Abstract
The contribution of oxidative stress to diabetic complications including neuropathy is widely known. Mitochondrial and cellular damage are associated with the overproduction of reactive oxygen species and decreased levels or function of the cellular antioxidant mitochondrial manganese superoxide dismutase (SOD2). We hypothesized that targeted SOD2 deletion in the peripheral nervous system using cre-lox technology under control of the nestin promoter would accelerate neuropathy in a type 2 model of diabetes, the BKS.db/db mouse. SOD2-deficient mice, however, demonstrated severe gait deformities and seizures and died by 20 days of age. Examination of SOD2 expression levels revealed that SOD2 was lost in brain and reduced in the spinal cord, but appeared normal in dorsal root ganglia and peripheral nerves in SOD2-deficient mice. These findings indicate incomplete targeted knockout of SOD2. Morphological examination revealed cortical lesions similar to spongiform encephalopathy in the brain of SOD2-deficient mice. No lesions were evident in the spinal cord, but changes in myelin within the sciatic and sural nerves including a lack of cohesion between layers of compact myelin was observed. Together, these results indicate that targeted neuronal SOD2 knockout using the nestin promoter results in severe central nervous system degeneration and perinatal lethality in mice. A specific peripheral nervous system-targeting construct is required to examine the consequences of SOD2 knockout in diabetic neuropathy.
Keywords: ROS, SOD2, oxidative stress, diabetic neuropathy, BKS.db/db mice
1. Introduction
Oxidative stress and mitochondrial dysfunction are implicated in the pathogenesis of multiple diseases of the central and peripheral nervous systems, including Parkinson’s and Huntington’s disease, amyotrophic lateral sclerosis, nervous system aging and diabetic neuropathy (DN). Substantial evidence implicates impaired mitochondrial energy production and increased mitochondrial oxidative damage early in the pathology of neurodegeneration (Moreira et al., 2010). Normally, cellular respiration results in the production of reactive oxygen species (ROS) that are neutralized by mitochondrial manganese superoxide dismutase (MnSOD or SOD2). In disease states, however, changes in mitochondrial respiration or a decreased cellular antioxidant capacity results in increased ROS levels and subsequent mitochondrial and cellular damage (Leinninger et al., 2006, Vincent et al., 2007, Naoi et al., 2009, Schon and Przedborski, 2011). It remains unclear, however, whether oxidative stress is the primary defect in the pathology of these diseases or a result of cellular dysfunction associated with neurodegenerative disease (Andersen, 2004).
The pathological consequences of increased ROS accumulation has previously been examined using mice with inactive or deleted SOD2 (Vincent et al., 2007). Complete loss of SOD2 in mice, however, results in embryonic or early postnatal lethality. This has been observed across genetic backgrounds, including CD-1 (Li et al., 1995), C57BL/6 (Ikegami et al., 2002) and DBA/2J (Huang et al., 2001), and is most likely due to cardiomyopathy. SOD2+/− heterozygous mice which have reduced SOD2 expression levels, on the other hand, have a normal lifespan, indicating that under normal conditions these mice are relatively healthy; however, they do exhibit an increased incidence of tumors (Elchuri et al., 2005) and significantly elevated measures of oxidative stress. Therefore, we previously examined the development of DN in diabetic C57Bl/6 SOD2+/− mice (Vincent et al., 2007). While no significant neuropathy was observed in a type 1 diabetes model, type 2 diabetic C57Bl/6.db/db SOD2+/− mice exhibited increased manifestations of DN compared to C57Bl/6.db/db SOD2+/+ control mice, suggesting that oxidative stress is important in the pathogenesis of DN (Vincent et al., 2007).
In the current study, we were interested in examining the specific effects of oxidative stress in neurons on DN onset and progression in BKS.db/db mice, a spontaneous and well-established model of type 2 diabetes which exhibits diabetes onset around 4 weeks of age and neurological deficits after 8 weeks (Sullivan et al., 2007). To circumvent the embryonic lethal effects of complete SOD2 deficiency, we utilized cre-lox technology to generate nervous system-specific SOD2 knockout BKS.db/db mice. We discovered, however, that targeted neuronal deletion of SOD2 under the nestin promoter is perinatally lethal and animals only survived for up to 3 weeks. Given the standard onset of diabetes and DN in BKS.db.db mice of 4 and 8 weeks, respectively, this prohibits any conclusions regarding the role of oxidative stress on the development of DN in these mice. Therefore, we performed a comprehensive morphological assessment to gain further insight into the effects of increased ROS in the central and peripheral nervous systems. We observed complete SOD2 deficiency in the brain of these mice, with select regions demonstrating increased oxidative stress and resulting pathology. SOD2 expression was also lost in a subset of motor neurons in the spinal cord and reduced in Schwann cells, but no major impact on SOD2 expression was seen in dorsal root ganglion neurons. These tissues were further characterized for physiological and morphological features, and our findings suggest that targeted deletion of SOD2 results in severe central nervous system degeneration characterized by mitochondrial damage within the brain and seizures that lead to perinatal lethality. Future studies examining the contribution of increased ROS to DN require a more specific targeting construct to delete SOD2 in the peripheral nervous system and circumvent the early lethality of central nervous system SOD2 deletion in BKS.db/db mice.
2. Experimental Procedures
2.1 Animals and genetic analyses
In collaboration with Jackson Laboratories (Bar Harbor, Maine), cre-lox technology was used to generate nervous system-specific SOD2 knockout mouse lines on a diabetic background. Cre expression was driven by the nestin promoter in B6.Cg-Tg(Nes-cre)1Kln/J mice (nes-cre+ or nes-cre−) to knock out floxed SOD2 (SOD2fl/fl or SOD2fl/+) in neuronal tissues (Tronche et al., 1999) of mice on the BKS.Leprdb/db background (BKS.db/db, BKS.db+ or BKS.+/+). To verify the genotype of all mice, genomic DNA preparation and PCR genotyping were performed using the Jackson Laboratory protocol (Bar Harbor, ME). Breeding colonies consisting of BKS.db+ SOD2fl/fl mice and BKS.db+ SOD2fl/+ nes-cre+/− mice were established at the University of Michigan to provide the animals used in this study. All animals were cared for following the University of Michigan Committee on the Care and Use of Animals guidelines.
The following groups were planned for our initial analysis: BKS.db/db SOD2fl/fl cre+, BKS.db/db SOD2fl/fl cre−, BKS.db+ SOD2fl/fl cre+ and BKS.db+ SOD2fl/fl cre−. Surprisingly, genotyping at weaning revealed no pups expressing the SOD2fl/fl cre+ genotype regardless of leptin receptor mutation status. These findings lead us to suspect a perinatal lethal effect of the targeted SOD2 deletion; therefore, subsequent litters were genotyped and checked daily from birth. Genotyping confirmed that pups dying between birth and weaning age were SOD2fl/fl cre+. Due to the early lethality of the SOD2fl/fl cre+ phenotype, and the fact that diabetes onset in BKS.db/db mice occurs around 4 weeks of age (Sullivan et al., 2007), we revised our experimental paradigm to investigate the impact of neuronal-specific SOD2 deficiency in these mice. Initial findings using hematoxylin & eosin staining (Carson and Hladik, 2009) to examine general histology revealed no gross anatomical abnormalities in liver, cardiac or skeletal muscle and no detectable differences in survival or neuronal pathology between BKS.db/db, BKS.db+ or BKS.+/+ mice (data not shown); therefore, for all subsequent studies, SOD2fl/fl or SOD2-deficient refers to BKS.+/+ SOD2fl/fl nes-cre+ mice expressing the targeted deletion of SOD2, and SOD2fl/+ refers to BKS.+/+ SOD2fl/+ nes-cre+ mice that express normal SOD2 levels.
2.2 Histological Assessment, SOD2 Expression Levels and Immunohistochemistry Analyses
SOD2-deficient pups became moribund between postnatal days 15–20; therefore, moribund pups and healthy littermate controls were euthanized at 10–14 d for tissue collection and morphological analyses. For general morphological examination and analysis of SOD2 expression levels, mice were euthanized by CO2 inhalation and brain, spinal cord, dorsal root ganglia (DRG), sciatic and sural nerves, liver, skeletal muscle and heart were dissected and immediately immersed in 10% neutral buffered formalin (Fisher) for histological studies or snap frozen in liquid nitrogen for storage at −80 C until needed for immunoblotting. For morphology studies, tissues were processed on a Tissue Tek VIP processor (Sakura Finetek), dehydrated and embedded in paraffin for sectioning. Sections were processed for standard hematoxylin and eosin staining or bright field immunohistochemistry (IHC) (Carson and Hladik, 2009). Briefly, IHC was performed using a Biocare automated stainer. Sections were treated with an antigen deblocker (RD913, Biocare) followed by peroxidase for 5 min. A rodent blocking solution (RBM961, Biocare) was applied for 30 min and sections were incubated with antibodies against GFAP (1:1000, ab7260, Abcam), neurofilament (NF; 1:500, ab40796, Abcam) and a marker of microglia (1:300, CP290, Biocare). Secondary rabbit anti-mouse horseradish peroxidase (HRP) was applied for 30 min and staining was revealed with 3′3-diaminobenzidine (DAB). Sections were counterstained with hematoxylin.
For immunoblotting, tissue lysates were prepared as previously described (Kim et al., 2009). The lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes for incubation with the desired primary and appropriate HRP-conjugated secondary antibodies. Antibodies against SOD2 (1:1000, ADISOD110, Enzo Life Sciences), Actin (1:1000, ab8227, Abcam) and GAPDH (1:1000, MAB374, Millipore) were used. The signal was visualized using enhanced chemiluminescence reagents (ECL; Amersham Bioscience, Piscataway, NJ) and images were captured using the Chemidoc XRS system (BioRad). Denstiometric analysis was performed using Quantity One software (BioRad).
Alternatively, for immunofluorescence (IF), mice were anesthetized by intraperitoneal injection of sodium pentobarbital (Fatal-plus; Dearborn, MI) and perfused through the left ventricle with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB, pH 7.4). Dissected brain, spinal cord and DRG, were fixed with 4% PFA, gradually cryoprotected in PB containing sucrose (10, 20, and 30%) and embedded for cryosectioning as previously described (Sullivan and Feldman, 1994, Cheng et al., 1996). Frozen sections were incubated with antibodies against SOD2 (1:200, ADISOD110, Enzo Life Sciences), NF (1:250, MAB5256X, Millipore), choline acetyltransferase (ChAT; 1:250, AB144P, Millipore) to identify motor neurons, and pyruvate dehydrogenase complex (PDH; 1:100, MSP06, Mitosciences) to label mitochondria, using a Mouse-On-Mouse kit (FMK2201, Vector). Sections were counterstained with fluorescent Nissl (1:50, N21480, Invitrogen). Motor neuron numbers in cervical and lumbar spinal cord sections were determined from every fourth 16 μm serial section for a total 10 sections each.
2.3 Transmission Electron Microscopy (TEM) Analysis
Brain, brain stem and sciatic and sural nerves were immersion fixed with 2.5 percent glutaraldehyde in 0.1 M Sorensen’s buffer, pH 7.4. After several buffer rinses, tissues were immersed for 1 h in 1% osmium tetroxide in the same buffer. To remove phosphate, double distilled water was used followed by en bloc staining with aqueous 3% uranyl acetate for 1 h. Tissues were then dehydrated in ascending concentrations of ethanol, transitioned through propylene oxide, and embedded in Epon epoxy resin (Edwards et al., 2010). Semi-thin sections were stained with toluidine blue for tissue orientation. Cross-sectional areas were ultra-thin sectioned (70 nm) and stained with uranyl acetate and lead citrate. They were examined using a Philips CM100 electron microscope at 60 kV. Images were recorded digitally using a Hamamatsu ORCA-HR camera system operated using AMT software (Advanced Microscopy Techniques Corp., Danvers, MA).
3. Results
3.1 General Physical Assessment and Tissue Analysis
SOD2-deficient pups developed an abnormal gait, had difficulty righting themselves between postnatal days 10–14 and became moribund between postnatal days 15–20. Genotyping confirmed that pups dying between birth and weaning age were SOD2fl/fl and cre+. These pups were small compared to their littermates; at 20 days of age the body weight of SOD2fl/fl pups averaged 3.51 g, compared to an average of 7.5 g for SOD2fl/+ pups.
Previous observations of animals in which SOD2 was globally deleted revealed severe cardiac myopathy, lipid accumulation in muscle and liver and metabolic acidosis (Li et al., 1995); therefore, cardiac muscle, skeletal muscle and liver paraffin sections were stained with hematoxylin and eosin to examine the general effects of targeted SOD2 deletion. Hearts from SOD2fl/fl and SOD2fl/+ mice both exhibit a normal ventricular size and wall thickness, and cardiac muscle histology is normal with no evidence of degeneration, atrophy or hypertrophy (data not shown). SOD2fl/fl and SOD2fl/+ mice also demonstrate normal skeletal muscle histology, and liver from both groups was normal with no evidence of steatosis (data not shown). Overall, no abnormalities in cardiac and skeletal muscle or liver in SOD2fl/fl and SOD2fl/+ mice were revealed.
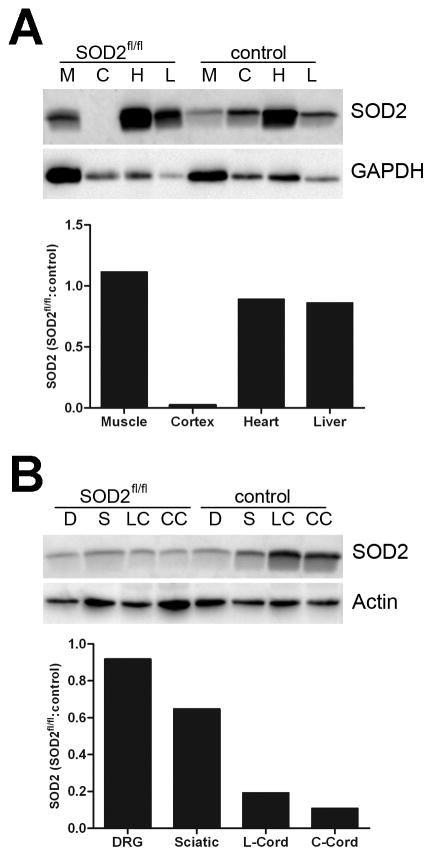
SOD2 expression levels were examined by western immunoblotting in brain, cardiac muscle, skeletal muscle and liver, and confirmed that SOD2 knockdown was restricted to nervous tissue in these mice (Figure 1A). However, further examination of neuronal tissues including DRG, cervical and lumbar spinal cord sections and sciatic nerve by western immunoblotting revealed variable effects of SOD2 knockdown in these tissues (Figure 1B). Both cervical and lumbar spinal cord tissue exhibited a considerable decrease in SOD2 levels in SOD2-deficient mice, whereas sciatic nerve tissue exhibited a slight decrease in SOD2 expression levels in SOD2-dificient mice. Little to no effects on SOD2 levels were observed in DRG. Together, these data reveal that SOD2 knockdown is inconsistent across neuronal tissues, thus supporting further investigation to identify the cell types affected and determine the extent and consequences of nestin-induced SOD2 knockdown in the central and peripheral nervous systems.
Fig. 1. SOD2 expression levels in SOD2fl/fl mouse tissues.
Various SOD2fl/fl mouse tissues were analyzed by western immunoblotting to determine SOD2 expression levels. Densitometry results are normalized to GAPDH or actin and then presented as a relative ratio to SOD2 expression levels in control littermates without SOD2 knockdown. A. Examination of skeletal muscle (M), cortex (C), heart (H) and liver (L) tissue indicate that SOD2 knockdown in SOD2fl/fl mice is restricted to neuronal tissue. B. Examination of DRG (D), sciatic nerve (S), and lumbar (LC) and cervical (CC) spinal cord tissue demonstrates incomplete SOD2 knockdown in peripheral nervous tissues, and more robust SOD2 knockdown within the spinal cord.
3.2 Central Nervous System
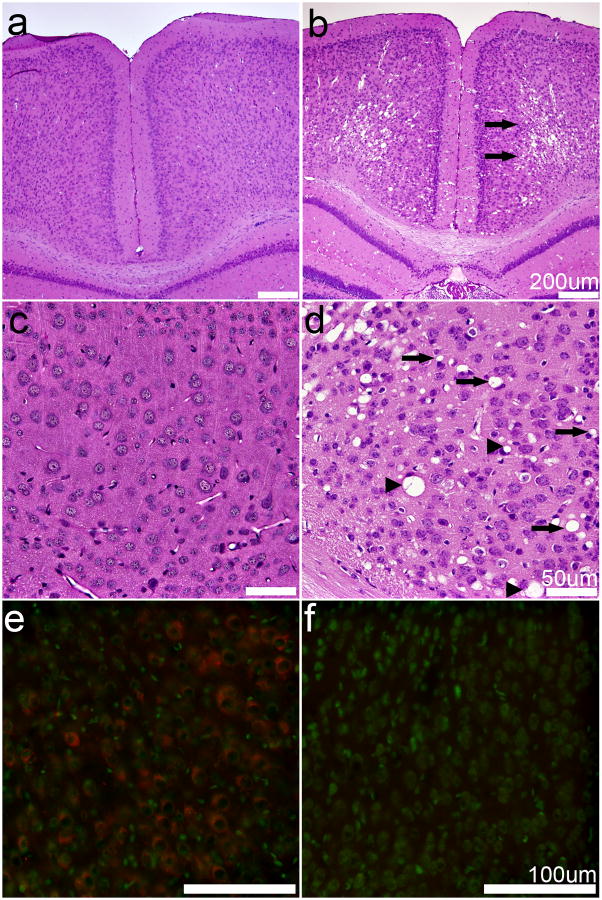
At approximately 10–15 days of age, SOD2fl/fl mice develop severe seizures and motor deficits; therefore, we first examined pathology within the central nervous system. Hematoxylin and eosin staining of brains from SOD2-deficient mice revealed varying degrees of vacuolization within the cortical neuropil (Figure 2). These bilateral symmetrical cortical lesions were detected in all SOD2fl/fl mice (Figure 2B, D) and never in the SOD2fl/+ mice (Figure 2A, C). Bilateral vacuolar lesions were also present in the hippocampus, thalamus and brain stem (data not shown). At the level of light microscopy, the vacuoles appear extra-neuronal and were often surrounded by compressed but intact neurons. SOD2 IF was localized within neurons and the neuropil in the SOD2fl/+ mice, but was absent in all cell types of the SOD2fl/fl mice (Figure 2E, F), although on very rare occasions, a SOD2-positive neuron was present.
Fig. 2. Spongiform degeneration and SOD2 expression in the cortex.
A–D. Hematoxylin and eosin staining of SOD2fl/+ (A, C) and SOD2fl/fl (B, D) mouse cortex reveals that SOD2fl/fl mice exhibit severe, bilateral, symmetrical, spongiform degeneration of the middle and deep layers of cerebral cortex (B, arrows). The SOD2fl/fl cortical lesions are characterized by extracellular vacuoles (B, D, arrows) with many neurons compressed by vacuoles (D, arrowheads). No specific lesions are observed in SOD2fl/+ mice (A, C). E–F. SOD2 IF is widespread in the cortex of SOD2fl/+ mice (E) but is not detected in SOD2fl/fl mice (F). Red, SOD2; green, Nissl. Scale bars equal 200 μm (A, B), 50 μm (C, D), or 100 μm (E, F).
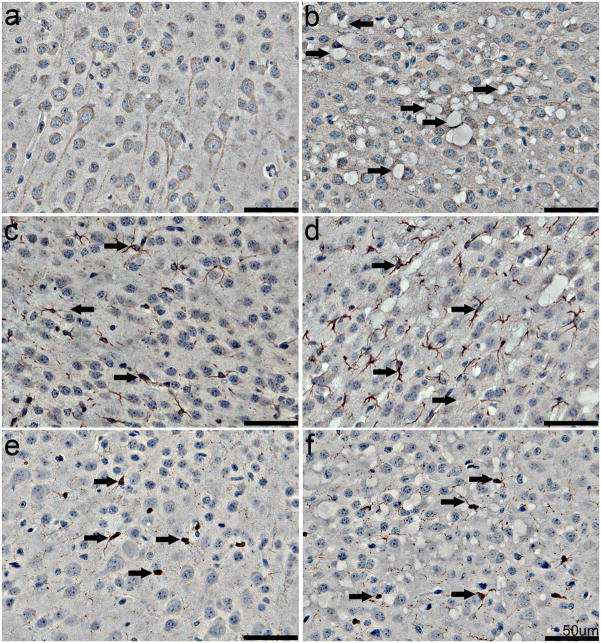
Further examination of the cortex from SOD2fl/fl (Figure 3B, D, F) and SOD2fl/+ (Figure 3A, C, E) mice included IHC for NF, the astrocyte marker GFAP and the microglial marker CP290. Intact cortical neurons surrounding many of the vacuoles are apparent in the cortex of SOD2fl/fl mice, but these vacuoles do not appear to be internal to any cortical neurons (Figure 3A, B). Similarly, numerous astrocytes with normal morphology are observed immediately adjacent to the vacuoles, with some cortical regions in the SOD2fl/fl mice exhibiting a trend for slightly increased astrocyte numbers (Figure 3C, D). Microglial staining was not indicative of any inflammatory changes associated with vacuolar lesions (Figure 3E, F).
Fig. 3. IHC examination of neurons and glia in the cortex.
IHC was performed on brain sections from SOD2fl/+ (A, C, E) and SOD2fl/fl (B, D, F) mice. A–B. NF IHC reveals the presence of vacuoles in the neuropil (arrows) and compression of neurons by vacuoles (arrowheads) in SOD2fl/fl cortex. C–F. Astrocytes labeled with GFAP (C, D, arrows) and microglia stained with CP290 (E, F, arrows) appear normal in both the SOD2fl/+ and SOD2fl/fl mice, although there is a trend towards increased numbers of astrocytes in SOD2fl/fl mice (D). Scale bars equal 50 μm.
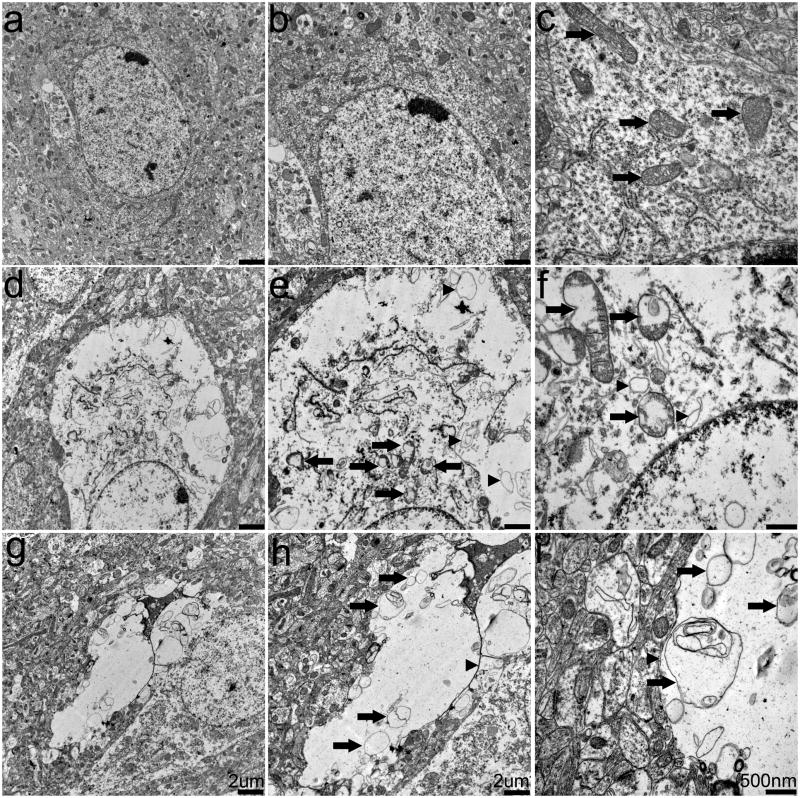
To determine whether the vacuoles were truly extracellular, we examined the cortex of SOD2fl/fl and SOD2fl/+ mice using TEM (Figure 4) which revealed both extra- and intracellular changes in the SOD2fl/fl mouse cortex (Figure 4D–I). Consistent with our observations using NF IHC (Figure 3), many neurons were compressed by extracellular vacuoles. Some vacuoles contained what appeared to be the membranous remnants of cellular organelles (Figure 4G–I; arrowheads), indicating that they could be representative of ruptured cells, as they were occasionally surrounded by small myelinated fibers. Intraneuronal changes included disrupted endoplasmic reticulum and swollen mitochondria with disrupted or missing cristae (Figure 4D–F; arrows). Apoptotic ultrastructure including nuclear condensation and blebbing was only rarely detected (data not shown). Similar lesions were observed in the hippocampus, thalamus and brain stem, and no abnormal pathologies were detected in astrocytes (data not shown). Overall, analysis of the central nervous system in SOD2fl/fl mice revealed cortical vacuolization and mitochondrial disruption.
Fig. 4. Ultrastructural analysis of cortex.
Cortical tissue from SOD2fl/+ and SOD2fl/fl mice was examined by TEM. A–C. Neurons from SOD2fl/+ mice exhibit no pathology and contain normal mitochondria (arrows) and organelles. D–F. SOD2fl/fl mouse cortical areas contain spongiform lesions and neurons that contain dilated and disrupted mitochondria (arrows) and membranous debris (arrowheads). G–I. Extracellular vacuoles in SOD2fl/fl− mice also contain membranous debris (arrows). Scale bars equal 2 μm (A, B, D, E, G, H) or 500 nm (C, F, I).
3.3 Spinal Cord
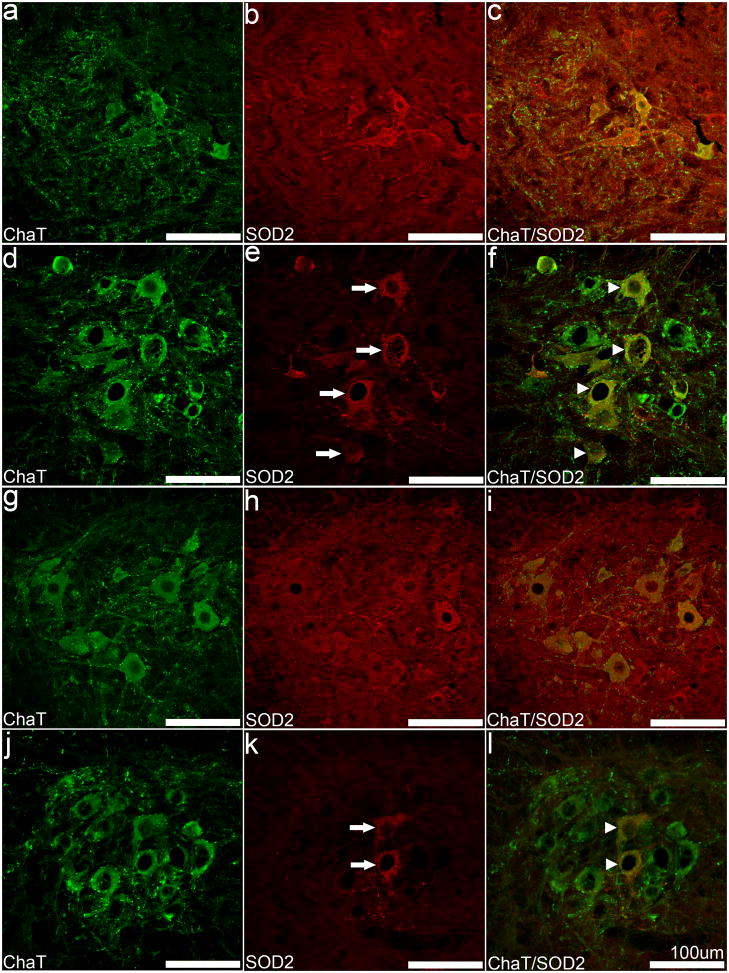
Cortical pathology has been reported in SOD2 knockout animal models; however, none of these reports examined the spinal cord or any aspect of the peripheral nervous system; therefore, we next examined SOD2 expression and PDH-labeling of mitochondria in the cervical and lumbar spinal cord (Figure 5). Neither motor neuron loss nor vacuolar changes were observed in the cervical or lumbar spinal cord of the SOD2fl/fl mice (Figure 5D–F, J–L) or the SOD2fl/+ mice (Figure 5A–C, G–I). SOD2 expression was lost in a number of motor neurons in the ventral horn region (Figure 5F, L); however, ChAT co-localization clearly depicts a subpopulation of SOD2-positive motor neurons (Figure 5F, L). While there was no apparent decrease in the total number of motor neurons, the number of SOD2-positive cells decreased in spinal cord. We also detected a general trend for greater numbers of SOD2-positive neurons within the cervical spinal cord (Figure 5E) compared to the lumbar spinal cord (Figure 5K); 33.7% of the total motor neurons in the cervical spinal cord and 11.2% of total motor neurons in the lumbar spinal cord were SOD2-positive in SOD2fl/fl mice. Motor neurons that did not express SOD2 had no obvious pathology and appeared normal.
Fig. 5. Differential loss of SOD2 in motor neurons from SOD2fl/fl mouse spinal cord.
Sections from the ventral horn of the cervical (A–F) and lumbar cord (G–L) were double-labeled with anti-SOD2 (B, E, H, K) and anti-ChAT (A, D, G, J). SOD2 IF was detected in motor neurons of the ventral horn of SOD2fl/fl mice (E, K, arrows) and co-localized with ChAT IF (F, L, arrowheads), indicating incomplete loss of SOD2 in motor neurons. The cervical cord appears to contain more SOD2-positive motor neurons (arrowheads). Scale bars equal 100 μm.
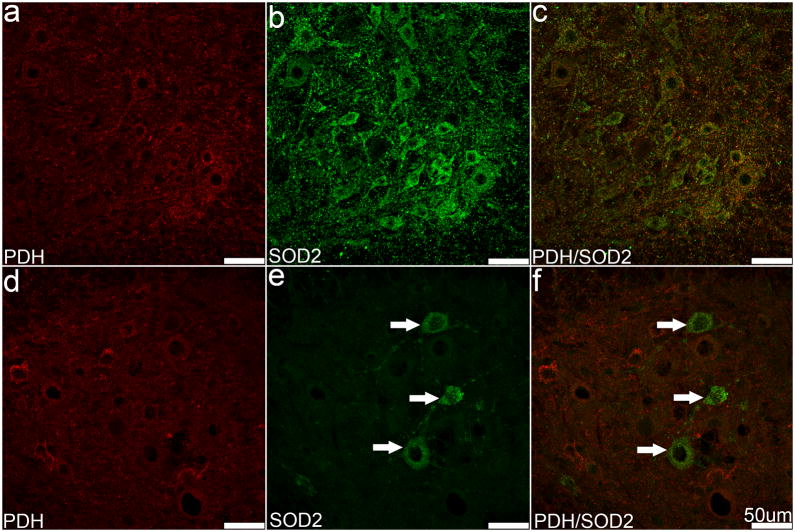
We next examined the effects of SOD2 expression levels on mitochondria in motor neurons in the lumbar spinal cord. The overall level of PDH IF appears slightly reduced in SOD2fl/fl mice compared to SOD2fl/+ mice, but there were no detectable differences in the distribution of mitochondria in the ventral horn (Figure 6A, D). In the dorsal horn, generally lower levels of SOD2 and PDH IF were detected within neurons and the neuropil, however, no significant differences between the SOD2fl/fl and SOD2fl/+ mice were identified (data not shown). Together, these data suggest that there is no significant neuronal pathology within the spinal cord of SOD2fl/fl mice, and indicate that SOD2 knockdown is not complete in the motor neuron population.
Fig. 6. Mitochondria in lumbar spinal cord motor neurons.
Sections from SOD2fl/+ (A–C) and SOD2fl/fl (D–F) mice were immunolabeled with PDH and SOD2. No significant difference in intensity or localization of PDH IF was detected between SOD2fl/+ and SOD2fl/fl mice (A, D); however, fewer SOD2 IF positive neurons were observed in the SOD2fl/fl mouse (E, F, arrows). Scale bars equal 50 μm.
3.4 Peripheral Nervous System
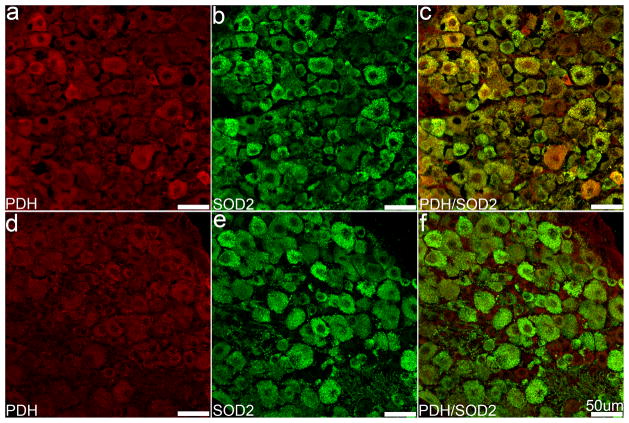
DN involves the degeneration and loss of sensory neurons of the peripheral nervous system; therefore, we next examined the impact of nes-cre-induced SOD2 knockout on DRG and peripheral nerves. Hematoxylin and eosin staining revealed no differences in DRG neuronal morphology between SOD2fl/fl and SOD2fl/+ mice (data not shown). SOD2 IF was similar between groups, and appeared as evenly distributed punctate fluorescence throughout the cytoplasm (Figure 7B, C, E, F). We did not observe any significant differences in the amount or distribution of mitochondrial PDH IF between SOD2fl/fl and SOD2fl/+ mice (Figure 7A, D). Differences were apparent, however, when the fluorescent signals were combined; SOD2fl/+ mice exhibited substantial overlap of SOD2 and PDH fluorescence, while the SOD2-deficient mice exhibited considerably less co-localization (Figure 7C, F).
Fig. 7. Mitochondria and SOD2 expression in DRG.
Sections from SOD2fl/+ (A–C) and SOD2fl/fl (D–F) mouse DRG were immunolabeled with PDH and SOD2. Both markers were evenly distributed in the cytoplasm and punctate labeling is common. Significant differences in either marker were not obvious (A, B, D, E); however, an apparent decrease in co-localization of these markers appears in the merged images (C, F). Scale bars equal 50 μm.
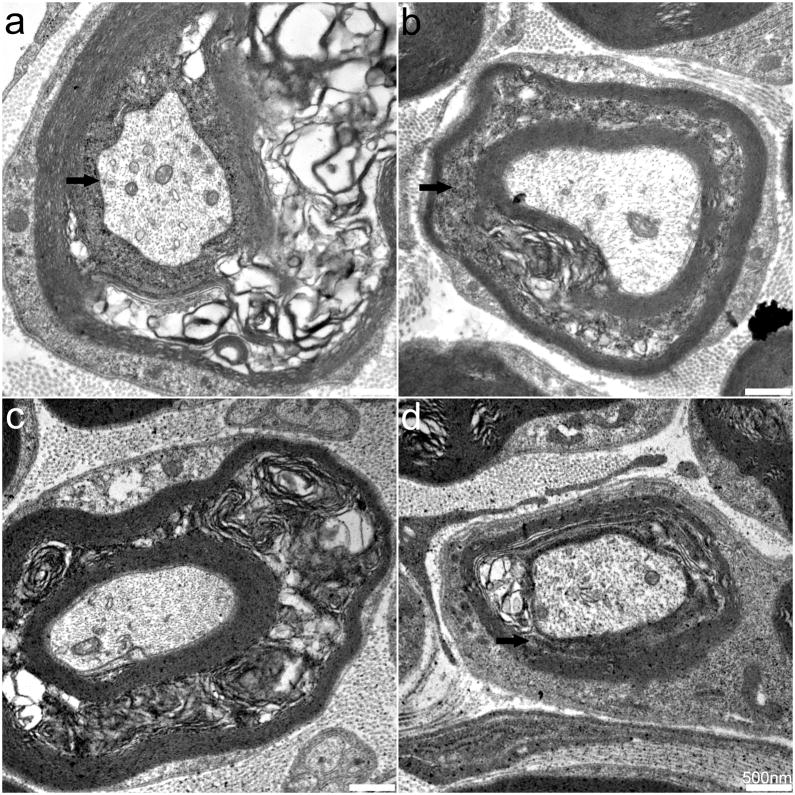
We next examined sciatic and sural nerves. No significant differences in axonal structure or general fiber density were observed between SOD2fl/fl and SOD2fl/+ mice (data not shown). Both PDH and SOD2 IF were prominent in Schwann cell cytoplasm but were not detected within axons (data not shown). TEM of SOD2fl/fl mouse sciatic (Figure 8A–B) and sural (Figure 8C–D) nerves, however, revealed prominent changes including myelin delamination and disrupted mitochondria in myelinating Schwann cells. Few changes were detected within non-myelinating Schwann cells or in myelinated or unmyelinated axons. These data indicate that nes-cre expression does not result in complete knockout SOD2 expression in DRG, but examination of peripheral nerves reveals defects in myelination and in SOD2 expression in Schwann cells.
Fig. 8. Ultrastructural analysis of sciatic and sural nerves.
TEM of SOD2fl/fl mouse sciatic (A, B) and sural nerves (C, D). Myelin delamination was consistently detected in the peripheral nerves of SOD2fl/fl mice. Scale bars equal 50 μm.
4. Discussion
Oxidative damage disrupts multiple cellular processes from transcription and translation to mitochondrial function and axonal transport, and our laboratory has been instrumental in establishing the impact of oxidative stress on the development and progression of DN (Russell et al., 2002, Feldman, 2003, Vincent et al., 2004, Vincent et al., 2005, Vincent et al., 2007, Russell et al., 2008). Recently, we reported that elevated triglycerides correlate with DN progression in human patients with diabetes (Wiggin et al., 2009) and that oxidized lipids are toxic to sensory neurons (Vincent et al., 2009). Therefore, the current study was aimed at investigating increased oxidative stress in the context of type 2 diabetes, a condition characterized by hyperglycemia and dyslipidemia. Cre-lox technology was used to create a targeted nervous system knockout of the mitochondrial detoxifying enzyme SOD2 in BKS.db/db mice, a robust model of type 2 diabetes. Targeted knockout of SOD2, however, resulted in a spongiform pathology in the brain and was perinatally lethal, independent of the leptin receptor mutation. In addition to the effects of SOD2-knockout in the brain, we also report differences in SOD2 expression in the spinal cord and peripheral nervous system; to our knowledge this is the first description of these changes. Overall, these studies validate the link between oxidative stress and neurodegeneration, and provide novel insight into the role of SOD2 in various neuronal tissues.
SOD2 is an essential component of the cellular antioxidant defense against superoxide accumulation (Moreira et al., 2010), and numerous studies have altered SOD2 expression in animal models to investigate the role of oxidative stress on various tissues. Early studies examining whole-animal inactivation of SOD2 found that loss of SOD2 function resulted in perinatal lethality around P10 (Li et al., 1995, Melov et al., 1998). Brain histology was normal and no neurological phenotype was observed; therefore, the authors concluded that the mice died from cardiac myopathy resulting from oxidative stress and abnormal mitochondria within cardiac myocytes (Li et al., 1995). Since that time, whole-animal SOD2 deletion has also been examined on various genetic backgrounds (Huang et al., 2001). While SOD2 deletion in C57Bl/6 mice results in embryonic lethality (E15) due to cardiomyopathy, DBA/2J and B6D2F1 mice survived to 12 days and 3 weeks, respectively, and did not exhibit a cardiac phenotype – these mice likely died from metabolic acidosis, indicative of overall reduced antioxidant defense (Huang et al., 2001). Careful examination of SOD2 knockout mice revealed that oxidative phosphorylation markers are reduced in multiple tissues following whole-animal SOD2 deletion. Specifically, complex II and III in skeletal and cardiac muscle and citrate synthase in cardiac muscle were significantly decreased, as was aconitase in cardiac muscle and brain (Melov et al., 1999). These tissues all exhibited increased levels of oxidative stress.
The ability to detoxify ROS is of particular importance in any long-lived, non-mitotic cells, including neurons. Therefore, we were interested in investigating the role of ROS on neurodegeneration in DN. In collaboration with Jackson Laboratories, we developed a nervous system-specific SOD2-deficient mouse using cre-lox technology, with cre expression driven by the nestin promoter to drive expression in developing neuronal cells (Tronche et al., 1999). Our initial observations revealed that no SOD2fl/fl pups survived to weaning age; the animals developed motor abnormalities between 10–14 days and show seizures with death likely resulting from cardiac arrest and/or arrhythmias secondary to continuous convulsions at 3 weeks of age. General pathological findings and western immunoblotting confirmed that SOD2 deletion was confined to the nervous system in these mice (Figure 1), indicating that oxidative stress in other tissues was not a contributing factor to lethality. Furthermore, we detected no effect of the leptin receptor mutation on survival, SOD2 expression, or nervous system pathology at this age. Because diabetes and DN onset in BKS.db/db mice does not occur until well after SOD2-deficient mice died, we chose to perform a comprehensive analysis of neuronal tissues in two groups of mice for the current studies: SOD2fl/fl and SOD2fl/+ mice on the BKS.+/+ background.
Severe central nervous system defects were observed in SOD2fl/fl mice. Spongiform vacuolization and mitochondrial damage were evident in multiple regions, including layers 3–6 of the cortex, the hippocampus, thalamus and brain stem (Figures 2–4, data not shown). These spongiform changes are consistent with those previously described in the cortex and brainstem of complete SOD2 knockout animals (Melov et al., 1998). The majority of the damage appears to be extracellular in that it is not localized near neuronal nuclei; however, due to the appearance of organelle remnants, it is possible that the vacuoles represent ruptured neurons or cellular areas beyond the nucleus. No significant damage to the surrounding neuropil, including profiles of passing axons and dendrites or in astrocytes and microglia was found, and no degenerative changes were observed in the white matter. Examination of astrocytes and microglia revealed normal morphology and distribution indicating that these cells were not affected by SOD2 deletion at the time of death, although we observed a trend towards a slight increase in astrocyte numbers in SOD2fl/fl mouse brains. This is not surprising, given the degenerative changes observed in the cortex. Furthermore, these findings are also supported by previous reports of mostly normal glia and no changes in white matter morphology (Melov et al., 1998), and the identification of prominent changes in the M1 and M2 regions of the cortex and within the motor nucleus of the trigeminal nucleus (Melov et al., 2001). Together, these data support the conclusion that more metabolically active neurons are affected by SOD2 deletion, and it is possible that glial cell types would be equally affected if stressed.
Previous studies did not address the effects of SOD2 knockout on the spinal cord or peripheral nervous system; therefore, we examined the DRG, peripheral nerves and cervical and lumbar regions of the spinal cord in SOD2fl/fl and SOD2fl/+ mice. Basic histology generally appeared normal in the targeted SOD2fl/fl mice, and spongiform or degenerative lesions were not detected in any part of the DRG, peripheral nerves or spinal cord gray or white matter. These limited pathological findings in SOD2fl/fl mice reflect the fact that SOD2 knockout was incomplete in neuronal tissues outside of the brain. In the DRG (Figures 1B, 7), we detected no significant changes in the levels or localization of SOD2 in the SOD2fl/fl mice. Within peripheral nerve (Figures 1B, 8), SOD2 immunoreactivity was evident in the cytoplasm of Schwann cells of SOD2fl/fl mice and appeared moderately diminished compared to controls. Examination of peripheral nerve ultrastructure revealed disrupted mitochondria in myelinating Schwann cells, while non-myelinating Schwann cells were normal. Axonal mitochondria were also normal. Due to the early lethality of the targeted SOD2 deletion, we were not able to assess nerve conduction velocities for either motor or sensory modalities.
In the spinal cord, SOD2 expression markedly decreased, but was still evident in a subset of motor neurons in SOD2fl/fl mice (Figures 1B, 5, 6). Given the extent of SOD2 knockdown, however, significant repercussions on the motor neuron microenvironment are possible despite the incomplete nature of SOD2 knockout within that cellular population. We also observed a trend for increased levels of SOD2 expression and increased numbers of SOD2-expressing motor neurons within the cervical spinal cord compared to the lumbar spinal cord. These motor neurons within the ventral horn displayed normal morphology, and examination of mitochondrial PDH IF in the spinal cord demonstrated co-localization with SOD2 in the SOD2fl/+ mice, but an apparent lack of co-localization in the SOD2fl/fl mice. Previous attempts to investigate targeted SOD2 knockout in motor neurons revealed similar findings and exhibited incomplete SOD2 deletion in motor neurons (Misawa et al., 2006). These animals exhibited a normal lifespan, displayed no motor weakness, tremors or paralysis and maintained normal neuronal numbers. Motor neurons within brain stem and ventral spinal cord, however, did exhibit increased oxidative stress and accelerated Wallerian degeneration following axotomy (Misawa et al., 2006). A role for oxidative damage and mitochondrial dysfunction in motor neurons has also been previously examined by expressing mutated copper-zinc superoxide dismutase (SOD1) in SOD2-deficient mice (Andreassen et al., 2000). SOD1 mutations are implicated in the pathology of familial amyotrophic lateral sclerosis. These mutations do not affect the dismutase activity of SOD1 and neuronal death is likely due to a toxic gain of function (Ilieva et al., 2009). Expression of SOD1G93A in SOD2-deficient mice resulted in a reduced lifespan and earlier onset of motor neuron death compared to SOD1G93A mice without a SOD2 deficiency (Andreassen et al., 2000). Together, these findings support a connection between oxidative stress, mitochondrial dysfunction and neurodegeneration, but alternative approaches are required to investigate the consequences of SOD2 loss on the peripheral nervous system.
The cre-lox system provides an enticing approach to investigate the effects of tissue-specific targeted knockout of genes (Ray and Gage, 1992, Ray et al., 2000, Yu and Bradley, 2001), and multiple groups have used this technology in various tissues in an attempt to circumvent the early lethality of complete SOD2 knockout in mice (Ikegami et al., 2002, Misawa et al., 2006, Nojiri et al., 2006, Kuwahara et al., 2010, Shimizu et al., 2010, Lustgarten et al., 2011, Parajuli et al., 2011). SOD2 deletion in kidney, skeletal muscle and liver all induced increases in oxidative stress measures, but no drastic effects on the specific tissues or lifespan was observed (Ikegami et al., 2002, Kuwahara et al., 2010, Lustgarten et al., 2011, Parajuli et al., 2011). For the current studies, we chose to combine cre-lox technology with the nestin promoter for its ubiquitous expression within the developing nervous system (Dahlstrand et al., 1995, Lothian and Lendahl, 1997, Guo et al., 2005, Yu et al., 2005). At the time we began these experiments, no central or peripheral nervous system-specific SOD2 deletion had been reported. Since that time, targeted deletion of SOD2 in the spinal cord and brain stem has been reported, although these animals exhibit incomplete SOD2 knockout, a normal lifespan and remarkably, no motor deficits or neuronal loss (Misawa et al., 2006). Central nervous system SOD2 deletion has also been briefly examined, and similar to our findings, mice exhibit seizures and die at approximately 3 weeks of age (Shimizu et al., 2010); however, a thorough characterization of the model was not described. Neurons from these mice have been used to examine the contribution of SOD1 and SOD2 to oxidative damage following hypoxia and reoxygenation (Sasaki et al., 2011). Taken together, these data indicate that tissue-specific knockout of SOD2 does increase oxidative stress and elicits subsequent tissue damage, but alone is not enough to recapitulate specific disease phenotypes. It is possible that compensatory mechanisms may be involved, although SOD1 mRNA, protein or enzyme activity were not upregulated in many of these models where mechanistic studies were investigated (Saito et al., 1995, Rebelo et al., 2007, Kuwahara et al., 2010, Shimizu et al., 2010, Parajuli et al., 2011); this remains an area of intense investigation.
To investigate the impact of ROS and mitochondrial dysfunction on DN, and establish models for future therapeutic development studies, a more restricted and specific promoter to target SOD2 deletion to sensory neurons is required. There are a number of neural crest-specific promoters; however, loss of sympathetic innervation and loss of the enteric nervous system would complicate data interpretation due to effects on vascular tone and nutrition. DRG11 and Foxs1 are specifically expressed in developing sensory neurons (Saito et al., 1995, Montelius et al., 2007, Rebelo et al., 2007), with DRG11 preferentially expressed in small DRG neurons (Saito et al., 1995, Rebelo et al., 2007). Either of these promoters in combination with cre-lox technology would be useful in targeting all or a subset of DRG neurons in future experiments
Overall, the current study demonstrates that targeted deletion of SOD2 using the nestin promoter results in mitochondrial damage within the brain and seizures that lead to perinatal lethality. While the incomplete loss of SOD2 expression in peripheral nervous tissues and the early death of these animals precludes an in-depth analysis of how ROS accumulation relates to DN, our central nervous system findings enable us to speculate towards a mechanism for this pathology. We propose that increased neuronal activity results in increased demand for ATP, increased mitochondrial activity, and ultimately increased oxidative stress. Without SOD2 to detoxify ROS, mitochondrial and cellular damage ensue. This would also apply to actively myelinating Schwann cells, as peripheral myelination is one of the last maturation events in the nervous system, and is supported by our current peripheral nerve findings and a recent report of decreased energy capacity in cortical synaptosomes isolated from SOD2-null mice (Flynn et al., 2011). In conclusion, excess oxidative stress causes mitochondrial disruption and deficits in mitochondrial enzymes and/or decoupling which result in increased oxidative stress. Which event occurs first in the development of neurologic disease is the subject of enormous study and debate.
Highlights.
Cre-lox under the nestin promoter was used to knockout SOD2 in the nervous system.
SOD2 knockout in the nervous system was perinatally lethal due to severe seizures.
Spongiform pathology and mitochondrial damage are seen in the central nervous system.
Knockout of SOD2 was incomplete in the peripheral nervous system.
Acknowledgments
This work utilized the Morphology & Image Analysis (MIAC) of the Michigan Diabetes Research and Training Center funded by grant DK020572 from the National Institute of Diabetes and Digestive and Kidney Disease. This work was supported by the National Institutes of Health (NIH UO1 DK076160-05) and the Program for Neurology Research and Discovery (www.pfund.umich.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Klivenyi P, Klein AM, Shinobu LA, Epstein CJ, Beal MF. Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Annals of Neurology. 2000;47:447–455. [PubMed] [Google Scholar]
- Carson FL, Hladik C, editors. Histotechnology: A Self Instructional Text. Chicago, IL: American Society for Clinical Pathology Press; 2009. [Google Scholar]
- Cheng HL, Sullivan KA, Feldman EL. Immunohistochemical localization of insulin-like growth factor binding protein-5 in the developing rat nervous system. Brain Res Dev Brain Res. 1996;92:211–218. doi: 10.1016/0165-3806(96)00016-8. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;53:160–169. doi: 10.1007/s00125-009-1553-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. The Journal of clinical investigation. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JM, Choi SW, Day NU, Gerencser AA, Hubbard A, Melov S. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free Radic Biol Med. 2011;50:866–873. doi: 10.1016/j.freeradbiomed.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ZM, Xu K, Yue Y, Huang B, Deng XY, Zhong NQ, Hong X, Chen XG, Xiao D. Temporal control of Cre recombinase-mediated in vitro DNA recombination by Tet-on gene expression system. Acta Biochim Biophys Sin (Shanghai) 2005;37:133–138. [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Kozy HM, Mantha S, Goodman SI, Ursell PC, Epstein CJ. Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic Biol Med. 2001;31:1101–1110. doi: 10.1016/s0891-5849(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Ikegami T, Suzuki Y, Shimizu T, Isono K, Koseki H, Shirasawa T. Model mice for tissue-specific deletion of the manganese superoxide dismutase (MnSOD) gene. Biochemical and biophysical research communications. 2002;296:729–736. doi: 10.1016/s0006-291x(02)00933-6. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. The Journal of cell biology. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Backus C, Oh S, Hayes JM, Feldman EL. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–5301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. 2010;48:1252–1262. doi: 10.1016/j.freeradbiomed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Edwards JL, Lipshaw MJ, Feldman EL. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nature clinical practice. 2006;2:620–628. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature genetics. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. The European journal of neuroscience. 1997;9:452–462. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ, Wallace DC. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nature Genetics. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- Misawa H, Nakata K, Matsuura J, Moriwaki Y, Kawashima K, Shimizu T, Shirasawa T, Takahashi R. Conditional knockout of Mn superoxide dismutase in postnatal motor neurons reveals resistance to mitochondrial generated superoxide radicals. Neurobiology of Disease. 2006;23:169–177. doi: 10.1016/j.nbd.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Montelius A, Marmigere F, Baudet C, Aquino JB, Enerback S, Ernfors P. Emergence of the sensory nervous system as defined by Foxs1 expression. Differentiation. 2007;75:404–417. doi: 10.1111/j.1432-0436.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochimica et biophysica acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Yi H, Inaba K, Akao Y, Shamoto-Nagai M. Mitochondria in neurodegenerative disorders: regulation of the redox state and death signaling leading to neuronal death and survival. J Neural Transm. 2009;116:1371–1381. doi: 10.1007/s00702-009-0309-7. [DOI] [PubMed] [Google Scholar]
- Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. The Journal of biological chemistry. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- Parajuli N, Marine A, Simmons S, Saba H, Mitchell T, Shimizu T, Shirasawa T, Macmillan-Crow LA. Generation and characterization of a novel kidney-specific manganese superoxide dismutase knockout mouse. Free Radic Biol Med. 2011;51:406–416. doi: 10.1016/j.freeradbiomed.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Gage FH. Gene transfer into established and primary fibroblast cell lines: comparison of transfection methods and promoters. Biotechniques. 1992;13:598–603. [PubMed] [Google Scholar]
- Ray MK, Fagan SP, Brunicardi FC. The Cre-loxP system: a versatile tool for targeting genes in a cell- and stage-specific manner. Cell Transplantation. 2000;9:805–815. doi: 10.1177/096368970000900607. [DOI] [PubMed] [Google Scholar]
- Rebelo S, Reguenga C, Osorio L, Pereira C, Lopes C, Lima D. DRG11 immunohistochemical expression during embryonic development in the mouse. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:2653–2660. doi: 10.1002/dvdy.21271. [DOI] [PubMed] [Google Scholar]
- Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiology of disease. 2008;30:420–429. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- Saito T, Greenwood A, Sun Q, Anderson DJ. Identification by differential RT-PCR of a novel paired homeodomain protein specifically expressed in sensory neurons and a subset of their CNS targets. Molecular and Cellular Neurosciences. 1995;6:280–292. doi: 10.1006/mcne.1995.1022. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Shimizu T, Koyama T, Sakai M, Uchiyama S, Kawakami S, Noda Y, Shirasawa T, Kojima S. Superoxide dismutase deficiency enhances superoxide levels in brain tissues during oxygenation and hypoxia-reoxygenation. Journal of neuroscience research. 2011;89:601–610. doi: 10.1002/jnr.22581. [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Nojiri H, Kawakami S, Uchiyama S, Shirasawa T. Model mice for tissue-specific deletion of the manganese superoxide dismutase gene. Geriatr Gerontol Int. 2010;10(Suppl 1):S70–79. doi: 10.1111/j.1447-0594.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Feldman EL. Immunohistochemical localization of insulin-like growth factor-II (IGF-II) and IGF-binding protein-2 during development in the rat brain. Endocrinology. 1994;135:540–547. doi: 10.1210/endo.135.2.7518384. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376–2385. doi: 10.2337/db09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine reviews. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Experimental neurology. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL. Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes. 2009;58:1634–1640. doi: 10.2337/db08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Dandekar M, Monteggia LM, Parada LF, Kernie SG. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005;41:147–153. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]
- Yu Y, Bradley A. Engineering chromosomal rearrangements in mice. Nature reviews Genetics. 2001;2:780–790. doi: 10.1038/35093564. [DOI] [PubMed] [Google Scholar]