Abstract
Background
Oral immunotherapy (OIT) is a promising treatment for food allergy. Studies are needed to elucidate mechanisms of clinical protection, and to identify safer and potentially more efficacious methods for desensitizing patients to food allergens.
Objective
We established a mouse model of OIT in order to determine how dose or form of antigen may affect desensitization, and to identify mechanisms of desensitization.
Methods
Increasing doses of egg white or ovomucoid as OIT were administered orally to sensitized mice. Impact of OIT on anaphylaxis elicited by oral allergen challenge was determined. Allergen-specific antibody and cytokine responses, and mast cell and basophil activation in response to OIT was measured. Gene expression in the small intestine was studied by microarray and real-time PCR.
Results
OIT resulted in desensitization but not tolerance of mice to the allergen. OIT did not result in desensitization of systemic effector cells, and protection was localized to the gastrointestinal tract. OIT was associated with significant changes in gene expression in the jejunum, including genes expressed by intestinal epithelial cells. Extensively heated ovomucoid that does not trigger anaphylaxis when given orally to sensitized mice was as efficacious as native ovomucoid in desensitizing mice.
Conclusions
OIT results in clinical protection against food-induced anaphylaxis through a novel mechanism that is localized to the intestinal mucosa and is associated with significant changes in small intestinal gene expression. Extensively heating egg allergen decreases allergenicity and increases safety while still retaining the ability to induce effective desensitization.
Keywords: Oral immunotherapy, Food allergy, Heated egg allergen, Mucosal immunology
INTRODUCTION
Food allergies affect 3% of the overall population and up to 4–6% of children.1 From 1997 to 2007, the prevalence of reported food allergy increased 18% among children under 18 years of age.2 Currently, there is no cure for food allergy and management remains avoidance of the offending food.3 Interventions that would lessen the risk of anaphylaxis would have a major impact on the quality of life, morbidity, and mortality.
Subcutaneous immunotherapy has been used as treatment for other allergic diseases, such as allergic rhinitis, venom allergy and allergic asthma, but has not been successful in food allergy due to high rates of systemic reactions.4, 5 There is an increasing interest in oral immunotherapy (OIT) for the treatment of food allergy, and several clinical trials have shown promising results.6–10 Most subjects tolerate significantly more allergen by the end of the study compared to their baseline. Adherence suffers from the high prevalence of side effects and clinical reactivity returns after discontinuation of OIT in a majority of treated subjects, demonstrating desensitization but not tolerance.9,11
Extensive heating can render milk and egg allergens tolerable to the majority of milk- and egg-allergic patients. When tolerated, introducing extensively heated antigens into the diet of allergic individuals may accelerate resolution of clinical reactivity to foods. Inclusion of extensively heated egg or milk into the diet appears to be well-tolerated and the majority of subjects go on to develop tolerance to unheated egg or milk. 12–14
Immunologic changes have been associated with desensitization in OIT clinical trials, including decreased basophil reactivity, decreased skin prick test wheal size , increased serum antigen-specific IgG4, and salivary IgA, and increased peripheral CD4+CD25+FOXP3+ regulatory T cells.6, 8–10, 15 Similar immune changes have been observed after inclusion of extensively heated milk into the diet, supporting the hypothesis that this may be functioning as an immunotherapy facilitating development of immune tolerance.16, 17 However, it is not known to what extent these immunologic changes contribute to clinical protection, and this is difficult to test in human subjects.
OIT is a promising treatment and potential cure for food allergy, but issues of safety and long-term efficacy remain.18 Mechanisms of clinical protection are difficult to investigate in human subjects, and the gastrointestinal mucosa cannot be practically accessed for study. To address these needs, we established a murine model of OIT and herein use this model to study mechanisms of clinical protection by OIT and the use of heat-modified allergens.
METHODS
Mice
Female C3H/HeJ mice were purchased from NCI (Frederick, MD). Mice were maintained in filter-top cages under specific pathogen-free conditions. The Institutional Animal Care and Use Committee at Mount Sinai School of Medicine approved all procedures.
Sensitization of mice
Mice were sensitized by intragastric administration of 1 mg ovalbumin (OVA, grade V, Sigma Chemicals, St. Louis, MO) or ovomucoid (OM, Sigma) plus 10 μg cholera toxin (List Biologicals, Campbell, CA, USA)as adjuvant weekly for six weeks.
Administration of oral immunotherapy (OIT)
Pilot experiments were done comparing the administration of antigen as OIT by daily gavage or through the drinking water, with the two methods being equally effective. Egg white was obtained from organic eggs and protein content measured by Bradford assay. Egg white was used as OIT primarily due to cost, but for sensitization and challenge purified allergens provide more consistent responses. An increasing dose of egg protein was administered daily for 14 days: 1 mg (days 1,2), 5 mg (days 3,4), 10 mg (days 5–7), 25 mg (days 8,9), 50 mg (days 10–14). The dose was calculated based on a drinking volume of 5 ml/day. For experiments using extensively heated and native OM as OIT, mice received OM by daily gavage at half the dose of that used for whole egg white protein, (increasing doses from 0.5 mg to 25 mg). This dose of OM was equivalent to the dose of OVA provided in whole egg white. OM was heated as a solution in a boiling water bath for 30 min.19
Allergen challenge
Mice were orally challenged with 50 mg OVA or 25 mg OM one day or two weeks after discontinuation of OIT. Mice were intraperitoneally injected with 1–100 μg of OVA in 0.1 ml PBS. Anaphylaxis severity was graded by symptom score 20 and body temperature measured (by rectal thermometer, World Precision Instruments, Sarasota, FL) 30 minutes after challenge. The symptom score was: 0 = no symptoms; 1 = scratching around the nose and head; 2 = puffiness around eyes and mouth; 3 = wheezing, labored respiration; 4 = no activity after prodding.
Assessment of antigen-specific immunoglobulins
Blood samples were obtained before allergen challenge. OVA- and OM-specific IgE was measured by capture ELISA using DIG-labeled OVA or OM as detection.21 OVA-specific IgA, IgG1, and IgG2a were measured by ELISA using biotinylated monoclonal detection antibodies (all from BD Biosciences, San Diego, CA). Secreted intestinal antibodies were measured in lavage obtained by flushing the entire excised small intestine with 4 ml of PBS containing complete protease inhibitor (Roche, Indianapolis, IN).
Antigen-specific cytokine production
Spleens or mesenteric lymph nodes were removed after challenge and cells isolated and plated with OVA or OM (100 μg/ml) for 72 h. Cytokine production was measured by ELISA (eBioscience, San Diego, CA).
Mast cell and basophil activation assays
Blood was collected in heparinized tubes, diluted 1:1 with RPMI, and incubated at 37 ºC for 60 min with 10 – 100 μg/ml of OVA or media alone. As additional controls, cells were incubated in U-bottom 96-well plates that had been coated overnight with 2 μg of anti-IgE (R35–72, BD Biosciences) or rat IgG1 (eBioscience) as isotype control. Red blood cells were lysed, and cells were fixed and stained for CD49b and IgE to detect basophils, and CD200R as an activation marker. 22 CD3 and CD19 were used to gate out B and T cells. Peritoneal lavage was collected and cells were stimulated as above. Cells were stained for c-kit and IgE to detect mast cells, and CD107a (LAMP-1) as an activation marker.23 All antibodies were from eBioscience (San Diego, CA).
Assessment of intestinal permeability
One day after discontinuing OIT, mice were euthanized, and 10 cm of jejunum were excised starting from the ligament of Treitz. Tissue was mounted in Ussing Chambers (Physiologic Instruments, San Diego, CA). Resistance and mucosal-to-serosal flux of FITC-dextran (3–4 kD, Sigma) measurements were obtained as described previously.24
Intestinal gene expression
Intestine was harvested 24 h after discontinuation of OIT. Two pieces of jejunum obtained 0 and 10 cm from the Ligament of Treitz were obtained. Total RNA was isolated with Trizol (Invitrogen) followed by RNA clean-up with RNeasy mini-kit (Qiagen, Valencia, CA). Microarray analysis was performed by Miltenyi (Cologne, Germany) using Agilent whole mouse genome oligo microarrays (8 × 60K). Paired analysis on 5 control and 5 OIT-treated tissues was performed using Cy3-labeled and Cy5-labeled cRNA. Gene expression was verified by real-time PCR using SYBR green master mix and primers (all from Invitrogen). Epithelial cells from 10–15 cm of mid-small intestine (predominantly jejunum) were isolated by EDTA treatment and RNA was isolated with an RNeasy mini-kit.
Statistical analysis
Student’s t-test or Mann-Whitney Rank Sum test was used for determining statistical significance (p < 0.05) between continuous variables, and a paired t-test or Wilcoxon Signed Rank test was used when comparing different time points.
RESULTS
OIT reduces anaphylaxis by desensitization in a murine model of food allergy
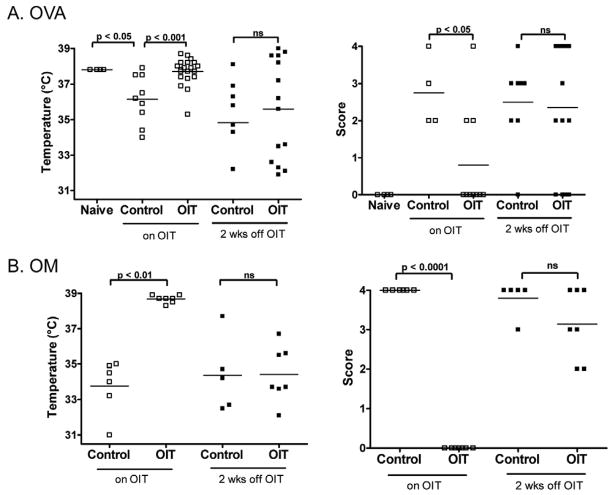
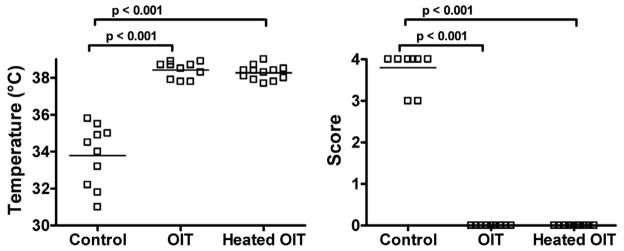
To determine the effect of OIT in a murine model of food–induced anaphylaxis, C3H/HeJ mice were orally sensitized to OVA or OM and administered OIT as described in Methods. Mice were then orally challenged with OVA or OM. OIT-treated mice were significantly protected from OVA- or OM-induced anaphylaxis as compared to controls (p<0.001 for OVA, p<0.01 for OM; based on body temperature and symptom scores). (Figure 1) After discontinuing OIT for two weeks, mice were re-challenged to assess tolerance. OIT-treated mice were no longer protected from anaphylaxis, indicating that OIT induced clinical protection through desensitization, not immune tolerance.
Figure 1. Oral immunotherapy (OIT) results in desensitization but not tolerance to OVA or OM.
OVA- and OM-sensitized mice were administered OIT, or left untreated (Control). Mice were orally challenged (on OIT), and again two weeks after discontinuation of OIT (off OIT). 30 minutes after challenge, body temperature was measured (left) and a clinical score was assigned (right).
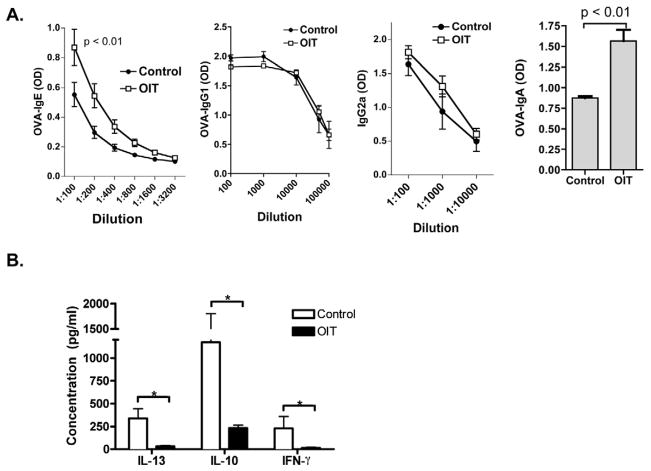
OIT induces an increase in OVA-specific serum IgE and IgA
OVA-specific serum immunoglobulins were measured after OIT treatment prior to challenge. A significant increase in OVA-specific IgE and IgA was observed in OIT-treated mice as compared to controls. (Figure 2a) Serum OVA-specific IgG1 and IgG2a, which have been shown to inhibit IgE-triggered mast cell activation 25, were not significantly different between OIT-treated mice and controls. Measurements of OM-specific serum IgE, IgA, IgG1, and IgG2a in OM OIT-treated mice were similar (data not shown). OVA-specific IgA in gut lavage could be readily detected in OVA-sensitized mice, but was not significantly increased by the administration of OIT (data not shown). It is possible that the high concentration of antigen in the gut during OIT interferes with our ability to detect OVA-specific IgA within the gut lumen, and therefore we do not rule out a role for mucosal IgA in protection against oral OVA- or OM-induced anaphylaxis.
Figure 2. Immunologic changes associated with OIT in mice.
Mice were orally sensitized to OVA, followed by administration of egg white OIT. (A) OVA-specific IgE, IgG1, IgG2a, and IgA measured in serum obtained on the last day of OIT. (B) Cytokine secretion from OVA-restimulated spleen cells obtained two weeks after OIT discontinuation. * p < 0.05
OIT is associated with a broad suppression of cytokines
The impact of OIT on antigen-specific T cell responses was assessed by re-stimulating splenocytes with OVA in vitro and measuring cytokine secretion. Antigen-specific IL-13, IL-10, and IFN-γ responses were all significantly reduced in OIT-treated mice as compared to controls, indicating broad suppression rather than skewing of T cell responses. IL-4 was near or below the level of detection. Suppression of IL-13 and IFN-γ was also observed in the mesenteric lymph node of mice receiving OIT (data not shown). When OIT was discontinued for two weeks, a sustained suppression of cytokines was observed in OIT-treated mice as compared to controls despite the return of clinical responsiveness. (Figure 2b)
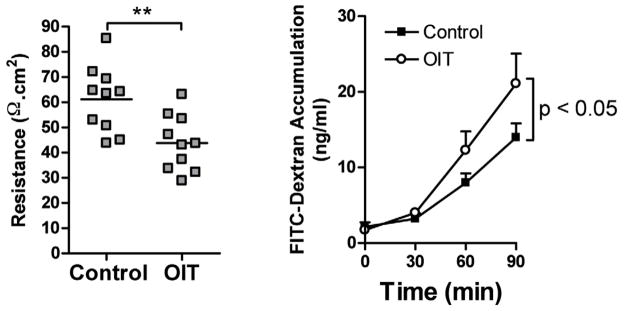
OIT results in decreased intestinal barrier function
Gastrointestinal side effects have been identified as a major early obstacle to OIT compliance.26 In order to determine the impact of OIT on intestinal physiology, segments of jejunum were collected from OIT-treated mice as well as controls and mounted in Ussing chambers. Epithelial barrier function was measured by electrical resistance as well as luminal-to-serosal flux of FITC-dextran. (Figure 3) OIT resulted in a significant decrease in resistance, and a significant increase in luminal to serosal flux of FITC-dextran, indicating a decrease in epithelial barrier function.
Figure 3. OIT decreases gastrointestinal epithelial barrier function.
Mice were orally sensitized to OVA, followed by administration of egg white OIT on days 0–14. On day 15, segments of jejunum were removed and mounted in Ussing chambers. Transmural resistance was measured at baseline (left), and luminal to serosal transport of FITC-dextran was measured over a 90-minute period (right). **p<0.01.
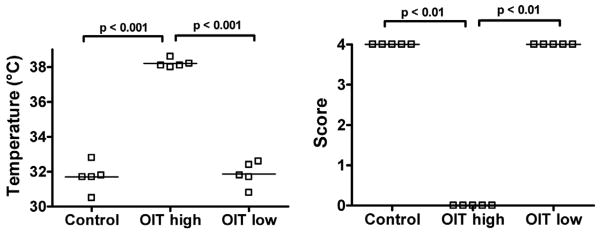
High-dose OIT is required for clinical protection
Low doses of antigen preferentially elicit regulatory T cells that can prevent systemic immune responses in classic oral tolerance studies.27 To determine if low-dose OIT could be effective, OVA-sensitized mice were administered either a daily low dose of 1 mg (the dose typically used in low-dose tolerance studies28) or escalating daily doses of up to 50 mg (high dose, or the standard OIT used in this model) for two weeks and then orally challenged with 50 mg of OVA. Upon oral challenge, high-dose OIT-treated mice were protected against anaphylaxis (p<0.001), while low-dose OIT-treated mice were not. (Figure 4)
Figure 4. High dose OIT is required for clinical protection against OVA-induced anaphylaxis in sensitized mice.
OVA-sensitized mice were administered high-dose or low-dose fresh egg white OIT on days 0–14, or left untreated (Control). Mice were orally challenged on day 15. 30 minutes after challenge, body temperature was measured (left) and a clinical score was assigned (right).
Extensively heated antigen effectively desensitizes mice
We have previously shown that heating of OVA or OM abolishes their capacity to trigger anaphylaxis when given orally, but not systemically, to sensitized mice.19 We investigated whether this non-reactogenic form of antigen could be effective when administered as OIT. Mice administered either heated or native OM as OIT were completely protected against anaphylaxis as compared to controls. (Figure 5) These data together with our previous work on heated egg allergens demonstrate that allergens can be modified to reduce risks of systemic anaphylaxis, yet still maintain their full desensitizing capacity.
Figure 5. Extensively-heated OM protects mice against anaphylaxis.
OM-sensitized mice were administered native or heated-OM OIT on days 0–14, or left untreated (Control). Mice were orally challenged with native OM on day 15. 30 minutes after challenge, body temperature was measured (left) and a clinical score was assigned (right).
OIT shifts the threshold of allergen reactivity without desensitizing systemic effector cells
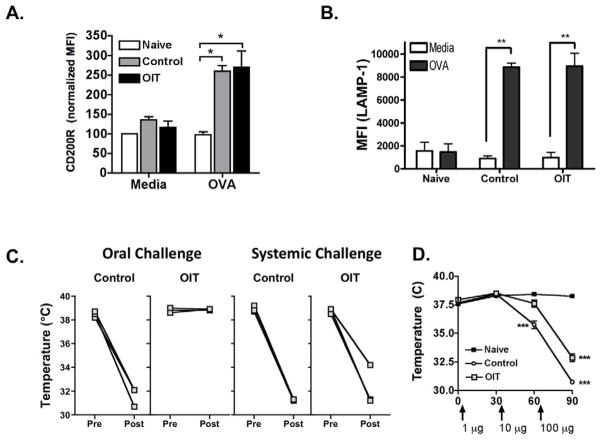
To test if OIT leads to desensitization of systemic effector cells, we performed activation assays with peripheral blood basophils and peritoneal mast cells. Blood from OIT-treated or control mice was incubated with OVA in vitro, followed by assessment of basophil activation by flow cytometry. Basophils were detected as CD49b+ IgE+ cells, and activation measured by up-regulation of CD200R. Activation of blood cells with plate-bound anti-IgE induces upregulation of CD200R on basophils, while plate-bound isotype control does not (Supplemental Figure). Basophils from untreated OVA-sensitized mice upregulated CD200R in response to OVA stimulation, and this was not suppressed in OIT-treated OVA-sensitized mice. (Figure 6a) Activation of peritoneal mast cells (c-kit+ FcεRI+) in response to OVA stimulation in vitro was measured by upregulation of the marker LAMP-1 (CD107a). Activation of peritoneal cells with plate-bound anti-IgE induces upregulation of LAMP-1 on mast cells, while isotype control does not (Supplemental Figure). LAMP-1 was upregulated in response to OVA stimulation in untreated sensitized mice, and to a similar extent in OIT-treated sensitized mice. (Figure 6b, and Supplemental Figure)
Figure 6. Clinical protection is localized to the gastrointestinal tract.
OVA-sensitized mice received egg white OIT on days 0–14. On day 15: (A) Peripheral blood basophil activation. *p<0.05 versus naïve. (B) Peritoneal mast cell activation. n=5 /group. **p<0.01 versus unstimulated. (C) Body temperature before and after challenge. (D) Body temperature after graded ip dose challenge to OVA.
Because of this lack of evidence for systemic effector cell desensitization, we hypothesized that clinical protection against food-induced anaphylaxis may be occurring locally within the intestinal mucosa. To test this, OVA-sensitized mice were challenged by either the oral route or systemically by low-dose intraperitoneal injection (using 0.2 % of the dose given orally). OIT-treated mice were protected against anaphylaxis induced by oral challenge, but were not substantially protected against anaphylaxis induced by systemic challenge. (Figure 6c)
To determine if OIT changes the threshold of reactivity to systemically administered allergen, we performed a graded allergen challenge given by the intraperitoneal route (1, 10, and 100 μg OVA given 30 min apart) (Figure 6D). Control mice responded to 10 μg of OVA with a significant drop in body temperature. At this dose, OIT-treated mice had cutaneous symptoms (symptom score of 2), but no significant drop in body temperature. At 100 μg of OVA, OIT-treated and control mice had significant drops in body temperature, but there was a statistically significant difference in the magnitude of the body temperature drop. These data indicate that there was a shift in the threshold of antigen required to induce symptoms in OIT-treated mice, although the level of protection was not as great as that in response to oral allergen challenge.
OIT induces significant changes in intestinal gene expression
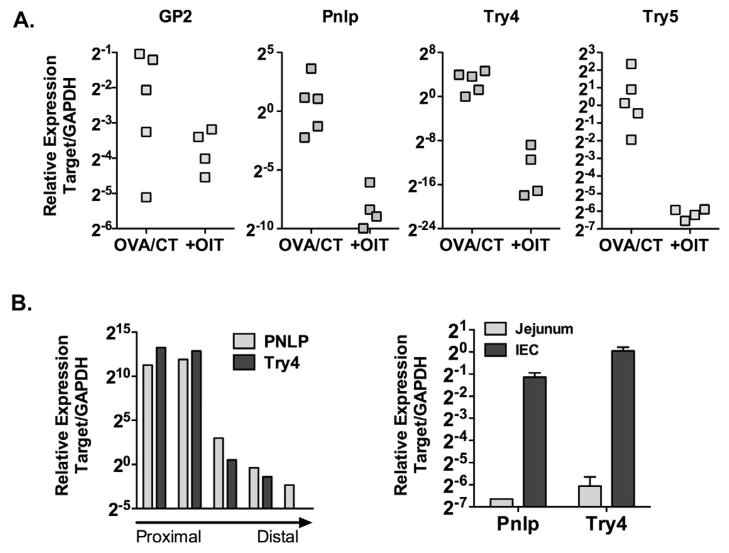
To begin to understand the impact of OIT locally within the gastrointestinal tract, we performed whole-genome microarray analysis on jejunum from sensitized control mice compared to those who had undergone OIT. Genes regulated at least 2-fold in at least 4 of 5 control:OIT comparisons were identified. 23 genes were found to be significantly down-regulated (Table 1). These could be broadly grouped into two categories: digestive enzymes such as trypsin, carboxypeptidase, lipase, and amylase; and antimicrobial peptides such as alpha-defensins. Down-regulation of representative genes was confirmed by real-time PCR (Figure 7). Several of these genes, such as alpha-defensins and trypsins, are expressed primarily by Paneth cells. To confirm small intestinal expression of these target genes, expression of two representative genes, pancreatic lipase (pnlp) and trypsin 4 (try4) was examined in segments from proximal to distal small intestine, carefully cleaned of mesentery. Gene expression was maximal in the duodenum, and decreased from jejunum to ileum. Small intestinal crypt epithelium was then isolated from 10–15 cm of mid-small intestine (predominantly jejunum), and expression of pnlp and try4 compared between full-thickness intestine and isolated epithelium. Expression was enriched > 60-fold for pnlp, and > 90-fold for try4 in isolated epithelial cells as compared to full-thickness intestine from the same region. These data show that administration of OIT leads to the regulation of a subset of genes in the intestine, including those expressed by intestinal epithelial cells, that have not previously been associated with food allergy.
Table 1.
List of genes regulated by OIT in 4 of 5 comparisons
| Gene | Fold Change | SEM |
|---|---|---|
| Trypsin 5 | −173.1 | 14.5 |
| Carboxypeptidase B2 | −143.9 | 66.2 |
| Amylase | −124.8 | 53.9 |
| Trypsin 4 | −114.5 | 15.8 |
| Pancreatic Lipase | −111.1 | 11.4 |
| Amylase 2a5 | −50.9 | 15.3 |
| Amylase 1 | −45.8 | 14.2 |
| Trypsin 1 | −38.8 | 7.8 |
| Elastase 2A | −31.1 | 9.5 |
| Elastase 3B | −29.9 | 5.4 |
| Carboxypeptidase A2 | −27.1 | 7.9 |
| Chymotrypsin C | −21.5 | 8.0 |
| Carboxypeptidase A1 | −19.8 | 1.2 |
| Syncollin | −18.3 | 8.6 |
| Phospholipase A2 | −12.7 | 3.4 |
| Protein disulfide isomerase a2 | −12.3 | 3.8 |
| Carboxyl Ester Lipase | −11.1 | 4.2 |
| GP2 | −10.1 | 2.6 |
| Chymotrypsinogen B1 | −8.2 | 1.1 |
| α-defensin, RS2 | −7.8 | 2.0 |
| α-defensin, RS12 | −5.5 | 1.5 |
| α-defensin, RS4 | −5.2 | 1.1 |
| VH mRNA | −3.0 | 0.6 |
Figure 7. OIT significantly alters intestinal gene expression.
(A) Jejunal gene expression of pancreatic lipase (pnlp), trypsin 4 (try4), trypsin 5 (try5) and GP2 was measured by PCR. (B) Expression of pnlp and try4 in proximal and mid-duodenum, jejunum, and proximal and distal ileum. Expression of pnlp and try4 in isolated intestinal epithelial cells (IEC) compared to full-thickness intestine (right).
DISCUSSION
OIT is a promising therapy for food allergy with mechanisms of clinical protection that are not well understood. We have developed a murine model of OIT for the treatment of food-induced anaphylaxis that induces clinical protection and is dependent on continued exposure to allergen. This response in sensitized mice closely resembles the typical human response to OIT. Using this model, we have generated two main novel findings. The first is that a major component of the mechanism of clinical protection is localized to the gastrointestinal tract and is associated with significant changes in intestinal gene expression. The second is that extensively heated antigen that is unable to elicit anaphylaxis can effectively desensitize mice.
Immunologic studies from human OIT trials have shown variable effects on antigen-specific IgE and consistent increases in antigen-specific IgG4. Our data indicates that clinical reactivity to food allergens can be abrogated without reducing allergen-specific IgE or increasing allergen-specific IgG. IgA is one potential mechanism of protection. We observed a significant increase in serum allergen-specific IgA associated with OIT, but could not confirm an increase in secreted allergen-specific IgA. Strait et al recently reported that IgA could suppress anaphylaxis by neutralizing absorbed antigen in the circulation rather than by preventing uptake of antigen. 29 We did observe a shift in the threshold of reactivity to allergen given systemically, suggesting that a component of clinical protection may be provided by neutralization by allergen-specific IgA. Kulis et al have recently found that changes in salivary IgA are correlated with the amount of tolerated peanut protein after sublingual immunotherapy to peanut in humans 15. Further studies are needed to determine if IgA is a marker or a mechanism of clinical protection.
Hypo-responsiveness of allergic effector cells, as measured by skin-prick test or in vitro basophil activation, has been variably reported in human OIT trials. It is theorized that mast cells and basophils are degranulated gradually by immunotherapy until they become unresponsive or are in a refractory state. 30 Skin-prick tests have been observed to be unchanged compared to placebo 8 or decreased 9, 10, and in vitro basophil activation tests have been shown to be unchanged 31 or decreased 32. Our current results showed that administration of OIT did not result in any changes in responsiveness of peripheral blood basophils or peritoneal mast cells to activation. Basophil activation assays were performed in whole blood and would therefore reflect the antibody milieu. Mast cells have been shown to be the primary cells responsible for systemic anaphylaxis in mice after sensitization with cholera toxin adjuvant33, 34, with basophils playing a minor role 34. Our results are consistent with those reported by Skripak et al, where significant clinical protection was observed despite a lack of significant effect of OIT on tissue mast cells compared to placebo.8 We observed that clinical protection in response to OIT was maximal in response to allergen challenge of the gastrointestinal tract. This may be in part due to neutralization by IgA, although we could not confirm an increase in secreted allergen-specific IgA. 34 Microarray studies showed that OIT was associated with a significant downregulation of gene expression in the proximal jejunum. Genes could be divided into two main categories: digestive enzymes such as proteases, lipases, and amylases; and anti-microbial peptides (α-defensin-related peptides). Expression was confirmed in the duodenum and proximal jejunum and at least for the two representative genes tested further was found to be expressed primarily by the epithelium. A number of digestive enzymes, including trypsin, are expressed by intestinal Paneth cells that are an important source of anti-microbial peptides including α-defensins. The function of these enzymes in the Paneth cell remains unknown, although some enzymes are involved in proteolytic processing of anti-microbial peptides.35 We did not address pancreatic expression of these enzymes, but the lack of chronic adverse reactions to OIT suggests that there was no global malabsorption due to downregulation of digestive enzymes. The link between these changes in gene expression and clinical protection needs to be determined, as does the applicability of these findings to the human response to OIT. These findings point to intriguing gastrointestinal mechanisms that may underlie clinical responsiveness to food allergens.
Persistent side effects are a roadblock to the adoption of OIT as a widespread therapy for food allergy. These include gastrointestinal side-effects, and consistent with this we have observed that OIT is associated with changes in epithelial barrier function. This is likely due to local effector cell activation that is known to modulate the epithelial barrier.36 Clinical studies have shown that a majority of egg- and milk-allergic individuals can tolerate baked egg or milk and it is anticipated that incorporating heated forms of allergen into their diet will accelerate resolution of their allergy.12–14 Furthermore, assessment of intestinal permeability in these subjects has shown no negative effects of incorporation of extensively heated egg or milk into the diet on intestinal barrier function.12, 13 We have previously shown that heated egg or milk allergens cannot elicit anaphylaxis by the oral route in sensitized mice due to changes in uptake of intact antigen across the intestinal mucosa.19,37 In this study, we found that heated egg allergen can nevertheless be used as OIT, and is as effective as native antigen in suppressing anaphylaxis in mice.
In summary, we have shown using a mouse model of OIT for experimental food allergy that desensitization is associated with a boosting of antigen-specific serum IgA as well as significant changes in gastrointestinal gene expression. These findings need to be verified in human subjects as the mouse model does not replicate all features of human food allergy, such as dependence on an experimental adjuvant to induce sensitization and the requirement for relatively high oral challenge doses to induce objective symptoms. Our results with heated egg allergens can be translated into an alternative form of OIT that may be safer, lead to fewer side effects and increase adherence to therapy in clinical trials. Modifications of allergens other than egg or milk to alter their handling in the intestinal mucosa may also be an effective approach to generating safer allergens for use in OIT.
Key Messages.
The mechanism of clinical protection induced by OIT in mice is localized to the gastrointestinal tract and is associated with significant changes in intestinal gene expression.
Extensively heated antigen that is unable to elicit anaphylaxis can effectively desensitize mice when used as OIT.
Acknowledgments
Funding: NIAID U19AI044236 (Project 2, to MCB); SL was supported by AAAAI/Elliot and Roslyn Jaffe Third Year Fellowship Food Allergy Research Award; GM was supported by the Spanish Research Council through the JAE program and by the Ministry of Science and Innovation through project AGL2008-01740
Abbreviations
- OIT
oral immunotherapy
- OVA
ovalbumin
- OM
ovomucoid
- CT
cholera toxin
- ip
intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008:1–8. [PubMed] [Google Scholar]
- 3.Boyce JA, Assa'a A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011;27:253–67. doi: 10.1016/j.nut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–9. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 8.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. 2008;122:1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005;60:1320–2. doi: 10.1111/j.1398-9995.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–83. e1. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008;122:342–7. 7 e1–2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. 2011;128:125–31. e2. doi: 10.1016/j.jaci.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol. 2009;123:789–94. e20. doi: 10.1016/j.jaci.2008.12.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, et al. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–2. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A. Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol. 2011;127:990–7. e2. doi: 10.1016/j.jaci.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 21.Blazquez AB, Mayer L, Berin MC. Thymic Stromal Lymphopoietin Is Required for Gastrointestinal Allergy but Not Oral Tolerance. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–9. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grutzkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–8. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- 24.Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1234–43. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 25.Uermosi C, Beerli RR, Bauer M, Manolova V, Dietmeier K, Buser RB, et al. Mechanisms of allergen-specific desensitization. J Allergy Clin Immunol. 2010;126:375–83. doi: 10.1016/j.jaci.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Vickery BP, Scurlock AM, Steele P, Kamilaris J, Hiegel AM, Carlisle SK, et al. Early and Persistent Gastrointestinal Side Effects Predict Withdrawal from Peanut Oral Immunotherapy (OIT) Journal of Allergy and Clinical Immunology. 2011;127:Ab26-Ab. [Google Scholar]
- 27.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CA, Jr, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci U S A. 1996;93:388–91. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, Finkelman FD. Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol. 2011;127:982–9. e1. doi: 10.1016/j.jaci.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castells M. Rapid desensitization for hypersensitivity reactions to medications. Immunol Allergy Clin North Am. 2009;29:585–606. doi: 10.1016/j.iac.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Frischmeyer-Guerrerio PA, Chichester K, Bieneman A, Keet C, Wood RA, Schroeder JT. Basophil Responses in Children Undergoing Immunotherapy for Milk Allergy. Journal of Allergy and Clinical Immunology. 2011;127:AB26-AB. [Google Scholar]
- 32.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 34.Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011;127:1552–61. e1. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–90. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 36.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–6. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63:882–90. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]