Abstract
By using the cDNA clone containing the sequence for the L1 ribosomal protein gene of Xenopus laevis as probe (1), we have isolated positive phages from a Drosophila melanogaster genomic library. The Drosophila genomic fragment, which gives the hybridization signal with the Xenopus cDNA, was sequenced: a region of 369 bp is 70% homologous to the sequence of X. laevis L1 cDNA. The gene was localized in situ at position 98AB of the right arm of the third polytene chromosome. By S1 mapping and heteroduplex analysis we have found that the gene is interrupted by three introns. A Drosophila cDNA embryonic library was screened and three cDNA clones were isolated (900, 1400 and 1500 nt long). By Northern analysis the cDNAs identify a 1400nt transcript present at every stage of development. By the features described, the clones we have isolated identify the Drosophila rp gene homologous to the L1 rp gene of Xenopus and could code for the L1 ribosomal protein described in D. melanogaster.
Full text
PDF




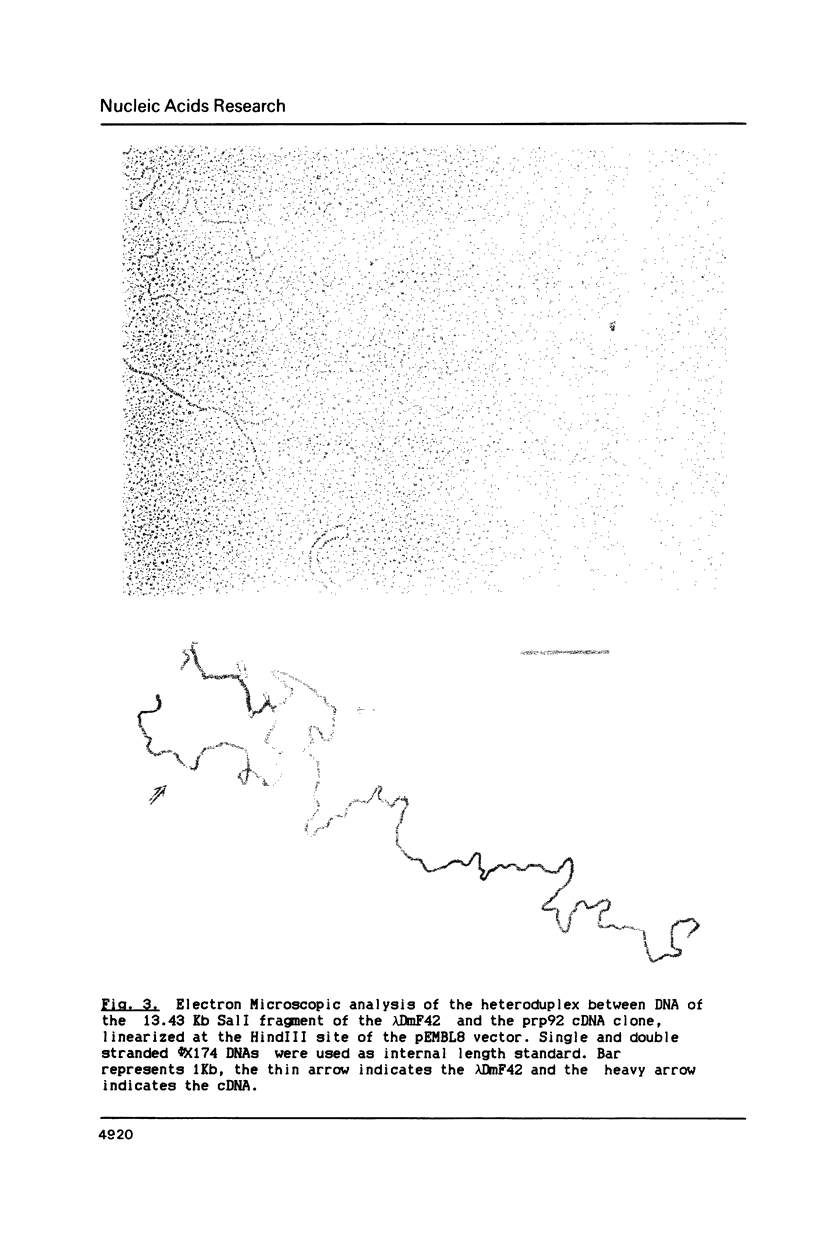
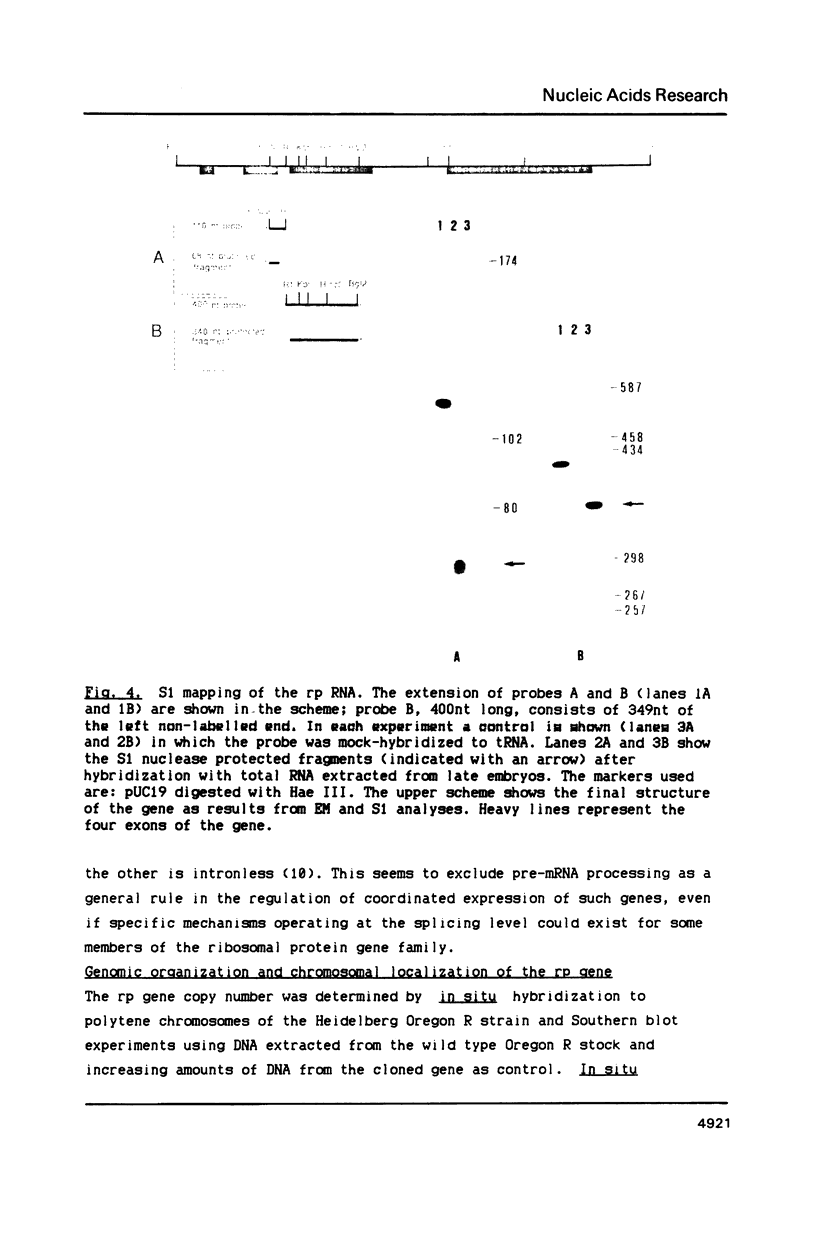





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berger E. The ribosomes of Drosophila. I. Subunit and protein composition. Mol Gen Genet. 1974;128(1):1–9. doi: 10.1007/BF00267290. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Beccari E., Luo Z. X., Amaldi F. Xenopus laevis ribosomal protein genes: isolation of recombinant cDNA clones and study of the genomic organization. Nucleic Acids Res. 1981 Mar 11;9(5):1069–1086. doi: 10.1093/nar/9.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzoni I., Fragapane P., Annesi F., Pierandrei-Amaldi P., Amaldi F., Beccari E. Expression of two Xenopus laevis ribosomal protein genes in injected frog oocytes. A specific splicing block interferes with the L1 RNA maturation. J Mol Biol. 1984 Dec 25;180(4):987–1005. doi: 10.1016/0022-2836(84)90267-5. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Tognoni A., Pierandrei-Amaldi P., Beccari E., Buongiorno-Nardelli M., Amaldi F. Isolation and structural analysis of ribosomal protein genes in Xenopus laevis. Homology between sequences present in the gene and in several different messenger RNAs. J Mol Biol. 1982 Nov 5;161(3):353–371. doi: 10.1016/0022-2836(82)90244-3. [DOI] [PubMed] [Google Scholar]
- Chooi W. Y., Sabatini L. M., Macklin M., Fraser D. Group fractionation and determination of the number of ribosomal subunit proteins from Drosophila melanogaster embryos. Biochemistry. 1980 Apr 1;19(7):1425–1433. doi: 10.1021/bi00548a025. [DOI] [PubMed] [Google Scholar]
- Cutruzzolá F., Loreni F., Bozzoni I. Complementarity of conserved sequence elements present in 28S ribosomal RNA and in ribosomal protein genes of Xenopus laevis and Xenopus tropicalis. Gene. 1986;49(3):371–376. doi: 10.1016/0378-1119(86)90373-2. [DOI] [PubMed] [Google Scholar]
- D'Eustachio P., Meyuhas O., Ruddle F., Perry R. P. Chromosomal distribution of ribosomal protein genes in the mouse. Cell. 1981 May;24(2):307–312. doi: 10.1016/0092-8674(81)90320-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Glover D. M. Differential replication of ribosomal gene repeats in polytene nuclei of Drosophila. Cell. 1979 Jul;17(3):597–605. doi: 10.1016/0092-8674(79)90267-8. [DOI] [PubMed] [Google Scholar]
- Fabijanski S., Pellegrini M. Isolation of a cloned DNA segment containing a ribosomal protein gene of Drosophila melanogaster. Gene. 1982 Jun;18(3):267–276. doi: 10.1016/0378-1119(82)90165-2. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Brown D. D. Co-ordinated synthesis of some ribosomal proteins and ribosomal RNA in embryos of Xenopus laevis. J Mol Biol. 1969 Dec 28;46(3):393–411. doi: 10.1016/0022-2836(69)90184-3. [DOI] [PubMed] [Google Scholar]
- Hattori M., Hidaka S., Sakaki Y. Sequence analysis of a KpnI family member near the 3' end of human beta-globin gene. Nucleic Acids Res. 1985 Nov 11;13(21):7813–7827. doi: 10.1093/nar/13.21.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K., Dellavalle R. P., Merriam J. R. Deficiency Analysis of the Tip of Chromosome 3R in DROSOPHILA MELANOGASTER. Genetics. 1986 Mar;112(3):539–550. doi: 10.1093/genetics/112.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K., Yu Q., Vincent A., Frisardi M. C., Rosbash M., Lengyel J. A., Merriam J. A Drosophila Minute gene encodes a ribosomal protein. Nature. 1985 Oct 10;317(6037):555–558. doi: 10.1038/317555a0. [DOI] [PubMed] [Google Scholar]
- Lambertsson A. G., Rasmuson S. B., Bloom G. D. The ribosomal proteins of Drosophila melanogaster. I. Characterization in polyacrylamide gel of proteins from larval, adult, and ammonium chloride-treated ribosomes. Mol Gen Genet. 1970;108(4):349–357. doi: 10.1007/BF00267772. [DOI] [PubMed] [Google Scholar]
- Lambertsson A. G. The ribosomal proteins of Drosophila melanogaster. II. Comparison of protein patterns of ribosomes from larvae, pupae and adult flies by two-dimensional polyacrylamide gel electrophoresis. Mol Gen Genet. 1972;118(3):215–222. doi: 10.1007/BF00333458. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreni F., Ruberti I., Bozzoni I., Pierandrei-Amaldi P., Amaldi F. Nucleotide sequence of the L1 ribosomal protein gene of Xenopus laevis: remarkable sequence homology among introns. EMBO J. 1985 Dec 16;4(13A):3483–3488. doi: 10.1002/j.1460-2075.1985.tb04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Beccari E., Bozzoni I., Amaldi F. Ribosomal protein production in normal and anucleolate Xenopus embryos: regulation at the posttranscriptional and translational levels. Cell. 1985 Aug;42(1):317–323. doi: 10.1016/s0092-8674(85)80127-6. [DOI] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Campioni N., Beccari E., Bozzoni I., Amaldi F. Expression of ribosomal-protein genes in Xenopus laevis development. Cell. 1982 Aug;30(1):163–171. doi: 10.1016/0092-8674(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Pierandrei-Amaldi P., Campioni N., Gallinari P., Beccari E., Bozzoni I., Amaldi F. Ribosomal-protein synthesis is not autogenously regulated at the translational level in Xenopus laevis. Dev Biol. 1985 Feb;107(2):281–289. doi: 10.1016/0012-1606(85)90311-2. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bröckl C. Transcription of the Drosophila white locus and some of its mutants. EMBO J. 1984 Mar;3(3):563–568. doi: 10.1002/j.1460-2075.1984.tb01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Zhang J. Y., Kay M. A., Jacobs-Lorena M. Structural analysis of the Drosophila rpA1 gene, a member of the eucaryotic 'A' type ribosomal protein family. Nucleic Acids Res. 1987 Feb 11;15(3):987–1003. doi: 10.1093/nar/15.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teem J. L., Abovich N., Kaufer N. F., Schwindinger W. F., Warner J. R., Levy A., Woolford J., Leer R. J., van Raamsdonk-Duin M. M., Mager W. H. A comparison of yeast ribosomal protein gene DNA sequences. Nucleic Acids Res. 1984 Nov 26;12(22):8295–8312. doi: 10.1093/nar/12.22.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaslet C. A., O'Connell P., Izquierdo M., Rosbash M. Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature. 1980 Jun 26;285(5767):674–676. doi: 10.1038/285674a0. [DOI] [PubMed] [Google Scholar]
- Warner J. R. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa cells are synthesized normally and degraded rapidly. J Mol Biol. 1977 Sep 25;115(3):315–333. doi: 10.1016/0022-2836(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]