Introduction
Physical activity is essential to the overall health and general wellbeing in people of all ages. In addition to aiding in the prevention of a myriad of illnesses, exercise has also been proven to be an effective and indispensible component in the rehabilitation of a variety of physical and psychological ailments including chronic low back pain, anxiety, and osteoarthritis (1,2,3). In particular, exercise therapy has been shown to improve the symptoms of persons suffering from claudication, a symptom of progressive leg or back pain that progresses with ambulation, that can be caused by nerve or vascular compromise (4,5).
Neurogenic claudication is associated with lumbar spinal stenosis, a pathological narrowing of the spinal canal. The overall prevalence of lumbar spinal stenosis in the United States remains unclear, but it has become one of the most common indications for surgery on the spine (6). About 85% of individuals with spinal stenosis report having some degree of leg pain, and 62% of stenosis patients have the classic signs of neurogenic claudication (7). While the pathophysiology of neurogenic claudication is not entirely clear, there appears to be a vascular connection as some suggest that an inadequately oxygenated cauda equina may be contributing to the leg pain experienced by patients with lumbar spinal stenosis (8).
Peripheral arterial disease, an artherosclerotic condition, affects 8 to 12 million people living in the United States (9). Intermittent claudication is a classic symptom of this disease; however, only 22% of individuals report having this symptom, as peripheral arterial disease can often be asymptomatic (10).
If an individual with neurogenic or vascular claudication becomes less willing to engage in beneficial physical activity due to pain or the anticipation of pain, this may have deleterious consequences when it comes to rehabilitation and improvement of symptom severity. It has already been well established that fear of movement/(re)injury and activity avoidance are associated with poorer outcomes due to decreased levels of physical activity among younger persons with non-specific low back pain (11,12).
Clinical decision making in regards to exercise rehabilitation may also benefit from a deeper understanding of the complex relationship between pain, functional impairment, depression, and fear-avoidance tendencies in people suffering from claudication due to either peripheral vascular disease or lumbar spinal stenosis. The current study examines these tendencies in three groups of individuals between the ages of 55 and 90: patients with neurogenic claudication, vascular claudication, or no leg pain at all.
Methods
Subjects
Subject Identification
As part of a larger study, medical records were examined to identify potential subjects who fit the study criteria. Individuals with neurogenic claudication were recruited from neurosurgery and orthopedic surgery clinics. They were invited to participate in the study only if they had ambulation-limiting leg pain, and their treating physician had made the diagnosis of lumbar spinal stenosis (based on clinical and imaging impressions). These inclusion criteria met the definitions of the 2007 North American Spine Society guidelines on spinal stenosis (NASS) (13). To further ensure that the population had the diagnosis and that it was at some level of severity, potential subjects had to have been offered surgery by their treating physicians.
Individuals with vascular claudication were recruited from the vascular surgery clinic and vascular lab. They were required to have a clinically performed abnormal ankle-brachial index and leg pain that limited their walking; and their treating physicians must have commented that their diagnosis is vascular claudication or peripheral artery disease. This meets modern criteria for vascular claudication (9). Asymptomatic volunteers were recruited from the surrounding communities through postings in the community and were accepted on a consecutive basis. All subjects underwent ankle-brachial index testing by the investigators and were examined by two study physicians. Subjects were paid $100 for participation in this part of the study.
Exclusion Criteria
After identifying potential candidates through the use of medical records, potential subjects were contacted and rigorously screened over the phone. All potential subjects with prior back surgery, known peripheral neuropathy, or previous significant lower limb nerve injury were excluded. Subjects who consumed twelve or more alcoholic drinks per week were excluded, as this would indicate an increased risk of peripheral neuropathy. In addition, subjects were excluded if they had severe cardiovascular disease, poor balance, or any other condition that may have impaired ambulation to a greater degree than the claudication alone. Any subject younger than 55 or older than 90 years of age was excluded. Subjects with diabetes were excluded if they were recruited as asymptomatic volunteers or neurogenic claudication patients. We found that in our vascular clinics it was exceedingly uncommon for vascular claudication patients to not be on anticoagulation and not be diabetics; therefore we liberalized the criteria for the vascular population to allow diabetics. Potential asymptomatic subjects were excluded if they had any back or leg pain, known peripheral vascular disease or insufficiency, or if they felt limited in walking for six minutes without an assistive device for any reason. Potential subjects with neurogenic claudication were excluded if they had any known vascular disease or insufficiency, or if they could walk over two hundred yards without difficulty. Finally, potential subjects with vascular claudication were excluded if they had back pain or known spinal stenosis, or if they could walk over two hundred yards without difficulty. Potential subjects who met eligibility criteria were enrolled consecutively and scheduled for participation. Informed consent was obtained from each subject under an Institutional Review Board approved protocol, and all subjects were compensated for their participation in the study.
Measures
Subjects completed a comprehensive questionnaire that included measures related to pain, activity, and disability. Subjects were also asked questions pertaining to their medical history and exercise habits.
Ankle-brachial index blood pressure measures were performed by the research team on all subjects. An ankle-brachial index of less than 0.90 was used as cutoff. This measure is 95% sensitive and 99% specific for angiographically diagnosed peripheral artery disease (14). The vascular subjects were required to have abnormal ankle-brachial index, while the asymptomatic and neurogenic claudication groups were required to have normal ankle-brachial indexes. All subjects underwent extensive history and physical examinations by faculty level neurosurgeons and vascular surgeons, who were masked to any previous physician's diagnosis or to any previous test results.
Visual Analog Scale (VAS)
In order to assess pain levels, a visual analog scale was used. Subjects were asked to rate their best, worst, and average pain levels during a typical week by placing a mark on a 10 cm line with 0 cm corresponding to no pain and 10 cm corresponding to the most extreme pain. It has been established that using a visual analog scale is an adequate and reliable way to assess an individual's level of pain (15). The data (including all other measures) from any asymptomatic subject reporting an average pain level of ≥ 5 on the visual analog scale were not included in the final analyses as these subjects could no longer be considered “asymptomatic” for the purposes of this study.
Quebec Back Pain Disability Scale (Quebec)
Many individuals with neurogenic claudication due to lumbar spinal stenosis also suffer from low back pain (16); therefore subjects were required to complete the Quebec Back Pain Disability Scale (17). Subjects rated their ability to perform twenty common tasks such as opening doors or carrying groceries by using a scale of 0-5 with 0 corresponding to “not difficult at all” and 5 corresponding to “unable to do”. A high score was indicative of a high level of disability. This scale is specific for persons with low back pain and therefore it was not used as the primary measure for assessing functional impairment among all three subject pools.
Short Form 36 Physical Functioning Scale (SF-36 Physical Functioning)
The Short Form 36 is a comprehensive questionnaire that was originally developed to gain insight into the patient's point of view regarding their medical outcomes (18). Eight sub-scores can be derived from the SF-36; however, only the physical functioning score was used for this particular study. The SF-36 Physical Functioning scale measures functional impairment on a scale of 0-100. Scoring is inverted relative to the Quebec as a high score corresponds to less functional impairment and a score of 0 corresponds to the most functional impairment. The SF-36 Physical Functioning scale has proven to be an effective measure in determining mobility disability (19). The SF-36 Physical Functioning scale is not specific for a particular disease process and therefore it was suitable for assessing functional impairment across all three subject pools.
Maximum Ambulation Distance
Subjects were also asked to estimate the maximum distance they were able to walk before having to stop due to leg pain or fatigue. This estimation was given in feet and later converted to meters. Although it has previously been shown that individuals with lumbar spinal stenosis tend to underestimate their maximum walking distance (20), this clinically relevant measure was still found to be useful in examining correlations with the other variables.
Tampa Scale for Kinesiophobia (Tampa)
In order to assess the psychometric properties of pain-related fear and activity avoidance in the subjects, the 13 item version of Tampa Scale for Kinesiophobia was employed (21). Subjects answered questions regarding their attitude toward function, pain, and exercise by using a numerical score of 1-4 with 1 corresponding to “strongly disagree” and 4 corresponding to “strongly agree”. Five of the questions were added to obtain a score for somatic focus i.e. fear of movement/(re)injury. The sum of the remaining eight questions represented a score for activity avoidance. A high score was indicative of a high level of fear or avoidance. This two dimensional model has previously been shown to be effective in evaluating fear and avoidance tendencies in persons with a variety of conditions that involve chronic pain including fibromyalgia and lower back pain (22). A total score (somatic focus sub-score + activity avoidance sub-score) was also obtained and used in the data analysis. Data from subjects who left more than one question per subsection blank were discarded. A mean imputation was used to fill in missing values if no more than one value was missing per subsection.
Center for Epidemiological Studies Depression (CES-D) Scale
Subjects were asked to fill out the Center for Epidemiological Studies Depression Scale (CES-D) (23). Fear-avoidance tendencies are inherently psychological phenomena based on the misguided belief that physical activity will cause (re)injury or further aggravate pain. Due to the fact that the current study is examining cognitive variables related to pain, it is important to explore any potential correlations between fear-avoidance beliefs and depression levels in the study participants. Subjects were asked to rate how often they experienced various symptoms commonly associated with depression such as poor appetite and poor mood using a scale of 0-3 with 0 corresponding to rarely/none of the time and 3 corresponding to most/all of the time. Four questions on the CES-D scale refer to positive moods and those scores were inverted. A higher score was indicative of a higher level of depression. The CES-D scale has been shown to have strong predictive validity and is an effective tool in assessing depression (24,25).
Data analysis
SPSS version 18.0.0 (SPSS Inc. Chicago, Illinois) software was utilized for the purpose of statistical analysis. A one-way analysis of variance (ANOVA) was used to assess differences in the means of numerous variables across the three groups including total Tampa scores and SF-36 Physical Functioning scores. Pearson's correlation analysis was used to determine the strength of any relationships among the variables. A p-value of less than .05 (two tailed) indicated statistical significance. Finally, a standard multiple regression model was used to assess the strength of selected variables in predicting the variance in the total score from the Tampa, the somatic focus sub-score, and the activity avoidance sub-score.
Results
Subject Demographics and Characteristics
166 potential spine and 123 potential vascular subjects were identified and offered participation. Of these, 71 potential spine and 65 potential vascular subjects declined to participate, and 38 spine and 35 vascular agreed but were excluded based on eligibility screening. 57 spine and 23 vascular subjects fully qualified and were accepted into the study. However, 12 spine and 6 vascular subjects withdrew from the study prior to completion, and 15 subjects left more than one answer blank on one or both Tampa subsections. The final neurogenic claudication pool was composed of 35 individuals; the vascular claudication pool was composed of 12 individuals. To these a pool of 20 asymptomatic subjects was added.
ANOVA showed differences in age across the three groups as approaching significance level (p = 0.049), 64.3, 69.2, and 61.8 years respectively for the neurogenic claudication, vascular claudication, and asymptomatic groups. Tukey post-hoc analysis showed a difference in age between the asymptomatic and vascular groups at the p=.038 level before a Bonferroni correction. Multiple pairwise comparisons using a Bonferroni correction where p < 0.0167 (0.05/3) was considered statistically significant did not detect significant difference in age between each pair of the groups. Correlation analysis did not find significant association between age and Tampa Scale scores, p > 0.05.
There was a slightly higher percentage of male participants in both symptomatic pools when compared to the asymptomatic pool. Subjects were asked about their exercise habits, and 56.7% of all subjects reported exercising at least once per week. As expected, the vascular group had the highest percentage of persons reporting cigarette usage within the past five years. All 12 vascular subjects reported having high blood pressure while only 20% of the asymptomatic control group reported high blood pressure. The majority of subjects were residents of the local region during the time of the study and represented a spectrum of socioeconomic and education levels. See table 1 for full subject demographics
Table 1.
Subject Demographics and Characteristics
| Neurogenic Claudication (n= 39) | Vascular Claudication (n= 15) | Asymptomatic (n= 28) | |
|---|---|---|---|
| Male (n, %) | 23 (59 %) | 10 (66.7 %) | 12 (42.9%) |
| Female (n, %) | 16 (41 %) | 5 (33.3 %) | 16 (57.1%) |
| Mean age (years, SD) | 64.67 (8.63) | 69.53 (8.41) | 61 (6.08) |
| Age Range (years) | 55 - 85 | 57 - 88 | 55 - 77 |
| Exercise every week (n, %) | 19 (48.7 %) | 11 (73.3 %) | 17 (60.7 %) |
| Smoker in past 5 years (n, %) | 8 (20.5 %) | 6 (40 %) | 2 (7.1 %) |
| Diabetes (n, %) | 0 (0 %) | 4 (26.7 %) | 0 (0 %) |
| High blood pressure (n, %) | 19 (48.7%) | 15 (100 %) | 7 (25 %) |
Fear and Avoidance of Movement
Two factors were derived from the Tampa for each participant: somatic focus corresponding to fear of movement/(re)injury and activity avoidance. A total score was also obtained. A one-way between-group analysis of variance was performed to assess the impact of neurogenic and vascular claudication on pain-related fear and activity avoidance as compared to an asymptomatic control population. A significant difference among the groups with a large effect size was observed for activity avoidance [F(2,66)=18.58, η2=.37, p<.001], somatic focus [F(2,66)=8.82, η2=.22, p<.001], and total score [F(2,66)=21.55, η2=.40, p<.001].
Tukey post hoc comparisons were used to further explore these relationships. The somatic focus score for patients with neurogenic claudication (M=11.56, SD=3.68) was significantly higher than the score for asymptomatic volunteers (M=7.65, SD=3.05, p<.001). This score for patients with vascular claudication (M=10.85, SD=2.82) differed significantly from the asymptomatic group at p<.05, but did not differ significantly from the neurogenic claudication group. When assessing the activity avoidance score, we found that the score for patients with neurogenic claudication (M=30.22, SD=4.49) was significantly higher than the score of the vascular group (M=15.33, SD=4.27, p=.003) as well as the asymptomatic group (M=13.20, SD=3.87, p<.001). The mean activity avoidance score was not found to differ significantly between the vascular and asymptomatic groups. We found the neurogenic group's total Tampa score (M=30.95, SD=7.22) to also be higher than that of both the asymptomatic group's score (M=18.57, SD=6.08, p<.001) and the vascular group's score (M=25.48, SD=6.30, p=.047). In addition, the score of the vascular group was significantly higher than the asymptomatic group at the p<.05 level.
Functional Impairment and Fear- Avoidance
The Quebec was used to determine if there was a correlation between fear-avoidance and functional impairment due to back pain in individuals with neurogenic claudication. Although any potential vascular and asymptomatic candidates with back pain were screened out, they were still required to complete the questionnaire. The Quebec score was positively correlated to the total Tampa score in individuals with neurogenic claudication (r=.367, p=.03). Interestingly, significant differences in Quebec scores were observed among the groups after running a one-way analysis of variance [F(2,63)=25.40, η2=.45, p<.001], suggesting that individuals with vascular claudication substituted back pain for leg pain when filling out the Quebec. The score for the neurogenic group (M=43.66, SD=22.35) was significantly higher than the asymptomatic group (M=6.26, SD=7.74, p<.01), but was not significantly higher than the vascular group (M=28.5, SD= 7.4) although it approached significance. In addition, the vascular group had a significantly higher score than the asymptomatic group at p<.05.
The SF-36 Physical Functioning scale was used as the main measure for determining mobility disability/functional impairment across the groups due to its non-specific nature. Scores from the SF-36 Physical Functioning scale was strongly correlated with total Tampa score among the subjects (r=-.598, p<.001). The negative correlation is due to the fact that a lower score corresponds to more disability on the SF-36 Physical Functioning scale which is opposite of the Quebec. Significant differences in functional impairment as measured by the SF-36 were observed across the groups [F(2,64)=30.15, η2=.49, p<.001]. The score of the neurogenic group (M=38.71, SD=21.33) was significantly lower than the asymptomatic group (M=83.95, SD=17.21, p<.001) but was not significantly lower than the vascular group (M=50.91, SD=22.90) although it approached significance. In addition, the vascular group had a significantly lower score than the asymptomatic group at p<.001.
The scores from the Quebec and SF-36 were strongly correlated among the subjects (r=-.816, p<.001). The strength of this correlation, along with the evidence that vascular claudication patients substituted back pain for leg pain when filling out the Quebec, suggests that the Quebec may be a valid tool in measuring functional impairment among groups with other disabling conditions besides low back pain.
Correlating Depression, Pain, Functional Impairment, and Fear-Avoidance
Upon utilizing a one-way analysis of variance to investigate differences in mean CES-D scores between the groups, we observed a statistically significant result [F(2,61)=3.94, η2=.12, p=.025]. When looking at the Tukey post-hoc analysis, it was determined that the significant difference in groups lies primarily between the neurogenic (M=13.03, SD=10.63) and asymptomatic groups (M=5.63, SD=4.27, p=.018). There was not a significant difference between the vascular claudication subjects (M=10.55, SD=10.32) and the other two groups.
A one-way analysis of variance was also utilized to investigate differences in average pain and self-reported maximum ambulation distances. A statistically significant result was obtained upon investigating differences in average pain levels among the groups [F(2,59)=21.90, η2=.43, p<.001]. The neurogenic group (M=4.69, SD=2.41) had a significantly higher pain score than the asymptomatic group (M=.49, SD=.91, p<.001). The vascular group (M=3.72, SD=2.54) had a significantly higher pain score than the asymptomatic group at the p=.001 level. There were no differences in average pain levels among the two symptomatic groups. We also found a statistically significant result when looking at differences in self-reported maximum ambulation distances [F(2,48)=8.78, η2=.28, p=.001]. There were no significant differences in ambulation distance between the two symptomatic groups. Distances reported by the neurogenic (M=584.12, SD=1419.02) and the vascular groups (M=82.97, SD=89.55) were both significantly lower at the p<.01 level than distances reported by the asymptomatic group (M=2138.74, SD=1606.09).
In order to gain more insight into the relationships between the numerous variables, Pearson's correlation analysis was used to explore the strength of any possible relationships among all subjects. Self-reported maximum ambulation distance was not correlated to any measures from the Tampa, although it was correlated with depression (r=-.307, p=.038), average pain (r=-.299, p=.041), and disability (r=.512, p<.001). The somatic focus score correlated with both depression (r=.557, p<.001) and disability (r=-.495, p<.001) as well as pain (r=.519, p<.001). In addition, the activity avoidance score was also correlated with both depression (r=.395, p<.001) and disability (r=-.549, p<.001) as well as pain (r=.434, p=.001). The average pain score from the visual analog scale was significantly correlated with all other variables. See table 2 for comprehensive results.
Table 2.
Relationships between fear, avoidance, depression, pain, walking distance, and global physical function for all subjects. r values given.
| Tampa Total | CES-D | Average VAS | Ambulation Distance | Somatic Focus | Activity Avoidance | SF-36 Physical Functioning | |
|---|---|---|---|---|---|---|---|
| Tampa Total | 1 | ||||||
| CES-D | .515** | 1 | |||||
| Average VAS | .461** | .498** | 1 | ||||
| Ambulation Distance | -.204 | -.267* | -.360** | 1 | |||
| Somatic Focus | .854** | .565** | .442** | -.097 | 1 | ||
| Activity Avoidance | .941** | .400** | .397** | -.240 | .628** | 1 | |
| SF-36 Physical Functioning | -.632** | -.488** | -.740** | .518** | -.540** | -.593** | 1 |
p<.05
p<.01
Contribution of Selected Variables to Variance in Fear-Avoidance Scores
A standard multiple regression model was used to determine which measures had the strongest predictive validity in determining fear-avoidance among the subjects. The three independent variables were the SF-36 Physical Functioning score, CES-D scale score, and average VAS score, while the dependent variable was the total score from the Tampa. This model explained 40.3% of the variance in the dependent variable [R=.635, R2=.403, adjusted R2=.366, F(3,52)=11.01, p<.001]. We found that disability as measured by the SF-36 Physical Functioning was the best predictor in determining the variance in the overall level of fear-avoidance i.e. the total score from the Tampa. The level of pain from the VAS did not make a significant contribution to the variance in the overall fear-avoidance score according to our model. See table 3a
Table 3a.
Contribution of predictive variables in determining variance in the total Tampa score.
| Predictive Variable | B | Std. Error | β | t | p |
|---|---|---|---|---|---|
| CES-D | .228 | .111 | .241 | 2.05 | .045 |
| “average” VAS | .430 | .567 | .141 | .920 | n.s. |
| SF-36 PS | -.113 | .045 | -.371 | -.252 | .014 |
To further explore these results, two more standard multiple regression models were constructed to elucidate the contributors to the variance in the somatic focus and activity avoidance sub-scores derived from the Tampa. The same three independent variables were used in these additional models. When looking at somatic focus, the model explained 36.2% of the variance in the dependent variable [R=.601, R2=.362, adjusted R2=.322, F(3,52)=9.251, p<.001]. Depression as measured by the CES-D scale was the only significant predictor in explaining the variance in the somatic focus score. When looking at activity avoidance, the model explained 34.3% of the variance in the dependent variable [R=.578, R2=.334, adjusted R2=.294, F(3,52)=8.21, p<.001]. Functional impairment or disability as measured by the SF-36 Physical Functioning Scale was the only significant predictor in explaining the variance in the activity avoidance score. See tables 3b and 3c
Table 3b.
Contribution of predictive variables in determining variance in the Tampa somatic focus score
| Predictive Variable | B | Std. Error | β | t | p |
|---|---|---|---|---|---|
| CES-D | .165 | .051 | .398 | 3.26 | .002 |
| “average” VAS | .266 | .212 | .299 | 1.255 | n.s. |
| SF-36 PS | -.014 | .020 | -.107 | -.697 | n.s. |
Table 3c.
Contribution of predictive variables in determining variance in the Tampa activity avoidance score
| Predictive Variable | B | Std. Error | β | t | p |
|---|---|---|---|---|---|
| CES-D | .063 | .080 | .097 | .786 | n.s. |
| “average” VAS | .163 | .337 | .078 | .485 | n.s. |
| SF-36 PS | -.099 | .032 | -.474 | -3.06 | .003 |
N.S. = not statistically significant
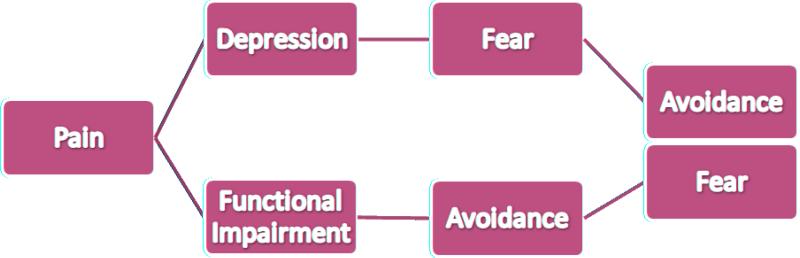
Based on these results, a schematic representation of the general progression from pain to fear-avoidance was constructed. This model is simplified and the relationships presented here are much more complex in reality. See fig.
Figure.
Schematic representation of the general progression from pain to fear-avoidance
Discussion
To our knowledge, this is the first study to elucidate fear-avoidance tendencies in persons with either neurogenic or vascular claudication. The findings from this study indicate that individuals with neurogenic claudication tend to experience a higher degree of fear of movement/(re)injury and activity avoidance than the asymptomatic control group. Individuals with neurogenic claudication also report a higher degree of activity avoidance than individuals with vascular claudication, as well as a higher total score on the Tampa Scale for Kinesiophobia; however, there were no major differences in the somatic focus score i.e. fear of movement/(re)injury between the two symptomatic groups. Individuals suffering from vascular claudication had a significantly higher somatic focus score compared to the asymptomatic group. Also, the total score from the Tampa Scale for Kinesiophobia was significantly higher in the vascular group when compared to the asymptomatic group.
Although the number of subjects in both the neurogenic claudication and asymptomatic groups was larger than the vascular claudication group, we do not believe this had a substantial impact on our results. One of the greatest strengths of the current study lies in the strict eligibility criteria that were employed when recruiting subjects; however, these stringent criteria did make recruiting vascular subjects particularly challenging. For example, potential subjects with cardiac problems were excluded from study participation, and people with peripheral arterial disease tend to have a greater risk of cardiovascular morbidity compared to the general population (26). Also, because of procedures performed in another part of the study, subjects on warfarin were excluded from the vascular population.
There are theoretical downsides to these strict criteria. Some (including the authors) may challenge the idea that a surgeon-even an academic surgeon-offering an operation is not a hard criteria for severity or certainty of diagnosis (27,28). However, the standard for publication on spinal stenosis at this time appears to be expert opinion plus radiological findings (although no specific measurement or cutoff measure is advocated) (13). The current study exceeds these criteria by using the point of an expert choosing to offer a procedure. This is not considered any kind of gold standard for diagnosis, but likely decreases the chance of a wrong diagnosis, or of stenosis that is only mildly disabling, or of including persons whose picture is clouded by other disabling conditions.
It is also possible that fear of pain actually biases stenosis patients away from the surgical inclusion criteria (e.g. afraid of a knife wound) or towards the inclusion criteria (e.g. afraid of pain enough to want someone to make it go away with surgery). To our knowledge this issue has not been addressed.
There are a variety of potential factors that may explain the differences in fear-avoidance tendencies among persons with different types of claudication. The challenge of equating the ‘severity of illness’ between the populations is not easy. Some may argue for ‘equivalent levels of pain’ or ‘equivalent risk of dying’ or ‘equivalent impact on health related quality of life’ or some other measures. In this study individuals with neurogenic claudication had to have been recommended for surgery in order to qualify for the study, while individuals with vascular claudication did not. Therefore, it is possible that our neurogenic claudication subjects were more homogenous in terms of symptom severity. On the other hand, the leg pain experienced by our vascular subjects may have occurred on a wide spectrum ranging from mild cramping in the calves to critical ischemia. This is in line with the notion that persons with peripheral vascular disease may experience a wide range of leg symptoms (29). Persons with neurogenic claudication did have slightly elevated pain scores compared to the vascular claudication group, but these scores were not high enough to be considered significantly different on a clinical basis. Therefore, it is probably overly simplistic to attribute differences in Tampa scores to differing levels of leg pain. It is likely that some other factor(s) is contributing to the differences that we have observed between the two symptomatic populations.
An intriguing possibility has to do with the notion of functional impairment. Most individuals suffering from neurogenic claudication due to lumbar spinal stenosis also have some degree of disabling lower back pain while none of our vascular subjects experienced this. Low back pain is very common among lumbar spinal stenosis patients with an estimated 87% suffering from this kind of pain (7). Of great importance, the average score on the SF-36 Physical Functioning scale which measures mobility disability was higher at a value approaching statistical significance in the neurogenic claudication patients when compared to the vascular group. This may explain why the activity avoidance score as well as the total Tampa of our neurogenic population was higher than the vascular population. The SF-36 Physical Functioning score correlates more strongly with the activity avoidance score than the somatic focus score, although both correlations are significant. Disability has also been correlated to depression as measured by the CES-D scale (30). Our own data has shown the CES-D scale and SF-36 Physical Functioning scores correlate at a statistically significant level among our subjects.
A 2006 study has indicated that up to 20% of individuals with lumbar spinal stenosis suffer from symptoms of depression (31). In contrast, recent estimates by the CDC indicate that the prevalence of depression among the general population is 6.6% (32). According to this data, patients with lumbar spinal stenosis are over three times more likely to suffer from depression than someone from the general population. It has been shown that depression can be predictive of fear of movement/(re)injury, although pain catastrophizing may have a greater predictive value (33). At the same time, a link between vascular disease and adverse mood states has been shown as patients with atypical leg symptoms and pain at rest do appear to be at a greater risk for developing anxiety and depression (34). Our own data showed that on average, individuals with neurogenic claudication reported significantly higher CES-D scores when compared to an asymptomatic control population, although the CES-D scores were not significantly different between the neurogenic and vascular groups. We see a similar pattern when looking at the somatic focus scores between the groups. The somatic focus component of the fear-avoidance model correlates more strongly with the CES-D scale score than does the activity avoidance component.
Among people with low back pain, it has been shown that pain catastrophizing and kinesiophobia are more correlated with the level of disability than the level of pain itself (35). Based on our data, we can also conclude that the level of pain as measured by the visual analog scale does not make a direct contribution to fear-avoidance in individuals with claudication resulting from either lumbar spinal stenosis or peripheral vascular disease. We have shown that pain is strongly correlated to both mobility disability and depression, and may therefore indirectly lead to fear-avoidance.
These findings, along with additional research, may lead clinicians to predict the success of an exercise rehabilitation program in persons with neurogenic or vascular claudication based on individual Tampa Scale for Kinesiophobia scores. Counseling should be used in conjunction with exercise rehabilitation in order to address any psychological issues in persons reporting high scores with the goal of increased participation in daily activities. If these often overlooked psychometric factors are properly addressed, individuals suffering from claudication of either vascular or neurogenic origin could see better outcomes from more optimized therapy.
Conclusion
Persons with neurogenic claudication appear to have increased fear and avoidance of pain compared to age-matched controls, and even in comparison to persons with vascular claudication; a similar symptom caused by a different pathology. Just as fear and avoidance have become important factors in rehabilitation of working-aged persons with mechanical back pain, an understanding of their etiology and encouragement to confront the fear and inactivity may prove important to the older population who suffer from spinal stenosis.
Acknowledgements
“The project described was supported by Award Number R01HD059259 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bischoff HA, Roos EM. Effectiveness and safety for strengthening, aerobic, and coordination exercises for patients with osteoarthritis. Curr Opin Rheumatol. 2003;15:141–144. doi: 10.1097/00002281-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Herring MP, O'Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170:321–333. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 3.Middelkoop MV, Rubinstein SM, Verhagen AP, Ostelo RW, Koes BW, van Tulder MW. Exercise therapy for chronic nonspecific low-back pain. Best Prac and Res Clin Rhematol. 2010;24:193–204. doi: 10.1016/j.berh.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain: A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 5.Goren A, Yildez N, Topuz O, Ardic F. Efficacy of exercise and ultrasound in patients with lumbar spinal stenosis: a prospective randomized control study. Clin Rehabil. 2010;24:623–631. doi: 10.1177/0269215510367539. [DOI] [PubMed] [Google Scholar]
- 6.Lurie JD, Birkmeyer NJ, Weinstein JN. Rates of advanced spinal imaging and spine surgery. Spine. 2003;28:616–620. doi: 10.1097/01.BRS.0000049927.37696.DC. [DOI] [PubMed] [Google Scholar]
- 7.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Porter Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 10.Stoffers H, Rinkens P, Kester A, Kasier V, Knottnerus JA. The prevalence of asymptomatic and unrecognized peripheral arterial occlusive disease. Int Journal of Epidemiol. 1996;25:282–290. doi: 10.1093/ije/25.2.282. [DOI] [PubMed] [Google Scholar]
- 11.Elfving B, Andersson T, Grooten W. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother Res Int. 2007;12:14–24. doi: 10.1002/pri.355. [DOI] [PubMed] [Google Scholar]
- 12.Werneke MW, Hart DL, George SZ, Stratford PW, Matheson JW, Reyes A. Clinical outcomes for patients classified by fear-avoidance beliefs and centralized phenomenon. Arch Phys Med Rehabil. 2009;90:768–777. doi: 10.1016/j.apmr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 13.North American Spine Society . Evidence Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis. North American Spine Society; Burr Ridge, IL: 2007. [Google Scholar]
- 14.Bernstein EF. Current status of noninvasive tests in the diagnosis of peripheral arterial disease. Surg Clin North Am. 1982;62:473–487. doi: 10.1016/s0039-6109(16)42739-8. [DOI] [PubMed] [Google Scholar]
- 15.Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anesthesia. 1976;31:1191–1198. doi: 10.1111/j.1365-2044.1976.tb11971.x. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez JA, Hardy RH., Jr Lumbar spine stenosis: a common cause of back and leg pain. Am Fam Physician. 1998;57:1825–1834. [PubMed] [Google Scholar]
- 17.Kopec JA, Esdaile JM, Abrahamoeicz M, et al. The Quebec Back Pain Disability Scale Measurement Properties. Spine. 1995;20:341–352. doi: 10.1097/00007632-199502000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survery (SF-36) I. Conceptual Framework and Item Selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Syddall HE, Martin HJ, Harwood RH, Cooper C, Aihie Sayer A. The SF-36: A simple, effective measure of mobility-disability for epidemiological studies. J Nutr Health Aging. 2009;13:57–62. doi: 10.1007/s12603-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomkins CC, Battie MC. Validity and reproducibility of self-report measures of walking capacity in lumbar spinal stenosis. Spine. 2010;35:2097–2102. doi: 10.1097/BRS.0b013e3181f5e13b. [DOI] [PubMed] [Google Scholar]
- 21.Clark ME, Kori SH, Brockel J. Kinesiophobia and chronic pain: psychometric characteristics and factor analysis of the Tampa Scale. Presented at 15th Annual Scientific Meeting of the American Pain Society, Abstracts. 1996;15:77. [Google Scholar]
- 22.Roelofs J, Goubert L, Peters M, Vlaeyen J, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain. 2004;8:495–502. doi: 10.1016/j.ejpain.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Radloff LS. The CES-D Scale: A self report depression scale for research in the general population. App Psych Measurment. 1977;1:385–401. [Google Scholar]
- 24.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the center for epidemiological studies depression (CES-D) scale. J of Clin Psych. 2006;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Turk DC, Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behav Res Ther. 1994;32:9–16. doi: 10.1016/0005-7967(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. New Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 27.Haig AJ. The need versus the choice for treatment of spinal disorders: Does our science help us to differentiate? Spine J. 2006;6:355–356. doi: 10.1016/j.spinee.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 28.Haig AJ, Tomkins CC. Diagnosis and Management of Lumbar Spinal Stenosis. JAMA. 2010;303:71–72. doi: 10.1001/jama.2009.1946. [DOI] [PubMed] [Google Scholar]
- 29.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 30.Alschuler KN, Theisen-Goodvich ME, Haig AJ, Geisser ME. A comparison of the relationship between depression, perceived disability, and physical performance among persons with chronic pain. Eur J Pain. 2008;12:757–764. doi: 10.1016/j.ejpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Sinikalio S, Aalto T, Airaksinen O, et al. Depression and associated factors in patients with lumbar spinal stenosis. Disab and Rehab. 2008;28:415–422. doi: 10.1080/09638280500192462. [DOI] [PubMed] [Google Scholar]
- 32.Center for Disease Control and Prevention Epidemiological Study Current Depression Among Adults, United States 2006 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1229–1235. [PubMed] [Google Scholar]
- 33.Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 34.Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14:297–304. doi: 10.1177/1358863X09104658. [DOI] [PubMed] [Google Scholar]
- 35.Thomas EN, Pers YM, Mercier G, et al. The importance of fear, beliefs, catastrophizing, and kinesiophobia in chronic low back rehabilitation. Ann of phys med and rehab. 2010;53:3. doi: 10.1016/j.rehab.2009.11.002. [DOI] [PubMed] [Google Scholar]