Abstract
Intermittent social defeat stress exposure augments behavioral response to psychostimulants in a process termed cross-sensitization. Brain-derived neurotrophic factor (BDNF) mediates synaptic plasticity and cellular responses to stress and drugs of abuse. We previously showed that repeated social defeat stress persistently alters BDNF and activates ΔFosB expression in mesocorticolimbic regions. Here, we hypothesized that social defeat stress would increase ΔFosB expression in BDNF-containing mesocorticolimbic neurons at a time when cross-sensitization is evident. Because the ventral tegmental area (VTA) is critical for cross-sensitization, we similarly hypothesized that repeated social defeat stress would induce ΔFosB in neurons of mesocorticolimbic terminal regions that innervate the VTA. We induced social defeat stress in rats by short confrontations with an aggressive resident rat every third day for 10 days. Control rats were handled according to the same schedule. Defeated rats exhibited sensitized locomotor response to amphetamine (1.0 mg/kg, i.p.) 10 days after termination of stress exposure. Separate rats, which underwent stress procedures without amphetamine challenge, were used for histological assessments. Rats received intra-VTA infusion of the retrograde tracer, Fluorogold, and brain tissue was collected 10 days after stress or handling for immunhistochemistry. Stress exposure increased BDNF immunoreactivity in anterior cingulate, prelimbic and infralimbic regions of the prefrontal cortex, medial amygdala, nucleus accumbens and VTA; ΔFosB labeling in anterior cingulate cortex and nucleus accumbens; and ΔFosB/BDNF co-expression in prelimbic cortex, nucleus accumbens and medial amygdala. Infralimbic ΔFosB-labeling was enhanced by stress in neurons innervating the VTA. Increased ΔFosB/BDNF co-expression and persistent functional activation of corticolimbic neurons after stress may contribute to mechanisms underlying cross-sensitization to psychostimulants.
Keywords: social defeat stress, vulnerability, amphetamine, cross-sensitization, prefrontal cortex, amygdala
Pre-clinical and clinical data point to stress exposure as a risk factor for addictive behavior (Sinha, 2007). Social stress resulting from defeat after aggressive confrontations with conspecific counterparts is a powerful stressor for both humans and animals (Koolhaas et al., 1999, Björkqvist, 2001). In rodent models, social defeat stress induces profound and long-lasting alterations of function in mesocorticolimbic circuits accompanied by persisting enhancement of drug-related behaviors. This includes augmented behavioral responses to low doses of psychostimulants, a process termed cross-sensitization, and enhanced psychostimulant self-administration (Covington and Miczek, 2001, Nikulina et al., 2004, Covington et al., 2005).
Behavioral sensitization is a consequence of drug-induced neuroadaptive changes and is thought to underlie certain aspects of drug addiction, such as craving and relapse (Robinson and Berridge, 2001). A neural circuit involving dopaminergic and glutamatergic interconnections between the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC) is essential for the induction and expression of behavioral sensitization (Vanderschuren and Kalivas, 2000). Brain-derived neurotrophic factor (BDNF) is a neurotrophin important for synaptic plasticity (Kang and Schuman, 1995, Horch et al., 1999) that is expressed within these regions (Seroogy et al., 1994, Conner et al., 1997) and may represent a critical molecular stimulus for persisting psychomotor cross-sensitization.
Social defeat stress induces activation of the mesocorticolimbic dopamine system (Tidey and Miczek, 1996), and stimulation of dopamine synthesis promotes the expression of BDNF (Okazawa et al., 1992). Recently we observed that repeated social defeat stress increases short-term BDNF expression in prefrontal cortical regions and delayed, prolonged BDNF expression in medial amygdala (AMY) and VTA (Fanous et al., 2010). Similarly, repeated exposure to psychostimulants both produces behavioral sensitization and increases BDNF in the PFC, NAc, and AMY (Meredith et al., 2002, Le Foll et al., 2005, Fumagalli et al., 2007, Fanous et al., 2011). These lines of evidence suggest that stress-induced alteration of BDNF signaling in the these brain regions could regulate the function of this reward circuit (Ghitza et al., 2010).
Additionally, inducible transcription factors of the Fos family are involved in neuroadaptations resulting from stress or psychostimulant administration (Hope et al., 1994, Vanderschuren et al., 2002, Perrotti et al., 2004, Hope et al., 2006, Perrotti et al., 2008). ΔFosB, a stable protein of the Fos family induced by chronic drug treatments, has been proposed as an important mediator of long-term plasticity in the brain (Nestler et al., 1999, McClung et al., 2004, Nestler, 2008). We previously observed that repeated social defeat stress increases ΔFosB expression in mesocorticolimbic terminal regions such as the PFC, NAc, and AMY, which persists up to 14 days after stress termination (Nikulina et al., 2008). Because enhanced mesocorticolimbic BDNF and ΔFosB represent lasting molecular consequences of both repeated social defeat stress and chronic drug treatments, expression of both together may contribute to cross-sensitization. However, whether ΔFosB and BDNF are co-expressed in mesocorticolimbic neurons during cross-sensitization is unknown.
Our present aim was to examine anatomical substrates for prolonged molecular consequences of social defeat stress. We hypothesized that repeated activation of mesocorticolimbic neurons by social defeat stress would increase ΔFosB and BDNF in an overlapping population of neurons. Thus, ΔFosB and BDNF co-expression was examined in mesocorticolimbic regions 10 days after exposure to intermittent social defeat stress at a time when behavioral cross-sensitization to psychostimulants is known to be present. Additionally, we hypothesized that stress exposure would increase ΔFosB in prefrontal cortical neurons innervating the VTA, which is implicated in the development of behavioral sensitization (Kalivas and Weber, 1988, Perugini and Vezina, 1994) and is reciprocally connected to prefrontal cortex (Geisler and Zahm, 2005). To investigate this, we infused the retrograde tracer Fluorogold (FG) into the VTA and measured ΔFosB/FG co-labeling in mesocorticolimbic terminal regions 10 days after repeated social defeat stress exposure.
EXPERIMENTAL PROCEDURES
Subjects
Twenty nine male Sprague-Dawley rats (Charles River Laboratories, Hollister, CA, USA) were acclimated to laboratory conditions for one week prior to the start of experimentation. Rats weighed 270 – 300 g at the beginning of experimental manipulations, and were singly housed in standard plastic cages (55 × 31 × 21 cm) prior to behavioral procedures and during recovery from surgery. Rats were maintained under a reverse 12-h light-dark cycle (lights off at 0900 h) with free access to food (Purina Rodent Chow) and water. Male hooded Long-Evans rats (weighing 550-700 g), termed “residents,” were continuously pair-housed with an individual female in large plastic cages (37 × 50 × 20 cm), and were used to induce social defeat stress in experimental Sprague-Dawley male rats. All females underwent tubal ligation prior to pair-housing with males to maintain cycling and prevent pregnancy. Residents were screened repeatedly for reliable performance of aggressive behavior toward an intruder rat. All experimental procedures were approved by the University of Arizona and Arizona State University Institutional Animal Care and Use Committees, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2003). In addition, all efforts were made to minimize animal suffering and the number of subjects.
Experimental Design
General procedure
After adaptation to laboratory conditions, rats were randomly assigned to either the “social defeat stress” experimental group or to a non-stressed handled control group. Experimental rats were exposed to intermittent social defeat stress every third day for 10 days (4 defeats total), and control rats were handled at the same times, but not exposed to resident rats. Two experiments were conducted in parallel. One set of rats (n=17) was subjected to social defeat stress to induce behavioral cross-sensitization or to control handling, and 10 days later was challenged with amphetamine. We chose this time-point because we and others have demonstrated both behavioral cross-sensitization and alterations in Fos and BDNF response in various mesocorticolimbic regions 10 days after repeated social defeat (Nikulina et al., 2004, Miczek et al., 2008, Miczek et al., 2011). In order to assess persistent protein changes resulting from stress without the confound of an acute amphetamine challenge, another set of rats (n=12) received injections of FG into the VTA one week before the same social defeat stress or handling procedure, and were euthanized 10 days after stress termination, a time point corresponding to the expression of cross-sensitization. Brain tissue was collected in the absence of amphetamine challenge in order to assess long-term effects of repeated social defeat stress on BDNF and ΔFosB expression at a time when cross-sensitization to amphetamine would normally be expressed.
Surgeries
Rats used for histological assessments received unilateral FG injections into VTA at the following stereotaxic coordinates: AP = -5.1, DV = -8.8, ML = -0.6 (Paxinos and Watson, 2005). FG produces reliable and long-lasting retrograde labeling from discrete injection sites without disrupting behavior or subsequent histochemical procedures (Cheung and Hammer, 1995). Briefly, FG (4% in 0.1M sodium cacodylate buffer; Fluorochrome, LLC, Denver, CO) was infused into rats anesthetized with isofluorane by stereotaxic iontophoresis using a 20-30 μm tip diameter micropipette and 6 μamp, 7 sec alternating current for 10 min. Withdrawal of the micropipette was accompanied by application of -1 μamp current to avoid FG diffusion along the pipette track. Wounds were closed with bone wax and surgical staples. Behavioral manipulation with social defeat stress or handling began after one week of recovery.
Social defeat stress
Experimental rats were defeated as described previously (Tornatzky and Miczek, 1995, Nikulina et al., 2004). After removing the female from the resident's cage 30 min prior to social stress exposure, the experimental intruder rat was placed into the home cage of a resident male rat. For the first 5 min, the intruder rat remained under a stainless steel protective cage (25 × 15 × 15cm) in order to expose the intruder to threats from the resident. The protective cage was then removed, and the resident attacked the intruder within 1-2 min. Defeat of the experimental rat was identified as the display of a submissive supine posture for at least four seconds, which usually occurred following 4-5 bites within a maximum of 5 min. Following display of this supine posture, the intruder was placed under the protective cage within the resident's cage and remained there for an additional 20 min. Intruder rats were not exposed to the same resident on consecutive days or more than twice during the experiment. After each social defeat stress exposure, intruder rats were immediately returned to their home cages.
Locomotor activity
Amphetamine (1.0 mg/kg, i.p.) injections were given and locomotor activity was tracked in a separate testing room in a home cage. Rats habituated to the testing room, testing chamber, and injection procedure for 2 days prior to the amphetamine or saline challenge day. Locomotor response to amphetamine was measured 10 days after the last social stress exposure using Videotrack software (Viewpoint Life System, Montreal, Canada) and a CCD video camera mounted above a platform where four cages were placed for behavioral monitoring. The software determines a centroid point around the subject shape. This allows differentiation of smaller and larger movements. Smaller movements, which we operationally defined as centroid movements of 4-10 cm, encompas smaller, stereotyped movements but may also include other small movements. Larger ambulatory movements were defined as movements greater than 10 cm. The number of each type of movement and total distance traveled were measured. Locomotor activity was recorded both 30 min before and 40 min after saline injection, then for 60 min after amphetamine challenge. The total tracking time for each rat was 130 min. To avoid measuring non-specific movement (e.g. respiration in a stationary animal), a minimal threshold for movement detection was set. Locomotor activity data were collected and analyzed in 10 min intervals.
Perfusion and tissue preparation
Ten days after repeated social defeat stress, rats were deeply anesthetized with sodium pentobarbital (Euthasol; Virbac, St. Louis, MO). Animals were perfused transcardially with heparinized saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS); brains were post-fixed in the same fixative for 1.5 h at 4° C, placed in graded sucrose solutions in PBS at 4° C, and sectioned at 20 μm on a sliding microtome. Coronal sections were taken from the PFC (AP= +3.0 to + 2.7 mm), NAc (AP= +1.0 to + 1.5 mm), AMY (AP= -2.3 to -2.8 mm), and VTA (AP= -4.9 to -5.4 mm; Paxinos and Watson, 2005). Sections were collected in chilled 0.05 M phosphate buffer (pH 7.4), mounted onto glass slides (Superfrost Plus; Fisher, Waltham, MA), dried, and stored at -35°C until processing. Sections from stressed and control handled rats were processed at the same time.
Double-label immunohistochemistry
Double immunostaining was performed using a sequential procedure that involves first staining for nuclear ΔFosB, then for either BDNF or FG. ΔFosB immunolabeling was performed using an antibody that recognizes both ΔFosB and full-length FosB (Perrotti et al., 2004). Because FosB is induced by acute stimuli, and ΔFosB accumulates for weeks following repeated stimulation with drugs or stress (McClung et al., 2004), only ΔFosB should be labeled in our assay due to the absence of an acute stimulus. PFC, NAc, AMY, and VTA tissue were assessed for ΔFosB/BDNF co-labeling, while PFC tissue was assessed for ΔFosB/FG co-labeling. After washing sections in 0.05 M potassium phosphate-buffered saline (KPBS) to remove any fixative, sections were incubated for one hour in 5% normal goat serum in 0.05M KPBS/0.4% Triton X-100, followed by incubation with a primary antibodies. Sections were first incubated with rabbit polyclonal antisera directed against FosB (sc-48, 1:7,500 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 48 hours at 4°C, then with biotinylated goat anti-rabbit serum for 1 hour and processed by the avidin-biotin-peroxidase method (Vector Laboratories, Burlingame, CA). ΔFosB was developed using diaminobenzidine as the peroxidase substrate that produces dark gray nuclear staining. After 1 hour incubation with 0.001M biotin (Sigma-Aldrich, St. Louis, MO), sections were incubated with either the BDNF or FG primary antibody for 48 hours at 4°C. BDNF immunohistochemistry used a rabbit polyclonal antibodies specific for BDNF over other neurotrophins (AB1779SP, 1:3,000 dilution; Chemicon/Millipore, Temecula, CA). To detect FG, rabbit polyclonal antibodies were utilized (AB153, 1:10,000 dilution; Chemicon). The sections were triple washed in 0.05 M KPBS between incubation steps. Immobilized antigens within cells were visualized by incubation with the ABC complex, and BDNF or FG labeling was developed using the VIP peroxidase substrate kit (Vector Laboratories, Burlingame, CA) that generates a purple cytoplasmic stain. Control procedures included preadsorption of the primary antibody using the corresponding peptide, and conducting procedures in the absence of the primary antibody. There was no detectable labeling after either procedure (data not shown).
Image analysis
Slides were coded and the investigator was blind to the treatment groups during microscopical analysis. Immunohistochemical labeling was analyzed using a Zeiss Axioskop microscope and Stereo Investigator software (MBF Bioscience, Williston, VT). A systematic random sampling approach using a stereological grid was employed to analyze at least 3 sections in each of the anterior cingulate (ACG), prelimbic (PL), and infralimbic (IL) subregions of the PFC; NAc shell and core; medial, central and basolateral AMY; and the VTA (Fig. 1). Images of selected areas in each region were digitized using a camera interfaced to the microscope using a 20x objective. Stereo Investigator software partitioned each image into 16 equal counting frames (100 × 75 μm each), half of which were randomly selected and analyzed. The number of single- and double-labeled neurons was counted separately for each frame, excluding any overlapping labeled profiles on the left and bottom borders. The density of labeled profiles was averaged together for three sections of each brain region by dividing the estimated total density of labeled profiles by the numbers of analyzed areas. A single value for density of labeled profiles per region per rat was used as an n = 1 for statistical analysis. A few cases were excluded from analysis of PFC, NAc and AMY due to poor quality of processed sections, and the identity of these cases differed across regions. ΔFosB-labeled profiles were identified by a dark gray stained nucleus, whereas FG- or BDNF-labeled profiles exhibited a purple cytoplasmic stain. A profile was considered labeled if its pixel luminance was more than 2 standard deviations different from the background luminance as calculated by Stereo Investigator software. The numbers of ΔFosB, and BDNF immunolabeled profiles, and ΔFosB/BDNF and ΔFosB/FG double-immunolabeled profiles were counted. ΔFosB-labeled profiles were identified by dark grey nuclear labeling, whereas BDNF-labeling exhibited a purple diffuse cytoplasmic stain with empty nucleus. Cells were considered double-labeled with ΔFosB/BDNF or ΔFosB/FG - when a nuclear ΔFosB label was present in a cell surrounded by purple BDNF- or FG-stained cytoplasm as evidenced by a purple “halo” and projection fibers around a dark stained nucleus. Labeling density was calculated by dividing the estimated total number of cells by the total area measured for conversion to the number of labeled cells per mm2.
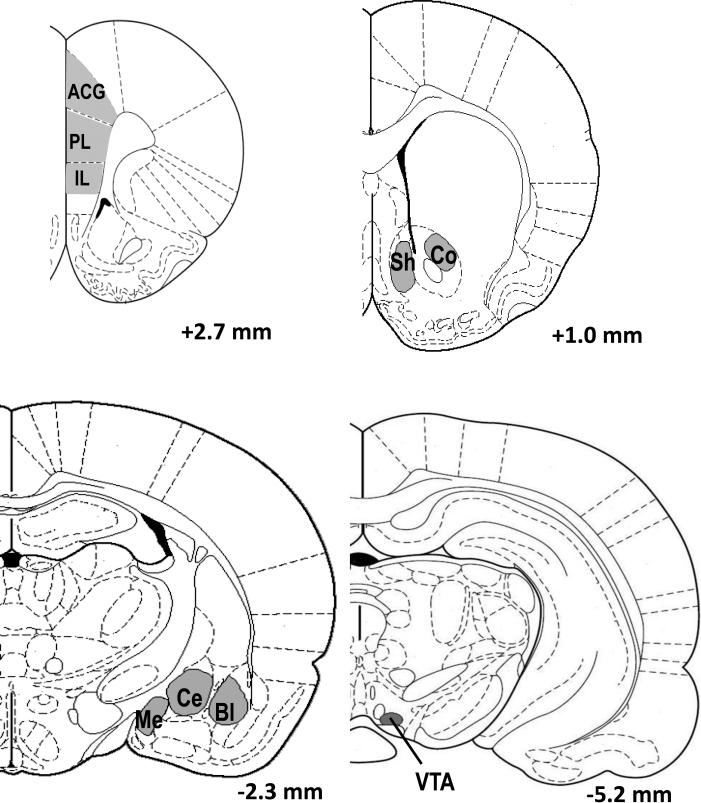
Figure 1. Schematic of regions examined for BDNF, ΔFosB, and FG immunolabeling.
Images adapted from the Paxinos and Watson brain atlas (2005) depict specific locations analyzed for immunolabeling: ACG – anterior cingulate cortex; PL – prelimbic cortex; IL – infralimbic cortex; Sh – nucleus accumbens shell; Co – nucleus accumbens core; Me – medial amygdala; Ce – central amygdala; Bl – basolateral amygdala; VTA – ventral tegmental area.
Statistics
Locomotor activity was quantified in two ways: total locomotor activity and locomotor activity over time (each 10 min. bin). Total locomotor activity data were evaluated by one-way ANOVA (handled vs. stressed) and locomotor activity over time by two-way mixed ANOVA (time as within-subjects factor and behavioral condition [handled or stressed] as between-subjects factor). Each of the 3 time frames (14 bins, 10 min each, before and after saline injection, and following drug challenge) was treated with a separate one-way (total locomotor activity) or two-way (locomotor activity over time) ANOVA. The immunohistochemical data were analyzed using t-test (comparison between handled and stressed groups). P ≤ 0.05 was considered significant, and all data are expressed as mean ± SEM.
RESULTS
Cross-sensitization
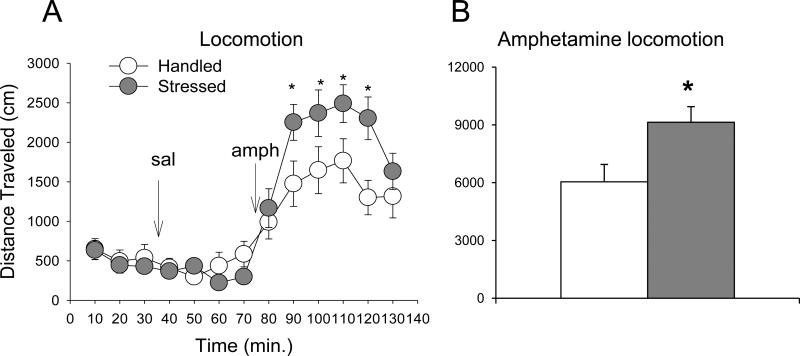
Total distance traveled and distance traveled over time after amphetamine challenge were significantly greater in rats exposed to social stress, as shown by one- and two-way mixed ANOVA, respectively (one-way ANOVA, F(1,16) = 6.5; two-way ANOVA, F(1,16) = 4.8, P ≤ 0.05; Fig. 2). The total number of ambulations and time spent in stereotypy-like movements after amphetamine injection did not differ between the handled and stressed groups (Table 1). Spontaneous locomotor activity and locomotor response to saline injection did not differ between handled and stressed rats in any aspect of locomotion examined (Fig. 2A).
Figure 2. Effect of intermittent social defeat stress on amphetamine-induced locomotion.
A – Distance traveled during baseline, after saline, and after amphetamine (1.0 mg/kg) challenge over time in animals exposed to stress or handling 10 days prior (n = 8-9, P ≤ 0.05 between stress and handling after amphetamine). B – Total distance traveled over 40 min. after amphetamine in animals exposed to stress or handling 10 days prior (n = 8-9). *P ≤ 0.05 vs. handled group. Values represent mean ± SEM.
Table 1.
Amphetamine-induced locomotor activity 10 days after social defeat stress or handling (Mean ± SEM)
| Handled | Stressed | P | |
|---|---|---|---|
| Time in stereotypy-like movements (s) | 779 ± 68 | 700 ± 28 | 0.3 |
| Number of ambulations | 1288 ± 163 | 1389 ± 100 | 0.6 |
ΔFosB, BDNF and double-labeled cells in the PFC
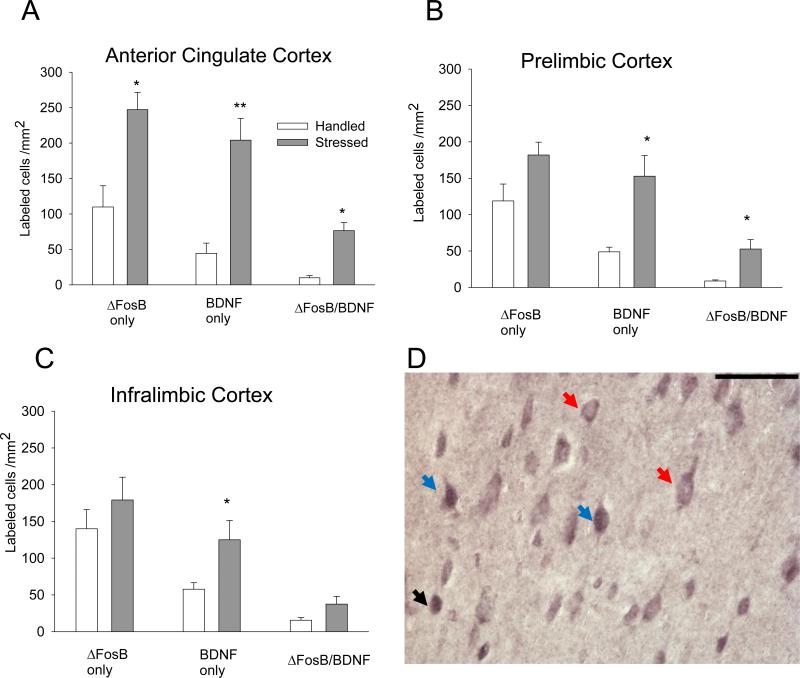
ΔFosB expression in the ACG was significantly enhanced after social defeat stress exposure in comparison with the handled control group (t(7)= 3.43; P = 0.011; Figs. 3A and D). No differences in ΔFosB expression between handled and stressed rats were observed in the PL (t(7) = 2.07; P > 0.05; Fig. 3B) or IL (P > 0.05; Fig. 3C). However, repeated social defeat stress significantly increased BDNF expression in all regions of the PFC (ACG: t(7) = 5.09, P = 0.001; PL: t(7) = 3.99, P = 0.005; IL: t(7) = 2.68, P = 0.032). Furthermore, the number of BDNF/ΔFosB double-labeled cells significantly increased after stress exposure in the ACG and PL (ACG: t(7) = 6.15, P ≤ 0.001; PL: t(7) = 3.83, P = 0.007). Likewise, the percentage of BDNF neurons expressing ΔFosB was significantly higher in PL after social defeat stress (P = 0.05; Table 2), and approached significance in ACG (P = 0.06; Table 2).
Figure 3. Effect of intermittent social defeat stress on ΔFosB, BDNF, and ΔFosB/BDNF immunolabeling in the PFC.
ΔFosB, BDNF, and ΔFosB/BDNF double immunolabeling expressed as labeled cells/mm2 in the ACG (A), PL (B) and IL (C) regions of the mPFC (n = 4-5). All values represent mean ± SEM. *P ≤ 0.05; ** P ≤ 0.01 vs. handled group. D – Representative photomicrograph showing ΔFosB and BDNF single and ΔFosB/BDNF labeling in ACG. ΔFosB profiles are represented by a dark grey nuclear label, and BDNF cells are labeled with a diffuse, purple cytoplasmic stain with an empty nucleus. ΔFosB/BDNF cells contain a dark nuclear ΔFosB label surrounded by diffusely labeled purple cytoplasm. Black arrow: ΔFosB; red arrow: BDNF; blue arrow: ΔFosB/BDNF double-labeling. Scale bar 100 μm.
Table 2.
Percentages of ΔFosB/BDNF co-labeled cells in different brain regions 10 days after social defeat stress and handled control rats
| Handled | Stressed | P | |
|---|---|---|---|
| ACG | 29.4 ± 7.4% | 37.4 ± 2.2% | P = 0.06 |
| PRL | 24.7 ±3.5% | 34.5 ± 3.4% | P ≤ 0.05 |
| IL | 34.3 ± 6.5% | 30.0 ± 3.3% | P > 0.05 |
| NAc shell | 29.8 ± 5.4% | 39.9 ± 2.2% | P > 0.05 |
| NAc core | 25.2 ± 2.4% | 30.7 ± 2.5% | P > 0.05 |
| Medial AMY | 13.1 ± 3.5% | 18.1 ± 1.3% | P > 0.05 |
| Central AMY | 14.5 ± 3.5% | 15.1 ± 3.7% | P > 0.05 |
| Basolateral AMY | 10.7 ± 3.4% | 9.8 ± 3.1% | P > 0.05 |
| VTA | 31.6 ± 2.7% | 23.3 ± 1.6% | P > 0.05 |
Fluorogold-labeled cells in PFC
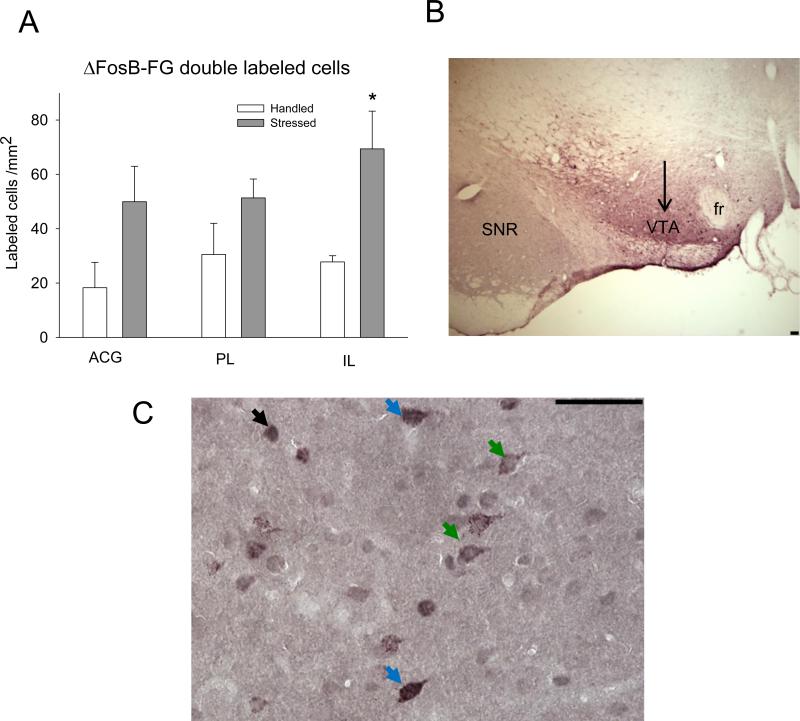
Neurons retrogradely labeled with FG exhibited granular staining restricted to cytoplasm. ΔFosB/FG double-labeling in the IL cortex was significantly greater 10 days after stress termination (t(6) = 2.96; P = 0.025; Fig. 4A and C). Thus, IL neurons which directly innervate the VTA were more functionally active following repeated social defeat stress. ΔFosB/FG double-labeling in the ACG and PL areas did not differ significantly between handled and stressed groups (P > 0.05). The total number of FG labeled cells was not statistically different between handled and stressed rats across three cortical sub-areas (ACG 140±11.4 cell/ mm 2 for handled and 190±27.4 for stressed rats; PL 179±9.7 cell/ mm2 for handled and 205±17.4 for stressed animals; IL 152±11.5 cell/ mm2 for handled and 212±16.4 for stressed rats; all comparison are p>0.05).
Figure 4. Effect of intermittent social defeat stress on ΔFosB/FG labeling in PFC regions.
ΔFosB/FG immunolabeling expressed as labeled profiles/mm2 (n = 5-6). *P ≤ 0.05 vs. handled group. B. – Representative low magnification microphotograph of FG immunolabeling in the VTA. The arrow is directed at the purple FG labeling in the VTA region. SNR –substania nigra reticulata; fr –fasciculus retroflexus. C – Representative photomicrograph of ΔFosB and FG immunolabeling in IL region of the PFC. ΔFosB profiles are represented by a dark grey nuclear label, and FG cells are labeled with a granular purple cytoplasmic stain with an empty nucleus. ΔFosB/FG cells contain contain a dark nuclear ΔFosB label surrounded by diffusely labeled purple cytoplasm. Black arrow: ΔFosB; green arrow: FG; blue arrow: ΔFosB/FG double-labeling. Scale bar 100 μm.
ΔFosB, BDNF, and double-labeled cells in the NAc
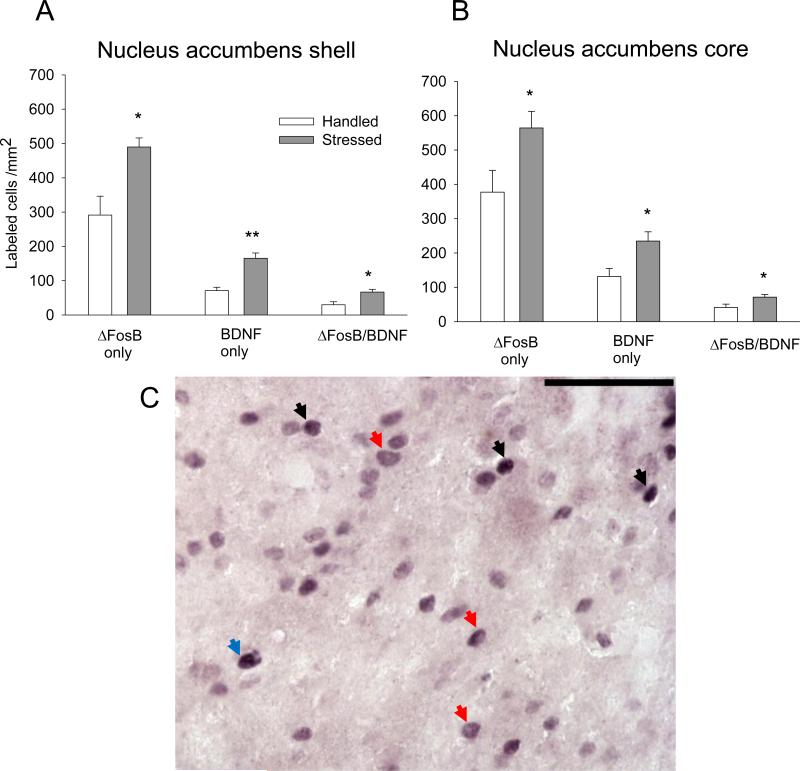
Repeated social defeat stress significantly increased ΔFosB expression (t(9) = 3.45; P ≤ 0.005; Fig. 5), the number of BDNF labeled cells (t(9) = 4.89; P ≤ 0.001), and co-localization of ΔFosB/BDNF labeling (t(9) = 3.1; P ≤ 0.05) in the NAc shell. Similarly, more ΔFosB cells were observed in the NAc core in rats exposed to stress (t(9) = 2.39; P ≤ 0.05). In the NAc core, BDNF expression and ΔFosB/BDNF co-expression was also higher (BDNF, t(9) = 2.83; P ≤ 0.05; ΔFosB-BDNF, t(9) = 2.43; P ≤ 0.05) after repeated social defeat stress. However, the percentage of ΔFosB/BDNF neurons was not differ significantly in NAc shell or core between handled and social defeat stressed rats (Table 2).
Figure 5. Effect of intermittent social defeat stress on ΔFosB, BDNF, and ΔFosB/BDNF in the NAc.
ΔFosB, BDNF, and ΔFosB/BDNF double immunolabeling expressed as labeled cells/mm2 in the shell (A), core (B) regions of the NAc (n = 5-6). All values represent mean ± SEM. *P ≤ 0.05; ** P ≤ 0.01 vs. handled group. C – Representative photomicrograph showing ΔFosB and BDNF single and ΔFosB/BDNF labeling in NAc shell. ΔFosB profiles are represented by a dark grey nuclear label, and BDNF cells are labeled with a diffuse, purple cytoplasmic stain with an empty nucleus; ΔFosB/BDNF cells contain a dark nuclear ΔFosB label surrounded by diffusely labeled purple cytoplasm. Black arrow: ΔFosB; red arrow: BDNF; blue arrow: ΔFosB/BDNF double-labeling. Scale bar 50 μm.
ΔFosB, BDNF and double-labeled cells in the AMY
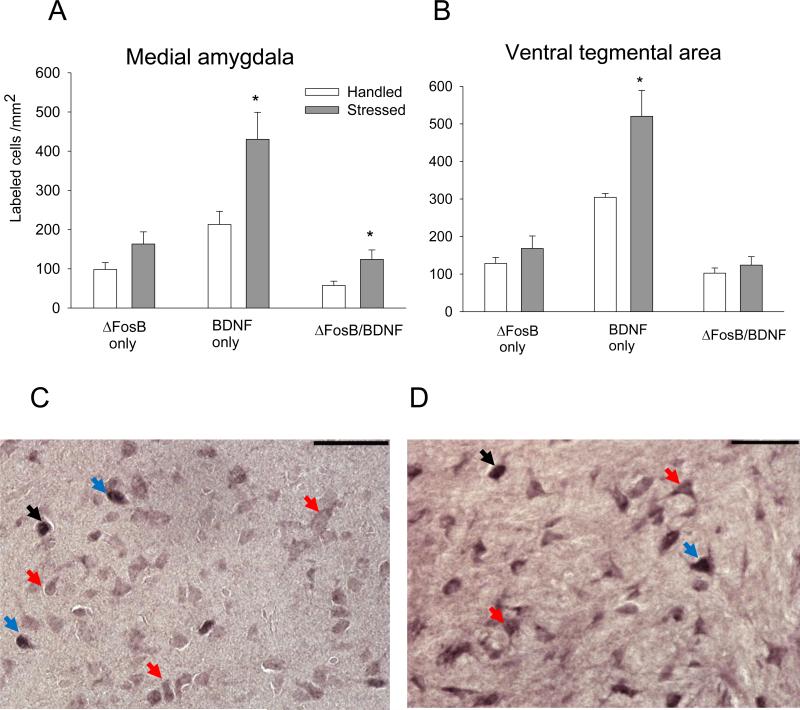
Increased BDNF expression (t(9) = 2.66; P ≤ 0.05; Fig. 6A and C) and increased ΔFosB/BDNF co-localization (t(9) = 2.3: P ≤ 0.05) were observed in medial AMY 10 days after stress termination. However, the percentage of BDNF-labeled cells also expressing ΔFosB was not significantly different from the handled control group (P > 0.05; Table 2). ΔFosB expression, BDNF expression, and ΔFosB/BDNF co-expression in the central and basolateral AMY were not altered after repeated stress exposure (Table 3; P > 0.05).
Figure 6. Effect of intermittent social defeat stress on ΔFosB, BDNF, and ΔFosB/BDNF in the medial amygdala (A) and VTA (B).
ΔFosB, BDNF, and ΔFosB/BDNF double immunolabeling expressed as labeled cells/mm2 (n = 5-6). All values represent mean ± SEM. *P ≤ 0.05 vs. handled group. Representative photomicrographs showing ΔFosB and BDNF single and ΔFosB/BDNF labeling in the medial amygdala (C) and VTA (D). ΔFosB profiles are represented by a dark grey nuclear label, and BDNF cells are labeled with a diffuse, purple cytoplasmic stain with an empty nucleus; ΔFosB/BDNF cells contain a dark nuclear ΔFosB label surrounded by diffusely labeled purple cytoplasm Black arrow: ΔFosB; red arrow: BDNF; blue arrow: ΔFosB/BDNF double-labeling. Scale bar 100 μm.
Table 3.
Mean numbers (± SEM) of labeled cells/mm2 in the central and basolateral amygdala 10 days after social defeat stress or handling. P > 0.05 for all.
| ΔFosB only | BDNF only | ΔFosB/BDNF | ||||
|---|---|---|---|---|---|---|
| Handled | Stressed | Handled | Stressed | Handled | Stressed | |
| Central Amygdala | 126.9 ± 20.1 | 135.0 ± 10.1 | 397.1 ± 77.7 | 456.2 ± 50.7 | 92.1 ± 24.2 | 96.2 ± 7.9 |
| Basolateral Amygdala | 77.8 ± 15.2 | 108.5 ± 9.9 | 326.3 ± 23.6 | 377.7 ± 54.1 | 65.8 ± 10.4 | 79.7 ± 11.5 |
ΔFos, BDNF and double-labeled cells in the VTA
Social defeat stress enhanced BDNF expression in the VTA 10 days after stress termination (t(10) = 3.1; P ≤ 0.01; Fig. 6B and D), though ΔFosB and ΔFosB/BDNF co-labeling were unchanged after repeated stress exposure.
DISCUSSION
In this study, we measured co-expression of ΔFosB and BDNF in mesocorticolimbic regions to identify potential anatomical and molecular substrates for vulnerability to the effects of psychostimulants after social defeat stress. We demonstrated that repeated social defeat stress induces a distinct pattern of ΔFosB and BDNF expression in reward regions. While increased BDNF expression was observed in all areas of prefrontal cortex, medial AMY, NAc and VTA, the number of ΔFosB-expressing cells were increased only in ACG and NAc. In the prefrontal cortex, these cellular alterations occurred in an overlapping population of cells as evidenced by increased percentage of stress-induced ΔFosB/BDNF co-labeling in PL cortex. Repeated stress also increased ΔFosB-labeling in the IL cortex, and notably, this enhancement was observed preferentially in neurons innervating the VTA.
These cellular findings were accompanied by behavioral cross-sensitization to amphetamine. In our experiments, repeated social defeat stress promoted cross-sensitization as demonstrated by enhanced locomotion upon challenge with a low dose of amphetamine ten days after the final episode of stress. Amphetamine-induced stereotyped behavior was not affected by stress, consistent with the finding that low doses of amphetamine augment locomotor activity without affecting stereotypy (Nordquist et al., 2008). Our previous studies have shown that repeated exposure to social defeat stress induced locomotor cross-sensitization to amphetamine that was evident up to two months after social stress (Nikulina et al., 2004, Covington et al., 2005). Indeed, only repeated social defeat stress produced such long-lasting cross-sensitization to psychostimulants, since a single social defeat induced short-lived cross-sensitization (Miczek et al, 1999; de Jong et al, 2005). It should be noted that although behavioral sensitization is not a requisite part of addictive behavior (Ahmed and Cador, 2006), it is thought to reflect neural sensitization, an important component of the addiction process (Vezina and Leyton, 2009).
It is well established that neuroadaptations underlying behavioral sensitization involve dopaminergic and glutamatergic transmission in the mesocorticolimbic system (Vanderschuren and Kalivas, 2000). Our finding that BDNF expression is enhanced in cortical ΔFosB-containing neurons following stress exposure is important because BDNF modulates NMDA glutamate receptors and activates glutamate transmission and plasticity in the PFC (Madara and Levine, 2008). BDNF is necessary for glutamate-dependent synaptic plasticity in midbrain dopamine neurons during cocaine withdrawal (Pu et al., 2006). This is likely relevant to behavior, as direct BDNF infusion into the NAc or VTA enhances the initial stimulant effects of cocaine and facilitates the development of sensitization after repeated cocaine use (Horger et al., 1999). However, BDNF effects are site-specific and depend on time interval after drug treatment (Ghitza et al., 2010). For example, cocaine seeking is suppressed by BDNF infusion into the medial PFC, but augmented by BDNF administration into the NAc (Berglind et al., 2007, Graham et al., 2007).
BDNF expression is generally considered to be dynamic and time-dependent, as has been observed in response to social defeat stress (Pizarro et al., 2004, Berton et al., 2006, Fanous et al., 2010). We recently showed that BDNF mRNA and protein expression were induced in the PFC immediately after termination of repeated social defeat stress, but returned to control levels four weeks later (Fanous et al., 2010). In contrast, BDNF protein was reduced in the medial AMY and unchanged in the VTA immediately after social defeat stress, but increased in these regions four weeks later. The present data reveal that BDNF expression increased in the PFC, medial AMY and VTA ten days after social defeat stress termination. Elevated PFC BDNF might be a consequence of dopaminergic stimulation resulting from social defeat (Tidey and Miczek, 1996, Miczek et al., 2011). However, dopamine transporter knockout mice, which have hyperdopaminergic tone, show reduced BDNF expression in the frontal cortex and diminished BDNF response in this area after chronic restraint stress (Fumagalli et al., 2003). Therefore, increased dopamine alone may not account for the stress-induced increase of mesocortical BDNF.
BDNF is expressed by pyramidal projection neurons, so endogenous increases of PFC or VTA BDNF could also result in enhanced anterograde transport of BDNF (Altar et al., 1997, Conner et al., 1997) to the NAc shell and core, where BDNF signaling plays a crucial role in psychomotor sensitization and neuroadaptation to psychostimulants (Bahi et al., 2008, Crooks et al., 2010, McGinty et al., 2010). Following social defeat stress exposure, activation of the TrkB receptor by BDNF leads to transduction of neurotrophic signals from the cell surface to the cell nucleus via extracellular signal-regulated kinase (ERK), and hyperphosphorylated ERK results in induction of immediate early genes, including the fos family (Sgambato et al., 1998). Prolonged induction and phosphorylation of ERK1/2 in PFC dendrites after chronic stress (Trentani et al., 2002) could represent an activated pathway for development of cross-sensitization after social defeat stress.
A significant finding of the present study is the sustained activation of the IL-VTA connection after social defeat stress. ΔFosB expression following repeated social defeat stress occurs primarily in IL neurons that directly innervate the VTA. VTA dopamine neurons receive glutamate inputs from the PFC (Omelchenko and Sesack, 2007), which might be activated after social defeat stress. Our results complement recent observations that prolonged social defeat exposure increases the number of ΔFosB-labeled cells selectively in the IL cortex, which also displays increased histone acetylation (Hinwood et al., 2011). Such chromatin remodeling associated with ΔFosB in IL cortex provides a substrate for long-term cellular response to social stress. Recently we reported that repeated amphetamine enhanced Fos/BDNF co-expression in the ACG, PL, and IL regions, as well as Fos labeling, in PFC neurons projecting to the VTA (Fanous et al., 2011), suggesting that overlapping substrates for stress and amphetamine sensitization may be present in PFC regions.
Our data show that intermittent exposure to social defeat stress induced ΔFosB protein in the ACG, NAc shell and core 10 days later. We previously reported that the same social stress paradigm enhanced ΔFosB expression for up to 14 days in the PL and IL regions of the PFC, medial AMY and NAc shell, but not core (Nikulina et al., 2008). This variability of ΔFosB expression in the PFC and NAc might depend on natural variation in the intensity of defeat. Similarly, ΔFosB accumulation in the ventral striatum following cocaine administration is variable and dependent on both the strength and duration of the drug stimulus (Larson et al., 2010). ΔFosB protein is thought to function as either a transcriptional activator or repressor depending on the target gene involved (Nestler, 2008). Notably, neuronal ΔFosB was specifically co-labeled with vGluT1 in the PFC after repeated restraint stress (Perrotti et al., 2004), which supports the involvement of PFC glutamatergic neurons in the process of stress-induced sensitization.
Repeated exposure to drugs of abuse also induces ΔFosB expression in the NAc (Hope et al., 1994, Chen et al., 1997, Larson et al., 2010), and this ΔFosB induction occurs selectively in dynorphin-containing medium spiny neurons of the dorsal and ventral striatum (Nye et al., 1995, Zachariou et al., 2006). Social stress-induced ΔFosB expression in the NAc of mice leads to enhanced expression of GluR2 AMPA receptors subunits (Vialou et al., 2010). In their study, enhanced expression of ΔFosB in the NAc was shown after chronic continuous social defeat in mice and may underlie depressive-like behavior in a susceptible group of mice. However, ΔFosB expression in the NAc may function differently in continuous vs. intermittent social stress, particularly given that these models of stress produce opposing effects on drug response (Miczek et al, 2011). In fact, drugs of abuse induce ΔFosB expression in the subclass of medium spiny neurons expressing D1 dopamine receptors (Nestler, 2008) while stress exposure stimulates dynorphin (D1)- and enkephalin (D2)-containing cells almost equally (Perrotti et al., 2004). Thus, ΔFosB could contribute to a mechanism of prolonged sensitization to drug exposure by regulating the expression of specific genes in brain reward circuit (Nestler, 2008).
Finally, the observed increase in ΔFosB/BDNF labeling in medial AMY cells after social defeat stress might be a result of a neuroendocrine stress response. The medial AMY, but not the central AMY is involved in response to an emotional stressor (Dayas et al., 1999) and social defeat stress increases Fos expression in medial AMY neurons containing corticotrophin-releasing factor receptors (Fekete et al., 2009). Our previous work shows that the AMY displays a sensitized Fos response specifically during amphetamine challenge two months after repeated social defeat (Nikulina et al., 2004), with persistent increase of ΔFosB and BDNF expression in the medial AMY several weeks after repeated social defeat stress (Nikulina et al., 2008, Fanous et al., 2010). Together, these findings suggest that persistent changes in ΔFosB and BDNF after repeated social defeat stress in AMY may have functional consequences which contribute to cross-sensitization, and thus drug-related behavior.
Overall, we report increased ΔFosB expression in IL cortical neurons innervating the VTA after intermittent social defeat stress, and increased BDNF/ΔFosB co-expression in the PL cortex, NAc, and medial AMY, which may contribute to the long-term behavioral sensitization observed after intermittent social defeat stress. These molecular alterations in mesocorticolimbic regions could provide potential targets for the development of fundamentally novel treatments for stress-induced drug dependence.
Highlights.
Episodic social defeat induced prolonged mesocorticolimbic ΔFosB/BDNF co-expression
Episodic defeat persistently enhanced ΔFosB in infralimbic corticotegmental neurons
Molecular changes in defeated rats occurred parallel with amphetamine sensitization
These findings may contribute to mechanisms of psychostimulant cross-sensitization
Acknowledgments
Support contributed by: USPHS awards DA024817, DA026451, and MH073930
Abbreviations
- BDNF
brain-derived neurotrophic factor
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- FG
fluorogold
- ACG
anterior cingulate cortex
- PL
prelimbic cortex
- IL
infralimbic cortex
- AMY
amygdala
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. European Journal of Neuroscience. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiology & Behavior. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung S, Hammer RP., Jr. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in arcuate neurons that innervate the medial preoptic area of the rat. Neuroendocrinology. 1995;62:283–292. doi: 10.1159/000127015. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr., Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Crooks KR, Kleven DT, Rodriguiz RM, Wetsel WC, McNamara JO. TrkB signaling is required for behavioral sensitization and conditioned place preference induced by a single injection of cocaine. Neuropharmacology. 2010;58:1067–1077. doi: 10.1016/j.neuropharm.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. European Journal of Neuroscience. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- de Jong J, Wasilewski M, van der Vegt B, Buwalda B, Koolhaas J. A single social defeat induces short-lasting behavioral sensitization to amphetamine. Physiol Behav. 2005;83:805–811. doi: 10.1016/j.physbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr., Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous S, Lacagnina MJ, Nikulina EM, Hammer RP., Jr. Sensitized activation of Fos and brain-derived neurotrophic factor in the medial prefrontal cortex and ventral tegmental area accompanies behavioral sensitization to amphetamine. Neuropharmacology. 2011;611:558–564. doi: 10.1016/j.neuropharm.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete ÉM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Racagni G, Colombo E, Riva MA. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol Psychiatry. 2003;8:898–899. doi: 10.1038/sj.mp.4001370. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm D. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Day TA, Walker FR. Repeated social defeat selectively increases deltaFosB expression and histone H3 acetylation in the infralimbic medial prefrontal cortex. Cereb Cortex. 2011;21:262–271. doi: 10.1093/cercor/bhq080. [DOI] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Horch H, Kruttgen A, Portbury S, Katz L. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- Kang H, Schuman E. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. NeuroReport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr., Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Miczek K, Nikulina E, Kream R, Carter G, Espejo E. Behavioral sensitization to cocaine after a brief social defeat stress: c-Fos expression in PAG. Psychopharmacology. 1999;141:225–235. doi: 10.1007/s002130050829. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of ΔFosB. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP., Jr. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr., Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nordquist R, Vanderschuren L, Jonker A, Bergsma M, de Vries T, Pennartz C, Voorn P. Expression of amphetamine sensitization is associated with recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology. 2008;198:113–126. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Hope BT, Kelz MB, Iadarola M, Nestler EJ. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680. [PubMed] [Google Scholar]
- Okazawa H, Murata M, Watanabe M, Kamei M, Kanazawa I. Dopaminergic stimulation up-regulates the in vivo expression of brain-derived neurotrophic factor (BDNF) in the striatum. FEBS Lett. 1992;313:138–142. doi: 10.1016/0014-5793(92)81430-t. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos A, Watson C. The Rat Brain in Stereotactic Coordinates. Elsevier Academic Press; New York, NY: 2005. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994:690–696. [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu Q, Poo M. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nature Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Lundgren K, Tran T, Guthrie K, Isackson P, Gall C. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Alcohol, anxiolytics and social stress in rats. Psychopharmacology (Berl) 1995;121:135–144. doi: 10.1007/BF02245600. [DOI] [PubMed] [Google Scholar]
- Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci. 2002;15:1681–1691. doi: 10.1046/j.1460-9568.2002.02000.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Van Leeuwen SD, Hof L, Jonker AJ, Voorn P. Compartment-specific changes in striatal neuronal activity during expression of amphetamine sensitization are the result of drug hypersensitivity. Eur J Neurosci. 2002;16:2462–2468. doi: 10.1046/j.1460-9568.2002.02308.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]