Abstract
Pyometra is an inflammatory disease of the uterus that can be caused by chronic exposure to estrogens. It is unknown whether weakly estrogenic endocrine disruptors can cause pyometra. We investigated whether dietary exposures to the estrogenic endocrine disruptor bisphenol A (BPA) induced pyometra. Pyometra did not occur in CD1 mice exposed to different dietary doses of BPA ranging from 4.1 to >4000 µg/kg/day or 17α-ethinyl estradiol (EE; 1.2 to >150 µg/kg/day). In the C57BL/6 strain, pyometra occurred in the 15 µg/kg/day EE and 33 µg/kg/day BPA treatment groups. At the effective concentration of BPA, histological analysis revealed pathological alterations of uterine morphology associated with a >5.3-fold increase in macrophage numbers in non-pyometra uteri of C57BL/6 mice exposed to BPA. These results suggest that BPA enhances immune responsiveness of the uterus and that heightened responsiveness in C57BL/6 females is related to increased susceptibility to pyometra.
Keywords: Bisphenol A, macrophage, pyometra, uterus, mouse estrogen, endocrine disruptor, immune response
1. Introduction
Inflammatory conditions of the uterus occur in specific strains of rats and mice receiving extended exposures to estrogens [1]. Pyometra is literally defined as “puss in the uterus.” This potentially life-threatening infectious disease is characterized by inflammation and intraluminal accumulation of purulent material in the uterus. Pyometra is clinically most well-known to veterinary medical science, where it is a common occurrence in unspayed female dogs; nearly 25% of bitches will be affected by pyometra before the age of 10 years [2, 3]. In addition to dogs, pyometra, while less common, is also a significant disease in domestic cats and captive large felids, some livestock species, and laboratory animals such as rabbits and some strains of mice and rats. In humans, pyometra is a relatively uncommon condition with rare but significant mortality resulting from spontaneous uterine perforation or rupture leading to septicemia. In the general population, it is estimated to account for about 0.04% of gynecological admissions; however, the incidence becomes increased to >13% in the elderly [4].
Hormonal factors, particularly the actions of estrogen on the uterus, play a central role in the etiology of pyometra. Along with hormonal status and age, genetic factors related to cellular infiltration of leukocytes into the uterus play a major role in regulating sensitivity to estrogen-induced uterine inflammation and pyometra [6–8]. In sensitive strains of laboratory rats and mice, it is well established that chronic exposure to estradiol or the highly efficacious non-steroidal estrogen, diethylstilbestrol (DES), can induce pyometra [1, 6, 9–12]. These findings suggest the possibility that the highly prevalent environmental estrogenic endocrine disrupting chemical, bisphenol A (BPA), may have a similar effect. Bisphenol A is a synthetic monomer used in the production of epoxy resin-based sealants and polycarbonate plastics. Measurable levels of BPA have been found in >90% of humans tested at levels that affect fecundity, embryonic development and carcinogenesis in experimental models [13–15]. The ability of endocrine disrupting chemicals such as BPA, that are considered weak estrogens based on potency in a uterotrophic bioassay or binding affinity at the nuclear estrogen receptors, to cause pyometra is essentially unknown. However, BPA can cause morphological changes in the CD1 mouse uterus at doses considered to be safe in humans, suggesting mechanisms other than those assessed by the rodent uterotrophic bioassay are involved with some actions of BPA in the uterus [16]. As part of a larger effort to determine the physiological consequences of dietary exposure to the endocrine disrupting chemical BPA, we investigated whether BPA might act in an estrogen-like fashion to induce pyometra in mice exposed to BPA or 17α-ethinyl estradiol (EE) as a positive control for estrogenic activity in their diet.
2. Materials and Methods
2.1 Animal husbandry and dietary exposures
All animal procedures were performed in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Animals were maintained on a 14 h light, 10 h dark cycle and provided food and water ad libitum in single-use polyethylene caging certified BPA-free (Innovive, San Diego California). Sani-chip bedding (Irradiated Aspen Sani-chip; P.J. Murphy Forest Products Corp. Montville, NJ) was used to avoid corncob-derived mycoestrogen exposure. Sterile drinking water was generated by a dedicated water purification system (Millipore Rios 16 with ELIX UV/Progard 2) that reduces organic contaminants to less than 1% of source levels. Drinking water was dispensed from BPA-free polyethylene water bottles (Innovive, San Diego California).
A modified open standard diet (supplemental data table 1; Product #10010504 Research Diets) was used to eliminate possible dietary phytoestrogen contamination. Test compounds were directly incorporated into the extruded pellet diets that were unsupplemented (control) or supplemented with 17α-ethinyl estradiol (EE; 1,3,5(10)-estratrien-17α-ethinyl-3,17β-diol, CAS No. 57-63-6, Batch No. H923; Steraloids Inc., Newport, RI) or bisphenol A (2,2-bis(4-hydroxyphenyl)propane; BPA; CAS 80-05-7; Lot 11909; USEPA/NIEHS standard) at desired final concentrations (supplemental data table 1). For BPA, all three doses used were designed to fall below the NOAEL (50 mg/kg/da). The high dose (30 ppm) was between the NOAEL and the reference dose (50 µg/kg/da), the middle dose (0.3 ppm) approximated the reference dose, and the low dose (0.03 ppm) fell below the reference dose [17]. Diets containing EE were estimated to be at or below an effective dose previously reported to decrease fecundity in mice (1.3 ppm, 0.1 ppm and 0.01 ppm) [18].
Seventy (35 of each sex) C57BL/6NHsd and seventy Hsd:ICR (CD1 Swiss) mice aged six to seven weeks were received from Harlan Laboratories, Inc. (Indianapolis, IN). Males and females from each strain were group housed five per cage with the same sex and evenly divided among control or one of the six dietary treatments and maintained on those treatments from arrival until necropsy. Mice were allowed to acclimate to housing and treatment for two weeks. Body weight and food consumption were monitored weekly, while general health and vaginal cytology were monitored daily. Following the acclimation period, males and females in the same group were randomly paired in individual housing and observed daily within the first 3 hr of the light cycle for copulation plug. Upon observation of a copulation plug, assessment of vaginal cytology ceased, the male was removed, and females were individually housed and observed twice daily. Dams were supplied with nestlets, monitored for general health, and observed for parturition. Body weights of both males and females were monitored weekly until necropsy. Dams and their offspring were housed together until postnatal day 21, at which time the offspring were separated and dams were group housed with F0 females from the same treatment group.
2.2 Monitoring of estrous
Progression of estrous cycles was monitored in F0 females by morphological analysis of daily vaginal lavage prior to copulation. Briefly, approximately 200 µl of sterile NaCl solution (0.09%) was used to flush the vulva using a fire polished pipette. Stage of the estrous cycle was determined by microscopic assessment of the vaginal cell morphology in each lavage sample. Samples with epithelial cells in various stages of nuclear degeneration and cytoplasmic shrinkage and polymorphonuclear leukocytes indicated diestrus; samples with nucleated endothelial cells were indicative of proestrus; and samples with cornified, the presence of non-nucleated cellular debris was characteristic of estrus.
2.3 Necropsy and tissue preparation
Female F0 mice were euthanized by CO2 asphyxiation and complete necropsy was performed between the ages of 19 and 23 weeks. Digital images of animals, uteri, and ovaries were captured as part of gross examination during necropsy (Nikon D3000 with 50 mM Nikkor macro lens). Tissues were excised and cleaned of excess connective tissue and fat, blotted of excess fluids, and weighed. Isolated tissues were fixed with several changes of 5% paraformaldehyde or 10% neutral buffered formalin. Tissues were blocked by sectioning with a sterile razor blade and washed several times in 70% ethanol prior to automated tissue processing and embedding in paraffin (Histocenter 3; Thermo-Shandon Kalamazoo, MI). Microtome sections were cut at 5 µm thickness from blocks at 4°C and placed on positively charged slides for hematoxylin and eosin (H&E) staining and immunohistochemistry. Standard H&E staining was performed in order to examine tissue structure and morphology.
2.4 Immunohistochemistry
Paraffin sections were dewaxed and rehydrated through graded series of ethanol concentrations into phosphate buffered saline (PBS, pH 7.4) and endogenous peroxidase blocked by treatment with 3% H2O2 in PBS. After washing, heat-mediated epitope retrieval was performed at 100°C in 10 mM citric acid with 0.05% Tween-20 (pH 6.0). Sections were incubated at room temperature for >30 min with 3% normal goat serum in PBS, washed, and then incubated overnight at 4°C with 4 µg/mL of a macrophage specific F4/80 rat monoclonal antibody (Cl:A3-1; Abcam Cambridge, Mass). Immunoreactivity was visualized with 0.05% 3’3-diaminobenzidine by the avidin-biotin peroxidase complex method (Vector Laboratories). Stained sections were lightly counterstained in hematoxylin for 30 seconds (Richard Allan; Kalamazoo, MI) and mounted in Permount (Fisher Scientific, Fair Lawn NJ). Internal controls for specificity of immunostaining included replacement of primary or secondary antibodies with non-specific serum. Sections prepared from spleen served as positive controls. Microscopic examination of immunostained material was carried out using a Nikon Eclipse 55i microscope using a DS-Fi1 CCD camera controlled with Digital Sight software (Nikon Instruments, Inc., Melville, NY). For quantitative assessments of immunopositive cell numbers, five random 40x fields of the myometrium and five random 40x fields of the endometrium were collected from a single section for each animal. The number of immunopositive cells per field was manually counted by an investigator blinded to treatment and strain. To account for possible region specific differences in infiltration, the mean number of cells in each of five 40x fields for the myometrium and the endometrium was determined for one section per individual. The sum of those values was taken to be representative of the number of F4/80 positive cells for that individual. Final photomicrograph graphics were generated and labeled using Adobe Photoshop CS3 (Adobe Systems, Inc., San Jose, CA).
2.5 Statistical analysis
Analysis of weight and cell count data was performed using one-way ANOVA followed by Tukey’s Multiple Comparison post-hoc test with Cramer’s correction or Dunnett’s multiple comparison test using GraphPad Prism v5 software (GraphPad Prism®, GraphPad Software, Inc., La Jolla, CA). Significance between differences in values was defined by a p-value of < 0.05.
3. Results
3.1 Diet consumption
The amount of chow consumed by each female animal was determined immediately after mating. From this consumption data, estimations of caloric intake and oral dose of EE and BPA were calculated (Table 1).
Table 1.
Estimated Food Consumption, Caloric Intake, and Dose
| C57BL/6 | Control |
EE 0.01 ppm |
EE 0.1 ppm |
EE 1.3 ppm |
BPA 0.03 ppm |
BPA 0.3 ppm |
BPA 30 ppm |
| Food Consumption (g/mouse/day) | 2.7 ± 0.4 | 2.2 ± 0.5 | 1.8 ± 0.5a | 1.6 ± 0.2a | 2.5 ± 0.4 | 2.5 ± 0.4 | 2.6 ± 0.4 |
| Caloric Intake (kcal/mouse/day) | 9.7 ± 1.5 | 7.9 ± 1.7 | 5.4 ± 1.9a | 5.8 ± 1.0a | 9.2 ± 1.5 | 9.2 ± 1.6 | 9.6 ± 1.4 |
| Dose (µg/kg/day) | 0.0 | 1.4 ± 0.4 | 14.7 ± 6.4 | 164.3 ± 49.5 | 4.0 ± 1.1 | 32.8 ± 10.3 | 4,168.0 ± 1,358.0 |
| Sample Size | 4 | 5 | 4 | 4 | 5 | 5 | 4 |
| CD-1 | Control |
EE 0.01 ppm |
EE 0.1 ppm |
EE 1.3 ppm |
BPA 0.03 ppm |
BPA 0.3 ppm |
BPA 30 ppm |
| Food Consumption (g/mouse/day) | 4.2 ± 1.9 | 4.8 ± 1.8 | 4.0 ± 2.3 | 3.2 ± 2.0 | 4.6 ± 1.6 | 3.8 ± 0.8 | 4.7 ± 1.6 |
| Caloric Intake (kcal/mouse/day) | 11.2 ± 1.5 | 12.7 ± 2.0 | 11.1 ± 4.3 | 8.5 ± 2.6 | 12.3 ± 2.1 | 11.4 ± 3.7 | 13.2 ± 2.4 |
| Dose (µg/kg/day) | 0.0 | 1.2 ± 0.3 | 11.6 ± 3.6 | 158.8 ± 25.3 | 4.1 ± 1.1 | 41.7 ± 10.7 | 4182.0 ± 1218.0 |
| Sample Size | 5 | 5 | 4 | 4 | 5 | 5 | 5 |
Values represent mean of all food consumption measurements ± SD
significantly different from control by one-way ANOVA (p < 0.01).
For C57BL/6 animals, the presence of 0.1 and 1.3 ppm EE in diet resulted in a significant decrease in food consumption and the intake of significantly fewer calories per day as compared to control diet. While EE dose dependently decreased consumption and caloric intake in CD1 animals, the difference in values for any one treatment group were not different from control. No differences in consumption or caloric intake were detected in additional studies comparing control diet and diet supplemented with 0.001 and 0.0001 ppm EE (data not shown). The presence of BPA was not associated with any change in consumption or caloric intake for either strain.
3.2 Effects on body weight
Body weights were monitored from the onset of study until study completion when animals were between 19 and 23 weeks of age. Not surprisingly, EE treatment dose dependently impacted the body weight (Table 2). In the 1.3 ppm EE dose group, body weight was significantly decreased at the end of the first study week. Lower concentrations of EE similarly decreased body weight following an inverse correlation with dose (Table 2). There was also a dose responsiveness observed in the onset of detectable differences in body weight of control and EE dose groups. After the second week on treatment, both 0.1 and 1.3 ppm EE groups weighed significantly less than controls. Those trends continued through the end of study. No difference in either the amount or the rate of weight gained was observed in females of any of the BPA groups (Table 1; Table 2). It thus appears that there is a dose dependent aversion to consuming diet containing EE and that the sensitivity to EE diet was strain dependent. After mating, body weight measurements for mated gravida and nulligravida females were analyzed separately to avoid confounders on body weight related to metabolic consequences of pregnancy, parturition, and lactation status. While impacts on body weight related to treatment and dose remain evident during those later time points, the resulting reduction in sample size prevented meaningful statistical analysis post copulation.
Table 2.
Female Body Weight
| C57BL/6 | ||||||||||||||
| Wks | Control | EE 0.01ppm | EE 0.1ppm | EE 1.3ppm | BPA 0.03ppm | BPA 0.3ppm | BPA 30ppm | |||||||
| 0 | 14.4 ± 0.6 | 14.1 ± 0.4 | 14.4 ± 0.4 | 14.7 ± 0.6 | 14.3 ± 0.8 | 13.7 ± 0.9 | 14.2 ± 0.9 | |||||||
| 1 | 15.5 ± 0.6 | 15.4 ± 0.9 | 14.0 ± 0.4 | 13.1 ± 0.4a | 16.1 ± 0.8 | 15.8 ± 0.6 | 16.4 ± 1.8 | |||||||
| 2 | 16.5 ± 0.6 | 15.7 ± 0.9 | 14.7 ± 0.9a | 13.2 ± 1.0a | 16.6 ± 0.7 | 16.1 ± 0.7 | 16.4 ± 0.6 | |||||||
| 3 | 17.5 ± 1.0 | 16.3 ± 1.3 | 14.6 ± 0.7a | 13.3 ± 0.7a | 17.8 ± 0.9 | 17.3 ± 0.4 | 17.2 ± 0.3 | |||||||
| Status | gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravid |
nulli gravida |
| 4 | 17.9 ± 0.9 | 17.1 ± 0.4 | 15.2 | 16.3 ± 1.1 | NA | 14.7 ± 0.8 | NA | 12.8 ± 0.9 | 18.2 ± 0.6 | 17.0 ± 0.5 | 17.7 ± 0.4 | 17.2 ± 0.6 | 17.6 ± 0.3 | 16.4 |
| 5 | 19.8 ± 1.1 | 19.0 ± 0.8 | 15.9 | 17.3 ± 1.5 | NA | 15.4 ± 0.4 | NA | 13.1 ± 1.5 | 19.9 ± 0.8 | 18.1 ± 1.2 | 19.7 ± 0.7 | 18.8 ± 0.0 | 19.7 ± 0.6 | 17.6 |
| 6 | 26.4 ± 1.7 | 20.4 ± 0.2 | 17.1 | 19.2 ± 0.6 | NA | 15.4 ± 1.1 | NA | 13.1 ± 0.6 | 25.5 ± 0.2 | 19.3 ± 0.1 | 26.6 ± 2.6 | 20.1 ± 0.1 | 26.7 ± 3.0 | 18.9 |
| 7 | 24.3 ± 0.8 | 21.2 ± 1.1 | 18.2 | 20.2 ± 2.0 | NA | 16.2 ± 0.6 | NA | 13.5 ± 1.3 | 32.3 ± 8.5 | 21.4 ± 0.2 | 27.8 ± 6.1 | 21.7 ± 1.2 | 26.3 ± 4.7 | 19.6 |
| CD1 | ||||||||||||||
| Wks | Control | EE 0.01ppm | EE 0.1ppm | EE 1.3ppm | BPA 0.03ppm | BPA 0.3ppm | BPA 30ppm | |||||||
| 0 | 20.1 ± 1.4 | 20.8 ± 0.6 | 19.9 ± 0.7 | 20.2 ± 1.7 | 20.3 ± 0.9 | 19.6 ± 1.3 | 19.3 ± 1.4 | |||||||
| 1 | 22.5 ± 1.2 | 24.0 ± 1.2 | 20.8 ± 1.7 | 18.6 ± 1.4a | 23.3 ± 1.0 | 23.4 ± 2.7 | 23.7 ± 1.6 | |||||||
| 2 | 24.6 ± 1.5 | 26.6 ± 1.8 | 21.8 ± 1.7 | 18.7 ± 1.3a | 25.4 ± 2.0 | 26.4 ± 4.2 | 27.0 ± 3.4 | |||||||
| 3 | 23.6 ± 1.0 | 25.0 ± 1.6 | 21.3 ± 1.3 | 18.3 ± 1.1a | 24.0 ± 1.8 | 25.5 ± 3.2 | 25.6 ± 2.3 | |||||||
| Status | gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
gravida |
nulli gravida |
| 4 | 26.0 ± 1.3 | NA | 27.9 ± 1.5 | 26.4 ± 2.0 | NA | 22.5 ± 1.2 | NA | 19.0 ± 1.4 | 25.2 ± 2.3 | 27.3 ± 2.6 | 27.4 ± 3.0 | 25.6 ± 2.8 | 26.8 ± 0.5 | 29.7 |
| 5 | 33.6 ± 2.4 | NA | 35.7 ± 6.2 | 31.2 ± 0.5 | NA | 23.0 ± 1.3 | NA | 19.3 ± 1.3 | 32.3 ± 5.1 | 29.0 ± 3.2 | 32.1 ± 1.3 | 26.5 ± 2.3 | 34.3 ± 1.9 | 29.6 |
| 6 | 53.4 ± 5.2 | NA | 33.7 ± 3.4 | 32.9 ± 3.5 | NA | 24.1 ± 2.4 | NA | 19.4 ± 1.4 | 47.6 ± 13.3 | 31.8 ± 4.2 | 45.6 ± 8.2 | 27.6 ± 3.2 | 33.7 ± 1.6 | 32.7 |
| 7 | 35.5 ± 2.7 | NA | 36.0 ± 0.8 | 32.2 | NA | 29.1 ± 1.9 | NA | 19.9 | 37.1 ± 1.6 | 34.8 ± 4.5 | 38.5 ± 1.0 | 38.5 ± 3.9 | 36.5 ± 2.8 | 37.9 |
Values represent group mean ± SD;
Significantly different from Control by one-way ANOVA (p < 0.05). Weeks 4–7 of treatment are separated by those F0 females that produced a live litter (Gravida) and those that did not produce a live litter (Nulligravida).
3.3 Fertility and fecundity
In both strains, diets containing 0.1 and 1.3 ppm EE resulted in no productive mating (Table 3). This is considered likely to have resulted from insufficient nutritional intake, as is the marked delay in mating and decreased percentage of mating that was also observed. In the C57BL/6 mice in the low dose EE group, a single productive mating occurred that resulted in a small litter with 4 pups. In the low dose EE CD1 group, a modest reduction in fertility and fecundity was also observed (Table 3). In contrast, no effect on fertility or fecundity in the C57BL/6 strain for any dose of BPA was observed (Table 3). In the CD1 strain 0.03 ppm (4 µg/kg/da) and 0.3 ppm (41 µg/kg/da) BPA treatment groups, an increase in latency of mating and a decrease in productive mating was noted.
Table 3.
Measures of Fertility and Fecundity
| C57BL/6 | |||||||
| Control | EE 0.01 | EE 0.1 | EE 1.3 | BPA 0.03 | BPA 0.3 | BPA 30 | |
| No. Breeding Pairs | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| No. litters born | 3 | 1 | 0 | 0 | 3 | 3 | 4 |
| Mating index (%) | 100 | 100 | 20 | 100 | 100 | 100 | 100 |
| Fertility index (%) | 60 | 20 | 0 | 0 | 60 | 60 | 80 |
| Precoital time (da) | 2.0 ± 0.8 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.8 ± 3.0 | 2.2 ± 0.8 | 2.2 ± 1.5 | 1.8 ± 0.5 |
| Gestation (da) | 19.0 ± 0.0 | N/A | N/A | N/A | 19.0 ± 0.0 | 18.7 ± 0.6 | 19.0 ± 1.0 |
| Pups per litter | 7.7 ± 1.2 | 4.0 | 0.0 | 0 | 8.0 ± 0.0 | 7.0 ± 0.0 | 6.8 ± 3.2 |
| % Males | 50.0 ± 10.1 | 50 | N/A | N/A | 50.0 ± 17.68 | 40.0 ± 18.7 | 37.2 ± 10.2 |
| CD1 | |||||||
| Control | EE 0.01 | EE 0.1 | EE 1.3 | BPA 0.03 | BPA 0.3 | BPA 30 | |
| No. Breeding Pairs | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| No. litters born | 5 | 3 | 0 | 0 | 3 | 3 | 4 |
| Mating index (%) | 100 | 80 | 60 | 40 | 80 | 80 | 100 |
| Fertility index (%) | 100 | 75 | 0 | 0 | 75 | 75 | 80 |
| Precoital time (da) | 2.6 ± 0.5 | 2.3 ± 0.5 | 7.2 ± 3.3 | 7.3 ± 6.5 | 4.2 ± 3.3 | 4.4 ± 3.1 | 2.2 ± 0.8 |
| Gestation (da) | 18.6 ± 0.5 | 18.5 ± 0.7 | N/A | N/A | 19.0 ± 0.0 | 19.0 ± 0.0 | 19.0 ± 0.8 |
| Pups per litter | 8.4 ± 3.5 | 10.0 ± 3.0 | N/A | N/A | 10.0 ± 4.0 | 10.0 ± 1.7 | 8.0 ± 4.8 |
| % Males | 34.9 ± 12.7 | 44.0 ± 18.7 | N/A | N/A | 50.5 ± 29.3 | 45.4 ± 30.0 | 31.7 ± 19.9 |
Values represent mean ± SD where relevant; Mating index (%) = No. copulation plug positive females / No. females paired X 100; Fertility index (%) = No. females with live litters / No. copulation plug positive females X 100; Precoital time = days from introduction of mating pairs to observation of copulation plug.
3.4 Necropsy and organ weights
Because of morbidity, the C57BL/6 mice in the EE treatment groups were euthanized, and necropsy was performed one to two weeks prior to necropsy of the control or BPA treated groups. Similarly, one C57BL/6 female was sacrificed prematurely (~wk 4) due to an undefined neurological pathology; pyometra was not observed in that individual. Due to the small sample size, variability of some endpoints, and pathology associated with differences in body weights, statistical assessments of differences in these endpoints compared to controls are of limited value in determining impact of treatments on gross organ weights. However, these values clearly illustrate the dramatic strain specific differences observed in the uterine weights in response to treatment with 0.1 ppm EE and 0.3 ppm BPA (Table 4). An average 23.5 fold increase in uterine weight compared to controls was observed in the C57BL/6 0.1 ppm EE treatment group. In contrast, the uterine weights for the CD1 0.1 ppm and 1.3 ppm EE treated groups were not different than the control groups. Spleen weights for the C57BL/6 0.1 ppm EE treatment group were significantly increased compared to controls, a result consistent with a substantial immune response in females with affected uteri (p<0.05). With the exception of uterus and spleen weights in each of the affected C57BL/6 treatment groups, there were no additional changes in organ weights.
Table 4.
Organ Weights
| C57BL/6 | 17α-Ethinyl Estradiol | Bisphenol A | |||||
| Control | 0.01 ppm | 0.1 ppm | 1.3 ppm | 0.03 ppm | 0.3 ppm | 30 ppm | |
| Sample Size | 4 | 3 | 5 | 4 | 4 | 5 | 4 |
| Liver | 1.53 ± 0.32 | 1.07 ± 0.02 | 1.18 ± 0.28 | 1.61 ± 0.16 | 1.53 ± 0.15 | 1.34 ± 0.17 | 1.39 ± 0.22 |
| Kidney | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 |
| Spleen | 0.09 ± 0.00 | 0.07 ± 0.01 | 0.17 ± 0.11 | 0.11 ± 0.03 | 0.09 ± 0.01 | 0.09 ± 0.00 | 0.10 ± 0.02 |
| Heart | 0.19 ± 0.02 | 0.16 ± 0.04 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.16 ± 0.02 | 0.21 ± 0.06 | 0.19 ± 0.05 |
| Brain | 0.44 ± 0.01 | 0.45 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.44 ± 0.01 | 0.46 ± 0.01 | 0.44 ± 0.01 |
| Bladder | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Uterus | 0.13 ± 0.03 | 0.16 ± 0.02 | 2.96 ± 0.59 | 0.63 ± 0.09 | 0.12 ± 0.02 | 1.21 ± 1.23 | 0.13 ± 0.01 |
| CD1 | 17α-Ethinyl Estradiol | Bisphenol A | |||||
| Control | 0.01 ppm | 0.1 ppm | 1.3 ppm | 0.03 ppm | 0.3 ppm | 30 ppm | |
| Sample Size | 5 | 4 | 5 | 5 | 4 | 4 | 5 |
| Liver | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.5 ± 0.3 | 1.8 ± 0.2 | 2.1 ± 0.4 | 1.6 ± 0.1 | 1.9 ± 0.4 |
| Kidney | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.20 ± 0.03 | 0.17 ± 0.02 | 0.19 ± 0.02 |
| Spleen | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.03 |
| Heart | 0.20 ± 0.04 | 0.23 ± 0.03 | 0.13 ± 0.02 | 0.10 ± 0.01 | 0.22 ± 0.05 | 0.18 ± 0.03 | 0.21 ± 0.03 |
| Brain | 0.50 ± 0.03 | 0.50 ± 0.02 | 0.48 ± 0.03 | 0.45 ± 0.02 | 0.50 ± 0.03 | 0.49 ± 0.03 | 0.47 ± 0.03 |
| Bladder | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Uterus | 0.21 ± 0.04 | 0.23 ± 0.08 | 0.23 ± 0.09 | 0.30 ± 0.13 | 0.25 ± 0.06 | 0.34 ± 0.13 | 0.23 ± 0.09 |
Bold indicates p<0.05 compared to control values.
3.5 Uterine Pathology: C57BL/6 pyometra
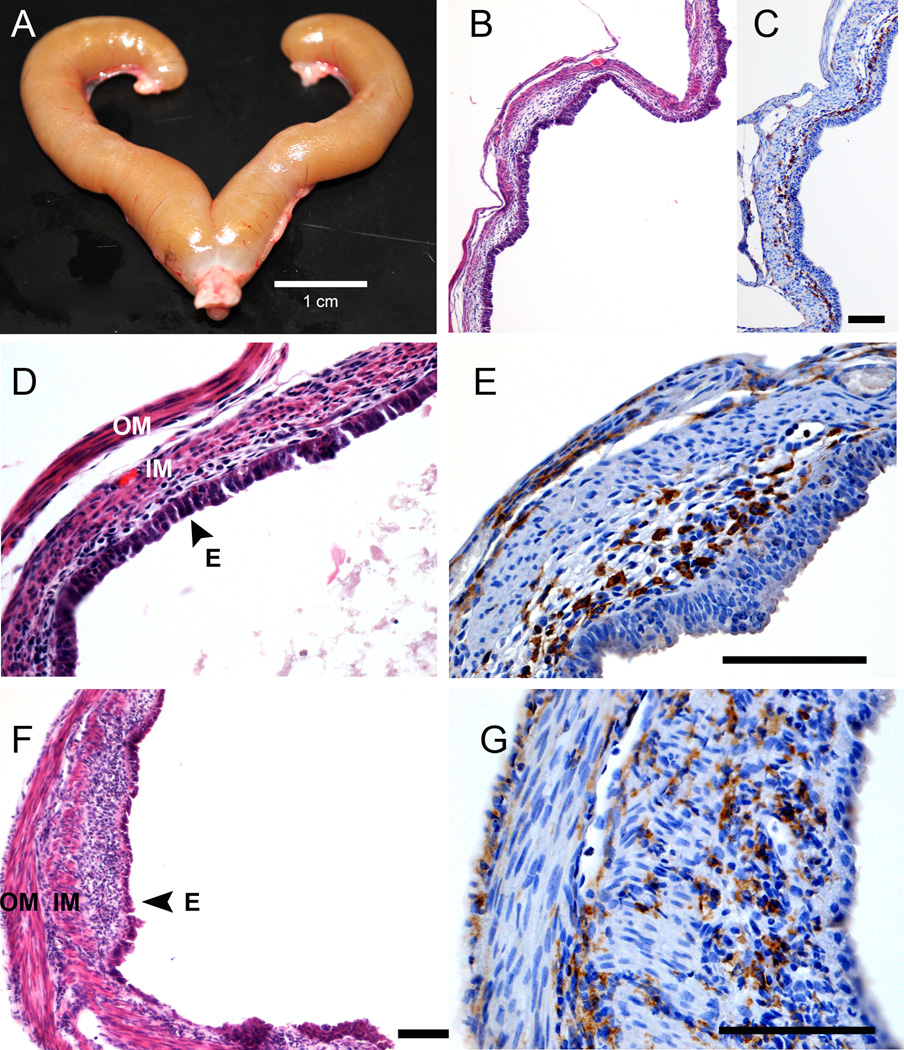
During the course of the study, it was observed that four of the C57BL/6 females in the 0.1 ppm EE group and one in the 0.3 ppm BPA group (none of which produced a live litter) developed a moribund phenotype characterized by lethargy and highly distended abdomens. Upon necropsy, the uterus of each of the affected animals was found to be grossly enlarged and filled with purulent fluid, indicative of severe pyometra (Fig. 1A). No pyometra was observed in any CD1 mice from any treatment group, indicating an increased sensitivity of estrogen-induced pyometra for C57BL/6 mice. Histological examination of the pyometic uteri revealed thin remnants of the myometrium and endometrium. The remaining cells of the endometrium were in a disorganized and ulcerated layer that was highly necrotized (Fig. 1B-G). Immunostaining for the macrophage specific antigen F4/80 revealed a high degree of macrophage infiltration associated with the outer layer of myometrium and localized to the inner border of the endometrial-like cell layer remnant (Fig. 1C; 1E; 1G).
Figure 1.
Histological and immunohistochemical assessments of C57BL/6 uterus affected by severe pyometra. (A-E) Representative images of a pyometric uterus from a C57BL/6 mouse in the 0.3 ppm BPA treatment group. (A) Shown is a photograph of the uterus dissected from the C57BL/6 female in the 0.3 ppm BPA treatment group that developed pyometra. The luminal space of the uterus was filled with purulent material. The scale bar is 1 cm. (B) Representative photomicrographs of H&E stained sections of the affected uterus, showing extreme metaplasia and a high degree of necrosis of the endometrium, features characteristic of severe pyometra. (C) Shown is a representative section of tissue from the affected uterus stained with a monoclonal antibody raised against the macrophage specific antigen F4/80. (D) Shown are representative sections of a uterus from a C57BL/6 female with pyometra from the 0.1 ppm EE treatment group stained with H&E or (E) stained with a monoclonal antibody raised against the macrophage specific antigen F4/80. Scale bar for paired images is 100 µm. (F) Shown are representative images from a section of a uterus from a C57BL/6 female with pyometra from the 0.1 ppm EE treatment group stained with H&E or (G) stained with a monoclonal antibody raised against the macrophage specific antigen F4/80. Scale bars for images are 100 µm. OM, outer myometrium; IM, inner myometrium; E, endometrium.
3.5 Uterine Pathology: effects on uterine morphology and macrophage infiltration
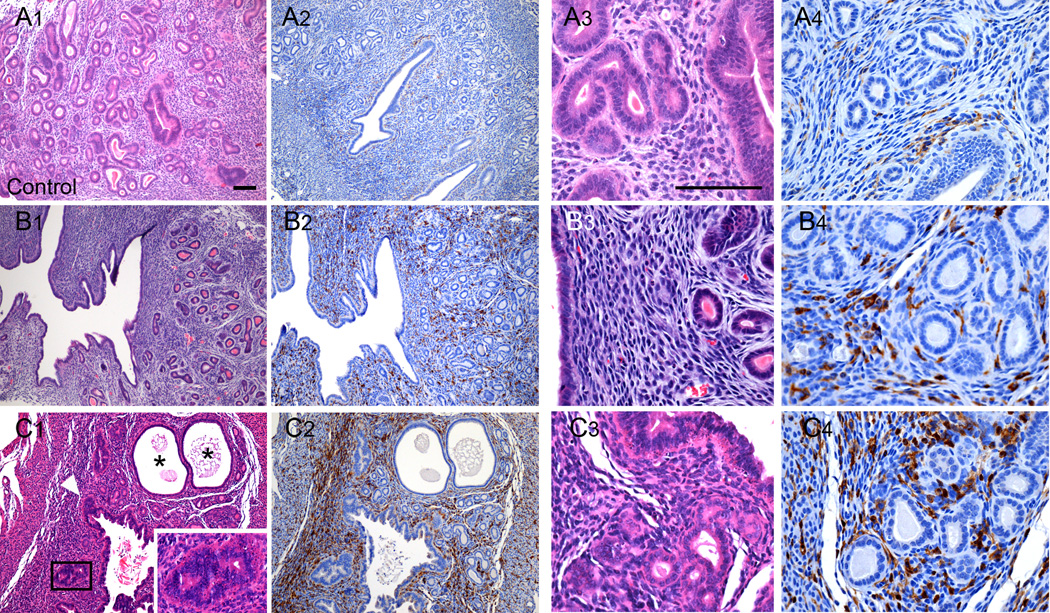
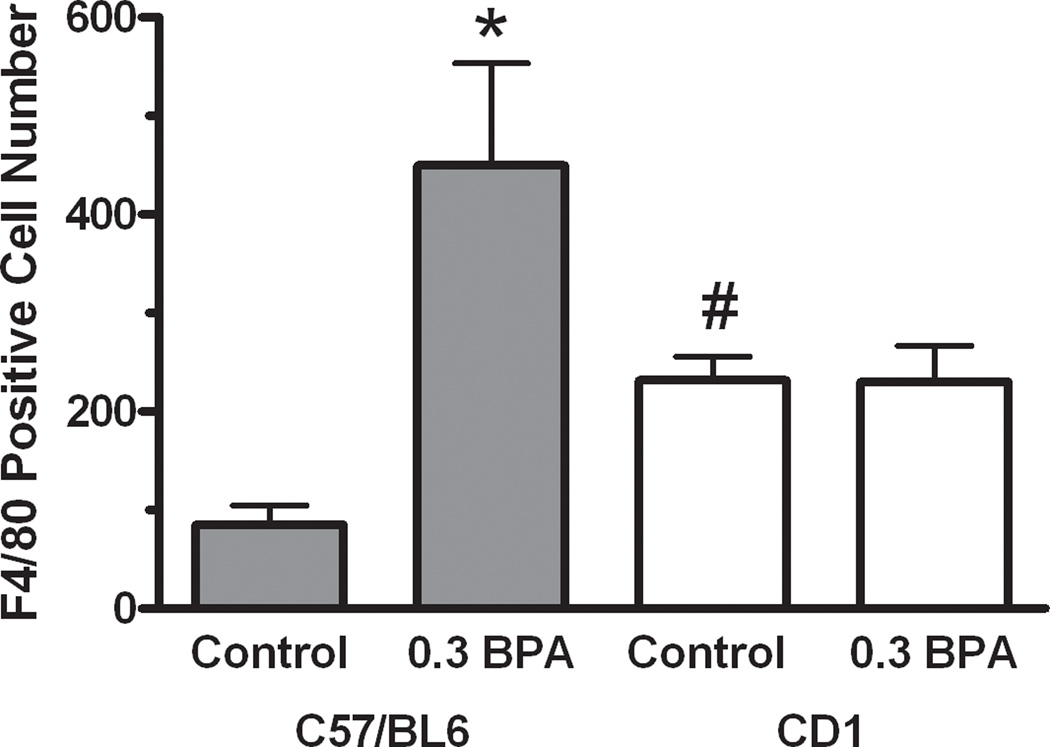
To investigate whether exposures to EE or BPA were associated with uterine pathology and increased macrophage infiltration, even in the absence of severe overt pyometra, histological comparison of uteri from C57BL/6 females in the control group with those from the 0.3 ppm BPA group without pyometra was performed. In the BPA treatment group, the general structural composition of the uterus including the myometrium, luminal and glandular epithelium, and endometrial stroma was intact. However, disorganization in these uterine structures was evident (compare Fig. 2A with Fig. 2B; 2C). The myometrium contained two distinctive smooth muscle layers each of proper thickness, and the endometrium contained groups of glands surrounding the luminal epithelium. However, hypertrophic luminal epithelial cells were evident. In some cases, cells of the stroma were tightly packed and disorganized (Fig. 2C). Cystic endometrial hyperplasia (CEH) was evident in the uterus of one of the C57BL/6 female in the 0.3 ppm BPA group (Fig. 2C1). Compared to control (Fig. 2A2), a marked increase in F4/80 positive immunostaining was observed in all uteri of the 0.3 ppm BPA treatment group (Fig. 2 B2; C2). Quantitative assessment of F4/80 immunopositive cell numbers revealed a statistically significant 5.3-fold increase in the number of macrophages present in the uterus of C57BL/6 mice from the 0.3 ppm BPA treatment group (Fig. 4). There were no detectable differences in the density of immunopositive cells localized to the endometrium or the myometrium in either control or 0.3 ppm BPA groups. Comparison of the numbers of F4/80 immunopositive cells in uteri from primagravida and nulliparous F0 C57BL/6 females without pyometra, revealed no differences within a given treatment group.
Figure 2.
Effects of oral exposure to BPA on uterine morphology and macrophage infiltration in C57BL/6 mice without overt pyometra. Shown are representative photomicrographs of uterine sections from a control female C57BL/6 mouse (Panels A1-A4) and from two different animals in the 0.3 ppm BPA treatment group without symptomatic pyometra (Panels B1-B4; C1-C4). The uteri of BPA treated animals displayed metaplasia (C1, inset) throughout the various regions of the uterus with glandular disorganization and apparent luminal epithelial hyperplasia (C1, arrowhead). Cystic endometrial hyperplasia was also observed in one uterus (C1; denoted by *). Comparison of the density of F4/80 immunopositive cells present in sections from controls (Panels A2; A4) with those from the BPA treatment groups (Panels B2; B4 and C2; C4) demonstrates increased macrophage infiltration. Scale bars for each high magnification and low magnification photomicrograph is 100 µm.
Figure 4.
Quantitative comparison of F4/80 immunoreactive cell numbers in the uterus of control and BPA treated C57BL/6 and CD1 mice. Immunopositive macrophage cell numbers present in the myometrium and endometrium were counted by an observer blinded to treatment and strain. Values indicated are mean cell numbers present in one 40x field of the myometrium and one field of the endometrium of a single section per animal. Error bars represent the SEM. For C57BL/6, n=4 animals, and for CD1, n=5 animals. The level of significance between values was assessed using a one-way ANOVA. * indicates the level of differences between values of same strain control group and BPA treated group are significant (P< 0.01); # indicates that the level of difference between values for each control group is significant (P< 0.01). No difference was observed between C57BL/6 and CD1 mice treated with 0.3 ppm BPA.
To assess whether heightened immune responsiveness with BPA and EE exposure was specific to the uteri from C57BL/6 animals that developed pyometra, we also assessed uterine morphology and the numbers of macrophages present in uterine tissue from CD1 mice (Fig. 3A-H). In contrast to uteri from C57BL/6 animals, the overall morphology of the uterus was generally normal for the CD1 females in the 0.3 ppm BPA (Fig. 3B; E) and 0.1 ppm EE (Fig. 3F-H) treatment groups. However, CEH was detected in one of four CD1 females in the 0.3 ppm BPA group, and three of four uteri in the 0.1 ppm EE group. Compared to the C57BL/6 control group, increased numbers of F4/80 immunopositive cells were evident in uterine tissues from CD1 controls (Fig. 3A; D; Fig 4). Similar increases in macrophages were apparent for the 0.3 ppm BPA (Fig 3B; E) and 0.1 ppm EE treated females (Fig. 3F-H). Quantitative comparison of the numbers of macrophages present in the uteri showed 2.7-fold higher F4/80 positive cells in CD1 controls compared to C57BL/6 control females (Fig. 4). Compared to CD1 controls, a significant increase in the number of F4/80 positive cells was not observed in the uteri of CD1 mice in the 0.3 ppm BPA treatment group (Fig. 4).
Figure 3.
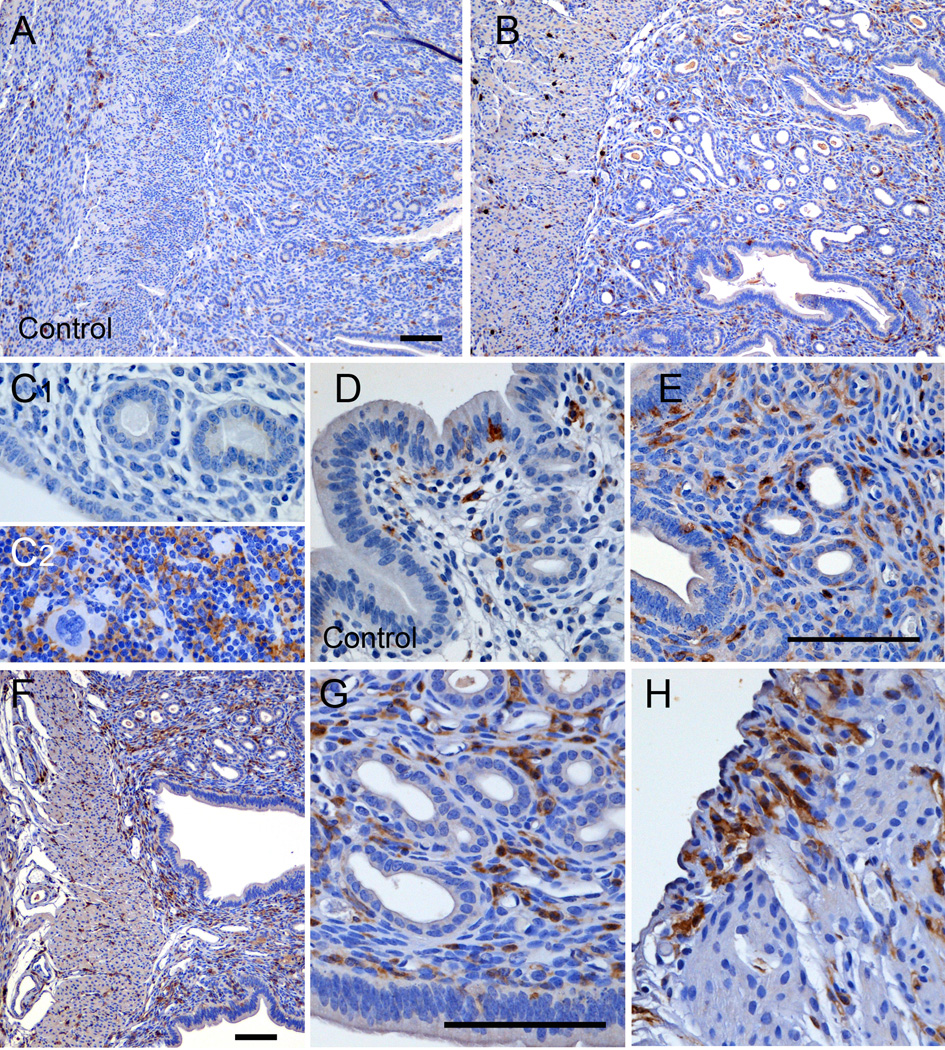
Effects of oral exposure to BPA on uterine morphology and macrophage infiltration in CD1 mice, which did not display pyometra. Shown are representative low and high magnification photomicrographs of uterine sections stained for F4/80 positive macrophages from female CD1 mouse in the control group (A; D), the 0.3 ppm BPA treatment group (B; E) or the 0.1 ppm EE treatment group (F-H). Control for specificity of immunostaining included replacement of primary antibodies with non-specific serum (C1). Immunostaining of sections prepared from spleen served as positive controls (C2). Scale bars represent 100 µm.
Discussion
It should first be pointed out that the intent of this study was to compare appropriate concentrations of dietary estrogenic chemicals that would allow reproduction and not to determine whether or not BPA had specific effects upon reproductive or other endpoints. As a result, the observed changes in those endpoints (e.g. fertility and fecundity, organ weights, etc.) must not be over interpreted. However, in the case where food consumption is markedly altered and reproduction was completely blocked, some interesting and valuable insight to the impact of these dietary estrogens is possible. A clear and significant differential sensitivity to estrogens between the CD1 and C57BL/6 strains was evident. This difference in sensitivity was dose dependent and extended to an apparent aversion for consuming EE. That effect was dramatic and statistically significant for two related, though independent, endpoint assessments (body weight and food consumption), even in a study with limited statistical power. Additionally, the development of pyometra, a well characterized uterine pathology related to estrogen exposure, was found to occur in the C57BL/6 strain but not in the CD1 strain. The relatively increased sensitivity of the C57BL/6 strain to impacts of estrogens on endpoints as varied as decreased food consumption and a uterine immunopathology could be interpreted to suggest that the differential sensitivity of these strains to estrogens is related to a common fundamental aspect of estrogen signaling rather than isolated tissue-specific differences between strains. However, the apparent trend toward decreased fecundity observed in the BPA exposed CD1 mice suggests there could be increased reproductive sensitivities to some actions of BPA. The phenomenon of strain differences in endpoint- and tissue-specific responses to estrogens in vivo has been extensively supported in the EDC literature [19–24]. The effects observed here suggest about a 10-fold increase in estrogen sensitivity of the C57BL/6 mice and support the notion of differential sensitivity of these experimental animal models. However, statements regarding strain specific estrogen sensitivity should take into account the specific endpoints analyzed.
In contrast to the relatively well-documented cases in veterinary medicine, estrogen-induced pyometra has received relatively little attention from researchers investigating the actions of endogenous estrogens and estrogenic endocrine disrupting chemicals. The first reported observation of pyometra occurring in response to long periods of estrogen exposure in laboratory rodents was made by Burrows [1]. A similar study indicated that some strains of rat remained resistant to follicular hormone-induced pyometra; a phenomenon not seen in other species tested [12]. Later studies demonstrated that estrogenic hormones altered bacterial content of the murine uterus, suggesting that there could be a link between the ability of estrogens to modify the structure of the uterus and the observed changes in the uterine microbiome [25]. Studies investigating the actions of the non-steroidal estrogen, DES, clearly demonstrated strain specific differences in the susceptibility to developing pyometra. Those strain-related differences may result from direct, estrogen receptor-mediated, activation of the innate immune response [10, 26–28]. During long-term DES exposure, Sprague-Dawley rats, but not ACI rats, developed DES-induced pyometra [11]. Subsequently, the Brown Norway rat was identified as sensitive to developing pyometra, which allowed demonstration, via quantitative trait loci mapping, that the strain specific differences in susceptibility to developing estrogen-induced pyometra were, in part, genetically controlled [6].
The results of this study clearly demonstrate strain differences in susceptibility as dietary exposure to approximately 15 µg/kg/day EE results in essentially quantitative induction of pyometra in C57BL/6 mice, while the CD1 mice did not develop pyometra at that dose or even at a 10-fold greater dose. Further, one of the five female mice that consumed diet containing 0.3 ppm BPA, resulting in exposure of about 33 µg/kg/day, developed pyometra. Again, no pyometra was observed in any CD1 animals or in C57BL/6 mice maintained on control diet lacking estrogens. The development of pyometra in a BPA-treated animal is important in that it is the first report of a weakly-estrogenic xenoestrogen inducing pyometra; this demonstrates that, in regard to its pathological actions on the uterus, BPA is presumably acting as an estrogen mimetic. This may be of concern in humans (especially sensitive subpopulations) given that >90% of Americans tested have measurable levels of BPA in their urine [13, 15]. It is also notable that the dose of BPA that induced pyometra in this study was below the dose considered safe for human exposure (50 µg/kg/day), which was based on a NOAEL of 50 mg/kg/day from rodent dietary studies with a BPA concentration of 1000 ppm [17].
To assess the role of the immune response in the differential strain susceptibility to develop pyometra with exposure to estrogens, we investigated the degree of uterine macrophage infiltration in each strain. Regardless of treatment, we found high levels of macrophages in the remnants of the necrotic myometrium and the endometrial-like cells of the pyometric uteri. Based on those findings we hypothesized that BPA and EE were increasing the basal inflammation in the uteri and reasoned that BPA-exposed C57BL/6 mice without overt pyometra would have an increased immune response, as indicated by increased presence of F4/80 positive macrophages. Comparison of the numbers of macrophages present in the uteri of control CD1 and C57BL/6 mice revealed a higher number of F4/80-staining macrophages in CD1 samples, suggesting that there was an elevated basal level compared to C57BL/6. However, there was a significant increase in macrophage counts in C57BL/6 mice treated with 0.3 ppm BPA compared to control with no increase seen in 0.3 ppm BPA treated CD1 mice. Along with increased numbers of macrophages in the mice that did not develop pyometra, significant structural differences were observed in the C57BL/6, but not the CD1 strain. The C57BL/6 mice exposed to 0.3 ppm BPA displayed stromal and glandular metaplasia, with no structural differences observed in the corresponding CD1 treatment group. Those differences in uterine phenotype further support the differential sensitivity to estrogens between the C57BL/6 and CD1.
Thus, it seems likely that these immunologic and structural differences between the strains indicate a potential key difference in susceptibility to developing overt pyometra which is related to the immune response. While the basal (control) number of macrophages in the CD1 uterus was significantly higher than that in the C57BL/6 mice, the increase with BPA exposure was very robust in the C57BL/6 mice. It would appear that the magnitude of the uterine immune response represents a possible reason for increased susceptibility in C57BL/6 mice, and may also explain the difference in structural response to BPA treatment in C57BL/6 mice. Therefore, the heightened responsiveness of the C57BL/6 animals is considered a probable contributing factor for the sensitivity of this strain to developing estrogen-induced pyometra. Previous studies in “sensitive” strains of rat have identified quantitative trait loci associated with estrogen-induced pyometra; however, there is no understanding of the mechanisms involved with sensitivity or resistance to pyometra. This pathology may be related to interactions between the uterine immune response and the uterine microbiome. The results here suggest that pyometra in the C57BL/6 strain might serve as a sensitive endpoint for understanding the mechanisms responsible for the impact of estrogen and estrogenic endocrine disrupting chemicals on immunity. Further, the C57BL/6 strain might serve as a useful model of sensitive subpopulations at risk for developing immunological disorders related to exposures to estrogenic EDCs.
Highlights.
Sensitivity for aversion to diet containing EE was identified
C57BL/6 mice are susceptible to estrogen-induced pyometra
CD1 mice do not develop pyometra when exposed to estrogens or Bisphenol A
Bisphenol A presumably acts as an estrogen to cause pyometra at a “low dose”
Bisphenol A exposure correlates with an increase in uterine macrophage infiltration
Supplementary Material
Acknowledgements
This work was supported by National Institute of Health grants R01 ES015145, RC2 ES018765, T32 ES016646, and the University of Cincinnati Center for Environmental Genetics (P30-ES06096).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burrows H. Leucocytic invasion as an accompaniment of epithelial metaplasia. The Journal of Pathology and Bacteriology. 1935;41:43–49. [Google Scholar]
- 2.Egenvall A, Hagman R, Bonnett BN, Hedhammar A, Olson P, Lagerstedt AS. Breed risk of pyometra in insured dogs in Sweden. J Vet Intern Med. 2001;15:530–538. doi: 10.1892/0891-6640(2001)015<0530:bropii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Smith FO. Canine pyometra. Theriogenology. 2006;66:610–612. doi: 10.1016/j.theriogenology.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Yildizhan B, Uyar E, et al. Spontaneous Perforation of Pyometra. Infectious Diseases in Obstetrics and Gynecology. 2006:26786. doi: 10.1155/IDOG/2006/26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keskin A, Yilmazbas G, Yilmaz R, Ozyigit MO, Gumen A. Pathological abnormalities after long-term administration of medroxyprogesterone acetate in a queen. J Feline Med Surg. 2009;11:518–521. doi: 10.1016/j.jfms.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould KA, Pandey J, Lachel CM, Murrin CR, Flood LA, Pennington KL, et al. Genetic mapping of Eutr1, a locus controlling E2-induced pyometritis in the Brown Norway rat, to RNO5. Mammalian Genome. 2005;16:854–864. doi: 10.1007/s00335-005-0070-7. [DOI] [PubMed] [Google Scholar]
- 7.Hagman R, Lagerstedt AS, Hedhammar A, Egenvall A. A breed-matched case-control study of potential risk-factors for canine pyometra. Theriogenology. 2011;75:1251–1257. doi: 10.1016/j.theriogenology.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Roper RJ, Griffith JS, Lyttle CR, Doerge RW, McNabb AW, Broadbent RE, et al. Interacting Quantitative Trait Loci Control Phenotypic Variation in Murine Estradiol-Regulated Responses. Endocrinology. 1999;140:556–561. doi: 10.1210/endo.140.2.6521. [DOI] [PubMed] [Google Scholar]
- 9.Gardner WU, Allen E. Some Effects of Estrogens on the Uterus of the Mouse. Endocrinology. 1937;21:727–730. [Google Scholar]
- 10.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 11.Stone JP, Holtzman S, Shellabarger CJ. Neoplastic Responses and Correlated Plasma Prolactin Levels in Diethylstilbestrol-treated ACI and Sprague-Dawley Rats. Cancer Res. 1979;39:773–778. [PubMed] [Google Scholar]
- 12.Zondek B. The effect of prolonged application of large doses of follicular hormone on the uterus of rabbits. J Exp Med. 1936;63:789–794. doi: 10.1084/jem.63.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carcinogenesis Bioassay of Bisphenol A (CAS No. 80-05-7) in F344 Rats and B6C3F1 Mice (Feed Study) Natl Toxicol Program Tech Rep Ser. 1982;215:1–116. [PubMed] [Google Scholar]
- 18.Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Hum Reprod. 2001;16:988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 19.Brossia LJ, Roberts CS, Lopez JT, Bigsby RM, Dynlacht JR. Interstrain differences in the development of pyometra after estrogen treatment of rats. J Am Assoc Lab Anim Sci. 2009;48:517–520. [PMC free article] [PubMed] [Google Scholar]
- 20.Greenman DL, Dooley K, Breeden CR. Strain differences in the response of the mouse to diethylstilbestrol. J Toxicol Environ Health. 1977;3:589–597. doi: 10.1080/15287397709529591. [DOI] [PubMed] [Google Scholar]
- 21.Long X, Steinmetz R, Ben-Jonathan N, Caperell-Grant A, Young PC, Nephew KP, et al. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect. 2000;108:243–247. doi: 10.1289/ehp.00108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morozova OV. Effects of estrogen on the uterus of mice of different strains. Biull Eksp Biol Med. 1991;112:631–633. [PubMed] [Google Scholar]
- 23.Pepling ME, Sundman EA, Patterson NL, Gephardt GW, Medico L, Jr, Wilson KI. Differences in oocyte development and estradiol sensitivity among mouse strains. Reproduction. 2010;139:349–357. doi: 10.1530/REP-09-0392. [DOI] [PubMed] [Google Scholar]
- 24.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein L, Gardner WU, Allen E. The Effect of Estrogenic Hormones on the Bacterial Content of the Uterus. The Yale journal of biology and medicine. 1943;16:43–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 27.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180:7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 28.Hendry WJ, 3rd, Zheng X, Leavitt WW, Branham WS, Sheehan DM. Endometrial hyperplasia and apoptosis following neonatal diethylstilbestrol exposure and subsequent estrogen stimulation in both host and transplanted hamster uteri. Cancer Res. 1997;57:1903–1908. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.