Abstract
Following acute tissue injury action potentials may be initiated in afferent processes terminating in the dorsal horn of the spinal cord that are propagated back out to the periphery, a process referred to as a dorsal root reflex (DRR). The DRR is dependent on the activation of GABAA receptors. The prevailing hypothesis is that DRR is due to a depolarizing shift in the chloride equilibrium potential (ECl) following an injury-induced activation of the Na+-K+-Cl−-cotransporter. Because inflammatory mediators (IM), such as prostaglandin E2 are also released in the spinal cord following tissue injury, as well as evidence that ECl is already depolarized in primary afferents, an alternative hypothesis is that an IM-induced increase in GABAA receptor mediated current (IGABA) could underlie the injury-induced increase in DRR. To test this hypothesis, we explored the impact of IM (prostaglandin E2 (1μM), bradykinin (10 μM), and histamine (1μM)) on IGABA in dissociated rat dorsal root ganglion (DRG) neurons with standard whole cell patch clamp techniques. IM potentiated IGABA in a subpopulation of medium to large diameter capsaicin insensitive DRG neurons. This effect was dependent on the concentration of GABA, manifest only at low concentrations (< 10μM). THIP evoked current were also potentiated by IM and GABA (1 μM) induced tonic currents enhanced by IM were resistant to gabazine (20 μM). The present data are consistent with the hypothesis that an acute increase in IGABA contributes to the emergence of injury-induced DRR.
Keywords: primary afferent, nociceptor, inflammation, voltage-clamp, excitation-inhibition balance
Introduction
It has long been appreciated that afferent volleys into the spinal cord are followed by a depolarization of the central afferent terminals, a phenomenon referred to as primary afferent depolarization (PAD) [7]. Subsequent data indicated that PAD is due to GABAA receptor activation (for review [23, 29]), that is associated with membrane depolarization in primary afferent neurons because, unlike neurons in the central nervous system, the Cl− equilibrium potential (ECl) in primary afferents is maintained at a relatively elevated level into adulthood [2, 25]. The elevated ECl appears to be due to the fact the Na+-K+-Cl−-cotransporter, NKCC1 expression is maintained in sensory neurons with no compensatory increase in the K+-Cl−-cotransporter, KCC2 [20].
Following tissue injury or intense noxious stimulation, afferent volleys that would normally result in a PAD can actually initiate antidromically propagated action potentials, a phenomenon referred to as a dorsal root reflexes (DRR) [29]. The DRR is also dependent on GABAA receptor activation [21] and is blocked by the NKCC1 antagonist bumetanide [27]. Furthermore, tissue injury is associated with first an increase in the phosphorylation of NKCC1 and then an increase in the translocation of this co-transporter to the membrane [10]. It has therefore been hypothesized that the emergence of the DRR is due to an increase in NKCC1 activity resulting in a depolarizing shift in ECl [5, 19]. The shift in ECl would enable the GABA-induced depolarization to drive the membrane potential above the action potential threshold, resulting in a DRR.
Recent evidence, however, suggests that ECl for many sensory neurons is already above the action potential threshold [22]. Furthermore, there is evidence that inflammatory mediators (IM) such as prostaglandin E2 are released in the spinal cord following noxious stimulation [18], as well as evidence that GABAA receptors may be dynamically regulated via an increase in kinase activity [14]. Together, these observations suggested an alternative to the NKCC1 hypothesis, which is that the emergence of the DRR is due to an IM-induced increase in GABAA current. The present study was designed to test this hypothesis. Isolated DRG neurons were studied with whole cell patch clamp techniques to record GABAA currents before and after the application of a combination of inflammatory mediators.
Methods
Adult (180-280g) male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used for all experiments which were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines as well as guidelines established by the International Association of the Study of Pain for the use of laboratory animals in research. L4-5 DRG were harvested from the rats deeply anesthetize with a cocktail containing ketamine (55mg/kg), xylazine (5.5 mg/kg), and acepromazine (1.1mg.kg), enzymatically treated, mechanically dissociated, and plated on laminin and ornithine-coated (Life Technologies, Grand Island, NY) cover-slips as previously described [17]. After plating for two hours, cells were flooded with Minimal Essential Media (MEM) containing 10% fetal bovine serum, 1000 units of penicillin-streptomycin, and 1x MEM vitamins (Life Technologies) and were placed in a CO2 (3%) incubator at 37°C for 24 hours prior to study.
Cultured DRG neurons were used in the present experiment because 1) we wished to be able to detect the impact of IM on both low and high affinity GABA receptors and 2) recent data indicates that there is an increase in high affinity GABA receptors in DRG neurons with time in culture [15].
Whole-cell patch-clamp recordings were performed with a HEKA EPC10 amplifier (HEKA Eletronik GmbH, Lambrecht, Germany). Data were acquired at 10 kHz and filtered at 2 kHz unless otherwise noted. Borosilicate glass (WPI, Sarasota, FL) electrodes were 2-3 MΩ when filled with the following solution (mM): CsCl 140, MgCl2 1, EGTA 11, HEPES 10, Mg-ATP 2, and GTP 1; pH was adjusted to 7.2 with Tris-base and osmolality was adjusted to 310 mOsm with sucrose. The bath solution contained the following (in mM): NaCl 130, KCl 3, CaCl2 2.5, MgCl2 0.6, HEPES 10, and glucose 10; pH was adjusted to 7.4 with Tris-base and osmolality was adjusted to 320 mOsm with sucrose. All reagents used for the preparation of recording solutions were obtained from Sigma-Aldrich (St Louis MO).
Neurons were held at −60 mV. Currents were evoked by 3 or 60 seconds of focal application of GABA or GABAA receptor agonists. After establishing whole cell access and compensating capacitative transients with amplifier circuitry, a stable baseline evoked current was established with at least a 3min inter-test-interval. Following establishment of stable GABA or THIP evoked currents, current amplitude was determined during and after 5 min application of IM. Capsaicin (500 nM) was applied at the end of experiment to help identify the phenotype of the neuron studied. A neuron was considered responsive to capsaicin if current evoked was greater than 3 times the maximal deflection in baseline observed with vehicle application.
A local superfusion system (Fast Step, Warner Instruments, Hamden CT) was used for drug delivery. Drugs were diluted to final concentrations in bath solution from stock solutions at least 1000 times greater than the highest concentration employed. Drugs used were GABA, δ subunit containing GABAA receptor agonist (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP) and allosteric modulator tetrahydrodeoxycorticosterone (THDOC), and GABAA receptor antagonists gabazine (SR95531) and bicuculline. Inflammatory mediators consisted of a combination of histamine (1μM, dissolved in water, 100mM stock concentration), bradykinin (10 μM, dissolved in 1% acetic acid, 23.58 mM stock concentration), and prostaglandin E2 (1 μM, dissolved in 100% ethanol, 10 mM stock concentration). All drugs were obtained from Sigma-Aldrich and prepared as stock solution stored −20 then diluted in bath solution before use.
Data are expressed as mean ± SEM. Differences between groups were assessed with a one-way ANOVA or a Students t-test, where p < 0.05 was considered statistically significant.
Results
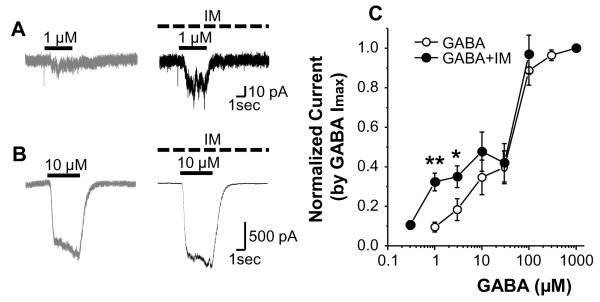
In initial experiments with GABA concentrations >60 μM (n = 12), there was no detectable change in IGABA in the presence of IM. However, in 7 neurons tested with concentrations of GABA < 10 μM, there was an increase in IGABA in the presence of IM. This suggested the impact of IM was dependent on the concentration of GABA used. To address this possibility, GABA concentration-response data were collected before, and during a 5 minute superfusion with IM. In 6 of 22 neurons tested, current evoked by 1 and 3 μM of GABA was significantly increased in the presence of IM from 2.5 ± 1.1 pA/pF to 9.9 ± 2.9 pA/pF (p < 0.01), and from 5.2 ± 1.7 pA/pF to 9.6 ± 1.2 pA/pF (p<0.05), respectively. Interestingly, a response (3.19 ± 1.59 pA/pF) to a previously ineffective concentration of GABA (0.3 μM) was detected when it was applied with IM. In contrast, there was no IM-induced increase in current at concentrations of GABA 10 μM (Fig 1B) or higher (Fig. 1C). This increase in current persisted following wash of IM, such that IGABA was still 89 ± 16.8 % greater than baseline at 5 minutes after IM wash. The absence of an increase in total IGABA in combination with an increase in current at low concentrations of GABA suggested IM was increasing the potency of GABA at a subpopulation of presumably high affinity GABA receptors.
Figure 1.
Impact of inflammatory mediators (IM) on GABA-evoked currents (IGABA). Representitive examples of IGABA evoked before (gray traces) and after (black traces) IM application in response to 1 μM (A) and 10 μM (B) GABA, respectively. GABA and IM application are indicated by solid and dashed bars, respectively. C: Concentration-response relationships for GABA before (open circle) and 5 minutes after (filled circle) IM application (n = 6). Data from each neuron were normalized with respect to maximal inward current (Imax). * is p < 0.05 and ** is p < 0.01.
In attempt to identify the putative phenotype of the neurons responsive to IM (i.e., in which IM induced an increase in IGABA), we compared cell body size and sensitivity to capsaicin of IM responders and non responders. The average membrane capacitance of the IM responders was 42.2 ± 3.8 pF, while that of non-responders was 25.2 ± 2.1 pF (p < 0.05). While 5 of 16 non-responders were responsive to capsaicin (500 nM), none of the 6 responders tested were responsive to capsaicin. These results suggest that IM responders may represent a subpopulation of non-nociceptive afferents.
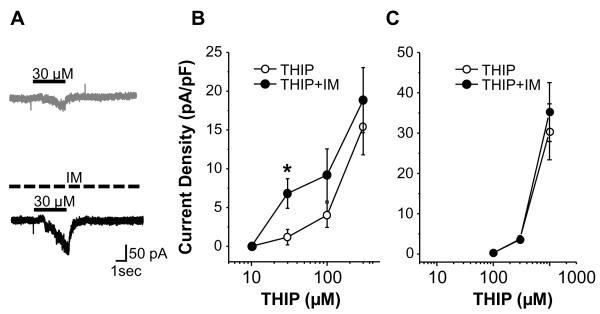
We recently obtained evidence indicating that δ-subunit containing receptors contribute to the subpopulation of high affinity GABAA receptors in DRG neurons [15]. The δ-subunit preferring agonist THIP was therefore used to determine whether δ-subunit containing receptors may be the target of IM actions. In a subpopulation of neurons tested (8 of 20), the currents evoked by 30μM of THIP were potentiated by IM (p < 0.05, n = 8, Fig. 2A, B). Neurons responsive to IM were all responsive to THIP at 30 μM or lower, while those unresponsive to IM were an order of magnitude less sensitive to THIP (Fig 2C). As with the results of the GABA current experiments, membrane capacitance of IM responsive neurons 81.9 ± 3.2 pF was significantly larger than that of neurons unresponsive to IM (28.8 ± 2.0 pF, p < 0.01). Furthermore, none of the IM responsive neurons tested with THIP were capsaicin sensitive while more than half of the IM unresponsive neurons tested (11 of 17) were capsaicin sensitive.
Figure 2.
Impact of inflammatory mediators (IM) on THIP evoked currents (ITHIP). A: Representitive example of current evoked by 30 μM of THIP in a DRG neuron with a large cell body diameter (65pF) that was capsaicin insensitive before (gray trace) and 5 minutes after (black trace) IM application. Solid and dashed bars indicate THIP and IM application, respectively. B: Concentration-response relationships for THIP before (open circle) and 5 minutes after (filled circle) IM application in medium to large diameter (>45 pF) capsaicin insensitive neurons (n = 8). C: There was no detectable influence of IM on THIP evoked currents in small diameter neurons at least half of which were capsaicin sensitive (n=17). * is p < 0.05.
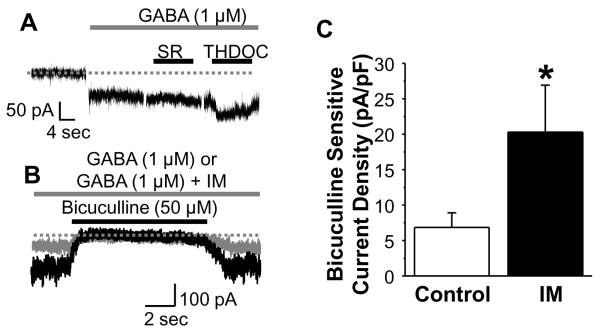
Three additional experiments were performed to further test the contribution of δ subunit containing receptors to the current potentiated by IM. Based on the biophysical and pharmacological properties of δ subunit containing receptors [8, 24, 31], we first determined whether the current potentiated by IM exhibited desensitization during prolonged (1 minute) GABA application. Second, we compared the impact of SR-95531 (20 μM) and bicuculline (50 μM) on tonic currents potentiated by IM. Finally, we assessed the impact of THDOC on IM potentiated tonic currents. Results of these experiments indicated that IM potentiates a tonic current evoked with 1 μM GABA that is resistant to SR-95531 (20 μM) and potentiated by THDOC (Fig 3A). These tonic currents are completely blocked by bicuculline (Fig 3B). To quantify the IM-induced increase in tonic current, we assessed the bicuculline sensitive current before and during application of IM. Pooled data (Fig 3C) indicate that the IM-induced increase in tonic current is also statistically significant (p < 0.05, n = 5).
Figure 3.
Pharmacology of inflammatory mediator (IM) potentiated high affinity tonic GABAA current in DRG neurons. A: Tonic GABA current was evoked with a prolonged application of GABA (1 μM, gray bar). This current was not blocked by SR95531 (20 μM), but was potentiated by THDOC (0.1 μM) which were applied as indicated by black horizontal bars.. B: Tonic currents evoked with 1 μM GABA were blocked by bicuculline (50 μM) whether (black trace) or not (gray trace) currents were potentiated by IM. Dotted lines in A and B indicate 0 current. C: Pooled data from 8 DRG neurons with a membrane capacitance ≥ 45 pF, in which the bicuculline sensitive current was used to quantify the magnitude of the tonic current evoked before (control) and after (IM) the application of IM. * is p < 0.05
Discussion
The present study was designed to test the hypothesis that the emergence of the DRR is due to an IM-induced increase in GABAA current. Consistent with our hypothesis, IM-induced an increase in IGABA in a subpopulation of neurons studied. This increase persisted after wash of IM and was associated with a partial shift in the GABA concentration response curve rather than an increase in total current. The IM sensitive current had biophysical and pharmacological properties of an extrasynaptic high affinity δ subunit containing receptor [8, 24, 31].
There is evidence that bradykinin activates a Ca2+-dependent Cl− current in DRG neurons [16] which was recently identified as being carried by TMEM16A, also known as ANO1 [16]. There is also evidence that inflammatory mediators, including bradykinin can drive an increase in intracellular Ca2+ in sensory neurons [28]. However, we have no evidence that this current contributed to actions of IM in the present study. That is, the only IM-induced increase in current observed, was observed in the presence of GABA, with no detectable increase in holding current in the absence of GABA. Furthermore, both the GABA evoked current and IM potentiated current were completely blocked by the GABAA receptor antagonist, bicuculline. Why we were not able to detect an IM-induced increase in Ca2+-dependent Cl− current in the present study is not entirely clear, but may be due to the fact that this outwardly rectifying current is minimized at −60 mV, the holding potential used for the study of GABA currents in the present experiments.
The selective influence of IM on putative non-nociceptive afferents was striking for several reasons. First, we have evidence that δ-subunit containing currents are dramatically increased under the culture conditions used in the present study, even in putative nociceptive afferents [15]. Second, it has been demonstrated that IM can drive the modulation of a number of ion channels in putative nociceptive DRG neurons (for review [12]), indicating that receptors for these mediators are both present and functional in putative nociceptive DRG neurons in culture. Third, the focus of prior studies of injury-induced changes in GABAA signaling [3], was to identify mechanisms that enable GABA to contribute to the increase in pain associated with acute tissue injury and/or inflammation. Thus, we anticipated that the inflammatory mediator-induced increase in GABAA current would be manifest in putative nociceptive afferents.
Because there is evidence for a subpopulation of nociceptive afferents with rapidly conducting axons [30], it is possible that the subpopulation of IM sensitive afferents observed in the present study are nociceptive and that the IM-induced increase in GABAA currents contributes to emergence of a pronociceptive role for GABA following tissue injury. However, if, as suggested by their phenotype, this subpopulation is non-nociceptive, the functional significance of an IM-induced increase in GABA current in this subpopulation is more complicated. On one hand, non-nociceptive afferents have TTX-sensitive Na+ currents subject to steady-state inactivation. An increase in GABAA mediated current in these neurons would result in a membrane depolarization-induced inactivation of channels necessary for action potential propagation, and thus, the inhibition of non-nociceptive input. This may, in turn, result in the facilitation of nociceptive input normally inhibited by activity in non-nociceptive afferents [11]. On the other hand, GABAA mediated depolarization of these neurons may be sufficient to facilitate low threshold afferent input to the superficial dorsal horn [4] which may attenuate nociceptive input into the dorsal horn. Additional studies would be needed to distinguish between these possibilities.
The mechanism(s) underlying the action of IM remain to be elucidated. However, in contrast to the increase in GABA potency at receptors already present on the membrane suggested by the results of the present study, an increase in receptor density has been far more commonly reported. The increase in receptor density appears to be due to changes in receptor trafficking, which has been shown to be mediated by tyrosine kinase [13], protein kinase C [1], and calcium calmodulin-dependent protein kinase II [6]. Thus, available evidence argues against second messenger pathways engaging these kinases as the mechanisms underlying the actions of IM in the present study. There is evidence from retina amicrine cells that protein kinase A-dependent (PKA) phosphorylation of GABAA receptors results in an increase in GABA potency with no change in efficacy [9], as was observed in the present study, although the GABAA receptors present in amicrine cells appear to have a relatively low affinity for GABA (i.e., ~70 μM). That said, it has long been appreciated that PKA is a major signaling pathway underlying the actions of inflammatory mediators including PGE2 [26].
Conclusions
The IM-induced potentiation of high affinity tonic GABAA currents in a subpopulation of putative non-nociceptive afferents suggests that the shift in the balance of nociceptive and non-nociceptive input to the CNS associated with tissue injury may be associated with changes in non-nociceptive input in addition to the well described increase in nociceptive input. This observation also suggests that there is subcellular compartmentalization of second messenger signaling cascades in primary afferents that it retained even in the isolated afferent cell body. Finally, given preliminary evidence that persistent inflammation is associated with an increase in GABAA current that is not due to a shift in GABA potency, but an increase in GABA functional receptors on the membrane [32], the acute IM-induced modulation of GABA currents suggest that there are time dependent changes in the regulation of GABAA receptors in sensory neurons following the induction of inflammation.
Highlights.
GABAA excites primary afferents in the presence of inflammation.
GABAA receptors are dynamically regulated in neurons in the central nervous system.
Inflammatory mediators induced an increase in GABA potency at high affinity receptors.
This is the first demonstration of the dynamic regulation of GABAA currents in primary afferents.
Acknowledgements
This work was supported by a grant from the National Institutes of Health: grant NS063010 (MSG)
Abbreviations
- DRG
dorsal root ganglion
- DRR
dorsal root reflex
- ECl
Cl− equilibrium potential
- GABA
γ-aminobutyric acid
- IGABA
GABAA current
- IM
inflammatory mediators
- KCC2
K+-Cl−-cotransporter
- NKCC1
Na+-K+-Cl−-cotransporter
- PAD
primary afferent depolarization
- SR-95531
gabazine
- THDOC
tetrahydrodeoxycorticosterone
- THIP
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–351. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- [6].Churn SB, DeLorenzo RJ. Modulation of GABAergic receptor binding by activation of calcium and calmodulin-dependent kinase II membrane phosphorylation. Brain Res. 1998;809:68–76. doi: 10.1016/s0006-8993(98)00834-8. [DOI] [PubMed] [Google Scholar]
- [7].Eccles JC, Schmidt R, Willis WD. Pharmacological Studies On Presynaptic Inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- [9].Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci U S A. 1994;91:10893–10897. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Galan A, Cervero F. Painful stimuli induce in vivo phosphorylation and membrane mobilization of mouse spinal cord NKCC1 co-transporter. Neuroscience. 2005;133:245–252. doi: 10.1016/j.neuroscience.2005.02.025. [DOI] [PubMed] [Google Scholar]
- [11].Garrison DW, Foreman RD. Effects of Transcutaneous Electrical Nerve Stimulation (TENS) Electrode Placement on Spontaneous and Noxiously Evoked Dorsal Horn Cell Activity in the Cat. Neuromodulation. 2002;5:231–237. doi: 10.1046/j.1525-1403.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- [12].Gold MS, Caterina MJ. Molecular Biology of Nociceptor Transduction. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference. Vol. 5. Academic Press; San Diego: 2008. pp. 43–74. [Google Scholar]
- [13].Kittler JT, Chen G, Kukhtina V, Vahedi-Faridi A, Gu Z, Tretter V, Smith KR, McAinsh K, Arancibia-Carcamo IL, Saenger W, Haucke V, Yan Z, Moss SJ. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor gamma2 subunit. Proc Natl Acad Sci U S A. 2008;105:3616–3621. doi: 10.1073/pnas.0707920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- [15].Lee KY, Charbonnet M, Gold MS. Upregulation of high-affinity GABA(A) receptors in cultured rat dorsal root ganglion neurons. Neuroscience. 2012;208:133–142. doi: 10.1016/j.neuroscience.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, Gamper N. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. J Physiol. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- [19].Pitcher MH, Cervero F. Role of the NKCC1 co-transporter in sensitization of spinal nociceptive neurons. Pain. 2010;151:756–762. doi: 10.1016/j.pain.2010.09.008. [DOI] [PubMed] [Google Scholar]
- [20].Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J Physiol. 1995;484(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl− Cotransport and Intracellular Chloride Regulation in Rat Primary Sensory Neurons: Thermodynamic and Kinetic Aspects. J Neurophysiol. 2008;100:169–184. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- [24].Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taiwo YO, Levine JD. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- [27].Valencia-de Ita S, Lawand NB, Lin Q, Castaneda-Hernandez G, Willis WD. Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95:3553–3561. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- [28].Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci. 2010;30:7878–7888. doi: 10.1523/JNEUROSCI.6053-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Willis WD., Jr. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- [30].Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci. 2003;23:601–610. doi: 10.1523/JNEUROSCI.23-02-00601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- [32].Zhu Y, Gold MS. Inflammation increases GABA-A currents in rat cutaneous DRG neurons via a persistent upregulation of tyrosine kinase activity. IASP Abstracts. 2010:PW252. [Google Scholar]