Abstract
Objective
To determine the effects of retinoic acid (RA) on establishment and growth of endometrial lesions, peritoneal IL-6 and MCP-1 concentrations, and CD38, CD11b, F4/80 expression on peritoneal macrophages in an immunocompetent mouse model of endometriosis.
Design
Experimental transplantation study using mice.
Setting
Academic medical center.
Animals
C57BL/6 recipient mice and syngeneic Green Fluorescent Protein transgenic (GFP+) mice.
Intervention(s)
Recipient mice were inoculated with GFP+ minced uterine tissue to induce endometriosis and treated with RA (400 nmol/day) or vehicle for 17 days (3 days before to 14 days after tissue injection).
Main Outcome Measure(s)
Total number of GFP+ implants in recipient mice, number of implants showing visible blood vessels, total volume of established lesions per mouse, concentrations of IL-6 and MCP-1 in peritoneal fluid, expression of CD11b, F4/80 and CD38 on peritoneal macrophages.
Results
17 days of RA treatment reduced the number of implants versus controls and decreased the frequency of lesions with vessels. Peritoneal washings in RA-treated animals had lower IL-6 and MCP-1 than controls 3 days after endometrial inoculation and lower levels of IL-6 on day 14 after inoculation. Concomitant with these effects on day 14, CD38, CD11b, and F4/80 were higher on macrophages from RA-treated mice vs. controls.
Conclusions
RA inhibits the development of endometriotic implants. This effect may be caused, at least in part, by reduced IL-6 and MCP-1 production and enhanced differentiation of peritoneal macrophages.
Keywords: endometriosis, retinoic acid, cytokines, GFP-transgenic mice
INTRODUCTION
Endometriosis is a chronic inflammatory syndrome, commonly associated with ectopic peritoneal implants of endometrial tissue (1). Its etiology appears to involve genetic and environmental factors, including aspects of diet and exercise (1–4). The gene-environment interaction contributes to local inflammatory responses in the uterine and peritoneal cavity, which are thought to play a key role in the establishment and maintenance of endometriosis (5). To this end, the pathogenesis and progression of the disease are promoted by the collective effects of cytokines, chemokines, proteases, and angiogenic factors in the peritoneal cavity (5). These mediators are derived from endometriotic implants, mesothelial cells, and particularly activated peritoneal macrophages (5). Studies have suggested that defects in macrophage activation/differentiation programs lead to chronic immune activation with accompanying reduction in scavenging (6–8). Both of these consequences are thought to contribute to the establishment, growth, and maintenance of endometriotic lesions (9, 10).
All-trans-retinoic acid (RA), the active metabolite of vitamin A, has many biologic effects, including a variety of immunomodulatory and anti-inflammatory activities (11, 12). RA has been shown to modulate inflammation in autoimmune disease via its ability to enhance regulatory T cell (T-reg) suppression of pro-inflammatory cells (13, 14). In model systems involving activated monocytes/macrophages, RA decreased pro-inflammatory cytokines while increasing anti-inflammatory proteins such as IL-10 (15). The role of retinoids in the female reproductive tract and in particular in the endometrium has been investigated in our laboratory and others’ (7, 16–19). Some studies suggest the possibility that the retinoid pathway is involved in the pathophysiology of endometriosis (7, 16, 17, 20).
Preclinical rodent models of endometriosis have been used to investigate the link between certain hormones (e.g. estrogen and progesterone) and the growth of endometriotic implants (21–23), and to test the efficacy of potential therapeutic agents (24, 25). In this context, immune-compromised mice (e.g., “nude” mice), have been extensively used as models for xenografting, whereas in rats, surgical implants of autologous endometrial tissue has been utilized (26–28). Unfortunately, immune deficient models are limited for investigating immune system-disease interactions, while surgical auto-transplantation models bypass the attachment/implantation phases of the disease. As a result, investigators have refined an immunocompetent mouse model whereby endometrial fragments from syngeneic donors are injected into the peritoneal cavity of estradiol-primed recipients to generate endometriotic lesions (29, 30). This non-surgical approach closely mimics the establishment of endometriosis in affected women via retrograde menses (31) and can be used to longitudinally study inflammatory signals associated with endometriotic lesion growth (32).
In this study, we examined the effects of RA on the growth and angiogenesis of endometriotic lesions in immunocompetent mice. We also evaluated the effects of RA on peritoneal macrophage activation/differentiation markers CD38, CD11b, and F4/80, and on pelvic washing concentrations of interleukin-6 (IL-6) and macrophage chemotactic factor-1 (MCP-1), cytokines known to be elevated in the peritoneal fluid of women with endometriosis (33–35).
MATERIAL AND METHODS
Chemicals and reagents
All-trans-retinoic acid (RA) was purchased from Sigma Chemical Co. (St. Louis, MO) and diluted in corn oil to the indicated concentrations for the experiments. IL-6 and MCP-1 ELISA kits were obtained from RayBiotech, Inc (Norcross, GA). Antibodies against CD38 and F4/80, and against CD11b were purchased from eBbioscience, Inc. (San Diego, CA) and from BD Pharmingen (Franklin Lakes, NJ), respectively. The reagents were equilibrated at room temperature for 0.5 hours before use.
Mice
This study was approved by the Emory Institutional Animal Care and Use Committee. All the procedures were performed according to the NIH Guidelines for Care and Use of Laboratory Animals. Wild-type C57BL/6 mice and transgenic C57BL/6-Tg(UBC-GFP) 30Scha/J donor mice (Tg-GFP) were purchased from Jackson Laboratory (Bar Harbor, ME). The Tg-GFP mice express enhanced Green Fluorescent Protein (GFP) under the direction of the human ubiquitin C promoter (36). Studies have demonstrated that C57BL/6 mice show little, if any, anti-GFP immune responses when transplanted with tissue from their Tg-GFP counterparts (37). Tg-GFP mice are viable, fertile, equivalent in size and do not display gross physical or behavioral differences. Food (standard pellet diet) and water were provided ad libitum. The mice were housed in a light/dark cycle of 12 h/12 h under standard conditions and allowed to acclimatize to these conditions for at least 1 week prior to inoculation of endometrial fragments.
Experimental Model
The mouse endometriosis model was established as previously described (30) with minor modifications using transplantation of Tg-GFP into syngeneic recipients, which yielded permanent expression of GFP in endometriotic tissue. This model provides the benefit of increased sensitivity in the identification of endometriotic lesions and more accurate quantification of lesion size and growth rates. Tg-GFP donor mice were treated subcutaneously (s.c.) with 100 μg/kg estradiol valerate (dissolved in corn oil) one week before sacrifice to stimulate proliferation of their endometrial tissue for transplantation. All procedures were conducted under aseptic conditions. Uteri were dissected, finely minced with iris scissors, and further fragmented by serial tituration through an 18-gauge adjuvant-mixing needle (Popper and Sons, Inc., New Hyde Park, NY) in 0.5 ml of sterile PBS. Each recipient mouse received an equal quantity of tissue (equivalent to one uterine horn [~35 mg]) via intraperitoneal (i.p.) injection with an 18-gauge needle through the abdominal wall just below the umbilicus. Recipient mice received 100 ug/kg/week estradiol valerate s.c. in corn oil each week, starting one week before i.p. inoculation of endometrial tissue, in order to synchronize their estrus cycles.
Experimental design and drug treatment
In the treatment groups, retinoic acid was administered by oral gavage. The dose of RA (0.1 ml of 4 mM/day) was previously shown to achieve therapeutic serum concentrations in anti-tumor studies without adverse side effects (38). Mice were treated daily with RA or vehicle control (corn oil) starting three days before inoculation with endometrial fragments (day-3) until sacrifice. Three - 14 days after endometrial fragment inoculation, we sacrificed the recipients and examined their peritoneum under 488 nm light (excitation wavelength peak of GFP) using interference filter eyeglasses to observe GFP+ endometrial implants (emission wavelength peak: 502 nm). Based on our experience, at 5 days after challenge endometriotic lesions are visible in all animals. The animals were killed by cervical dislocation 24 hours after the last administration of drug or vehicle. 3 ml of PBS was injected intraperitoneally (see below), and the abdominal skin and peritoneum were opened to harvest peritoneal washings and macrophages, examine the visceral organs and evaluate the presence of endometriotic implants and vascularization. The sizes of all the lesions were measured in situ with calipers. We confirmed the authenticity of the implants by verifying the histopathological criteria for endometriosis, including formation of endometriotic cysts with endometrial glands, stroma, vessels and hemorrhage (Fig. 1D).
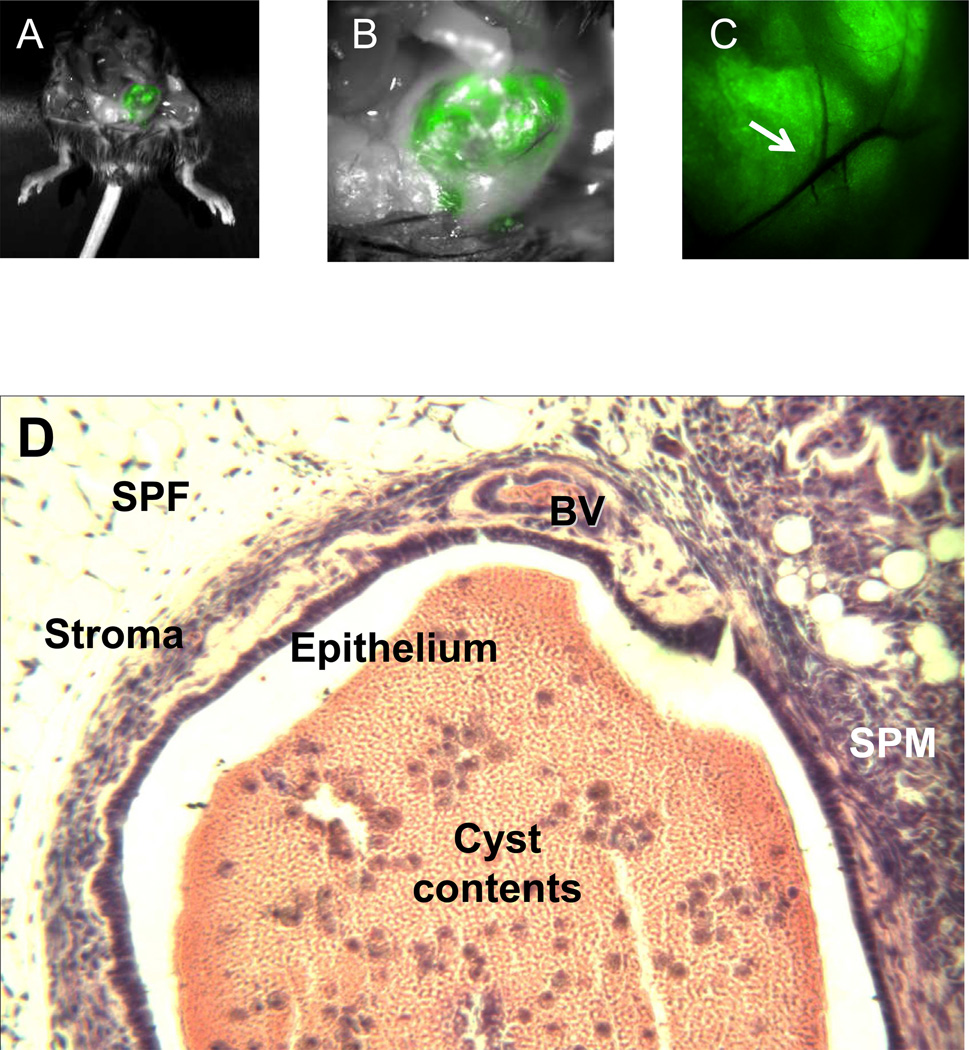
Figure 1.
Endometriotic lesions in recipient mice, 14 d after inoculation. A. GFP+ lesions were visible in situ using interference filter eyeglasses (excitation wavelength, 488 nm; emission wavelength, 502 nm). B. Higher power (5.6x) view shows large cystic and two small satellite GFP+ lesions invading anterior parietal peritoneum and preperitoneal fat in the left lower pelvis. C. High magnification (12x) view of GFP+ lesion shows vascular arcade of established GFP+ implant with arrow indicating vessel branchpoint. Pictures were taken with a Hamamatsu Multiplier CCD Camera (Bridgewater, NJ). D. H&E histology of murine endometriotic lesions was nearly identical to that observed in the human counterpart. This example of a cystic lesion was lined by dense epithelium and surrounded by endometrial stroma. The lesion invaded subperitoneal fat (SPF) and subperitoneal muscle (SPM). The cyst wall contained blood vessels (BV) and the cyst contents included erythrocytes (pink) and leukocytes (purple). Magnification = 40x.
Specimen Collection and flow cytometry
Peritoneal fluid washings were harvested for assessment of IL-6 and MCP-1 levels by ELISA, and peritoneal leukocytes were evaluated by flow cytometry for quantitation of macrophage markers (CD11b and F4/80) and the differentiation/activation marker CD38. Resident peritoneal cells were obtained as described (39). Briefly, after sacrifice, 3 ml of ice cold PBS was injected i.p. using an 18 g needle with 5 ml syringe. After gentle massage, the abdominal wall was carefully incised and peritoneal fluid-containing cells was harvested and centrifuged at 1000 rpm for 10 min at 4°C. The lavage fluid was concentrated 6-fold using Amicon Ultra-15 Centrifugal Filter Tubes (Millipore, Billerica, MA) and the concentrate then stored at −80°C for furthe r analysis of IL-6 and MCP-1 levels. The cell pellet was reconstituted with washing media (PBS with 2% FBS). After counting, 1×106 cells were suspended in 100 μl washing media and incubated at 4°C for 1 hour with appropriate dilutions of the following antibodies: FITC-conjugated rat anti-mouse CD11b (BD Pharmingen), PE-Cy5 conjugated rat anti-mouse CD38 (eBioscience), APC-conjugated rat anti-mouse F4/80 Antigen (eBioscience). Isotype control antibodies were used at the same dilution as their counterparts. The cells were washed and analyzed on a LSRII flow cytometer (Becton Dickinson, San Jose, CA) within 4 hours or fixed with 1% paraformaldehyde and run within 4 days after storage in dark/cold. For each experiment, 10,000 events were collected and analyzed using the Flowjo 7.6 program. The data were expressed as mean fluorescence intensity (MFI; arbitrary units).
Statistical analysis
Statistical analysis was performed using SPSS (release 19.0 SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± SEM. Differences between treatment groups were analyzed by t test (two tailed) where p < 0.05 was considered statistically significant.
RESULTS
Effects of RA on the establishment and growth of endometriotic lesions
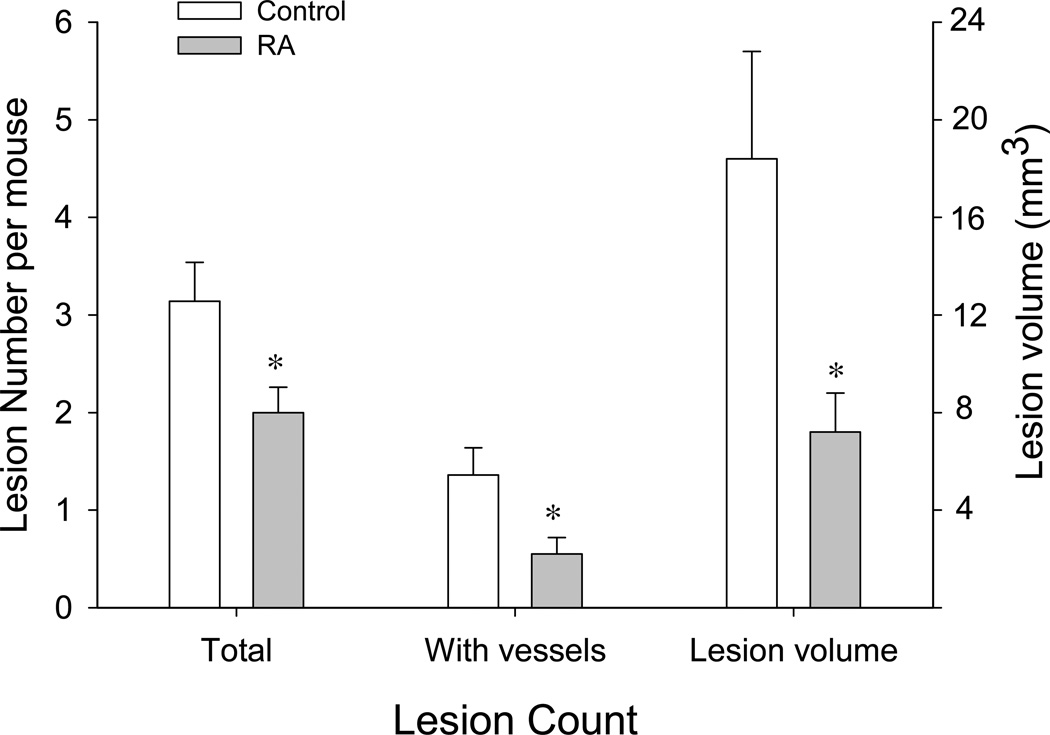
In a previous study of the effects of RA on tumor growth in a mouse model (38), we optimized an RA treatment protocol to achieve a mean peak serum concentration of 1 μM over extended periods (up to 30 days). We have utilized this protocol in the present study since this concentration of RA was shown to be optimal for inducing a variety of alterations in macrophages and endometrial cells that might be beneficial for reducing the invasive phenotype of ectopic endometrial tissue (7, 20, 40, 41). In pilot experiments, we determined that 100% of the animals had detectable peritoneal lesions by 5 days after challenge with uterine fragments. By 14 days, mature, GFP+ implants were prominent and many contained well-formed blood vessels. The presence of the latter tended to correlate with the invasive quality of the implant (depth of invasion into host tissue) (Fig. 1A-C). Therefore, our evaluation of lesion formation delineated both total number of invading lesions and those showing visible vessels in animals where RA or vehicle treatment began 3 days before intraperitoneal inoculation of endometrial cells and continued for 14 days until sacrifice. As shown in Fig. 2, RA-treated animals showed a reduction in the mean number of total implants (2.00 ± 0.26) vs. vehicle-treated controls (3.14 ± 0.40, P<0.03) and a decrease in the mean number of lesions with visible vessels (RA, 0.55 ± 0.17; controls, 1.36 ± 0.28, P<0.02). In addition, the average volume of all endometriotic lesions per mouse in the RA group (7.2 ± 1.6 mm3) was significantly less than those in the control group (18.4 ± 4.4 mm3, P<0.03). There was no significant difference between the mean weights of the control versus RA-treated animals at the time of sacrifice (control, 18.3 ± 0.3 g; RA-treated, 18.4 ± 0.3 g).
Figure 2.
Inhibition of lesion growth by retinoic acid. RA or vehicle control treatment began 3 days before intraperitoneal inoculation of endometrial cells and continued for 14 days until sacrifice. RA treatment significantly reduced the mean number of total lesions and number of vascularized lesions vs. vehicle-treated controls (n=22 for both groups). In addition, the average volume of established endometriotic lesions in the RA group was significantly less than those in the control group. Values represent the mean ± SEM of the indicated lesion count parameter. *, Significant decrease of the indicated lesion parameter compared with control-treated mice (p< 0.03).
Suppressive effects of RA treatment on cytokine secretion
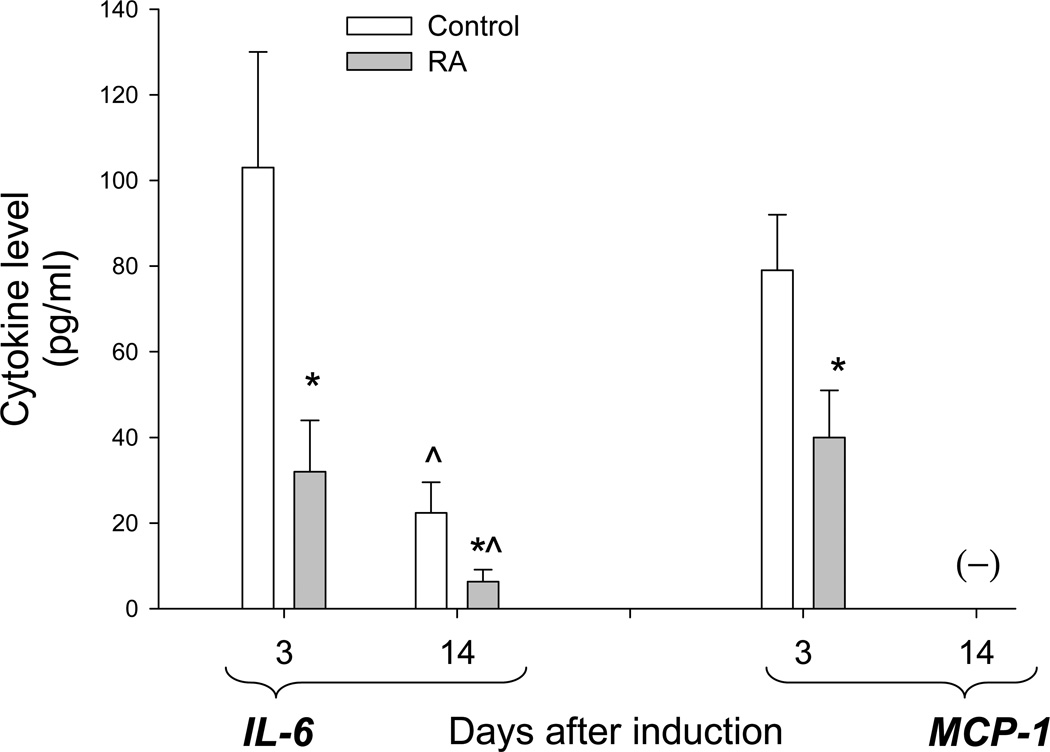
The proinflammatory cytokines IL-6 and MCP-1 are known to be elevated in the peritoneal fluid of women with endometriosis (33–35). Studies in mice have demonstrated that secretion of these cytokines peak within the first few days after i.p. injection of endometrial cells and subsequently decrease to near background levels within a week (42). Thus, after concentrating the peritoneal lavage fluid, we measured IL-6 and MCP-1 levels in short-term experiments before lesions were apparent (3 days after endometrium injection) as well as at the 14-day time point when the endometriotic lesions were quantified. Fig. 3 shows that the concentrations of IL-6 diminished over the 11-day interval, and were significantly lower in the RA-treated groups compared with the control groups. On day 3, MCP-1 was significantly lower in the in the peritoneal washings of RA-treated mice compared to controls, but had fallen below detectable concentrations by day 14.
Figure 3.
Suppressive effects of retinoic acid treatment on peritoneal fluid IL-6 and MCP-1 levels. Values represent the mean ± SEM of cytokine levels found in control and RA-treated animals 3 days (n=10, both groups) and 14 days (n=22, both groups) after induction of endometriosis. (-) indicates below the level of detection. *, Significant decrease in levels of the indicated cytokine compared with control-treated mice (p< 0.05) ^, Significant reduction in 14-day control and RA-treated samples compared with those from correspondingly treated groups at day 3 (p<0.01).
Effects of RA treatment on expression of macrophage activation/differentiation markers CD38, CD11b, and F4/80
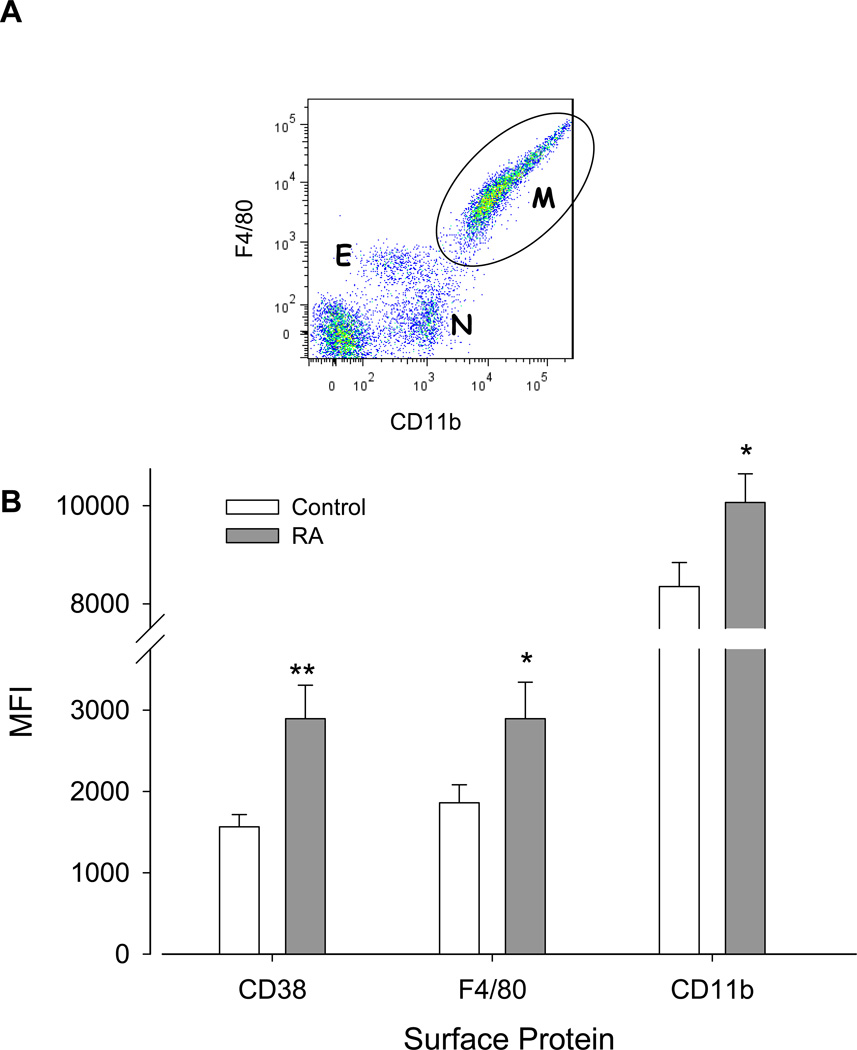
Mouse macrophages are typically characterized by surface F4/80 glycoprotein and CD11b expression, but these markers are also expressed to lesser degrees by eosinophils and neutrophils (Fig. 4, top) (43–45). Peritoneal cells were harvested from washings after 14 days. As a means of discriminating the macrophage population from eosinophils and neutrophils in flow cytometric analyses, we gated on the CD11bhighF4/80high populations (Fig. 4, top). There were no significant differences in the subpopulation profiles (i.e. relative number of macrophages, eosinophils, and neutrophils) between the control and RA-treated animals. Relative changes in differentiation between control and RA treatment groups were assessed by quantitation of CD38, CD11b, and F4/80 expression as mean fluorescence intensity (MFI). Fig. 4, bottom shows that in macrophages, all three surface markers were significantly increased in the RA-treated versus control mice.
Figure 4.
A. Representative dot plot showing CD11b and F4/80 fluorescent intensity of peritoneal cells from a control mouse 14 days after induction of endometriosis. Subpopulations of viable eosinophils (E, CD11bmediumF4/80medium), neutrophils (N, CD11bmediumF4/80negative), and macrophages (M, CD11bhighF4/80high) are indicated. Dot blot analysis of RA-treated mice showed similar population profiles. B. Cells gated on the macrophage (M) population were analyzed for CD11b, F4/80, and CD38 expression. Upregulation by RA of the macrophage markers (CD11b and F4/80) and the differentiation/activation marker CD38 was observed. Values represent the MFI ± SEM of the surface proteins as indicated in control (n=22) and RA-treated (n=22) mice. *, p< 0.03; **, p<0.002.
DISCUSSION
This study demonstrated that in vivo RA treatment suppressed the establishment and growth of endometriotic lesions in an immunocompetent mouse model of endometriosis. RA also decreased the peritoneal fluid levels of IL-6 and MCP-1, two inflammatory cytokines that are elevated in the peritoneal fluid of women with endometriosis and have been implicated in its pathogenesis (5, 33, 35). In addition, RA treatment modulated the “differentiated state” of peritoneal macrophages as reflected by increased expression of CD38, CD11b, and F4/80. On a quantitative bases, both F4/80 and CD11b increase with differentiation of monocytes to macrophages and with inflammatory reactions that are associated with maturity and increased macrophage function including phagocytosis (44–47). CD38 is a type II transmembrane glycoprotein widely used as a marker of lymphocyte and macrophage activation and differentiation (48). RA has been shown to upregulate CD38 on immune cells via an RA response element located in the first intron rather than the 5'-flanking region of the CD38 gene (49). In macrophages, RA induction of CD38 is associated with an increase in differentiated functions including antigen presentation and cell adhesion (48, 50). These findings reveal intergrated anti-inflammatory and immunomodulatory effects of RA, which we propose contribute to the suppression of endometriotic lesions, and suggest the therapeutic potential of agents that target the retinoic acid pathway for the treatment of clinical endometriosis.
Numerous studies provide evidence of the anti-proliferative effects of RA on cancer cells both in vitro and in vivo (51–53). Our results indicate that RA inhibited the growth of endometriotic lesions as reflected by decreases in the average number and volume of established implants in the retinoid-treated group. In contrast, in vitro studies of RA on primary endometrial cell cultures have not demonstrated direct antiproliferative activity (unpublished). This fact suggests that growth inhibition by RA on endometriotic implants is indirect, perhaps mediated by changes in macrophage function or altered production of cytokines involved in the survival of ectopic endometrial cells. To this end, aberrant regulation of IL-6 responses is thought to play a role in the development of endometriosis (54, 55). The mechanism of action of IL-6 on endometriotic cell growth is unknown, but may be mediated directly or through its ability to co-activate macrophages and lymphocytes, resulting in the sustained production of other cytokines involved in chemotaxis and cellular growth (7). Concomitant reduction of both IL-6 and MCP-1 levels in the peritoneal fluid of RA-treated mice is consistent with this latter hypothesis. In addition, both IL-6 and MCP-1 have been shown to be potent pro-angiogenic cytokines which can stimulate endothelial cell proliferation and migration (56, 57), and thereby promote the de novo vasculature necessary for the sustained growth of endometriotic lesions. To this end, the reduced number of lesions with vessels that formed in RA-treated animals is consistent with the reduced levels of these cytokines in their peritoneal fluid. Results of our in vivo studies support previous work showing that RA inhibited the expression and secretion of IL-6 from endometrial cells in vitro and suppressed MCP-1 expression in spleen cells from mice with collagen induced arthritis (20, 58).
Macrophages play an essential role in the establishment of endometriosis and we have suggested that pharmacologic manipulation of macrophage function may provide a novel mechanism for treating this disease (7). Peritoneal fluid macrophages are involved in the removal of red cells and endometrial tissue products arising via retrograde menstruation. In endometriosis, peritoneal macrophages have diminished capacity to scavenge cellular debris, which may lead to the persistence of endometriotic lesions (6). In addition, secretion of inflammatory cytokines from peritoneal macrophages is increased, leading to a positive feedback loop and the further propagation of a peritoneal inflammatory environment. As an example, we have shown that the expression of CCR1, a high affinity receptor for RANTES (Regulated upon Activation, Normal T cell Expressed and Secreted), is upregulated in peritoneal fluid macrophages from women with endometriosis (59).
RA treatment resulted in a more differentiated phenotype of peritoneal fluid macrophages as shown by upregulation of CD38, CD11b, and F4/80. These findings are consistent with in vitro studies showing that RA can upregulate CD38 and CD14 on human macrophages (60, 61). Additional promising anti-inflammatory actions of RA include induction of T-reg cells with resulting inhibition of IL17-producing helper cells (14), and stimulation of immunosuppressive CD8+ T cells (62) and myeloid-derived suppressor cells (63). Further studies are needed to delineate the salutary RA effects in our mouse model.
In conclusion, our results indicate that RA inhibits the development of endometriotic implants in vivo. We postulate that this effect is due, at least in part, to suppression of IL-6 and MCP-1 production, and to promotion of peritoneal macrophage differentiation. These preclinical in vivo findings emphasize the potential use of retinoids to treat women with endometriosis.
ACKNOWLEDGMENTS
The authors thank Jie Yu for her expert advice in animal handling and technical assistance in some of the experiments.
Support: NIH R01HD55379, 1R21HD065115-01, U54HD55787, UO1HD66439
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors have nothing to disclose
REFERENCES
- 1.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 2.Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25:1528–1535. doi: 10.1093/humrep/deq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitonis AF, Hankinson SE, Hornstein MD, Missmer SA. Adult physical activity and endometriosis risk. Epidemiology. 2010;21:16–23. doi: 10.1097/EDE.0b013e3181c15d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dun EC, Taylor RN, Wieser F. Advances in the genetics of endometriosis. Genome Med. 2010;2:75. doi: 10.1186/gm196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 6.Chuang PC, Wu MH, Shoji Y, Tsai SJ. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol. 2009;219:232–241. doi: 10.1002/path.2588. [DOI] [PubMed] [Google Scholar]
- 7.Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955:159–173. doi: 10.1111/j.1749-6632.2002.tb02777.x. discussion 99-200, 396-406. [DOI] [PubMed] [Google Scholar]
- 8.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y, Tsai SJ. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am J Pathol. 2010;176:850–860. doi: 10.2353/ajpath.2010.090551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebovic DI, Chao VA, Taylor RN. Peritoneal macrophages induce RANTES (regulated on activation, normal T cell expressed and secreted) chemokine gene transcription in endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:1397–1401. doi: 10.1210/jc.2003-031010. [DOI] [PubMed] [Google Scholar]
- 11.Kim CH. Retinoic acid, immunity, and inflammation. Vitam Horm. 2011;86:83–101. doi: 10.1016/B978-0-12-386960-9.00004-6. [DOI] [PubMed] [Google Scholar]
- 12.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74:1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 14.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Allen C, Ballow M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J Clin Immunol. 2007;27:193–200. doi: 10.1007/s10875-006-9068-5. [DOI] [PubMed] [Google Scholar]
- 16.Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, et al. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26:2157–2164. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95:E300–E309. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucco RA, Zheng WL, Davis JT, Sierra-Rivera E, Osteen KG, Chaudhary AK, et al. Cellular retinoic acid-binding protein(II) presence in rat uterine epithelial cells correlates with their synthesis of retinoic acid. Biochemistry. 1997;36:4009–4014. doi: 10.1021/bi962094o. [DOI] [PubMed] [Google Scholar]
- 19.Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, et al. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawatsri S, Desai N, Rock JA, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril. 2000;73:1012–1019. doi: 10.1016/s0015-0282(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 21.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 22.Monckedieck V, Sannecke C, Husen B, Kumbartski M, Kimmig R, Totsch M, et al. Progestins inhibit expression of MMPs and of angiogenic factors in human ectopic endometrial lesions in a mouse model. Mol Hum Reprod. 2009;15:633–643. doi: 10.1093/molehr/gap063. [DOI] [PubMed] [Google Scholar]
- 23.Cummings AM, Metcalf JL. Induction of endometriosis in mice: a new model sensitive to estrogen. Reprod Toxicol. 1995;9:233–238. doi: 10.1016/0890-6238(95)00004-t. [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Rosas F, Gomez R, Ferrero H, Gaytan F, Garcia-Velasco J, Simon C, et al. The effects of ergot and non-ergot-derived dopamine agonists in an experimental mouse model of endometriosis. Reproduction. 2011 doi: 10.1530/REP-11-0223. [DOI] [PubMed] [Google Scholar]
- 25.Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94:2489–2494. doi: 10.1210/jc.2008-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- 27.Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwad ST, Isaacson K. The SCID mouse: an experimental model for endometriosis. Hum Reprod. 1999;14:3107–3111. doi: 10.1093/humrep/14.12.3107. [DOI] [PubMed] [Google Scholar]
- 28.Tabibzadeh S, Miller S, Dodson WC, Satyaswaroop PG. An experimental model for the endometriosis in athymic mice. Front Biosci. 1999;4:C4–C9. doi: 10.2741/tabibzad. [DOI] [PubMed] [Google Scholar]
- 29.Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, Panina-Bordignon P. Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Hum Reprod. 1999;14:2944–2950. doi: 10.1093/humrep/14.12.2944. [DOI] [PubMed] [Google Scholar]
- 30.Hirata T, Osuga Y, Yoshino O, Hirota Y, Harada M, Takemura Y, et al. Development of an experimental model of endometriosis using mice that ubiquitously express green fluorescent protein. Hum Reprod. 2005;20:2092–2096. doi: 10.1093/humrep/dei012. [DOI] [PubMed] [Google Scholar]
- 31.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–469. [Google Scholar]
- 32.Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertil Steril. 2004;82(Suppl 3):999–1007. doi: 10.1016/j.fertnstert.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–597. doi: 10.1016/s0002-9378(97)70553-2. [DOI] [PubMed] [Google Scholar]
- 34.Tao Y, Zhang Q, Huang W, Zhu H, Zhang D, Luo W. The peritoneal leptin, MCP-1 and TNF-alpha in the pathogenesis of endometriosis-associated infertility. Am J Reprod Immunol. 2011;65:403–406. doi: 10.1111/j.1600-0897.2010.00920.x. [DOI] [PubMed] [Google Scholar]
- 35.Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–495. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 37.Tian C, Bagley J, Kaye J, Iacomini J. Induction of T cell tolerance to a protein expressed in the cytoplasm through retroviral-mediated gene transfer. J Gene Med. 2003;5:359–365. doi: 10.1002/jgm.363. [DOI] [PubMed] [Google Scholar]
- 38.Abemayor E, Chang B, Sidell N. Effects of retinoic acid on the in vivo growth of human neuroblastoma cells. Cancer Lett. 1990;55:1–5. doi: 10.1016/0304-3835(90)90057-5. [DOI] [PubMed] [Google Scholar]
- 39.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010 doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han S, Sidell N. Peroxisome-proliferator-activated-receptor gamma (PPARgamma) independent induction of CD36 in THP-1 monocytes by retinoic acid. Immunology. 2002;106:53–59. doi: 10.1046/j.1365-2567.2002.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wuttge DM, Romert A, Eriksson U, Torma H, Hansson GK, Sirsjo A. Induction of CD36 by all-trans retinoic acid: retinoic acid receptor signaling in the pathogenesis of atherosclerosis. FASEB J. 2001;15:1221–1223. doi: 10.1096/fj.00-0488fje. [DOI] [PubMed] [Google Scholar]
- 42.Chen QH, Zhou WD, Su ZY, Huang QS, Jiang JN, Chen QX. Change of proinflammatory cytokines follows certain patterns after induction of endometriosis in a mouse model. Fertil Steril. 2010;93:1448–1454. doi: 10.1016/j.fertnstert.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 43.McGarry MP, Stewart CC. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J Leukoc Biol. 1991;50:471–478. doi: 10.1002/jlb.50.5.471. [DOI] [PubMed] [Google Scholar]
- 44.Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leukoc Biol. 2010;88:169–180. doi: 10.1189/jlb.0809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J, Zhu N, Wang Q, Yu M, Feng J, Li Y, et al. p38 MAPK contributes to CD54 expression and the enhancement of phagocytic activity during macrophage development. Cell Immunol. 2009;256:6–11. doi: 10.1016/j.cellimm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman SM, Fleming SD. Natural Helicobacter infection modulates mouse intestinal muscularis macrophage responses. Cell Biochem Funct. 2010;28:686–694. doi: 10.1002/cbf.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prus E, Fibach E. Retinoic acid induction of CD38 antigen expression on normal and leukemic human myeloid cells: relationship with cell differentiation. Leuk Lymphoma. 2003;44:691–698. doi: 10.1080/1042819031000060564. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto H, Hoshino S, Ohori M, Kontani K, Nishina H, Suzawa M, et al. Molecular mechanism of human CD38 gene expression by retinoic acid. Identification of retinoic acid response element in the first intron. J Biol Chem. 1998;273:15429–15434. doi: 10.1074/jbc.273.25.15429. [DOI] [PubMed] [Google Scholar]
- 50.Musso T, Deaglio S, Franco L, Calosso L, Badolato R, Garbarino G, et al. CD38 expression and functional activities are up-regulated by IFN-gamma on human monocytes and monocytic cell lines. J Leukoc Biol. 2001;69:605–612. [PubMed] [Google Scholar]
- 51.Shentu J, Zhang B, Fan L, He Q, Yang B, Chen Z. Anti-proliferative activity of fenretinide in human hepatoma cells in vitro and in vivo. Anticancer Drugs. 2007;18:47–53. doi: 10.1097/CAD.0b013e32800feeb5. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann S, Rockenstein A, Ramaswamy A, Celik I, Wunderlich A, Lingelbach S, et al. Retinoic acid inhibits angiogenesis and tumor growth of thyroid cancer cells. Mol Cell Endocrinol. 2007;264:74–81. doi: 10.1016/j.mce.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Toma S, Isnardi L, Raffo P, Dastoli G, De Francisci E, Riccardi L, et al. Effects of all-trans-retinoic acid and 13-cis-retinoic acid on breast-cancer cell lines: growth inhibition and apoptosis induction. Int J Cancer. 1997;70:619–627. doi: 10.1002/(sici)1097-0215(19970304)70:5<619::aid-ijc21>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Wieser F, Fabjani G, Tempfer C, Schneeberger C, Sator M, Huber J, et al. Analysis of an interleukin-6 gene promoter polymorphism in women with endometriosis by pyrosequencing. J Soc Gynecol Investig. 2003;10:32–36. [PubMed] [Google Scholar]
- 55.Velasco I, Acien P, Campos A, Acien MI, Ruiz-Macia E. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol. 2010;84:199–205. doi: 10.1016/j.jri.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 58.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clin Immunol. 2006;119:272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Wieser F, Dogan S, Klingel K, Diedrich K, Taylor RN, Hornung D. Expression and regulation of CCR1 in peritoneal macrophages from women with and without endometriosis. Fertil Steril. 2005;83:1878–1881. doi: 10.1016/j.fertnstert.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 60.Drach J, Zhao S, Malavasi F, Mehta K. Rapid induction of CD38 antigen on myeloid leukemia cells by all trans-retinoic acid. Biochem Biophys Res Commun. 1993;195:545–550. doi: 10.1006/bbrc.1993.2080. [DOI] [PubMed] [Google Scholar]
- 61.Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- 62.Fleissner D, Frede A, Knott M, Knuschke T, Geffers R, Hansen W, et al. Generation and function of immunosuppressive human and murine CD8+ T cells by transforming growth factor-beta and retinoic acid. Immunology. 2011;134:82–92. doi: 10.1111/j.1365-2567.2011.03469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JM, Seo JH, Kim YJ, Kim YS, Ko HJ, Kang CY. The restoration of myeloid-derived suppressor cells as functional antigen-presenting cells by NKT cell help and all-trans-retinoic acid treatment. Int J Cancer. 2011 doi: 10.1002/ijc.26411. [DOI] [PubMed] [Google Scholar]