Abstract
Background
Studies of pediatric conduct disorder (CD) have described frontal and temporal lobe structural abnormalities that parallel findings in antisocial adults. The purpose of this study was to examine previously unexplored cortical thickness and folding as markers for brain abnormalities in “pure CD”-diagnosed adolescents. Based on current fronto-temporal theories, we hypothesized that CD youth would have thinner cortex or less cortical folding in temporal and frontal lobes than control subjects.
Methods
We obtained T1-weighted brain structure images from n=24 control and n=19 CD participants aged 12–18 years, matched by overall gender and age. We measured group differences in cortical thickness and local gyrification index (regional cortical folding measure) using surface-based morphometry with clusterwise correction for multiple comparisons.
Results
CD participants, when compared with controls, showed both reduced cortical thickness and folding. Thinner cortex was located primarily in posterior brain regions, including left superior temporal and parietal lobes, temporoparietal junction and paracentral lobule, right superior temporal and parietal lobes, temporoparietal junction and precuneus. Folding deficits were located mainly in anterior brain regions and included left insula, ventro- and dorsomedial prefrontal, anterior cingulate and orbitofrontal cortices, temporal lobe, right superior frontal and parietal lobes and paracentral lobule.
Conclusions
Our findings generally agree with previous CD volumetric studies, but here show the unique contributions of cortical thickness and folding to gray matter reductions in pure CD in different brain regions.
Keywords: Conduct disorder, cortical thickness, local gyrification index, cortical folding, somatic marker hypothesis, empathy
Introduction
Conduct disorder (CD) is characterized by aggression, property destruction, deceitfulness or theft, and serious rule violations occurring before age 18 (1) that occurs in as many as 16.0% of boys and 9.2% of girls (2). Neurodevelopmental theories suggest that early expression of brain abnormalities may increase the risk for lifelong antisocial behavior (3–5). According to these theories, pediatric structural brain abnormalities may persist into adulthood, thereby providing a substrate for adult antisocial personality (APD) or psychopathy. Studies of brain volume in CD found gray matter deficits in orbitofrontal cortex (OFC), hippocampus (6), amygdala (6–8), insula (7,8), cerebellum (6,9), dorsomedial prefrontal cortex, caudate nucleus, fusiform gyrus/occipital cortex (8) and bilateral temporal lobes (6,10). A recent study of boys with disruptive behavioral disorders found reduced cortical thickness in left anterior cingulate cortex (ACC) (11). In contrast, one study of youth with callous-unemotional traits found increases in gray matter concentration in frontal lobes and both volume and concentration in temporal lobes compared with controls (12). Despite emerging uniformity of abnormal brain volume findings across regions and ages, there is a need to determine with improved consistency which brain regions show structural abnormalities in antisocial disorders.
With one exception (11), all published structural brain studies of CD youth have focused exclusively on volume deficits (e.g., (13)). In recent years, surface-based measurements of cortical thickness (11,14) and folding (15) increasingly have been used to compare groups. Surface-based measurements are important because the two-dimensional folded laminar structure of the cerebral cortex is a function of both cortical thickness and surface area (where surface area is proportional to the degree of cortical folding for a fixed intracranial volume) (16–20). Cortical thickness and folding are increasingly viewed as important, separable endophenotypes for understanding the relationship of genetic influences on brain structure and function (16,19,21,22). Because volumetric techniques may obscure the degree to which each factor contributes to gray matter volume differences (19,20), focusing on these two more specific measures of brain structure abnormality might help clarify previously discrepant findings in CD.
Inconsistencies in CD structural findings might also be due to comorbid psychopathology, such as ADHD, depression, anxiety and substance use disorders. Notably, almost all CD published structural brain studies have examined samples with documented high ADHD comorbidity. While statistical control can mitigate the confounding effects of comorbid psychopathology, it is essential to examine CD individuals without comorbidity to ensure any brain structure abnormalities cannot be attributed to other disorders. However, CD without comorbidity is the norm, not the exception. The recent National Comorbidity Survey Replication study (23) found that less than 40% of adolescents met lifetime criteria for more than one disorder, with current comorbidity rates in community-recruited CD youth long known to be only between 7–28% for ADHD, depression or anxiety (24). Although childhood CD and ADHD have both been proposed to predict future adult antisocial behavior, Lahey and colleagues determined that only CD directly predisposes individuals to the development of antisocial personality disorder (APD) in adulthood (25). Because recent studies have provided evidence for gray matter volume deficits in adult APD (26–28) and psychopathy (29–31) in regions that overlap with structural brain abnormalities in CD, identification of such abnormalities in a more “pure” CD sample may elucidate specific neurobiological factors underlying the development of adult APD and psychopathy.
The primary purpose of this study was to identify cortical thickness and folding differences in a relatively pure CD adolescent sample. We hypothesized that CD adolescents without other significant psychopathology would show cortical thickness or folding deficits in prefrontal cortex (e.g., ventromedial, OFC, insular cortex, rostral cingulate) and bilateral temporal lobes. This prediction was based not only on previous CD brain volume abnormality findings, but also on findings of structural abnormalities in similar regions in adult APD and psychopathy. Although we were uncertain whether lateral prefrontal regions would show cortical thickness or folding deficits in a sample of pure CD youth because of inconsistencies in previous findings and due to questions about disorder specificity (i.e., comorbid ADHD) these regions were previously linked to antisocial behavior in functional and structural neuroimaging (32) and in lesion-based studies (33). Therefore, we also predicted CD cortical thickness or folding deficits in dorso- and ventrolateral prefrontal cortex.
Methods
Study participants
We selected n=19 adolescents (ages 12–18) diagnosed with CD and n=24 adolescents without psychiatric disorders from an original sample of 310 datasets collected in an NIMH-funded study comparing CD, ADHD, and control group participants (K23 MH070036). Participants were recruited by community advertisements, direct referral from clinical treatment programs at The Institute of Living (http://www.instituteofliving.org), and letters sent to families of youth on probation following arrest in the Connecticut Court Support Services Division. The CD group had one left-handed and one mixed-dominant participant. All participants were healthy as determined from responses on a parent-report medical questionnaire. Informed assent for study participation and parental permission were obtained jointly from the participants and their parent or legal guardian. The Hartford Hospital Institutional Review Board approved all consent and study procedures.
Clinical diagnoses for research purposes were made using the K-SADS-PL (34) conducted by trained bachelor’s- and master’s-level staff working under the supervision of a licensed clinical psychologist. The K-SADS-PL, based on DSM-IV, is a validated, reliable and widely-used semi-structured clinical research interview. Interviews were performed separately for both adolescents and parents. Information was synthesized and diagnoses confirmed in weekly research group meetings. All participants tested negative on a urine screen for marijuana, cocaine and heroin on the MRI day. No participants met lifetime criteria for ADHD and all CD participants, except one, reported zero or one current ADHD symptoms. By design, none of the CD sample had current co-morbid psychiatric diagnoses or substance dependence. However, one CD adolescent was diagnosed with cannabis abuse, another with past cannabis dependence, while another had past Major Depressive Disorder. (Primary analyses were re-run omitting the these three subjects, and no significant differences were found.) Seven CD participants also would have qualified for an Oppositional Defiant Disorder diagnosis if their behavior had not been better accounted for by Conduct Disorder. Healthy control participants were free of any DSM-IV Axis I psychopathology. Sample demographic and clinical characteristics are listed in Table 1. Verbal ability was estimated using the Wide Range Achievement Test (3rd Edition) (WRAT-3) (35) because numerous CD participants did not exert adequate effort on more challenging WISC-III/WAIS-III Vocabulary subtests originally intended to estimate verbal-conceptual ability. WRAT-3 is strongly correlated with verbal IQ (36,37). Consistent with previous research (38,39), CD verbal ability was significantly lower compared to controls (t39=5.09, p<.001). Nonverbal intelligence (estimated by available WASI Matrix Reasoning scores) also differed between groups. However, both groups were well within the average range on both measures.
Table 1.
Sample demographic and clinical characteristics
| Conduct Disorder Mean ± SD |
Healthy Controls Mean ± SD |
p | |
|---|---|---|---|
| Age | 16.3 ± 1.3 | 16.1 ± 1.4 | ns |
| Gender (M/F) | 10/9 | 14/10 | ns |
| WASI Matrix Reasoning subtesta | 46.9 ± 7.6 (33–59) | 51.4 ± 5.8 (39–62) | 0.049 |
| WRAT-3 Reading subtest Scaled Score | 89.3 ± 12.4 | 98.4 ± 9.2 | 0.010 |
| K-SADS-PL | |||
| CD symptoms | 5.7 ± 2.0 | 0.0 ± 0.0 | < 0.001 |
| ADHD Hyperactivity/Impulsivity symptoms | 0.3 ± 0.8 | 0.0 ± 0.2 | ns |
| ADHD Inattentive symptoms | 0.7 ± 1.4 | 0.1 ± 0.5 | ns |
WASI information was not collected for 2 non-CD and 5 CD participants. Subtest score range in parentheses.
WASI, Wechsler Abbreviated Scale of Intelligence; WRAT, Wide Range Achievement Test; ns, not significant
MRI Data Collection
We obtained MRI images on a Siemens 3T Allegra MRI machine at the Olin Neuropsychiatry Research Center at The Institute of Living/Hartford Hospital. T1-weighted brain structure images were collected using a 3D MPRAGE pulse sequence (TR/TE/TI=2300/2.74/900 ms, flip angle=8°, FOV=176×256 mm, matrix=176×256×176, voxel size=1×1×1 mm, pixel bandwidth=190 Hz; 7:09 minutes).
Image Processing
We prepared brain structure images for cortical reconstruction by correction of the estimated MRI bias-field using SPM5 software (http://www.fil.ion.ucl.ac.uk/), followed by noise reduction using FSL SUSAN filtering software (http://www.fmrib.ox.ac.uk/). We then performed anatomical reconstruction of the cortical surfaces using the FreeSurfer image analysis suite (v5.0; http://surfer.nmr.mgh.harvard.edu/). SPM5 and FSL preprocessing was used only to facilitate FreeSurfer analyses.
FreeSurfer surface-based cortical reconstruction and analysis has been described previously (17,40) and validated in a number of studies (41–44). The reconstruction estimated the white surface, comprised of the gray/white matter interface, and the pial surface, comprised of the gray matter/cerebrospinal fluid interface via two-dimensional mesh of triangular elements comprised of >100,000 vertices per hemisphere. The estimated white and pial surfaces were manually corrected for inconsistencies by visual inspection and addition of control points where necessary to aid gray and white matter differentiation. Typically, only the temporal poles required manual edits to improve reconstructed surface accuracy. Cortical thickness at each vertex was calculated by measuring the shortest distance between the white and pial surfaces at that vertex (41). Estimated total intracranial volume for each subject also was obtained.
Gyrification Index
Schaer and colleagues developed a measure of cortical folding called the local gyrification index (lGI) that is implemented in Freesurfer (45). The lGI is a surface-based, three-dimensional extension of the linear, two-dimensional coronal section gyrification measurement method of Zilles and colleagues (46). The lGI measures the ratio of a vertex-based, 25 mm radius circular region of interest (ROI) of folded pial surface area to the corresponding surface area of a tight-fitting contour enveloping the cortex’s outer perimeter. The resulting cortical surface maps of lGI represent the amount of cortical folding, i.e., extent of cortex buried within the sulcal folds, at each pial surface location.
Statistical Analyses
Surface-based group analyses were performed using FreeSurfer’s general linear model (GLM) tools. Prior to group comparison, each participant’s data were resampled into a common anatomical space. Surface-based measurements of cortical thickness and lGI for all subjects were smoothed using Gaussian kernels of 10 mm and 5 mm full-width/half-maximum, respectively.
Statistical significance of between-group cortical thickness and lGI differences was evaluated using a clusterwise correction for multiple comparisons from Monte Carlo z-field simulation (47). For each iteration, a z-field was synthesized, smoothed with a Gaussian filter (using residual FWHM value from GLM), thresholded at a user-chosen vertexwise value, and finally clusters were extracted and sized. This was repeated 10,000 times to derive the distribution of cluster sizes expected under the null hypothesis. Clusters were initially obtained using a p<0.05 (two-tailed) vertexwise threshold, and then reported only if they met additional clusterwise probability (Pcluster) of p<0.05 (two-tailed). The Pcluster is the probability of forming a cluster that size by chance.
We performed several post hoc analyses of CD data. We defined regions-of-interest (ROIs) as 3 mm radius spheres in volume space, with each ROI center located at the cluster vertex coordinate having the peak statistical group difference (HC > CD) in cortical thickness or lGI. We then transformed each spherical volume ROI into an area (cluster) ROI in surface-space and measured the mean cortical thickness or lGI at each cluster ROI for each subject.
The linear association of ROI values with symptom count for CD participants was assessed using Pearson correlation to determine whether structural deficits were linked to disorder severity. To ensure that our findings were not due to intellectual differences between groups, we also examined cluster ROI value correlations with WRAT-3 Reading or WASI Matrix Reasoning scores. Unavailable scores on WRAT-3 (CD/HC, n=2/5) and WASI-MR (CD/HC, n=5/2) were replaced by group means. We then performed linear regression of cluster ROI values with subjects’ WRAT-3 or WASI-MR scores, and statistically compared regression coefficients between CD and HC groups. To ensure our CD<HC findings were unrelated to gender, we examined ROI values into a two-way group-by-gender ANOVA. We also performed one “whole brain” post hoc analysis to explore potential CD differences between subgroups defined by age of disorder onset. Cortical thickness and lGI data differences between CD childhood-onset versus adolescent-onset classifications were compared using multiple regression, controlling for gender. Finally, we compared estimated total intracranial volume between groups. All post hoc findings were reported if they met p<.05 uncorrected threshold.
Results
Cortical thickness
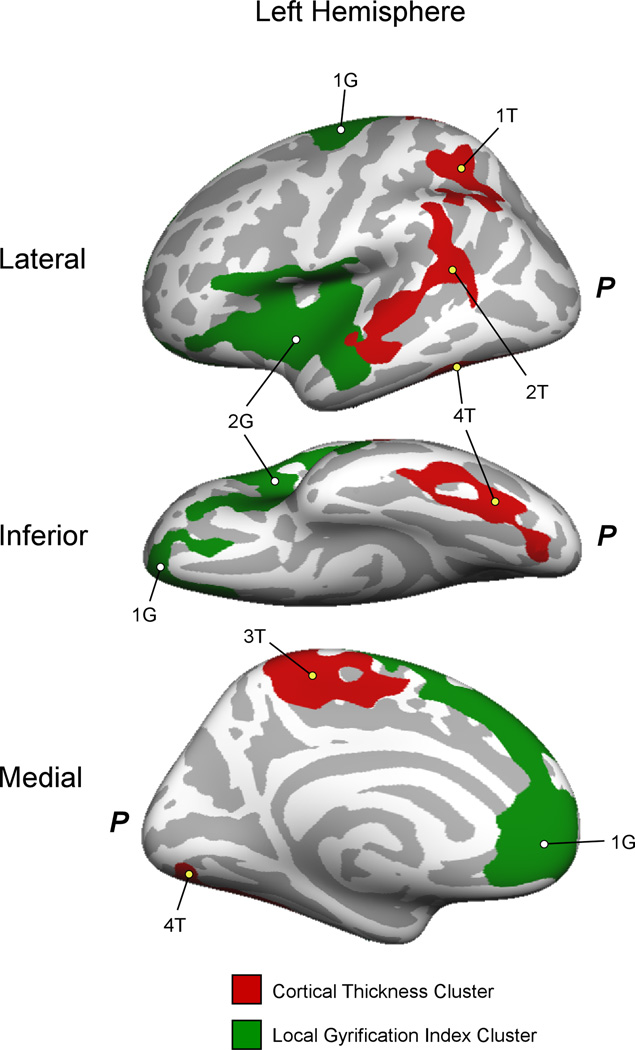
Compared with HC, CD adolescents had reduced cortical thickness in four clusters in the left hemisphere, including the supramarginal/angular gyri and superior temporal lobe (Pcluster=0.0003), superior parietal lobe (Pcluster=0.007), lingual, fusiform and inferior temporal gyri (Pcluster=0.007) and paracentral lobule (Pcluster=0.0018) (Figure 1).
Figure 1.
Left hemisphere clusters with significant cortical thickness (red) and lGI (green) differences (CD < HC). The lateral (top), inferior (middle) and medial (bottom) views of the left hemisphere are shown. Cluster labels (numbers) correspond to those provided in Table 2. P, Location of the posterior of the left hemisphere.
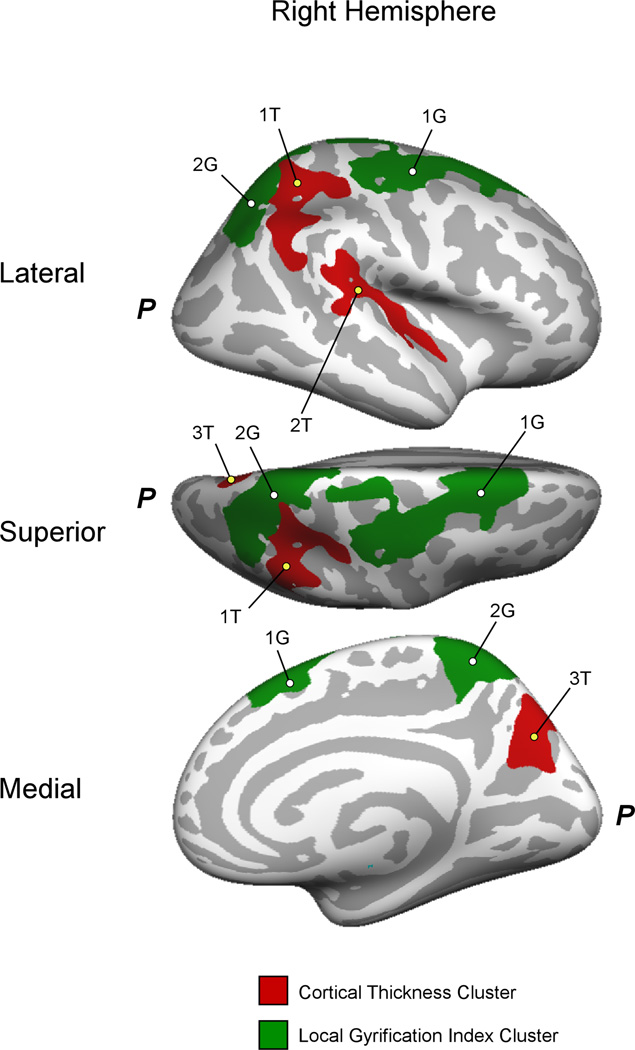
In the right hemisphere, cortical thickness was reduced in CD in three clusters, including the superior/inferior parietal lobe and postcentral gyrus (Pcluster=0.0004), supramarginal/angular gyri and superior temporal sulcus (Pcluster=0.0003) and precuneus (Pcluster=0.0497) (Figure 2). Tables 2 and 3, top, summarize left and right hemisphere cortical thickness cluster measurements, respectively. Cortical thickness was not found to be greater in CD than HC.
Figure 2.
Right hemisphere clusters with significant cortical thickness (red) and lGI (green) differences (CD < HC). The lateral (top), superior (middle) and medial (bottom) views of the right hemisphere are shown. Cluster labels (numbers) correspond to those provided in Table 3. P, Location of the posterior of the right hemisphere.
Table 2.
Left hemisphere cluster regions showing cortical thickness (top) and lGI (bottom) deficits in CD adolescents relative to HC in a whole brain analysis, clusterwise-corrected for multiple comparisons.
| Cortical Thickness: CD < HC | |||||||
|---|---|---|---|---|---|---|---|
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1T | superior parietal lobe | 3.51 | 1488 | −27.4 | −46.3 | 39.8 | 0.007 |
| 2T | superior temporal, supramarginal, & angular gyri | 3.50 | 2289 | −53.1 | −46.2 | 14.7 | 0.0003 |
| 3T | paracentral lobule | 2.83 | 1834 | −7.4 | −31.1 | 50.8 | 0.0018 |
| 4T | fusiform/inferior temporal gyri | 2.10 | 1488 | −13.8 | −82 | −6.7 | 0.007 |
| Local Gyrification Index (lGI): CD < HC | |||||||
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1G | vm/dmPFC & ACC, OFC, precentral gyrus | 3.97 | 5895 | −18.7 | −9.5 | 54.1 | 0.0001 |
| 2G | Insula, lateral OFC, anterior temporal lobe | 2.32 | 5650 | −55.7 | −11.2 | −18.0 | 0.0001 |
Pcluster, clusterwise probability; OFC, orbitofrontal cortex; vm/dmPFC, ventromedial and dorsomedial prefrontal cortex; ACC, anterior cingulate cortex
Table 3.
Right hemisphere cluster regions showing cortical thickness (top) and lGI (bottom) deficits in CD adolescents relative to HC in a whole brain analysis, clusterwise-corrected for multiple comparisons.
| Cortical Thickness: CD < HC | |||||||
|---|---|---|---|---|---|---|---|
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1T | superior & inferior parietal lobe | 4.82 | 2269 | 33.5 | −30.7 | 45.4 | 0.0004 |
| 2T | STS, supramarginal & angular gyri | 4.13 | 1546 | 39.2 | −19.3 | −4.6 | 0.0073 |
| 3T | precuneus | 2.93 | 1143 | 19.5 | −61.7 | 31.2 | 0.0497 |
| Local Gyrification Index (lGI): CD < HC | |||||||
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1G | Superior frontal lobe | 2.61 | 3929 | 20.8 | 18.1 | 51.4 | 0.0047 |
| 2G | Superior parietal lobe, paracentral lobule | 2.51 | 3303 | 9.9 | −44.7 | 66.0 | 0.0141 |
Pcluster, clusterwise probability; STS, superior temporal sulcus
lGI
When compared with HC, we found reduced lGI in CD participants in two relatively large left hemisphere clusters. The first included insula, lateral orbitofrontal and inferior frontal cortices and anterior temporal lobe (Pcluster=0.0001). The second cluster comprised ventro- and dorsomedial prefrontal regions including ACC (Pcluster=0.0001) (Figure 1).
In the right hemisphere, there were two significant clusters with reduced lGI in CD versus HC. The first cluster consisted of the superior frontal lobe, including frontal eye fields, pre- and postcentral gyrus as well as dorsomedial prefrontal lobe (Pcluster=0.0047); and the second cluster, the superior parietal lobe and precuneus (Pcluster=0.0141) (Figure 2). Tables 2 and 3, bottom, summarize left and right hemisphere lGI cluster measurements. lGI was not greater in CD than HC in any significant cluster.
Post Hoc Analyses
CD Severity
There were no significant correlations in each cluster ROI between CD symptom count and cortical thickness or lGI.
Verbal and Nonverbal Abilities
One cluster in the superior temporal sulcus, supramarginal and angular gyri showed a relationship between WRAT-3 verbal ability and cortical thickness (p=.041 uncorrected; cluster 2T; Table 3). Another cluster in the superior parietal lobe and paracentral lobule showed a WRAT-3 relationship with lGI (p=.034 uncorrected; cluster 2G; Table 3). These did not survive corrections for multiple comparisons.
Gender
An ROI analysis of group-by-gender interaction found a significant effect in only one cortical thickness ROI (p=.011 uncorrected; cluster 1T; Table 3). This interaction effect also did not survive corrections for multiple comparisons.
Age-of-Onset
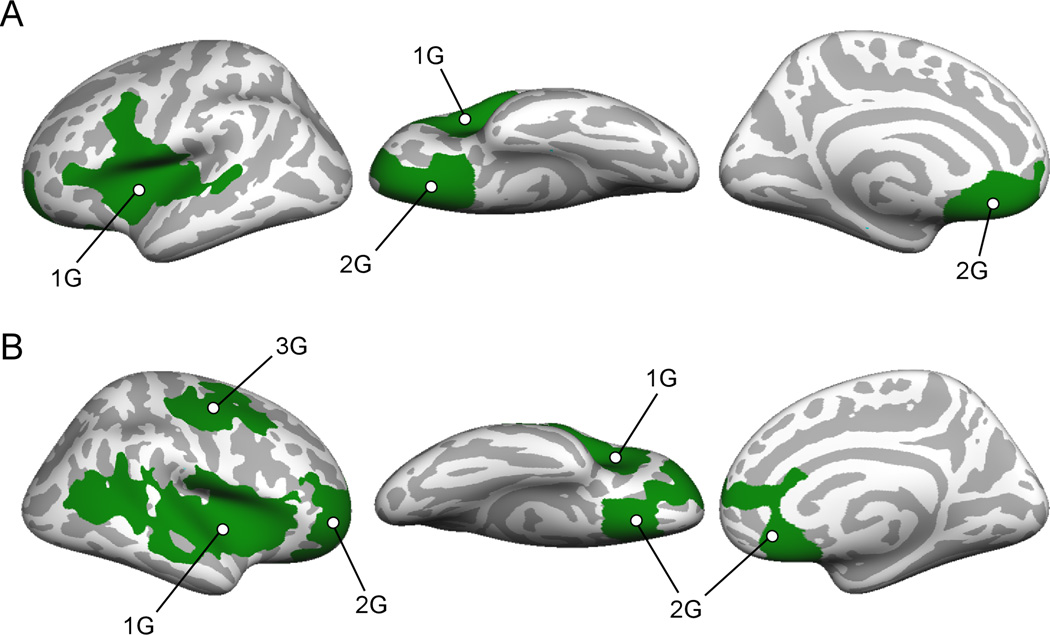
Cortical thickness differed between childhood-onset and adolescent-onset CD in several small clusters (p<.05 uncorrected), but none survived corrections for multiple comparisons. lGI values were greater for childhood-onset CD in two left and three right hemisphere clusters (Figure 3) that survived clusterwise correction for multiple comparisons (Table 4).
Figure 3.
Significant clusters where lGI is less in adolescent-onset than childhood-onset CD subjects. A, Left hemisphere, lateral, ventral and medial views. B. Right hemisphere, lateral, ventral and medial views.
Table 4.
Clusters in the left and right hemispheres where lGI is greater for CD-CO than CD-AO subjects (clusterwise-corrected for multiple comparisons).
| Left Hemisphere: Local Gyrification Index (lGI): Childhood-onset > Adolescent-onset | |||||||
|---|---|---|---|---|---|---|---|
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1G | insula, IFG, superior temporal | 4.03 | 7281 | −51 | 30 | 2 | 0.0001 |
| 2G | vmPFC, medial OFC, frontal pole | 3.36 | 4128 | −22 | 8 | −14 | 0.0001 |
| Right Hemisphere: Local Gyrification Index (lGI): Childhood-onset > Adolescent-onset | |||||||
| Cluster # | Anatomical Regions | Max −log10(p-val) | Area (mm2) | Talairach (x, y, z) maxima | Pcluster | ||
| 1G | insula, IFG, superior temporal, inferior parietal | 3.53 | 9752 | 31 | 21 | −3 | 0.0001 |
| 2G | vmPFC, rACC, medial OFC | 3.21 | 5324 | 6 | 20 | −16 | 0.0001 |
| 3G | precentral, postcentral | 2.91 | 3432 | 38 | −5 | 51 | 0.0003 |
Pcluster, clusterwise probability; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex; rACC, rostral anterior cingulate cortex; IFG, inferior frontal gyrus
Total Intracranial Volume
There were no significant group differences in mean estimated total intracranial volume.
Discussion
Cortical thickness
As hypothesized, we found reduced cortical thickness bilaterally in the posterior aspect of CD participants’ superior temporal lobes, including the superior temporal sulcus (STS). We also discovered cortical thickness deficits in CD in superior and inferior parietal lobe regions, left fusiform and inferior temporal gyrus, left paracentral lobule and right precuneus. Our bilateral superior temporal lobe findings agree not only with prior volumetric brain research showing bilateral temporal lobe abnormalities in CD youth (6,10), but also with temporal lobe abnormalities in antisocial adults (27,31,48,49) and gray matter volume deficits in bilateral mid- and posterior STS of adult psychopaths (50). Temporo-parietal junction, parietal lobe or angular and supramarginal gyri volume deficits generally have not been found in either CD or antisocial adults, with exception of De Brito et al. (12) who examined boys aged 10–13 years high on callous-unemotional traits. That report described gray matter volume and concentration increases, rather than decreases, in widespread regions including the parietal lobe. Differences between the De Brito et al. study and our study, however, might be attributed to different age ranges (10–13 vs. 12–18 years of age) and diagnostic groupings (e.g., high levels of callous-unemotional traits as well as conduct problems vs. “pure” CD) used in the two studies.
Recent functional imaging studies have shown that superior temporal lobe and temporo-parietal regions play an important role in social abilities such as empathy (51–53), perspective taking (54), attention to emotions (55) and moral reasoning (48,56). Decety et al. reported aggressive CD youth had lower neural responses versus controls in right temporo-parietal junction to visual images of pain inflicted on people (57). In particular, the posterior superior temporal sulcus (STS) was found to be crucial for understanding social cues and correct interpretation of the actions and behaviors of others, concepts central to “Theory of Mind” (ToM) research (50,58,59). Dolan and Fullam (60) found that antisocial adults had subtle impairments in brain regions that have been linked to ToM. They speculated such impairments may play a role in the antisocial individual’s apparent indifference to the suffering of potential victims. Although some early behavioral studies found either no difference in perspective-taking between antisocial youth and controls (61), or even antisocial youth superiority in this regard (62–64), more recent studies found CD youth were impaired in empathy and perspective-taking (65,66). Because the ToM construct originated from autism research, it is unlikely that it will be a sufficient explanatory framework for CD. However, such neurobiological studies no doubt will inform efforts to accurately describe socialization deficits in CD. CD cortical thickness deficits bilaterally in the superior temporal lobe including the STS, temporoparietal junction and angular and supramarginal gyrus might form a possible structural substrate for socialization impairments often associated with CD, including compromised empathy, perspective-taking, social functioning and the ability to attend to emotions.
Until this study, there have been no structural MRI studies in CD youth samples without significant co-morbidity. Rubia and colleagues, however, found non-comorbid CD boys (aged 9–16 years) had activation deficits relative to controls in bilateral temporo-parietal regions, superior temporal lobes and right precuneus (67–69). Rubia has postulated (69) that CD might be distinguished from ADHD by abnormalities in the “hot” paralimbic system, which includes superior temporal lobes. The impaired regions found in these recent functional studies in non-comorbid CD youth overlap well with our own structural findings of cortical thinning in the bilateral temporal-parietal regions, superior temporal lobes and right precuneus. This convergence of functional and structural evidence suggests that posterior temporo-parietal regions should be the focus of future inquiry in CD.
lGI
We also discovered reduced prefrontal cortex lGI (cortical folding) in prefrontal regions. In summary, in the left hemisphere, we detected lGI deficits in CD in insular cortex, inferior frontal gyrus, lateral OFC and a large cluster on the medial surface including ventro- and dorsomedial prefrontal cortices (vmPFC and dmPFC) and ACC. In the right hemisphere, clusters of CD lGI deficits were found in superior parietal lobe, precuneus, primary motor and somatosensory cortex, and superior prefrontal cortex including supplementary motor area (SMA) and premotor regions. Several recent fMRI studies of CD found abnormal activation in OFC (57,68–73) and insula (57,70,72,73), regions in which we found lGI deficits. Herpertz and colleagues, however, found no abnormalities in OFC or insula activation in CD youth (74). The medial regions in the left hemisphere exhibiting lGI deficits in CD include regions known to be critical for emotional regulation (ACC, vmPFC) (75,76), error detection and resolution (ACC, dmPFC) (77,78), attentional processes (ACC) (79) and risk evaluation (dmPFC) (80).
In the left hemisphere, a large cluster of reduced lGI in CD versus controls included nearly all insular cortex. Medford and Critchley have highlighted the joint role of the anterior cingulate and anterior insular cortices in both the experience of emotions and coordination of appropriate responses to events (79). According to the somatic marker hypothesis, insular cortex is essential to formation of subjective feeling states (79,81). In particular, joint action of these regions is postulated to be responsible for the awareness of self, in contrast to third-person awareness and empathy previously discussed as the function of the temporo-parietal regions. Dysfunction in CD in both these prefrontal regions, along with vmPFC dysfunction, might arise from a diminished capacity to learn from, as well as avoid, risky situations and negative consequences when prompted by internal emotional states. Recent behavioral studies in disruptive behavioral disorders (82–84) provide additional evidence supporting this hypothesis.
Topographical differences: Frontal versus posterior
In our CD subjects, cortical thickness deficits occurred chiefly in posterior brain regions, whereas in contrast, lGI deficits occurred primarily in the frontal lobe. Overlap of deficit types were surprisingly limited (Figures 1–2). Current theories propose that cortical folding (lGI) is determined largely prenatally, whereas in contrast, cortical thickness is determined postnatally and undergoes significant changes throughout the human life span (85,86). Therefore, in CD individuals, reduced lGI in the prefrontal cortex may be congenital, resulting in lifelong impairment in social and cognitive behavior. These observations are interesting in light of our post hoc comparison of CD age-of-onset subgroups (87). We found greater lGI deficits in CD youth whose symptoms appeared after age 10, suggesting that possibly congenital abnormalities did not result in demonstrable behavioral effects until puberty. These data agree with Fairchild et al. (8), who found greater insular cortex volume deficits in adolescent- versus childhood-onset CD. These findings raise intriguing possibilities for future gene-by-environment and developmental studies, particularly those that focus on pubertal influences on brain structure and function.
Additional topics
Comparing our CD results with published adult APD studies, we note that Yang et al. (30,88) found cortical thickness deficits in OFC, whereas we found lGI deficits in this same region. They also found thickness deficits in superior temporal lobe, in agreement with our findings. In violent APD subjects, Narayan et al. (28) discovered cortical thickness deficits in medial prefrontal cortex, whereas we found lGI deficits in similar regions. However, they also measured thickness deficits in sensorimotor regions that partially overlap with superior parietal regions found in our study. Our post hoc analysis of possible CD gender effects found only one significant interaction in the right hemisphere superior parietal cluster (1T) at p<.05 uncorrected. We therefore conclude that gender did not significantly impact study findings.
Study limitations
One study limitation is that structural measurements are confined to cortical regions only using surface-based methods. Other limitations include somewhat broad clusters that prevented fine-grained abnormality localization needed to most effectively advance neurobiological theories of antisocial behavior disorder, and a comparatively small sample size for a structural MRI study. Indeed, our failure to find any relationship post hoc between CD severity and structural deficits might simply be due to querying the wrong regions (i.e., our small sample size may have precluded an effective whole brain correlational analysis). Also, CD and non-CD verbal and nonverbal abilities differed significantly as in previous studies of verbal ability (38,39). Although post hoc analyses found no consistent relationships between verbal or nonverbal intellectual estimates and CD brain structure in the regions of CD deficits, we noted at significance levels uncorrected for multiple comparisons that verbal ability was linked to cortical thickness in one right-hemisphere superior temporal sulcus/supramarginal/angular gyri cluster and cortical folding in a left-hemisphere superior parietal lobe/paracentral lobule cluster. Although these clusters did not simply represent classic language-related areas, the implicated cortex does include regions where cortical thickness has been found to covary with Full Scale IQ (89–92) and verbal IQ (92). This suggests that factors related to reading achievement or verbal intelligence might contribute to these particular CD deficits. However, the majority of CD brain structure deficits were empirically unrelated to intelligence estimates. Finally, we did not collect measures of behavioral traits of high interest in CD research, including callous-unemotional traits, psychopathy and aggression. In particular, both aggression and psychopathy have been linked to structural abnormalities in the temporal lobe and associated limbic structures, the hippocampus and amygdala (27,93). Future studies should focus on examining the link between aggression and CD-related temporal lobe cortical thickness or gray matter abnormalities.
Summary
To our knowledge, this is the first examination of both cortical thickness and cortical folding (lGI) deficits in CD adolescents. Our study examined “pure” CD subjects to assess two dissociable facets of brain volume at the whole brain level with statistical correction for multiple comparisons. Our findings suggest that previously-reported gray matter volume abnormalities in CD youth reflect both cortical thickness and folding deficits each localized to generally different brain structures. We also found novel evidence for CD cortical thickness abnormalities in parietal lobe regions, left paracentral lobule and right precuneus. Psychiatric comorbidity in previous antisocial brain volume studies might have obscured this finding. Parietal lobe structural abnormalities in antisocial disorders require replication before being integrated into neurocognitive theories, but existing research suggests mechanisms through which these abnormalities could contribute to antisocial behavior. The anterior-posterior distinction of cortical folding versus thickness CD deficits implies that specific localized volumetric abnormalities in antisocial samples might result from different neurobiological factors, possibly with distinct genetic correlates. Future studies should attempt structural and functional measurements in the same samples, using integrative “fusion” methods to jointly examine relationships among the measures, particularly social neuroscience fMRI paradigms. Finally, longitudinal studies would help address the question whether structural brain abnormalities found in CD youth persist into adulthood in cases where antisocial behavior also endures.
Supplementary Material
Acknowledgements
This study was funded by K23 MH070036 (PI Stevens) and supported in part by R01 MH080956 (PI Stevens). Appreciation is offered to the Connecticut Court Support Services Division and Ms. Rena Goldwasser, as well as research staff who collected and helped to prepare the data for analysis, including Ms. Sandra Navarro, Laura Miller, Abigail Quish, and Sara Beyor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Hyatt, Ms. Haney-Caron, and Dr. Stevens have no biomedical financial interests or potential conflicts of interest.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Loeber R, Burke J, Pardini DA. Perspectives on oppositional defiant disorder, conduct disorder, and psychopathic features. J Child Psychol Psychiatry. 2009;50:133–142. doi: 10.1111/j.1469-7610.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Glenn AL, Schug RA, Yang Y, Raine A. The neurobiology of psychopathy: a neurodevelopmental perspective. Can J Psychiatry. 2009;54:813–823. doi: 10.1177/070674370905401204. [DOI] [PubMed] [Google Scholar]
- 4.Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Dev Psychopathol. 2009;21:1111–1131. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- 5.Crowe SL, Blair RJ. The development of antisocial behavior: what can we learn from functional neuroimaging studies? Dev Psychopathol. 2008;20:1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- 6.Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 7.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- 9.Bussing R, Grudnik J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: an MRI study in a community sample. World J Biol Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- 10.Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Fahim C, He Y, Yoon U, Chen J, Evans A, Perusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav. 2011;37:326–337. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- 12.De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 14.Silk TJ, Wood AG. Lessons About Neurodevelopment From Anatomical Magnetic Resonance Imaging. J Dev Behav Pediatr. 2010 doi: 10.1097/DBP.0b013e318206d58f. [DOI] [PubMed] [Google Scholar]
- 15.Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27:11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 18.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH. Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 2009;30:175–184. doi: 10.1002/hbm.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerasa A, Cherubini A, Quattrone A, Gioia MC, Tarantino P, Annesi G, et al. Met158 variant of the catechol-O-methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience. 2010;167:809–814. doi: 10.1016/j.neuroscience.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, et al. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry. 2010;67:493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 25.Lahey BB, Loeber R, Burke JD, Applegate B. Predicting future antisocial personality disorder in males from a clinical assessment in childhood. J Consult Clin Psychol. 2005;73:389–399. doi: 10.1037/0022-006X.73.3.389. [DOI] [PubMed] [Google Scholar]
- 26.Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128-119. [DOI] [PubMed] [Google Scholar]
- 27.Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behav Brain Res. 2006;169:239–247. doi: 10.1016/j.bbr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Narayan VM, Narr KL, Kumari V, Woods RP, Thompson PM, Toga AW, et al. Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry. 2007;164:1418–1427. doi: 10.1176/appi.ajp.2007.06101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, et al. Hippocampal structural asymmetry in unsuccessful psychopaths. Biol Psychiatry. 2004;55:185–191. doi: 10.1016/s0006-3223(03)00727-3. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–562. 555. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- 31.Muller JL, Ganssbauer S, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke T, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Res. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Vloet TD, Konrad K, Huebner T, Herpertz S, Herpertz-Dahlmann B. Structural and functional MRI- findings in children and adolescents with antisocial behavior. Behav Sci Law. 2008;26:99–111. doi: 10.1002/bsl.794. [DOI] [PubMed] [Google Scholar]
- 33.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Jastak J, Wilkinson G. WRAT-3: Wide range achievement test administration manual. Wide Range, Inc.; 1993. [Google Scholar]
- 36.Griffin SL, Mindt MR, Rankin EJ, Ritchie AJ, Scott JG. Estimating premorbid intelligence: comparison of traditional and contemporary methods across the intelligence continuum. Arch Clin Neuropsychol. 2002;17:497–507. [PubMed] [Google Scholar]
- 37.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 38.Moffitt TE, Lynam D. Neuropsychology of delinquent behavior: Implications for understanding the psychopath. In: Fowles D, Sutker P, Goodman S, editors. Psychopathy and antisocial personality: A developmental perspective. New York: Springer; 1994. pp. 233–262. [Google Scholar]
- 39.Moffitt TE. The neuropsychology of conduct disorder. Development and Psychopathology. 1993;5:133–151. [Google Scholar]
- 40.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 41.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 42.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 43.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 44.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 45.Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 46.Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- 47.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Soc Cogn Affect Neurosci. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber S, Habel U, Amunts K, Schneider F. Structural brain abnormalities in psychopaths-a review. Behav Sci Law. 2008;26:7–28. doi: 10.1002/bsl.802. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignacio F, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 51.Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 53.Hooker CI, Verosky SC, Germine LT, Knight RT, D'Esposito M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010;1308:100–113. doi: 10.1016/j.brainres.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 55.Blair RJ, Mitchell DG. Psychopathy, attention and emotion. Psychol Med. 2009;39:543–555. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young L, Camprodon JA, Hauser M, Pascual-Leone A, Saxe R. Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc Natl Acad Sci U S A. 2010;107:6753–6758. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 59.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolan M, Fullam R. Theory of mind and mentalizing ability in antisocial personality disorders with and without psychopathy. Psychol Med. 2004;34:1093–1102. doi: 10.1017/s0033291704002028. [DOI] [PubMed] [Google Scholar]
- 61.Happé FGE, Frith U. Theory of mind and social impairment in children with conduct disorder. British Journal of Developmental Psychology. 1996 [Google Scholar]
- 62.Sutton J, Smith PK, Swettenham J. Social cognition and bullying: Social inadequacy or skilled manipulation? British Journal of Developmental Psychology. 1999;17:435–450. [Google Scholar]
- 63.Sutton J, Smith PK, Swettenham J. Bullying and ‘Theory of Mind’: A Critique of the ‘Social Skills Deficit’View of Anti Social Behaviour. Social Development. 1999;8:117–127. [Google Scholar]
- 64.Silvern LE, Waterman JM, Sobesky W, Ryan VL. Effects of a developmental model of perspective taking training. Child Development. 1979;50:243–246. [Google Scholar]
- 65.de Wied M, Gispen-de Wied C, van Boxtel A. Empathy dysfunction in children and adolescents with disruptive behavior disorders. Eur J Pharmacol. 2010;626:97–103. doi: 10.1016/j.ejphar.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Anastassiou-Hadjicharalambous X, Warden D. Cognitive and affective perspective-taking in conduct-disordered children high and low on callous-unemotional traits. Child Adolesc Psychiatry Ment Health. 2008;2:16. doi: 10.1186/1753-2000-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubia K, Halari R, Smith AB, Mohammed M, Scott S, Giampietro V, et al. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- 68.Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Brammer MJ. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J Child Psychol Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 69.Rubia K. "Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 70.Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 73.Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49:781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 75.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simoes-Franklin C, Hester R, Shpaner M, Foxe JJ, Garavan H. Executive function and error detection: The effect of motivation on cingulate and ventral striatum activity. Hum Brain Mapp. 2010;31:458–469. doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19:1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 82.Fairchild G, van Goozen SH, Stollery SJ, Aitken MR, Savage J, Moore SC, et al. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biol Psychiatry. 2009;66:162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luman M, Sergeant JA, Knol DL, Oosterlaan J. Impaired decision making in oppositional defiant disorder related to altered psychophysiological responses to reinforcement. Biol Psychiatry. 2010;68:337–344. doi: 10.1016/j.biopsych.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 84.Schutter DJ, van Bokhoven I, Vanderschuren LJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. J Abnorm Child Psychol. 2011;39:333–339. doi: 10.1007/s10802-010-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frye RE, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp MS. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb Cortex. 2010;20:2625–2635. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapellou O, Counsell SJ, Kennea N, Dyet L, Saeed N, Stark J, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lahey BB, Loeber R, Quay HC, Applegate B, Shaffer D, Waldman I, et al. Validity of DSM-IV subtypes of conduct disorder based on age of onset. J Am Acad Child Adolesc Psychiatry. 1998;37:435–442. doi: 10.1097/00004583-199804000-00022. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J Abnorm Psychol. 2010;119:546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- 89.Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, et al. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 92.Joshi AA, Lepore N, Joshi SH, Lee AD, Barysheva M, Stein JL, et al. The contribution of genes to cortical thickness and volume. Neuroreport. 2011;22:101–105. doi: 10.1097/WNR.0b013e3283424c84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volkow ND, Tancredi LR, Grant C, Gillespie H, Valentine A, Mullani N, et al. Brain glucose metabolism in violent psychiatric patients: a preliminary study. Psychiatry Res. 1995;61:243–253. doi: 10.1016/0925-4927(95)02671-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.