Abstract
Angelman syndrome (AS) is a neurodevelopmental disorder largely due to abnormal maternal expression of the UBE3A gene leading to the deletion of E6-associated protein. AS subjects have severe cognitive impairments for which there are no therapeutic interventions. Mouse models (knockouts of the maternal Ube3a gene: ‘AS mice’) of the disorder have substantial deficits in long-term potentiation (LTP) and learning. Here we report a clinically plausible pharmacological treatment that ameliorates both deficits. AS mice were injected ip twice daily for 5 days with vehicle or the ampakine CX929; drugs of this type enhance fast EPSCs by positively modulating AMPA receptors. Theta burst stimulation (TBS) produced a normal enhancement of field EPSPs in hippocampal slices prepared from vehicle-treated AS mice but LTP decreased steadily to baseline; however, LTP in slices from ampakine-treated AS mice stabilized at levels found in wild-type controls. TBS-induced actin polymerization within dendritic spines, an essential event for stabilizing LTP, was severely impaired in slices from vehicle-treated AS mice but not in those from ampakine-treated AS mice. Long-term memory scores in a fear conditioning paradigm were reduced by 50% in vehicle-treated AS mice but were comparable to values for littermate controls in the ampakine-treated AS mice. We propose that AS is associated with a profound defect in activity-driven spine cytoskeletal reorganization, resulting in a loss of the synaptic plasticity required for the encoding of long-term memory. Notably, the spine abnormality along with the LTP and learning impairments can be reduced by a minimally invasive drug treatment.
Keywords: actin, AMPA receptor, hippocampus, learning and memory, LTP, UBE3A
Introduction
Angelman Syndrome (AS) is a rare (1 in ~15,000 live births) neurogenetic disorder associated with cognitive impairment, motor dysfunction, and frequent co-diagnosis of autism spectrum disorder. Microcephaly, seizures, and severe speech impairments are also prominent components of the AS phenotype (Oliver et al., 2007). AS most often arises from deletions in the 15q11–q13 region of the maternally inherited chromosome 15 (Williams et al., 1990). One of the deleted genes, UBE3A, encodes the E6-associated protein (E6AP), which functions both as an E3 ligase in the ubiquitin proteasome pathway (Greer et al., 2010; Margolis et al., 2010; Scheffner et al., 1993), and as a transcriptional co-activator for steroid hormone receptors (Nawaz et al., 1999). As mutations in UBE3A alone result in AS, deletion of E6AP is generally considered to be a primary contributor to the disorder (Lalande and Calciano, 2007).
A mouse model of AS has been developed by deletion of the maternal Ube3a gene; the mutant mice exhibit several features of the human disease, including abnormal dendritic spine morphology (Dindot et al., 2008) and learning deficits (Jiang et al., 1998; van Woerden et al., 2007). Possibly related to these two features, the animals also have a marked impairment of long-term potentiation (LTP). It has been proposed that the LTP problems are due to abnormal phosphorylation of CaMKII (van Woerden et al., 2007) and/or a deficiency in the activity related cytoskeletal protein Arc, which may be a substrate for E6AP (Greer et al., 2010). We recently hypothesized that the plasticity defects in several developmental disorders reflect a failure of activity-driven signaling pathways that lead to the reorganization of the spine cytoskeleton required for consolidation of LTP and memory (Baudry et al., 2011). In agreement with this, we report here that the spine actin polymerization that occurs shortly after LTP induction, and is critical to LTP stabilization (Kramar et al., 2006; Lin et al., 2005; Lynch et al., 2008), does not occur in AS mice. This result indicates that AS shares a specific spine dysfunction with rodent models of two other human conditions --- low estrogen levels and early stage Huntington’s Disease (Kramar et al., 2010; Simmons et al., 2009) --- associated with memory and cognitive problems.
Recent work demonstrated that actin polymerization defects in these two models are reversed by daily injections of ampakines (Kramar et al., 2010; Simmons et al., 2009). These drugs slow deactivation and desensitization of AMPA receptors, thereby increasing fast EPSCs in telencephalon (Arai and Kessler, 2007; Lynch et al., 2008 for reviews). The restorative effects of ampakine treatment on activity-driven filament assembly in the rodent models were obtained the day after the last injection, well after removal of the drug from the brain. Therefore, their positive actions are likely to be due to long-lasting changes induced by daily increases in excitatory transmission. In this study, we used the same ampakine strategy to (i) determine if mechanisms are present in AS spines that can be manipulated to offset the observed severe actin polymerization defect described here and (ii), if so, test the prediction that correcting these defects will be accompanied by a restoration of LTP and long-term memory.
Materials and Methods
Animal treatments
Experiments were done with 9–12 week-old male littermates housed in groups of 2 per home cage and maintained on a 12 h light/dark cycle with food and water ad libitum. Mice received two intraperitoneal (ip) injections per day, at 8:00 to 9:00AM and 5:00 to 6:00PM, for a total of 8 days as previously reported (Rex et al., 2006). For the first 3 days, mice received vehicle injections of normal saline plus 15–20% 2-hydroxypropyl-beta-cyclodextrin (Sigma). After this period of acclimatization, mice assigned to drug treatment were injected for the next 5 days with the ampakine CX929 (5 mg/kg, ip), whereas control-assigned mice continued to receive injections of vehicle. Animals were trained and tested for fear conditioning on day 4 and 5 of ampakine injections and hippocampal slices were prepared 2 days after the last injection.
Electrophysiology
Hippocampal slices were prepared and tested for LTP induction and maintenance according to standard methods (Kramar et al., 2006). fEPSPs were elicited by stimulation of the Schaffer-commissural projections and recorded from proximal stratum radiatum of CA1b; LTP was induced by a 10-burst theta train (TBS: 10 bursts of 4 pulses delivered at 100 Hz, with an interburst interval of 200 msec). Synaptic responses elicited by stimulation every 20 sec were recorded for 20 min before and up to 60 min after TBS. Amplitudes and slopes of fEPSPs were measured and normalized to the average of the values recorded during the 10-min pre-TBS baseline. One or two slices were evaluated for each mouse. Means and variances were calculated from the pooled values for all slices (Krause et al., 2008; Kung et al., 2007).
In situ labeling of F-Actin
Immediately after the recording session, Alexa568-phalloidin (6 μM/2–4 μL; Invitrogen) was applied topically 4 times at 3 min intervals. Slices were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), sectioned at 20 μm, and photographed at 40× (PlanApo; NA 1.4) with epifluorescent illumination (Zeiss Axioskop) using an Axiocam CCD camera. Quantitative analyses were performed on 3 serial sections as described (Kramar et al., 2006; Lin et al., 2005; Rex et al., 2007). Image z-stacks (2-μm steps) were collapsed by extended focal imaging (Axiovision 4.0 software), and intensity levels were cropped at values determined for each experiment to visualize low-intensity labeling. Labeled spine-like structures were identified automatically from a 550-μm2 sampling zone surrounding the recording site. Intensity thresholds (8-bit) were applied to identify spine-like structures at varying levels of label intensity. Spine counts were performed with a computerized system and averaged across the sections from each slice.
Fear-Conditioning Learning
All behavioral experiments were performed during the last 6 h of the light cycle. Experiments were run in a conditioning chamber consisting of a Plexiglas cage (29 cm × 29 cm × 29 cm) with a grid floor composed of stainless steel rods (Coulbourn Instruments, Allentown, PA) located in an isolated, sound-attenuating box. A computer controlled the experimental events and behavior recorded with a video camera system for offline scoring of freezing. On day 1 of training, mice were placed in the chamber and after 3 min received three episodes of tone presentation terminated with a foot-shock (tone: 20 s, 80 dB, 2 kHz; foot shock: 1 s, 0.8 mA; 1 min inter-trial intervals). The mice were returned to their home cages 30 sec after the final foot shock. Twenty-four hours after training, mice were tested for conditioning to the context by placing them into the conditioning chamber for 8 min without foot shocks or tones. Forty-eight hours after training, mice were tested for conditioning to the tone (cue test) by placing them into a new chamber that differed from the training chamber in its visual, tactile, and olfactory properties (30 cm × 20 cm × 13 cm). Following a 3 min acclimation period in the new chamber, the conditioning tone was presented for an 8 min test.
A time-sampling procedure performed by a trained observer blind to the experimental conditions was used to measure freezing. Briefly, every 10 s, each mouse was judged as either freezing or active. Freezing was defined as the absence of all visible non-respiratory movements of the body and vibrissae. The percent time freezing was calculated for the 8 min trial by dividing the number of freezing episodes.
Results
1. Ampakine treatment increases LTP in AS mice
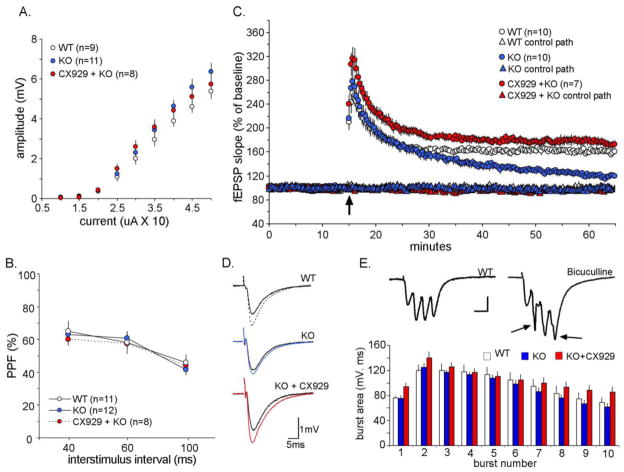
Baseline transmission in slices prepared from vehicle-treated AS mice was not significantly different from that found in wild-type (WT) slices. Input/output curves were comparable in the two groups (Fig. 1A) as was paired pulse facilitation (Fig. 1B), a measure that is sensitive to disturbances in transmitter release kinetics. A single TBS train produced a short-term potentiation effect in AS-vehicle slices comparable to that found in slices from WT mice: mean fEPSP slope (± SEM) at 2 min post-TBS was 246 ± 19% of baseline for the vehicle-treated AS mice and 256 ± 17% (p=0.68) for the WT mice. Moreover, the decay of potentiation in the mutants paralleled that for the WT mice for the first 10 min post-TBS (Fig. 1C). These results indicate that the complex collection of events required for the induction and normal expression of LTP is not substantially affected by deletion of the maternal Ube3a gene. In contrast, LTP consolidation was markedly impaired in the AS mice: instead of stabilizing at 15 min post-TBS, as found in WTs, LTP continued to decay to baseline over the following 30 min (% LTP at 50 min post-TBS was 20 ± 4% of baseline for vehicle-treated AS mice and 60 ± 5% for WT; p<0.0001).
Figure 1. LTP impairment in hippocampal slices from AS mice and the effects of CX929 treatment.
Hippocampal slices were prepared from vehicle-treated wild-type (WT), vehicle-treated AS mice (KO) and CX929-treated (CX929) AS mice and incubated at 30–31 C under continuous subfusion. A) Input/output curves. Amplitudes of the field EPSPs were determined for various intensities of stimulation. The results are means ± S.E.M. of the indicated number of experiments; there were no significant differences between the 3 groups of mice. B) Paired-pulse facilitation. The amplitude of the second response of a paired pulse was calculated as a percent of the amplitude of the first response for various interpulse intervals. The results are means ± S.E.M. for the indicated number of experiments. C) TBS-induced LTP. Theta burst stimulation (TBS) was delivered after 15 min of baseline recording and slopes of the fEPSPs monitored for an additional 55 min. The values (means ± S.E.M) for the indicated number of experiments are normalized to the baseline. D) Representative traces from each group before and 50 min after TBS. Scale bar = 1 mV, 5 ms. E) (Top) Composite responses to the first theta burst in a train for wild type slices under control conditions (left) and in the presence of the GABA-A receptor antagonist bicuculline (10 μM for 10 min; right). Note that the latter responses contained large population spikes (arrows). The distorted waveforms and spikes produced by the inhibitor were not present in any of the three groups of slices. (Bottom) Facilitation of theta burst responses during the train used to induce LTP was comparable for all three groups of slices.
A five-day treatment with CX929 did not measurably affect I/O curves or paired-pulse facilitation in AS slices prepared 2 days after the last injection (Fig. 1A,B). Moreover, the waveforms of the fEPSPs were not noticeably different between the ampakine-treated cases and the other two groups (Fig. 1D). Half-width and decay time constant measurements for the three groups confirmed this point (Table 1). The absence of changes in response duration strongly suggests that the ampakine pretreatment did not affect feedforward inhibition, a GABAergic effect known to shorten the decay phase of hippocampal EPSPs.
Table 1.
Characteristics of fEPSPs in field CA1 of hippocampus from littermates, AS mice and AS mice treated with CX929.
| Slope (mV/ms) | Half-width (mV) | Decay tau (ms) | |
|---|---|---|---|
| WT (n = 10) | 0.77 ± 0.04 | 7.4 ± 0.3 | 6.8 ± 0.5 |
| AS mice (n = 10) | 0.71 ± 0.08 | 7.4 ± 0.2 | 7.1 ± 0.5 |
| AS mice + CX929 (n = 7) | 0.82 ± 0.06 | 7.5 ± 0.4 | 6.9 ± 0.7 |
Results represent means ± S.E.M. for the different parameters calculated in the indicated number of slices from each animal group.
LTP in slices prepared from AS mice treated with the ampakine exhibited the normal decay for the first 15 min post-TBS but, in marked contrast to the results for the vehicle-treated AS mice, stabilized at a level (76 ± 6%) that was well above baseline and equivalent to WT values (Fig. 1C). The difference in percent LTP between slices from the vehicle- vs ampakine-treated AS mice at the conclusion of recording was highly significant (p<0.0001). The fiber volley elicited by single stimulation pulses in the ampakine AS group was not affected by TBS: the mean amplitude for the last 10 min of baseline recording was 0.24 ± 0.04 mV while the comparable value in the vehicle-injected group at 45–55 min post-TBS was 0.25 ± 0.04 mV (p=0.39, paired t-test). Pre- and post-TBS values were highly correlated (r=0.97) across individual slices, indicating that consistent amplitudes were maintained across the full extent of the recording session. Importantly, the waveforms of the composite responses elicited by the first of the theta bursts in a train were comparable in WT, AS-vehicle, and AS-ampakine groups, and did not include the large population spikes found when inhibition is pharmacologically reduced (Fig. 1E, top). Moreover, the facilitation of burst responses that occurs during the theta train did not appear to differ across the three groups of slices (Fig. 1E, bottom). We therefore conclude that ampakine treatment selectively ameliorates a discrete but nonetheless profound disturbance in the delayed consolidation of LTP.
2. TBS-induced actin polymerization is absent in AS mice and increased by ampakine pretreatment
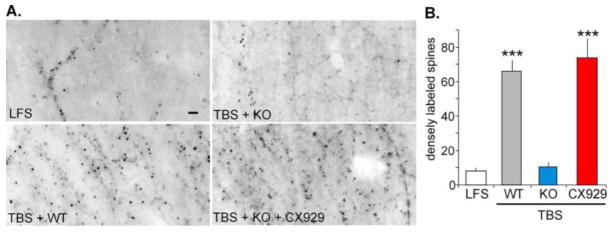
We next tested if Ube3a gene deletion affects the marked increase in spines containing high concentrations of filamentous (F-) actin normally found minutes after TBS. Alexa568-conjugated phalloidin, which binds selectively to F-actin, was topically applied to the field CA1 starting 10–15 min post-TBS and the slices subsequently processed for quantitative fluorescence microscopy. Survey micrographs showed that TBS caused an obvious effect in slices from WT mice (n=18) relative to low frequency simulation controls (n=26), but produced no evident change in slices from the vehicle-treated AS mice (Fig. 2A). However, the same stimulation triggered actin polymerization in a large number of spines in slices prepared from AS mice that had been treated with CX929 (Fig. 2A). Quantitative analyses showed the number of F-actin-labeled spines after TBS in slices from the ampakine-treated AS mice (n=12 slices) was markedly greater than that found in slices from the vehicle-treated AS mice (n = 16 slices, p<0.0001) and equal to the spine counts for slices from WT mice (p=0.27) (Fig. 2B). These results establish that the AS mutation blocks an essential element in the LTP consolidation process and that ampakine treatment has potent effects on activity-induced polymerization in the AS mice.
Figure 2. CX929 treatment promotes TBS-induced actin polymerization in the hippocampus of AS mice.
AlexaFluor tagged phalloidin was topically applied to slices at the conclusion of physiological recording to label spines with dense concentrations of F-actin. Counting was done by an automated system for a 550 μm2 sampling zone surrounding the recording electrode. A) Typical results for slices from four groups: LFS: low frequency stimulation in a WT case; TBS + WT; TBS+KO: slice from a mutant that had received vehicle injections; TBS+KO+CX929: slice prepared from a KO mouse given daily injections of the ampakine. Scale bar = 5 μm. B) Quantitative analysis of the data. Results are means ± S.E.M. (LFS: n=26; WT+TBS: n=18; KO+TBS: n=16; KO+CX929+TBS: n=12. *** p < 0.0001 as compared to LFS or to TBS in vehicle-treated AS mice.
3. CX929 treatment reduces a learning impairment in AS mice
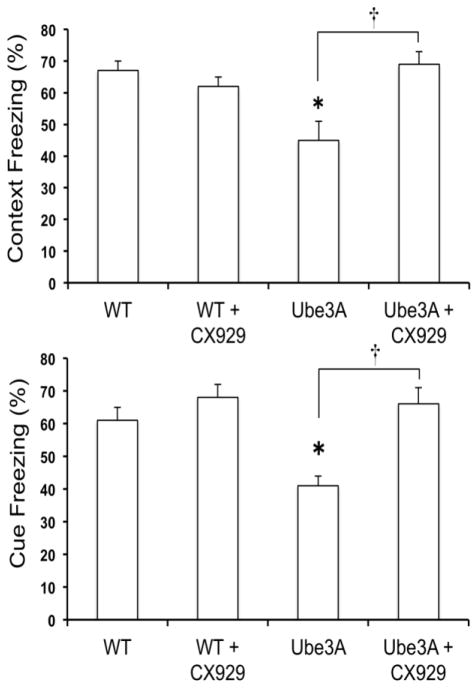
Mice with maternal deficiency of Ube3a are impaired in several behavioral paradigms testing motor function and learning and memory capability. We evaluated learning and memory in the mutants using a fear-conditioning paradigm. Compared with WT littermates, AS mice had a clear deficit in long-term memory, as indicated by a 50% reduction in freezing scores to the context on the day following training and to the tone 2 days following training (Fig. 3A,B). Under our experimental conditions, CX929 treatment did not significantly modify performance in WT mice for either context- or cue-learning, although the performance was slightly higher for cue-learning in CX929-treated mice. On the other hand, ampakine-treated AS mice exhibited performance similar to those observed in control or ampakine-treated WT mice: percent times freezing in these animals for both context and cue were very close to those for the WT mice. The differences between freezing scores for the vehicle-treated AS mice and ampakine-treated AS mice were highly significant (p<0.01). Notably, locomotor activity and responses to the foot-shock were not different in the four groups of mice, excluding the possibility that the changes in freezing levels observed during testing were due to differences in pain sensitivity of motor activity.
Figure 3. Effects of CX929 treatment on fear conditioning in WT and AS mice.
WT and AS (KO) mice were semi-chronically treated with CX929 (5 mg/kg) for five days, and were trained and tested in the fear-conditioning paradigm as described. Results are expressed as the percent time mice exhibited freezing behavior in the context (top) or the cue (bottom) condition during an 8-min observation period and are means ± S.E.M. of 4–12 animals. *p <0.01 as compared to WT controls; † Not significant from WT controls.
Discussion
The above results demonstrate that TBS-induced polymerization of spine actin, an effect that has been described in several studies using normal rats and mice, and that has been shown to be tightly correlated with LTP stabilization (Lynch et al., 2008; Simmons et al., 2009; Baudry et al., 2011) is severely impaired in AS mice. This effect provides a likely explanation for the impairment in LTP stabilization found in the mutants (see below) and possibly for the abnormal spine morphology associated with AS. Notably, baseline transmission, paired-pulse facilitation, and LTP induction were normal in the AS mice. This suggests that the impairment in synaptic plasticity in these mice lies in some event that promotes actin filament assembly during the minutes following the inducing stimulation. While the ubiquitin ligase E6AP encoded by Ube3a is present in dendritic spines and its depletion is associated with abnormal spine morphology (Dindot et al., 2008), its functions there have not yet been established. E6AP is reported to down-regulate Arc (Greer et al., 2010), a protein up-regulated by LTP induction and that functions as a negative regulator of AMPA receptors through increased endocytosis (refs in (Bramham et al., 2008)). These effects would be expected to negatively affect baseline transmission and, as described, we did not detect evidence for this. The apparent normalcy of excitatory synaptic physiology, including responses to afferent bursts, in AS mice has to be viewed as surprising given the abnormalities in synaptic structure produced by the mutation. In any event, our results favor a hypothesis in which the lack of Ube3a disturbs a step upstream of actin filament assembly, and current efforts are directed at identifying potential targets of the ligase that could fill this role. We found the previously reported LTP defects in AS mice to be unexpectedly discrete in that potentiation appeared normal in the mutants for the first 10 minutes post-TBS; this indicates that the machinery required to induce and express LTP is operative in the mutants but consolidation events, which include actin filament assembly, are not.
Previous studies showed that a five-day treatment with CX929 enhances TBS-induced actin polymerization and LTP consolidation in ovariectomized rats (Kramar et al., 2010) and multiple mouse models of Huntington’s Disease (Lynch et al., 2007; Simmons et al. 2011; Simmons et al., 2009). We found that this also holds true for AS mice. The number of spines containing high levels of F-actin at 10–15 min after LTP induction was not detectably different from the value measured in slices from littermate control mice tested under the same conditions, and greatly increased above the values obtained in slices from vehicle-treated AS mice. As expected from the actin results, the ampakine treatment also offset the striking impairment in LTP consolidation found in the AS mice. Although we cannot exclude other possibilities, results from the present study together with previous reports, suggest that enhanced actin polymerization mediates, at least partially, the effect of ampakine treatment on LTP. Short-term potentiation and the initial decay of LTP in the slices from ampakine-treated AS mice were comparable to WT values, and the EPSPs produced by the control input exhibited excellent stability over the 90 min of testing. Moreover, we found no evidence that ampakine treatment affected excitatory transmission, inhibition, or the complex responses elicited by a single theta burst. These points indicate that the effects of the treatment were discrete in nature and restricted to LTP consolidation.
We conducted the slice studies two days after the final injection of the short-half life (~15 min) drug, from which it follows that the underlying mechanism for enhanced actin polymerization and LTP is (i) induced by relatively brief periods of enhanced excitatory transmission and (ii) very persistent. It therefore presumably involves an enduring epigenetic event or the induction of genes whose products have long half-lives. Finally, the pertinent changes appear to act in discrete fashion, as evidenced by our physiological results, and to influence actin polymerization regulation. This is a severe set of constraints that are not likely to be met by many potential substrates. Changes in some aspects of BDNF (Brain Derived Neurotrophic Factor) signaling at synapses constitute one possibility: TBS activates synaptic TrkB receptors for the neurotrophin and there is evidence that this is required for polymerization of spine actin (Rex et al., 2007). Accordingly, it will be of interest in future studies to determine if ampakine treatment enhances TBS-induced TrkB activation in AS mice.
The present studies confirmed that AS mice are severely impaired in the encoding of long-term memory. We found that the ampakine treatment resulted in freezing scores equivalent to those measured in WT mice in a fear conditioning paradigm, a result that reinforces the hypothesis that the loss of synaptic plasticity in the mutants, which is also offset by the treatment, is responsible for the learning deficits. Importantly, ampakine treatment did not modify the basal level of motor activity or pain sensitivity, eliminating the possibility that the restoration of normal memory represented a side effect of the treatment. Ampakines have been used in a number of animal and human studies without producing significant adverse side effects, and have been shown to improve learning performance in a variety of behavioral studies (Arai and Kessler, 2007; Hamlyn et al., 2009; Zheng et al., 2011). While we did not observe a significant effect of CX929 treatment on learning and memory in WT mice, our experimental conditions were not optimized to detect such an effect, as vehicle-treated mice performed at a very high level. In addition, because of the very short half-life of CX929, the behavioral experiments were performed at a time when the levels of the drug in the brain would have been extremely low, and therefore not likely to directly modify the behavior.
We previously discussed the fact that ampakines have the potential to modify behavior and learning and memory through three routes, and therefore could act as broad-spectrum therapeutic agents for several psychiatric disorders (Lynch and Gall, 2006). While our results do not demonstrate that the effects of ampakine treatment on LTP and learning and memory in AS mice are specifically related to AS pathology, they nevertheless point to a minimally invasive (daily injections of a drug with a very short half-life), mechanism-based strategy for treating aspects of the memory and cognitive problems that accompany AS. Patients with the disorder commonly exhibit seizure activity, and ampakines, by increasing AMPA receptor-mediated synaptic responses, could potentially exacerbate this debilitating component of the condition. We are currently assessing the effects of the drug protocols used here on seizure thresholds in AS mice, as well as the duration of the beneficial effects of various treatment regimens.
Conclusion
Maternal deficiency of E6AP in mice results in defective synaptic plasticity, including impairment in LTP and in TBS-induced actin polymerization, an event that has been shown to be associated with LTP induction. AS mice also exhibit severe learning and memory impairment. A short-term treatment with an ampakine, CX929, ameliorates the deficits in TBS-induced actin polymerization, LTP induction and learning and memory. These results suggest that ampakine treatment could provide a minimally invasive procedure to treat some of the deficits found in AS patients.
Highlights.
LTP consolidation is impaired in a mouse model of Angelman syndrome (AS)
Actin polymerization following theta burst stimulation is impaired in AS mice
CX929 treatment promotes LTP consolidation and actin polymerization
CX929 treatment enhances behavioral performance in a fear-conditioning paradigm
Acknowledgments
The authors thank Cortex Pharmaceuticals Inc. for providing CX929. This work was supported by grant P01NS045260-01 from NINDS (PI: Dr. C.M. Gall). G. Lynch is a coauthor on a University of California held patent for the use of ampakines to support cognitive function and is a co-founder, shareholder, and member of the Scientific Board of Cortex Pharmaceuticals Inc, which has licensed the patent for development of ampakines for therapeutic purposes.
Footnotes
The other authors indicate that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Current drug targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bi X, Gall C, Lynch G. The biochemistry of memory: The 26-year journey of a 'new and specific hypothesis'. Neurobiol Learn Mem. 2011;95:125–133. doi: 10.1016/j.nlm.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn E, Brand L, Shahid M, Harvey BH. The ampakine, Org 26576, bolsters early spatial reference learning and retrieval in the Morris water maze: a subchronic, dose-ranging study in rats. Behavioural pharmacology. 2009;20:662–667. doi: 10.1097/FBP.0b013e328331ba1b. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Lauterborn JC, Simmons DA, Gall CM, Lynch G. BDNF upregulation rescues synaptic plasticity in middle-aged ovariectomized rats. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Yang Z, Rao G, Houston FP, Barnes CA. Altered dendritic integration in hippocampal granule cells of spatial learning-impaired aged rats. J Neurophysiol. 2008;99:2769–2778. doi: 10.1152/jn.01278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung VW, Hassam R, Morton AJ, Jones S. Dopamine-dependent long term potentiation in the dorsal striatum is reduced in the R6/2 mouse model of Huntington's disease. Neuroscience. 2007;146:1571–1580. doi: 10.1016/j.neuroscience.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Lalande M, Calciano MA. Molecular epigenetics of Angelman syndrome. Cell Mol Life Sci. 2007;64:947–960. doi: 10.1007/s00018-007-6460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. TRENDS Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch G, Kramar EA, Rex CS, Jia Y, Chappas D, Gall CM, Simmons DA. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington's disease. J Neurosci. 2007;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, Soskis MJ, Sahin M, Greenberg ME. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O'Malley BW. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–1189. doi: 10.1128/mcb.19.2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C, Horsler K, Berg K, Bellamy G, Dick K, Griffiths E. Genomic imprinting and the expression of affect in Angelman syndrome: what's in the smile? J Child Psychol Psychiatry. 2007;48:571–579. doi: 10.1111/j.1469-7610.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Mehta RA, Lauterborn JC, Gall CM, Lynch G. Brief ampakine treatments slow the progression of Huntington's disease phenotypes in R6/2 mice. Neurobiol Dis. 2011;41:436–444. doi: 10.1016/j.nbd.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Williams CA, Zori RT, Stone JW, Gray BA, Cantu ES, Ostrer H. Maternal origin of 15q11–13 deletions in Angelman syndrome suggests a role for genomic imprinting. Am J Med Genet. 1990;35:350–353. doi: 10.1002/ajmg.1320350308. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Balabhadrapatruni S, Masumura C, Darlington CL, Smith PF. Effects of the Putative Cognitive-Enhancing Ampakine, CX717, on Attention and Object Recognition Memory. Current Alzheimer Research. 2011;8:876–882. doi: 10.2174/156720511798192709. [DOI] [PubMed] [Google Scholar]