinical potential [9]. Furthermore, developing cocktails of monoclonal antibodies that interact with distinct functions of PA may allow for the most effective anthrax toxin neutralization [9, 25].
The focus of this study was to characterize the anti-PA response following vaccination with AVA on a per antibody basis and determine the mechanism of antibodies demonstrating neutralization. To this end, we characterized nine PA-specific, fully human monoclonal antibodies (hmAbs) using a technique that enables the production of full-length hmAbs following vaccination [26, 27]. The antibodies in this study were produced and characterized from individual antibody secreting cells that arose from a memory response of a fully primed, healthy volunteer. These antibodies bound PA epitopes across the molecule, but only two (20%) showed significant in vitro neutralizing activity, one of which neutralizes by blocking furin cleavage. Strikingly, antibodies which bound to domain 3 are capable of inhibiting oligomerization of PA63, yet are weakly or non-neutralizing in both in vitro and in vivo neutralization assays. Overall, this study simultaneously provides detailed information about the neutralizing antibodies produced by the donor and provides a novel approach for generating cocktails of monoclonal antibodies that neutralize by multiple mechanisms.
Materials and Methods
Human subjects
All studies requiring informed consent were pre-approved by the Institutional Review Board of the Oklahoma Medical Research Foundation. The donor was a Caucasian male, 54 years old who received his 7th AVA vaccination as standard of care. Peripheral blood was drawn seven days post-vaccination and antibody secreting cells were isolated and sorted using fluorescence activated cell sorting (FACS) as previously described [26, 27]. The presence of PA-specific ASCs was confirmed by standard ELISpot [27].
Antibody production
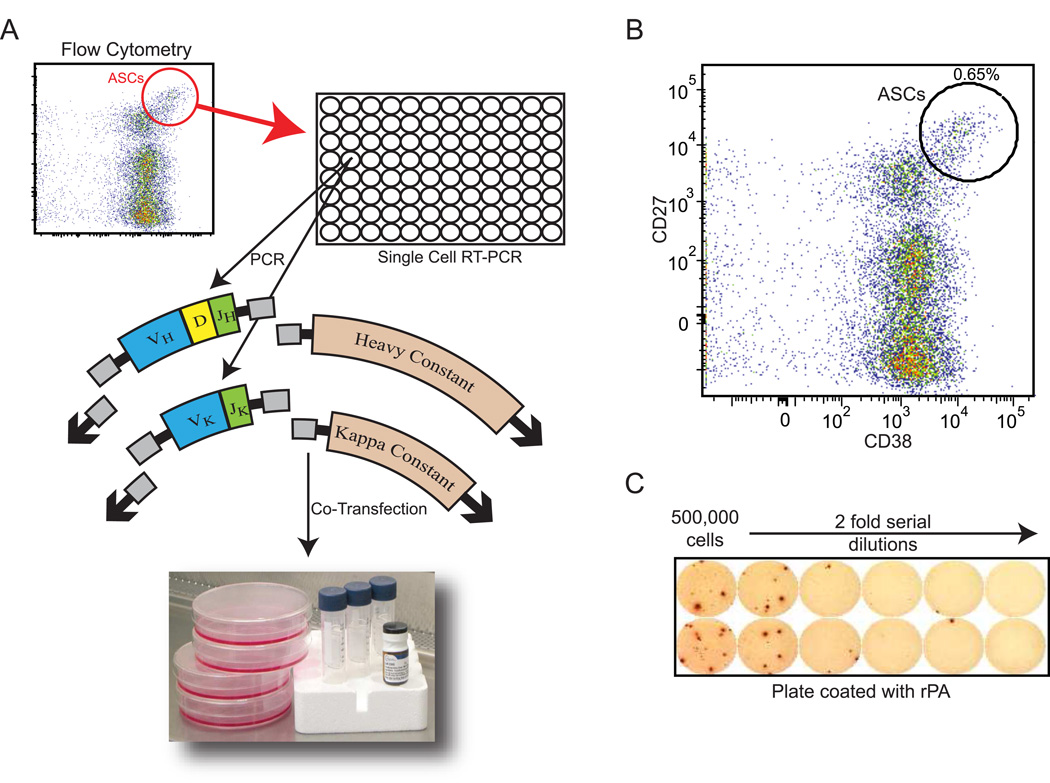
Briefly, CD3neg/CD19low/CD27high/CD38high cells were isolated from peripheral blood mononuclear cells (PBMCs) and bulk sorted by FACS using a FACSAria (BD Biosciences, San Jose, CA), and single cells were further sorted into 96-well plates using a MoFlo (DakoCytomation) sorter. VH and Vκ RNA from single B cells were amplified by RT-PCR and nested PCR reactions. The V regions were then prepared for restriction cloning by PCR and cloned into IgG1 or Igκ expression vectors as previously described [27]. Heavy- and light-chain plasmids were co-transfected into the HEK293 cell line for antibody expression and monoclonal antibodies were purified from culture supernatants by Protein A-Agarose beads (Pierce, Rockford, IL) (Figure 1a).
Figure 1. Generation of fully human monoclonal antibodies following vaccination with anthrax vaccine adsorbed (AVA).
A) VH and Vκ genes from single B cells, sorted into 96-well plates, were amplified by RT-PCR and nested PCR reactions. Heavy- and light-chain plasmids were co-transfected into HEK293 cells for antibody expression and antibodies were purified with Protein A-agarose beads. B) CD3negCD20lowCD19intCD27highCD38high cells were isolated from blood at day 7 post vaccination and single cells were sorted to isolate antibody secreting cells. A representative cell sorting is shown. C) PBMCs from each donor were tested for reactivity to rPA in a standard ELISPOT assay and the number of PA-specific cells were determined.
Recombinant Protein Reagents
Recombinant anthrax toxins, rPA, rLF, or rEF (List Biological Laboratories, Campbell, CA) were stored frozen in 1 mg/ml aliquots as recommended by the supplier. Recombinant human furin (R&D Systems, Minneapolis, MN) was reconstituted to a concentration of 60 nM (4 µg/ml).
Protective antigen, lethal factor, and edema factor ELISAs
Antibody specificity for toxin components was determined using standard ELISAs. High bind plates (Costar 3369) were coated overnight with 1 µg/well of rPA, rLF, or rEF. After washing with PBS plus 0.05% Tween-20, antibody was added to the wells using two-fold serial dilutions starting at 66.7 nM (10 µg/ml). Anti-human IgG conjugated to horseradish peroxidase (Jackson Immunoresearch, West Grove, PA) then Super Aqua Blue Substrate (eBioscience, San Diego, CA) were added after appropriate washing. The optical density (OD) was detected at 405 nm. Binding curves were generated with a saturation binding, non-linear curve fit using GraphPad Prism software. Equilibrium dissociation constants (Kd) values for each hmAb were calculated using the equation Y=Bmax*X/(Kd+X) where Bmax is the maximum number of binding sites, X is the concentration of the antibody and Y is the specific binding. Therefore the reported dissociation constants are equal to the concentration of antibody where half the binding sites are occupied at equilibrium. Each antibody was run in duplicate in at least three unique experiments. The results for each experiment were then averaged to obtain the reported Kd.
In vitro lethal toxin neutralization
Inhibition of LT activity by monoclonal antibodies was performed as previously described for sera [8, 28, 29]. Toxin-sensitive mouse macrophages (RAW264.7, ATCC, Manassas, VA) were plated into a 96-well flat bottom tissue culture plate (50,000 cells per well) and cultured overnight at 37°C with 5% CO2. Two-fold serial dilutions of hmAbs starting at 33.3 nM (5 µg/ml) were incubated with LT using 6.0 nM (50 ng) PA and 5.56 nM LF (50 ng) in 100 µl/well, empirically determined to induce 95% toxicity, for 1 hour. The media was removed from the cultured cells and the serum/toxin mix was added to each well. Cells alone, PA only, LF only, or cells with LT served as controls. After addition of the serum/toxin mixture, the cells were incubated at 37°C with 5% CO2 for 3.5 h, followed by addition of 10 µl of WST-8 (CCK8, Dojindo Molecular Technologies, Rockville, MD). The Optical Density (OD) at 450 nm was read at 3 hours and the percent viability was determined by the absorbance reading of the sample well divided by the absorbance reading from the control wells containing cells only. Each antibody was run on this assay at least three independent times.
Passive transfer and lethal toxin challenge
Six-week old female A/J mice received intraperitoneal injections of human monoclonal antibodies (30 µg in 500 µl volume), saline, or an anti-influenza monoclonal antibody (30 µg in 100 µl volume). Three hours later, mice were challenged with 3X LD50 of LT (empirically determined as 300 µg PA + 125 µg LF) injected i.p. (in 350 µl/mouse). Each challenge group consisted of 5–10 mice. Mortality was recorded, and survival curves and percent survival were generated using GraphPad Prism. All animal procedures were reviewed and approved by the OMRF Institutional Animal Care and Use Committee (IACUC).
Oligomerization and furin cleavage assays
Non-reducing SDS-PAGE-based assays for determining the capacity of hmAbs to inhibit oligomerization of PA63 or furin cleavage of PA83 were adapted from previous reports [30, 31]. Gels for both assays were visualized by incubation with Sypro Orange protein stain (Invitrogen, Carlsbad, CA) and imaged using the ethidium bromide filter on an Ultra-Lum imaging system (UltraLum, Inc, Claremont, CA).
The oligomerization assay was performed by adding 33 pmol (5 µg) of antibody to 48 pmol (3 µg) of PA63 and incubating for 30 minutes at room temperature. The total volume was increased to 15 µl of MES buffer at pH 6.0 to cause oligomerization (final concentration of 2.2 µM antibody and 3.2 µM PA). Laemmli buffer (without β-mercaptoethanol (BME)) was added and the samples were run on 7.5% polyacrylamide gels with 4% stacking gels. The furin cleavage assay was performed in a similar manner: Antibody (33 pmol, 5 µg) was incubated with 36 pmol (3 µg) of PA83 for 30 minutes at room temperature. The volume was then increased to 15 µl with TBS + 1 mM Ca+2, pH 8.5 (to retain monomeric PA63 after cleavage, final concentration of 2.2 µM antibody and 2.4 µM PA). Recombinant human furin (5 µl of 60 nM, 20 ng), was then added and the samples were incubated for 1 hour at 37 °C. Laemmli buffer (without BME) was then added and the gels were run as above (7.5%).
Recombinant protective antigen domains
cDNA sequences for PA domains 1A, 3, 3–4, and 4 were generated by RT-PCR [32] and cloned into a pGEX-6P-1 vector (GE Healthcare, Pittsburgh, PA). BL21 cells were transformed with PA/pGEX-6P-1 vectors. IPTG was added to the cultures to induce production of PA subunits at 16 °C. GST purification was used to purify the PA domains (Pierce; B-PER GST Fusion Protein Spin Purification Kit; Rockford, IL). For ELISAs performed using these domains, plates were coated with 1 µg/well of recombinant domain, antibodies were serially diluted from 10 µg/ml and the plates were developed as with full length PA above.
Statistical analysis
For the in vivo survival data, survival curves were generated and tested using a log-rank test (Mantel-Cox) to determine the overall p value. Mann-Whitney U tests were then used to compare each treatment group to the control group (those that received toxin only). All statistical analyses were performed using GraphPad Prism 4.0.
Results
Rapid generation of fully human monoclonal antibodies following anthrax vaccine adsorbed vaccination
We used the technology described by Smith et al. [27] to generate recombinant human monoclonal antibodies following AVA vaccination. A healthy volunteer received booster immunization and seven days after, antibody secreting cells (ASCs), (CD19low/intCD27highCD38high) were isolated. The percentage of ASCs as a fraction of total peripheral blood B cells was only 0.65% (Figure 1b). While this small number is close the baseline number of healthy donor ASCs [26], a fraction of the PBMCs was used in a standard ELISPOT assay [27] and clearly demonstrated the presence of PA-specific ASCs (Figure 1c). 4.5 PA spots were produced per 125,000 total PBMCs and 8.9 IgG spots were observed. Thus, seven days post AVA vaccination, 50.3% of the IgG producing cells were PA-reactive.
Monoclonal antibodies generated from a vaccinated individual strongly bind to protective antigen
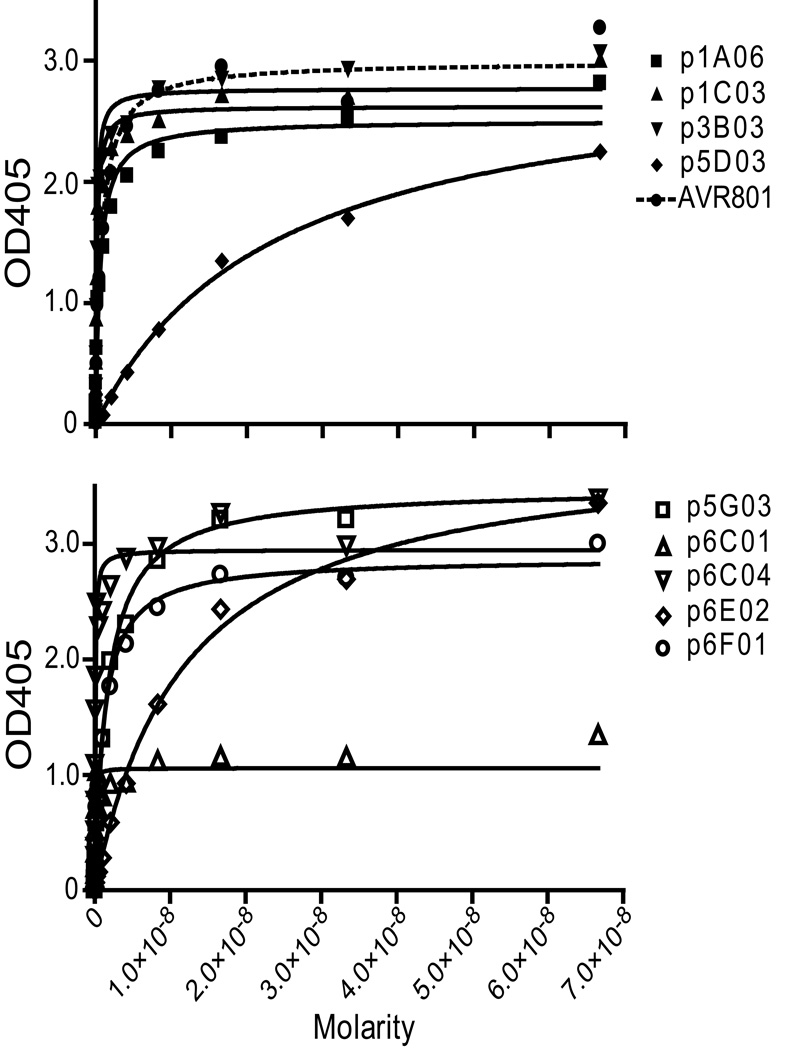
As the immune response to the vaccine could be directed to bacterial proteins present but not quantified in the vaccine, the specificity of these antibodies to the three toxin proteins, PA, LF, and EF, was tested. Of the 30 antibodies tested, nine bound to PA specifically, with no cross-reactivity to other toxin proteins. None were detected that bound to LF or EF. ELISA curves depicting the binding of these nine antibodies are shown (Figure 2). These PA-specific antibodies had a range of estimated Kd’s from 10−8M to 10−10M. For comparison, human standard anthrax reference serum, (AVR801, [33]) shows a Kd in this assay of 1.2×10−10M.
Figure 2. Identification of nine PA-specific antibodies.
Antibodies (and AVR801) were serially diluted at concentrations ranging from 66.7 nM to 0.00204 nM (10 to 0.000305 µg/ml and tested for reactivity to rPA using a standard ELISA. The ELISA curves are displayed on two panels to allow clearer presentation of the data.
Monoclonal antibodies generated from vaccinated individuals can provide protection from in vitro and in vivo lethal toxin challenge
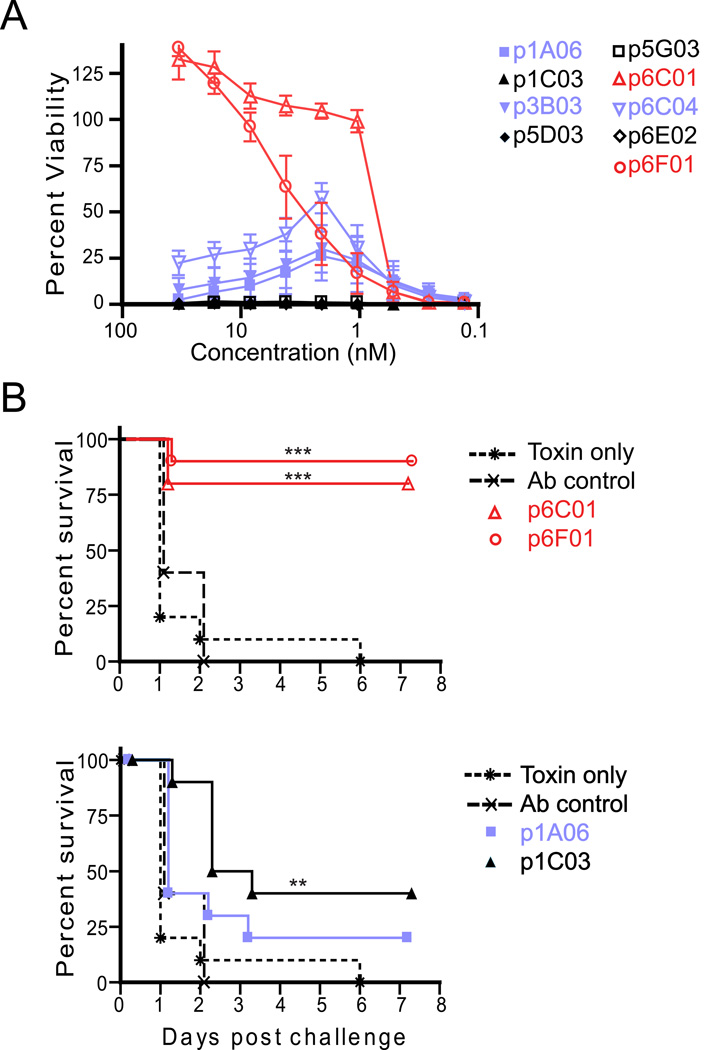
To determine the level of protection provided by the hmAbs, we used an in vitro lethal toxin neutralization assay, as previously described [8]. Five of the nine PA-specific antibodies showed no ability to neutralize toxin even at a high (66.7 nM, 10 µg/ml) concentration, (Figure 3A). However, two antibodies (red), p6C01 and p6F01, provided 100 percent protection using this assay (at 0.667 nM (0.1 µg/ml) and 16.7 nM (2.5 µg/ml) respectively). Indeed these antibodies provided 50% protection against lethal toxin mediated killing at low concentrations (IC50 = 0.8 nM (0.12 µg/ml) and 3.0 nM (0.45 µg/ml), Figure 3A). AVR801 was simultaneously tested in this assays as a quality control, resulting in an IC50 = 0.24 µg/ml (published as ~0.2 µg/ml in a similar assay, [34]) indicating that 10 µg/ml of p6C01 has a slightly higher neutralizing capacity than 10 µg/ml of standardized pooled hyperimmune serum. Three antibodies (blue), p1A06, p3B03 and p6C04, showed an interesting pattern of protection with moderate toxin neutralization at concentrations between 6.67 nM (1 µg/mL) and 0.667 nM (0.1 µg/mL) only (Figure 3A).
Figure 3. Two human monoclonal antibodies provide protection against lethal toxin challenge both in vivo and in vitro.
A) Antibodies were serially diluted and tested in duplicate in a standard in vitro lethal toxin assay. Shown is the average and SEM of the percent viability of the cells at the indicated concentrations from three independent experiments. Antibodies which are protective in this assay are shown in red (IC50: p6C01 = 0.8 nM (0.12 µg/ml) and p6F01 = 3.0 nM (0.45 µg/ml)), Three antibodies which showed slight protection at a small range of concentrations are shown in blue. B) In an in vivo assay, antibodies (30µg) were injected intraperitoneally into A/J mice (n= 10) three hours before challenge with lethal toxin. Shown is the percent survival following challenge. All antibodies were tested, but for clarity antibodies showing less than or equal to 20% protection at day 1 were omitted from the graphs. The first graph shows the two “red” antibodies protective in the in vitro assay, the second shows one “blue” antibody from the in vitro assay and another antibody showing slight protection only in this assay. The control lines in each graph are toxin only, no antibody (stars and dotted line), and an anti-influenza antibody used as a non-specific antibody control (x’s and dashed line). ** p < 0.01, *** p < 0.001 by log-rank test (Mantel-Cox) as compared to toxin only group.
The hmAbs were then tested for their ability to provide protection against in vivo lethal toxin challenge as well. These experiments were performed in A/J mice, which are sensitive to toxin in a dose-dependent manner and can be sufficiently protected from toxin or spore challenge by anti-toxin therapies [6]. A/J mice received 30 µg of the PA-specific antibody, a negative control influenza-specific monoclonal antibody, or saline. Three hours following passive transfer, the mice were injected with lethal toxin at three times the lethal dose (3xLD50) and survival was monitored daily for seven days. Two monoclonal antibodies were significantly protective in vivo, including p6F01 and p6C01 which were 90% and 80% protective, respectively (p < 0.001 as compared to toxin only group by log-rank, Figure 3B). For these antibodies, the in vitro neutralization data correlated with in vivo protection. One antibody (p1C03), which did not neutralize toxin in vitro, surprisingly conferred 40% protection in vivo (p < 0.01, Figure 3B). None of the other antibodies provided significant protection from lethal toxin challenge using this murine model.
The mechanism of protection by antibody p6C01 is inhibition of furin cleavage
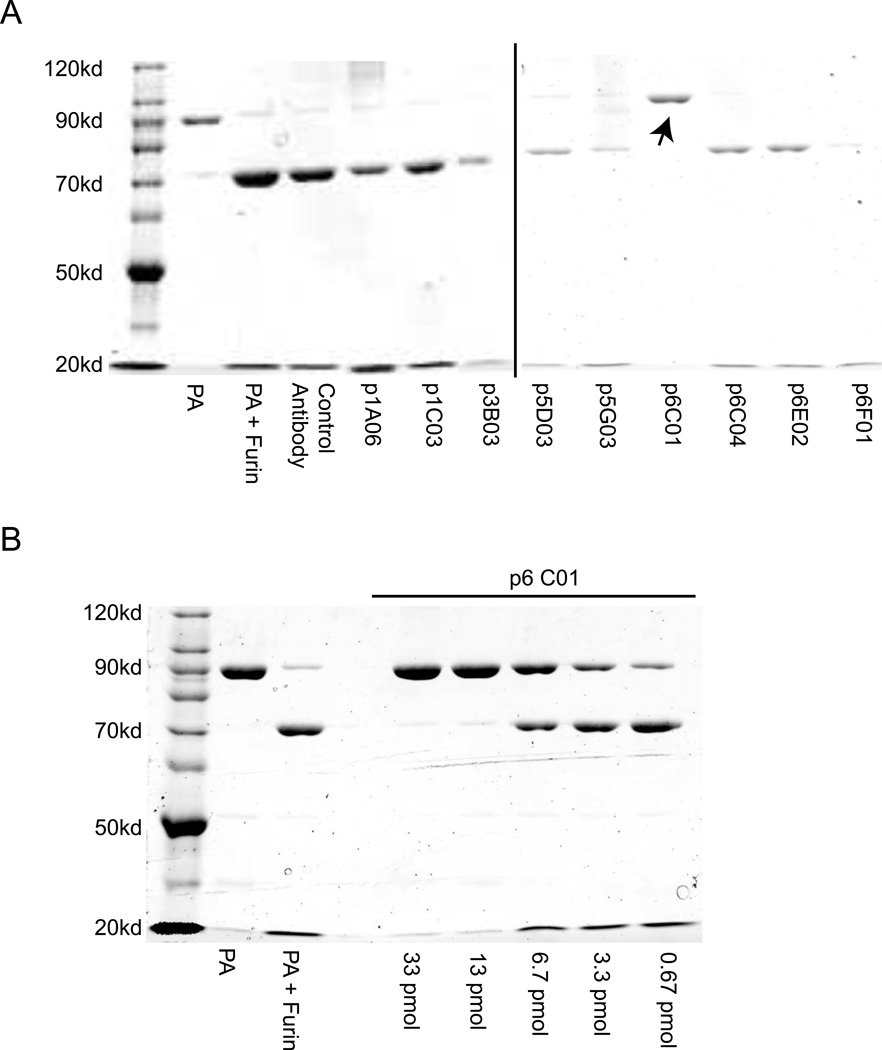
To determine whether any of the antibodies were able to inhibit the cleavage of PA by furin, PA83 was incubated with antibody and then reacted with recombinant human furin for one hour before being visualized by non-reducing PAGE. The only antibody that inhibited furin cleavage is p6C01 (Figure 4A), which is also protective in both the in vivo and in vitro assays (Figure 3). The furin inhibition is dose-dependent (Figure 4B), with 0.013 nmoles (2 µg) of antibody completely inhibiting the cleavage of 0.036 nmoles (3 µg) of PA83. Interestingly, p6C01 did not clearly bind to any subunit by ELISA or Western blot (Supp. Figure 1, Figure 5B).
Figure 4. Antibody p6C01 is protective by inhibiting furin cleavage.
A) Each antibody was tested in a furin cleavage assay, only p6C01 was able to block the activity of recombinant human furin to cleave PA83 to PA63 (the arrow indicates the presence of the PA83 band). B) This antibody protects in a dose-dependent manner, showing full protection with 0.013 nmoles (2 µg) of antibody completely inhibiting the cleavage of 0.036 nmoles (3 µg) of PA83
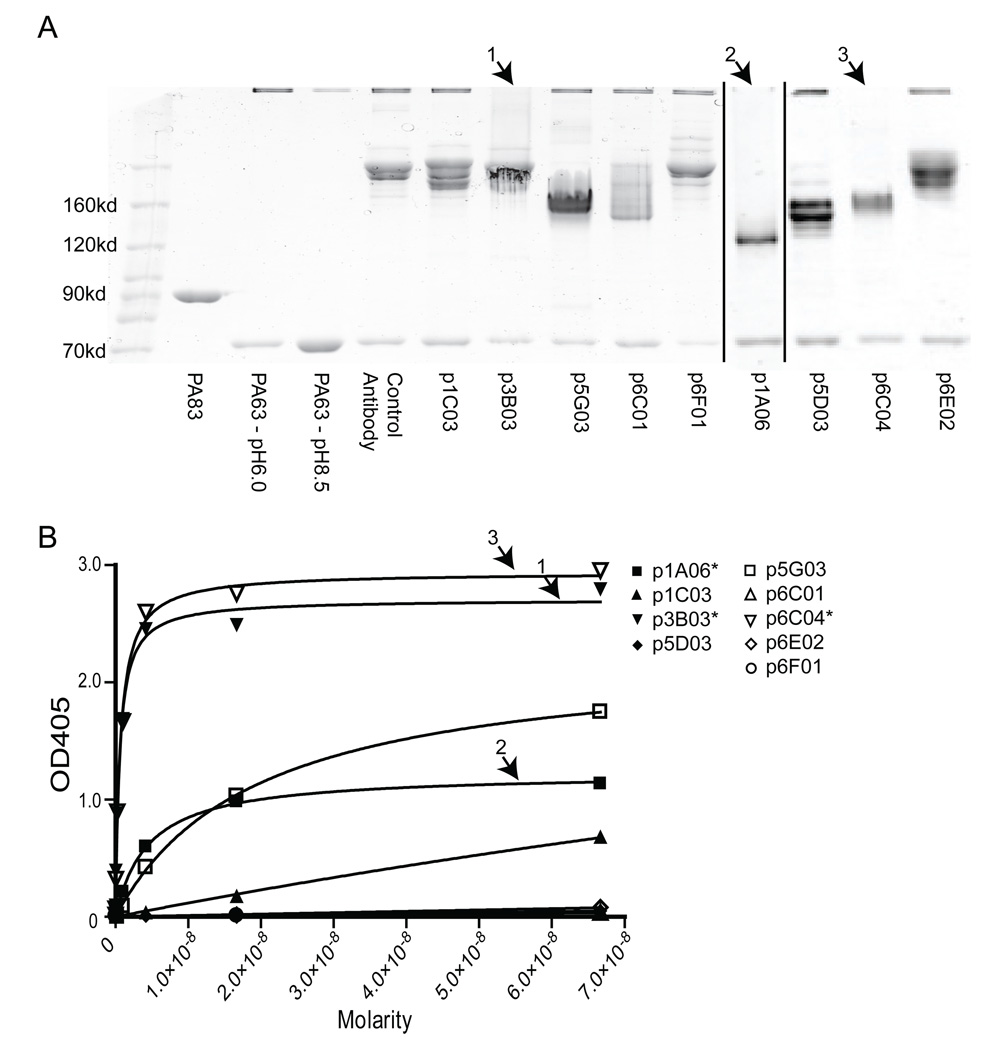
Figure 5. Three antibodies bind to domain 3 and prevent oligomerization.
A) Each antibody was tested in an oligomerization blocking assay. The three antibodies indicated by an numbered arrow show the lack of a high molecular weight oligomer band, indicating that they were capable of inhibiting oligomerization. B) The same three antibodies (marked with a numbered arrow and an asterisk in the legend), plus one other, show appreciable binding in a domain 3 subunit ELISA.
Three of the antibodies bind to domain 3 and inhibit oligomerization
To further characterize the antibodies, an assay was performed to determine which antibodies were able to inhibit oligomerization. As shown in Figure 5A, PA63 was incubated with each antibody at physiological pH, and the pH was then dropped to pH 6.0 to favor oligomerization. The samples were then examined by non-reducing PAGE. Lack of oligomers can clearly be seen with three antibodies, p1A06, p3B03, and p6C04, indicating that these antibodies are able to inhibit oligomerization. To confirm these results, ELISAs were performed using domain 3 (known as the heptamerization domain), and similarly we show that these three antibodies bind strongly to domain 3 (Figure 5B). It appears that p1A06 has a higher Kd for domain 3, and this may be reflected in the PAGE gel as a faint oligomer band can be detected. The other domain 3 weakly-binding antibody, p5G03, does not inhibit oligomerization. It is important to note that although neither p3B03 nor p6C04 were protective in the in vivo neutralization assay, they did show some protection at a certain range of concentrations in the in vitro assay, indicating that there may be a particular range of concentration required for neutralization by this mechanism.
Three other antibodies bind to domain 1A and/or 4
ELISAs were also performed using domain 1A (PA20) and domain 4 as substrates (data not shown). Three antibodies, p1C03, p5G03, and p6E02, bound strongly to domain 1A (better than 10−9M, see Supplemental Figure 1 for a domain Western blot of p1C03). One antibody, p5D03, binds weakly to domain 4 (9×10−8M, not shown). As p5D03 shows a much stronger binding to whole PA, it is likely that it binds a conformational epitope involving domain 4. Although p6F01 did not bind to any domains by ELISA, it bound weakly to domain 3 by Western blot (Supp. Figure 1).
Discussion
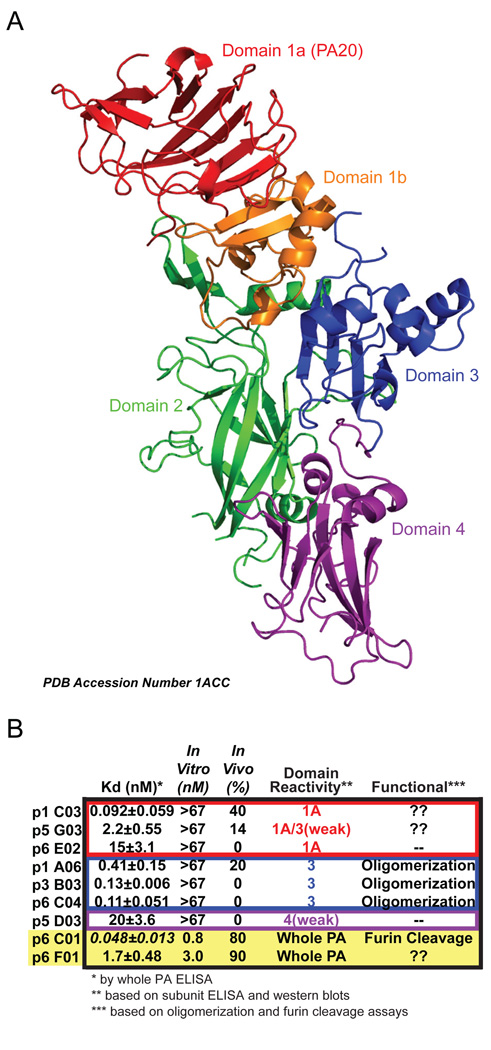
This study is the first to examine a group of full-length human monoclonal antibodies from an individual donor and determine the subunit specificities, Kd’s and mechanisms of neutralization of the antibodies. Although only 30% (9 of 30 total) of the antibodies we characterized bound to PA, it is clear that the vaccine elicited an antibody response to many different PA epitopes, neutralizing and non-neutralizing, including at least domains 1A and 3 (see Figure 6 for a ribbon diagram of PA and a summary of antibody characterization).
Figure 6. Summary of anti-PA human monoclonal antibodies.
A) Ribbon structure of PA (PDB accession number 1ACC) with domains color coded modeled using PyMOL (http://www.pymol.org/). B) Summary of the nine antibodies characterized in this study; the two with the highest neutralizing potential are highlighted in the yellow box. The color of the boxes matches the color of the domain bound as in part A. Each antibody is detailed with Kd (nanomolar with standard deviation), in vitro neutralization (IC50), in vivo neutralization (% survival), the domain to which the antibody binds and how the antibody provides neutralization (“??” indicates the antibody is at least partially neutralizing, but the epitope bound is unknown; “--” indicates that the antibody is not neutralizing). The Kd of p6C01 is in italics because although it is quite low, the Bmax for this antibody is low (see ELISA curve from Figure 2).
While we have only characterized the response of one donor, in the context of recent work the specificity of the PA response may be more important than the quantity of the anti-PA. Antibodies which block receptor binding, both of PA to the cell or EF/LF to PA, show the most promise of neutralization. In mouse models, domain 4 subunit vaccines have been shown to be effective at providing protection [35, 36]. Promising results have also been shown in a rabbit model of inhalation anthrax where the animals were protected by immunization with peptides mimicking known protective epitopes from domain 2 which are neutralizing yet absent in PA-immune animals [37, 38]. Interestingly, in non-human primates, post-challenge sera from animals that survived infection were absent of binding to domain 3 [39]. Thus, an inverse relationship exists in this model between survival and making antibodies to irrelevant portions of PA. Overall, our study, in the context of this recent work, suggests that immunization with whole PA may not be the most efficacious way to induce neutralizing epitopes.
Two antibodies, p6C01 and p6F01, were found to be strongly neutralizing in both in vitro and in vivo models of protection. It is curious that both of these neutralizing antibodies have intermediate affinities and their binding specificities were difficult to characterize using subunit assays. These hmAbs may bind domain 1b-2 which we cloned, but were unable to express as a subunit or which may span conformational epitopes spanning domains, thus providing ambiguous results or low affinities in subunit ELISAs and Western blots. As we were able to determine that p6C01 is able to inhibit furin cleavage, such antibodies should be considered as passive immunotherapeutics, especially as cocktails with antibodies that inhibit receptor binding. We can make the assumption that this particular donor had such a cocktail in their serum (p6C01 and p6F01) providing them efficient protection.
While p1A06, p3B03 and p6C04 are capable of preventing oligomerization, they only neutralize over a small range of concentrations. Classically, this prozone or prozone-like effect has been observed in a variety of systems, although it is not fully understood [40, 41]. These results verify that antibodies to domain 3, which seem to certainly prevent oligomerization in various assays, are poorly- or non-neutralizing in vivo. Such antibodies may be precluded as passive immunotherapeutics and further studies are needed to determine whether blocking oligomerization is an important mechanism of protection.
Conversely, p1C03 shows protection in the in vivo assay, but not in vitro. By both Western blot (Supplemental Figure 1) and ELISA (data not shown) p1C03 strongly binds to domain 1A. Perhaps through its strong binding (Kd<1×10−10M) this hmAb can aid in clearing the toxin, without showing any neutralization in the in vitro assay, but this is not proven. The overall observation that 80% of the PA binding antibodies that we have characterized have no or only partial neutralization potential strongly highlights the importance of having a properly directed immune response towards PA in order to prevent a mostly irrelevant response as a result of vaccination. For the same reasons, the use of hyperimmune serum from donors vaccinated with AVA is clearly not the most efficient method for passive immunotherapy.
A cocktail of hmAbs which demonstrate neutralization by a variety of methods is a strong choice for supplementing antimicrobial therapies. In this study, we demonstrate that novel hmAbs can be produced following AVA vaccination. Most of the antibodies characterized bind to either domain 3 or 1a, and those binding domain 3 are able to inhibit oligomerization, yet do not provide protection. The two antibodies strongly neutralizing in both in vitro and in vivo assays have intermediate affinities and do not show clear results in subunit assays. However, p6C01 inhibits furin cleavage in a dose-dependent manner. Taken together, these antibodies provide a wealth of information about the donor’s humoral response to vaccination and details about which specific PA epitopes are bound by vaccine-induced antibody, ultimately providing toxin neutralization and protection.
Highlights.
>We generated nine fully human monoclonal antibodies to anthrax protective antigen. >Two antibodies were able to neutralize lethal toxin in vitro at low concentrations. >Two of these antibodies confered protection to A/J mice challenged with lethal toxin. >Antibody p6C01 neutralized by preventing furin cleavage of PA. >Three antibodies bound to domain 3 of PA, but do not confer significant protection.
Supplementary Material
Purified PA1a (0.75 µg/lane), and PA3, PA3–4, and PA4 (1.5µg/lane), were electrophoresed on a 12% Tris/Glycine gel (Invitrogen, Carlsbad, CA). Proteins were then transferred to nitrocellulose membrane and blocked in 3% BSA blocking solution overnight. Monoclonal antibody (3 µg/mL) was applied to the membrane for 1 hour at RT. Next, alkaline phosphatase conjugated anti-human antibody was applied (1:50,000 dilution) at RT for 1 hour, and BCIP/NBT substrate (Sigma-Aldrich; St. Louis, MO) was added. Then, Western blots were scanned and analyzed for monoclonal binding.
Acknowledgments
We thank J. Donald Capra, MD for his helpful discussions and critical reading of this manuscript and Renata Engler for helpful discussions regarding anthrax vaccination. We also thank our donors for this study as well as our clinical staff, Virginia Roberts and Jeremy Levin. We would also like to thank Amanda Vineyard, Linda Ash, Erica Edwards, Angie Duke, Nai-Ying Zheng, Jacob Bass and Diana Hamilton for their technical assistance, as well as Melissa Nguyen for toxin production. Human anti-anthrax vaccine absorbed reference serum (AVR801) was obtained through the National Institutes of Health Biodefense and Emerging Infections Research Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This work was supported by the National Institute of Allergy and Infectious Diseases [U19 AI062629] and the National Center for Research Resources [P20 RR015577, P20 RR015577-10S1, and P30 RR031152].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AVA, anthrax vaccine absorbed; PA, protective antigen; ASC, antibody secreting cell; hmAb, human monoclonal antibody; LT, lethal toxin; LF, lethal factor; EF, edema factor; PBMCs, peripheral blood mononuclear cells
References
- 1.Mourez M. Anthrax toxins. Rev Physiol Biochem Pharmacol. 2004;152:135–164. doi: 10.1007/s10254-004-0028-2. [DOI] [PubMed] [Google Scholar]
- 2.Jang J, Cho M, Chun JH, Cho MH, Park J, Oh HB, et al. The poly-γ-D-glutamic acid capsule of bacillus anthracis enhances lethal toxin activity. Infect Immun. 2011;79:3846–3854. doi: 10.1128/IAI.01145-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 4.Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham K, Lacy DB, Mogridge J, Collier RJ. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc Natl Acad Sci USA. 2002;99:7049–7053. doi: 10.1073/pnas.062160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goossens PL. Animal models of human anthrax: the Quest for the Holy Grail. Mol Aspects Med. 2009;30:467–480. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Marano N, Plikaytis BD, Martin SW, Rose C, Semenova VA, Martin SK, et al. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA. 2008;300:1532–1543. doi: 10.1001/jama.300.13.1532. [DOI] [PubMed] [Google Scholar]
- 8.Crowe SR, Ash LL, Engler RJ, Ballard JD, Harley JB, Farris AD, et al. Select human anthrax protective antigen epitope-specific antibodies provide protection from lethal toxin challenge. J Infect Dis. 2010;202:251–260. doi: 10.1086/653495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Moayeri M, Purcell R. Monoclonal antibody therapies against anthrax. Toxins. 2011;3:1004–1019. doi: 10.3390/toxins3081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht MT, Li H, Williamson ED, LeButt CS, Flick-Smith HC, Quinn CP, et al. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect Immun. 2007;75:5425–5433. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altboum Z, Gozes Y, Barnea A, Pass A, White M, Kobiler D. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect Immun. 2002;70:6231–6241. doi: 10.1128/IAI.70.11.6231-6241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly-Cirino CD, Mantis NJ. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect Immun. 2009;77:4859–4867. doi: 10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little SF, Leppla SH, Burnett JW, Friedlander AM. Structure-function analysis of Bacillus anthracis edema factor by using monoclonal antibodies. Biochem Biophys Res Commun. 1994;199:676–682. doi: 10.1006/bbrc.1994.1281. [DOI] [PubMed] [Google Scholar]
- 14.Little SF, Leppla SH, Cora E. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracistoxin. Infect Immun. 1988;56:1807–1813. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abboud N, De Jesus M, Nakouzi A, Cordero R, Pujato M, Fiser A, et al. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect Immun. 1988;56:1807–1813. doi: 10.1128/iai.56.7.1807-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubbins MJ, Berry JD, Corbett CR, Mogridge J, Yuan XY, Schmidt L, et al. Production and characterization of neutralizing monoclonal antibodies that recognize an epitope in domain 2 of Bacillus anthracis protective antigen. FEMS Immunol Med Microbiol. 2006;47:436–443. doi: 10.1111/j.1574-695X.2006.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed N, Clagett M, Li J, Jones S, Pincus S, D’Alia G, Nardone L, et al. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect Immun. 2005;73:795–802. doi: 10.1128/IAI.73.2.795-802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld R, Marcus H, Ben-Arie E, Lachmi BE, Mechaly A, Reuveny S, et al. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus anthracis infection. PLoS One. 2009;4:e6351. doi: 10.1371/journal.pone.0006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada-Hirai R, Jiang I, Wang F, Sun SM, Nedellec R, Ruther P, et al. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J Immune Based Ther Vaccines. 2004;2:5. doi: 10.1186/1476-8518-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wild MA, Xin H, Maruyama T, Nolan MJ, Calveley PM, Malone JD, et al. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat Biotechnol. 2003;21:1305–1306. doi: 10.1038/nbt891. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Moayeri M, Zhou YH, Leppla S, Emerson S, Sebrell A, et al. Efficient neutralization of anthrax toxin by chimpanzee monoclonal antibodies against protective antigen. J Infect Dis. 2006;193:625–633. doi: 10.1086/500148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Moayeri M, Crown D, Emerson S, Gorshkova I, Schuck P, et al. Novel chimpanzee/human monoclonal antibodies that neutralize anthrax lethal factor, and evidence for possible synergy with anti-protective antigen antibody. Infect Immun. 2009;77:3902–3908. doi: 10.1128/IAI.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reason D, Liberato J, Sun J, Keitel W, Zhou J. Frequency and domain specificity of toxin-neutralizing paratopes in the human antibody response to anthrax vaccine adsorbed. Infect Immun. 2009;77:2030–2035. doi: 10.1128/IAI.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, et al. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med. 2009;361(2):135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 25.Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, et al. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A. 2002;99:11346–11350. doi: 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed N, Li J, Ferreira CS, Little SF, Friedlander AM, Spitalny GL, Casey LS. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect Immun. 2004;72:3276–3283. doi: 10.1128/IAI.72.6.3276-3283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen ML, Crowe SR, Kurella S, Teryzen S, Cao B, Ballard JD, et al. Sequential B-cell epitopes of Bacillus anthracis lethal factor bind lethal toxin-neutralizing antibodies. Infect Immun. 2009;77:162–169. doi: 10.1128/IAI.00788-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Ruther P, Jiang I, Sawada-Hirai R, Sun SM, Nedellec R, et al. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum Antibodies. 2004;13:105–110. [PubMed] [Google Scholar]
- 31.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63:82–87. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flick-Smith HC, Walker NJ, Gibson P, Bullifent H, Hayward S, Miller J, et al. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect Immun. 2002;70:1653–1656. doi: 10.1128/IAI.70.3.1653-1656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenova VA, Steward-Clark E, Stamey KL, Taylor TH, Jr, Schmidt DS, Martin SK, et al. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin Diagn Lab Immunol. 2004;11:919–923. doi: 10.1128/CDLI.11.5.919-923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Soroka SD, Taylor TH, Jr, Stamey KL, Stinson KW, Freeman AE, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333:89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Park YS, Lee JH, Hung CF, Wu TC, Kim TW. Enhancement of antibody responses to Bacillus anthracis protective antigen domain IV by use of calreticulin as a chimeric molecular adjuvant. Infect Immun. 2008;76:1952–1959. doi: 10.1128/IAI.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConnell MJ, Hanna PC, Imperiale MJ. Cytokine response and survival of mice immunized with an adenovirus expressing Bacillus anthracis protective antigen domain 4. Infect Immun. 2006;74:1009–1015. doi: 10.1128/IAI.74.2.1009-1015.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oscherwitz J, Yu F, Jacobs JL, Liu TH, Johnson PR, Cease KB. Synthetic peptide vaccine targeting a cryptic neutralizing epitope in domain 2 of Bacillus anthracis protective antigen. Infect Immun. 2009;77:3380–3388. doi: 10.1128/IAI.00358-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oscherwitz J, Yu F, Cease KB. A synthetic peptide vaccine directed against the 2β2–2β3 loop of domain 2 of protective antigen protects rabbits from inhalation anthrax. J Immunol. 2010;185:3661–3668. doi: 10.4049/jimmunol.1001749. [DOI] [PubMed] [Google Scholar]
- 39.Semenova V, Svoboda P, Pohl J, Quinn CP. Peptide epitope mapping of anthrax protective antigen using sera of rhesus macaques that survived inhalation anthrax [abstract 67-Mo]. The International Conference on Bacillus anthracis, B cereus & B thuringiensis; Bacillus ACT; Bruges, Belgium. 2011. p. 150. [Google Scholar]
- 40.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003;170:3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 41.Peeling R, Maclean IW, Brunham RC. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984;46:484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purified PA1a (0.75 µg/lane), and PA3, PA3–4, and PA4 (1.5µg/lane), were electrophoresed on a 12% Tris/Glycine gel (Invitrogen, Carlsbad, CA). Proteins were then transferred to nitrocellulose membrane and blocked in 3% BSA blocking solution overnight. Monoclonal antibody (3 µg/mL) was applied to the membrane for 1 hour at RT. Next, alkaline phosphatase conjugated anti-human antibody was applied (1:50,000 dilution) at RT for 1 hour, and BCIP/NBT substrate (Sigma-Aldrich; St. Louis, MO) was added. Then, Western blots were scanned and analyzed for monoclonal binding.